Abstract
Objective
Evidence-based practices are not consistently applied in the intensive care unit (ICU). We sought to determine if nurse-led remote screening and prompting for evidence-based practices using an electronic health record could impact ICU care delivery and outcomes in an academic medical center.
Design
Single-center, before-after evaluation of a quality improvement project.
Setting
Urban, academic medical center in the mid-Atlantic United States with 8 subspecialty ICUs and 156 ICU beds.
Patients
Adult patients admitted to the ICU between January 1, 2011 and August 31, 2012.
Intervention
Beginning on July 25, 2011, trained ICU nurses screened all ICU patients for selected evidence-based practices on a daily basis. The screening was conducted from a remote office, facilitated by the electronic health record. Selected practices included compliance with a ventilator care bundle, assessment of appropriateness of indwelling venous and urinary catheters, and concordance between sedation orders and documented level of sedation. When gaps were observed they were communicated to the point-of-care bedside nurse via telephone, page or facsimile.
Measurements and Main Results
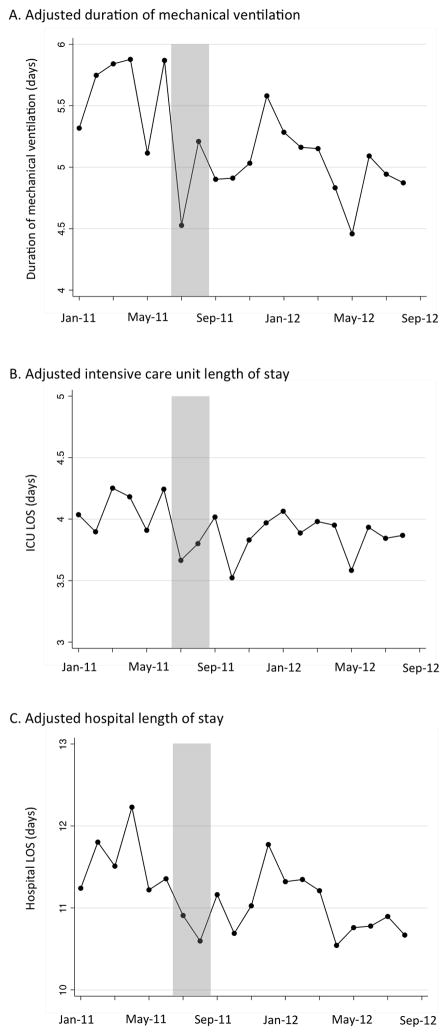
14,823 unique patients were admitted during the study period. We excluded 1,546 patients during a 2-month run-in period from July 1, 2011 to August 31, 2011, resulting in 4,339 patients in the 6-month pre-intervention period and 8,938 patients in the 12-month post-intervention period. Compared to patients admitted in the pre-intervention period, patients admitted in the post-intervention period were more likely to receive sedation interruption (incidence rate ratio: 1.57, 95% CI: 1.45 – 1.71) and a spontaneous breathing trial (incidence rate ratio: 1.24, 95% CI: 1.20 – 1.29). Hospital acquired infection rates were not different between the two periods. Adjusting for patient characteristics and illness severity, patients in the post-intervention period experienced shorter duration of mechanical ventilation (adjusted reduction: 0.61 days, 95% CI: 0.27 – 0.96, p <0.001), shorter ICU length of stay (adjusted reduction: 0.22 days, 95% CI: 0.04 – 0.41, p=0.02), and shorter hospital length of stay (adjusted reduction: 0.55 days, 95% CI: 0.15 – 0.93, p=0.006). In-hospital mortality was unchanged (adjusted odds ratio: 0.96, 95% CI 0.84 – 1.09, p=0.54). The results were robust to tests for concurrent temporal trends and coincident interventions.
Conclusions
A program by which nurses screened ICU patients for best-practices from a remote location was associated with improvements in the quality of care and reductions in duration of mechanical ventilation and length of stay, but had no impact on mortality.
Keywords: mechanical ventilation, intensive care units, quality improvement, organization and administration, nursing, telemedicine
INTRODUCTION
Quality improvement efforts in the intensive care unit (ICU) increasingly rely on innovations intended to simplify and standardize clinical practice, including care protocols, decision aids and checklists (1). These strategies can improve outcomes by prompting clinicians to regularly address key elements of critical care, facilitating evidence-based practice and preventing errors of omission (2). Early studies of these strategies appear promising (3–5). However, widespread adoption is hindered by the lack of available expertise at the bedside. ICU care protocols are often complicated and time consuming, and dedicated personnel to facilitate protocol and checklist adherence are typically unavailable (6).
One way to address these problems is to use technology to increase protocol and checklist adherence through remote screening and prompting of bedside clinicians (7). With the advent of the electronic medical record (EMR), clinicians need not be physically present in the ICU to screen patients for receipt of evidence-based practice and facilitate protocol utilization. Indeed, this idea is an underlying tenet of ICU telemedicine, which often makes use of remote screening for best-practices in addition to its more typical role of remote monitoring (8). To better understand the potential value of remote screening for evidence-based practice without remote monitoring, we studied the effect of a quality improvement initiative at an academic medical center based solely on remote screening. We hypothesized that remote screening of ICU patients enabled by an EMR would improve care processes and outcomes of critically ill patients.
METHODS
Study design and setting
We performed a retrospective, before-after, cohort study of an ICU quality improvement intervention at the University of Pittsburgh Medical Center (UPMC) Presbyterian University Hospital, an 866-bed, university-affiliated academic medical center located in Pittsburgh, Pennsylvania. UPMC Presbyterian contains 8 subspecialty ICUs: an 32-bed medical ICU, a 20-bed neurovascular ICU, a 10-bed neuro-trauma ICU, a 22-bed surgical trauma ICU, a 14-bed surgical ICU, a 28-bed abdominal transplant ICU, a 20-bed cardio-thoracic ICU, and a 10-bed coronary care unit. Each ICU is independently managed by a unique physician ICU director with oversight from a hospital-wide ICU clinical practice committee. All patients except those in the CCU are cared for by trained intensivist physicians and nurses under either a closed or a mandatory co-attending model with daily multidisciplinary rounds. At night there are two in-house intensivist physicians available for emergencies.
Intervention
In an effort to standardize ICU care practice and improve utilization of evidence-based care, on July 25, 2012 UPMC Presbyterian instituted an “ICU Command Center”. The primary goal of the command center was to use the hospital’s EMR (Cerner PowerChart, Cerner Corporation, Kansas City, MO) to screen all ICU patients for selected evidence-based practices (Table 1). These practices were chosen because they are identifiable in the EMR either through pharmacy records or nurse charting and are thought to be associated with improved clinical outcomes in ICU patients (9–11).
Table 1.
Care processes targeted by the remote screening team
Ventilator bundle adherence
|
| Indwelling central catheter necessity |
| Indwelling urinary catheter necessity |
| Continuity between sedation orders and sedation level |
| Appropriate charting of level and necessity of care |
Although this screening could in theory be done in-person at the bedside, UPMC decided to centralize the screening process in a single location, within the hospital but remote from the ICUs. Given that these care practices can be affected by a large number of providers, and that they may be implemented at varying times throughout a day, remote screening using an EMR was thought to both standardize the process and maximize its efficiency.
Screening was performed by registered nurses with at least three years direct beside experience in the ICU. From 7 AM to 7 PM the Command Center was staffed by one nurse and one supporting staff member. From 7 PM to 7 AM the Command Center was staffed by two to three nurses. Screening was performed on a rolling basis once per day, although screening could be repeated when time allowed or when omissions in practice were noted. When the screening nurses noted that patients had not received a certain evidence-based practice, the bedside nurse was notified by either a phone call, a page or a facsimile. We targeted the bedside nurse for these prompts because they were an easily identifiable, single point of contact for the screening team. In addition to screening patients daily, the Command Center nurses were available to answer questions and provide clinical assistance to bedside nurses in emergencies or in cases of staffing strain. For these instances, the Command Center nurses also had access to web-based continuous electrocardiogram tracking software, although this software was not routinely used to monitor patients for physiological deterioration. At night, the Command Center staff also assisted with rapid response team calls on the hospital wards.
Patients and variables
To evaluate the clinical impact of the Command Center we studied all adult patients admitted to UPMC Presbyterian ICUs from January 1, 2011 to August 31, 2012. This time period corresponded to a six-month “pre-intervention” period (1/1/2011 to 6/30/2011), a two-month “run-in” period (7/1/2011 to 8/31/2011) and a one-year “post-intervention” period (9/1/2011 to 8/31/2012). A two-month run-in period was selected not only to allow time for the program to reach full effectiveness but also to ensure exclusion of patients receiving care under both models. To avoid interdependence of observations we used only the first ICU admission for each patient during the study period. We excluded patients admitted during the run-in period and patients with missing values for key covariates.
All data elements were obtained from the UPMC electronic medical record. The primary exposure variable was the time period of ICU admission (pre-intervention versus post-intervention). Outcome variables of interest included the provision of selected evidence-based practices for mechanically ventilated patients (daily spontaneous breathing trials and daily interruption of continuous sedation), ICU-acquired infections (catheter associated blood stream infections, catheter associated urinary tract infections, and ventilator-associated pneumonia), duration of mechanical ventilation, ICU length of stay, hospital length of stay, and in-hospital mortality. Although we were interested in other evidence-based practices besides spontaneous breathing trials and sedation interruption, these were the only ones that were both available in the EMR and measured consistently throughout the study period.
Daily spontaneous breathing trials were charted by the bedside respiratory therapists and defined as at least 30 minutes of spontaneous breathing in patients receiving full ventilator support for at least 24 hours, excluding patients ineligible due to hemodynamic instability, intracranial hypotension, positive end-expiratory pressure >8, active myocardial ischemia, or the use of neuromuscular blockade. Daily interruption of continuous sedation was defined as interruption of either propofol, midazolam, lorezapam or dexmedetomidine, excluding patients ineligible due to hemodynamic instability, intracranial hypotension, positive end-expiratory pressure >10, fraction of inspired oxygen >0.7, active myocardial ischemia, high risk of wound dehiscence after recent surgery, the presence of an esophageal balloon or the use of neuromuscular blockade.
ICU acquired infectious were identified by hospital infection control personnel and defined according to the Centers for Disease Control National Healthcare Safety Network (NHSN) standards (12). Duration of mechanical ventilation was obtained using daily mechanical ventilation resource utilization codes as previously performed (13). ICU length of stay and hospital length of stay were obtained from the hospital patient-tracking system. In-hospital mortality was obtained from the hospital discharge location in the administrative record.
To control for differences in case-mix and severity of illness we developed a de novo severity of illness score using selected elements from the electronic health record. We based our approach on the electronic Simplified Acute Physiology Score (eSAPS), a validated-ICU risk-adjustment measure that maps electronic health data to the clinical data comprising the SAPS III score (14). For our study we used age, comorbidities, length of hospital stay prior to ICU admission, location prior to ICU admission, Glasgow coma score, temperature, Creatinine, heat rate, white blood cell count, arterial pH, platelet count, systolic blood pressure, and oxygenation as previously described (15). A logistic regression model for inhospital mortality using these variables resulted in a C-statistic of 0.81, indicating discrimination on par with other commonly used ICU risk-adjustment tools (16).
Analysis
Bivariate analyses comparing patient demographics, clinical characteristics and outcomes between the pre-intervention and post-intervention were performed using unpaired t-tests or chi-square tests, as appropriate. To compare evidence-based practice utilization and ICU-acquired infections across time periods we used bivariate Poisson regression. The exponentiated coefficients of these models are interpreted as incidence rate ratios (IRRs). These analyses did not adjust for differences in case-mix between time periods, since it is unclear the degree to which the care processes and complications are influenced by case-mix and the goal is either 100% adherence (for care processes) or 0% incidence (for infections). For catheter-related urinary tract infections, we excluded admissions after 2/28/12, since a change in the NEHN definition of this complication in March, 2012 made consistent comparisons of infection rates impossible.
To compare outcomes across time periods we performed a series of multivariate analyses using either logistic regression (for mortality) or linear regression (for duration of mechanical ventilation, ICU length of stay and hospital length of stay). These analyses adjusted for differences in case-mix and severity of illness between time periods using the de novo illness severity measure described above. For the linear regression analyses we chose not to transform duration of mechanical ventilation and length of stay even though these variables were not normally distributed, since our sample size was sufficient to rely on the central limit theorem to allow for valid inference on the adjusted means (17).
Testing for temporal trends
We performed two analyses to examine the degree in which our results might have been influenced by temporal trends that were independent of the intervention. First, we used direct standardization to calculate month-specific utilization rates, complication rates, and adjusted outcome rates; and plotted these outcomes over time. Visual inspection of these graphs could help indicate whether any changes in outcomes were observable as trends prior to the intervention. Second, we split the six-month “pre-intervention” period into two three-month periods (1/1/2011 to 3/31/2011 and 4/1/2011 to 6/30/2011) and compared outcomes between these two periods. Statistically significant differences between these two periods could indicate that observed trends in the main analysis may have been occurring prior to the screening intervention.
Sensitivity analyses
We also performed a series of sensitivity analyses to assess the robustness of our findings to study assumptions. First, we repeated our analyses after excluding each individual ICU in turn. The goal of this analysis was to examine the potential impact of other quality improvement initiatives that may have occurred in individual ICUs but were unrelated to the primary evaluation. To our knowledge, the only related intervention occurred in the surgical-trauma ICU, which implemented an independent checklist to improve spontaneous breathing trial and daily interruption of sedation on August 6, 2011. Thus our sensitivity analysis could assess whether our results were driven by quality improvements in this ICU, or any other ICU, during the study period.
Second, we repeated our analysis including a random ICU admission for each patient, rather than subsequent admissions after the first. The goal of this analysis was to ensure that excluding subsequent admissions did not bias our results, since by definition subsequent admissions were more likely to occur in the post-intervention period.
All analyses were performed with Stata 12.1 (StataCorp, LP, College Station, Texas). For all regression models we used robust Huber-White confidence intervals to adjust the standard errors for correlation within ICUs. All tests were two-tailed, and a p-value of ≤0.05 was considered significant. The intervention examined in this study was designated as a quality improvement by UPMC, since its primary goal was to improve local patient care. The use of patient records to evaluate the impact of the quality improvement intervention was reviewed and approved by the University of Pittsburgh Institutional Review Board.
RESULTS
During the study period there were 18,848 ICU admissions representation 16,097 unique patients. We excluded 2,751 readmissions, 884 patients with missing age, 6 patients with missing pre-ICU length of stay, and 384 patients with missing location prior to ICU admission, resulting in 14,823 unique patients in the sample. We also excluded 1546 patients admitted during the run-in period, leaving 13,227 patients in the final analyses. Of these 4,339 (32.8%) were admitted during the pre-intervention period and 8,938 (67.6%) were admitted during the post-intervention period. Patient characteristics were generally similar between time periods (Table 2).
Table 2.
Patient characteristics by study period
| Before (n=4,339) | After (n=8,938) | P-value | |
|---|---|---|---|
| Age | 59.9 ± 17.3 | 59.6 ± 17.8 | 0.42 |
| Female | 1,889 (43.5) | 3,943 (44.1) | 0.53 |
| Race/Ethnicity | |||
| White | 3,482 (80.2) | 6,928 (77.5) | <0.001 |
| Black | 370 (8.5) | 747 (8.4) | |
| Hispanic | 11 (0.35) | 8 (0.1) | |
| Other | 56 (1.29) | 82 (0.9) | |
| Unknown | 420 (9.7) | 1,173 (13.1) | |
| Comorbidities* | |||
| CHF | 221 (5.1) | 470 (5.26) | 0.69 |
| Lung disease | 1,063 (24.5) | 2,254 (25.2) | 0.64 |
| Cancer | 478 (11.0) | 972 (10.9) | 0.81 |
| Diabetes | 1,363 (31.4) | 2,772 (31.1) | 0.64 |
| ICU admission source | |||
| ED | 1,561 (36.0) | 3,480 (38.9) | <0.001 |
| OR/PACU | 1,005 (23.2) | 1,930 (21.6) | |
| Ward | 1,002 (23.1) | 1,963 (22.0) | |
| Other hospital | 174 (4.0) | 499 (5.6) | |
| Clinic | 597 (13.8) | 1,066 (11.9) | |
| MV at admission | 1,770 (40.8) | 3,380 (37.8) | 0.001 |
| ICU type | |||
| Coronary Care Unit | 341 (7.9) | 739 (8.3) | <0.001 |
| Cardio-thoracic | 456 (10.5) | 902 (10.1) | |
| Medical | 609 (14.0) | 1,437 (16.1) | |
| Neurovascular | 766 (17.7) | 1,791 (20.0) | |
| Neuro-trauma | 307 (7.1) | 697 (7.8) | |
| Surgical | 447 (10.3) | 886 (9.9) | |
| Trauma | 699 (16.1) | 1,504 (16.8) | |
| Transplant | 714 (16.5) | 982 (11.0) | |
| Predicated probability of in-hospital death** | 0.07 [0.03–0.14] | 0.07 [0.03–0.14] | 0.28 |
| Duration of MV (days) | 5.5 ± 6.3 | 5.1 ± 6.7 | 0.03 |
| Length of stay (days) | |||
| ICU | 4.1 ± 5.4 | 3.9 ± 5.0 | 0.005 |
| Hospital | 11.9 ± 12.5 | 10.8 ± 11.2 | <0.001 |
| Discharge location | |||
| Dead | 515 (11.9) | 1,015 (11.4) | <0.001 |
| Home | 1,370 (31.6) | 3,036 (34.0) | |
| Hospice | 32 (0.7) | 65 (0.7) | |
| Long-term acute care | 171 (3.9) | 248 (2.8) | |
| Other hospital | 31 (0.7) | 39 (0.4) | |
| Skilled nursing facility | 2,220 (51.2) | 4,534 (50.7) | |
Values are mean ± standard deviation or frequency (percent).
CHF = congestive heart failure; ICU = intensive care unit; ED = emergency department; OR = operating room; PACU = post-anesthesia care unit; MV = mechanical ventilation
Comorbidities are not mutually exclusive
Predicted probability of in-hospital death is presented as a marker of severity of illness, and was derived from the de novo logistic regression model.
Processes and complications of care are shown in Table 3. Patients in the post-intervention period were more likely to receive daily sedation interruptions (IRR: 1.57, 95% CI: 1.45 – 1.71, p<0.001) and daily spontaneous breathing trials (IRR: 1.24, 95% CI: 1.20 – 1.29). CAUTI, CLABSI, and VAP rates were not different between the pre-intervention and post-intervention time periods.
Table 3.
Processes and complications of care by study period.
| Before (N=4,339) | After (N=8,938) | IRR (95% CI) | P-value | |
|---|---|---|---|---|
| Processes | ||||
| Sedation interruption | ||||
| Days eligible | 1875 | 2964 | 1.57 | <0.001 |
| Days received | 824 | 2055 | (1.45 – 1.71) | |
| Rate | 0.44 | 0.69 | ||
| Spontaneous breathing trial | ||||
| Days eligible | 6881 | 9766 | 1.24 | <0.001 |
| Days received | 5297 | 9359 | (1.20 – 1.29) | |
| Rate | 0.77 | 0.96 | ||
| Complications | ||||
| CAUTI* | ||||
| Infections | 34 | 36 | 1.12 | 0.63 |
| Catheter days | 21,570 | 20,367 | (0.70 – 1.79) | |
| Rate/1000 catheter days | 1.58 | 1.77 | ||
| CLABSI | ||||
| Infections | 16 | 32 | 1.06 | 0.84 |
| Catheter days | 22,131 | 41,625 | (0.58 – 1.94) | |
| Rate/1000 catheter days | 0.72 | 0.77 | ||
| VAP | ||||
| Infections | 47 | 69 | 0.82 | 0.30 |
| Ventilator days | 14,493 | 25,887 | (0.57 – 1.19) | |
| Rate/1000 ventilator days | 3.24 | 2.67 | ||
IRR = incidence rate ratio; DVT = deep vein thrombosis; CAUTI = catheter-associated urinary tract infection; CLABSI = central-line associated blood stream infection; VAP = ventilator-associated pneumonia
Due to the change in CAUTI definition in March, 2012, the after period only contains data from 9/1/11 to 2/28/12 (n=4,499).
Clinical outcomes are shown in Table 4. In-hospital mortality was not different between the pre-intervention and post intervention period. However, patients in the post-intervention period experienced shorter mean duration mechanical ventilation, ICU length of stay, and hospital length of stay.
Table 4.
Adjusted effect of remote screening team on patient outcomes.
| Effect estimate* (95% CI) | P-value | |
|---|---|---|
| In-hospital mortality (odds ratio) | 0.96 (0.84 – 1.09) | 0.54 |
| Duration of MV, days (adjusted difference)† | −0.61 (−0.96 – −0.27) | <0.001 |
| ICU length of stay, days (adjusted difference) | −0.22 (−0.41 – −0.04) | 0.02 |
| Hospital length of stay, days (adjusted difference) | −0.55 (−0.93 – −0.15) | 0.006 |
MV = mechanical ventilation; ICU=intensive care unit
Effects estimated using multivariate regression adjusting for age, pre-intensive care unit length of stay, admission source, admission intensive care unit, severity of illness and comorbidities.
Calculated in 5,150 patients receiving mechanical ventilation.
Graphical analyses of care processes, complications and outcomes indicated no evident temporal trends that were independent of the intervention (in-hospital mortality shown in Figure 1; duration of mechanical ventilation, ICU length of stay and hospital length stay shown in Figure 2; processes and complications of care shown in eFigures 1 and 2 in the online supplement). Additionally, after splitting up the pre-intervention period into two time periods we found no statistically significant differences in outcomes to suggest that improvements were occurring prior to the intervention (eTables 1 and 2 in the on-line supplement).
Figure 1.
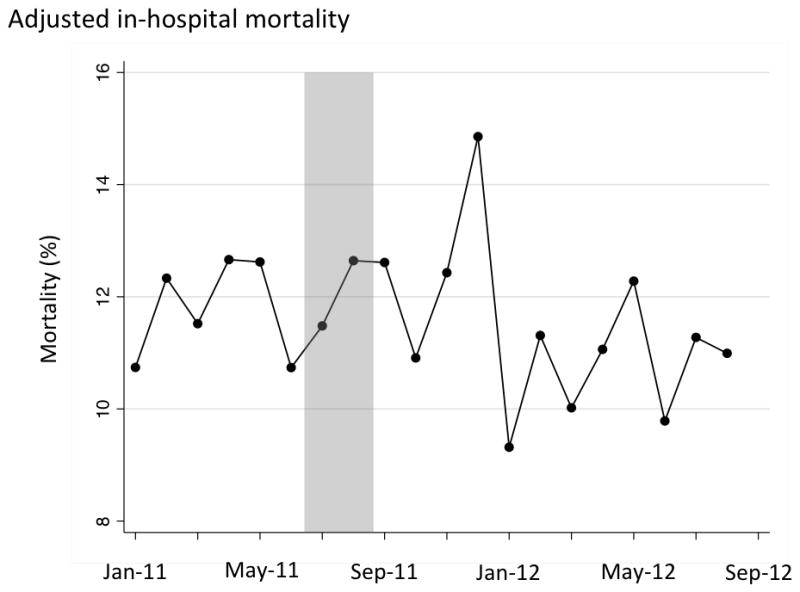
Adjusted in-hospital mortality over time. Shaded area represents excluded run-in period, with included patients to the left (pre-period) and right (post-period) of the shaded area.
Figure 2.
Adjusted duration of mechanical ventilation (Panel A), intensive care unit length of stay (Panel B) and hospital length of stay (Panel C) over time. Shaded area represents excluded run-in period, with included patients to the left (pre-period) and right (post-period) of the shaded area.
Sensitivity analyses on clinical outcomes after excluding each ICU in turn and after analyzing a random admission for each patient yielded similar results (eTables 3 and 4 in the on-line supplement).
DISCUSSION
A hospital-wide ICU quality improvement intervention involving remote screening and prompting for selected evidence-based practices was associated with decreased duration of mechanical ventilation, shorter ICU length of stay and shorter hospital length of stay. The effect was likely mediated in part by an increase in the use of daily interruption of continuous sedation and daily spontaneous breathing trials, rather than changes in ICU-acquired complications, which were not affected by the intervention.
This study demonstrates the potential value of remote screening as a quality improvement strategy in the ICU. Despite the existence of evidenced-based guidelines to aid decision making in the ICU, the gaps between clinical evidence and clinical practice remain wide (18). Remote screening and prompting appears to be a viable approach to close these gaps. By acknowledging that bedside providers cannot, and should not, be solely responsible for consistent provision of evidence-based practice, remote screening and prompting introduces redundancy into the complex system that is the ICU (19). This approach not only appears useful to improve quality within a large hospital like UPMC, but also may be useful to improve quality at small hospitals from a distance, in which dedicated evidence-based practice screeners may not be available.
Our findings complement those of a recent study of in-person “prompting” in an academic medical ICU (20). In that study, patients care teams were randomized to receive in-person prompting when they failed to address all the elements of a patient-care checklist on morning rounds. Patients in the prompting group experienced substantially lower odds of death and shorter ICU length of stay compared to patients in the unprompted group. That study demonstrated the potential value of bedside-prompting as a quality improvement strategy, but left unaddressed the question of how to deliver similar interventions on a large scale. A bedside human prompter in every ICU is neither feasible nor practical, and electronic prompting for evidence-based practice is still in evolution. We show that remote prompting using an EMR may be a viable way to achieve at least some of the quality gains seen in bedside prompting.
This study also provides insight into the potential value of ICU telemedicine, which in some cases involves not only continuous remote monitoring but also remote screening and prompting for evidence-based practice. Existing ICU telemedicine evaluations show mixed results, with some studies showing a large benefit and others showing no benefit (21). To date there are few data explaining this variation in program effectiveness (22). Importantly, the largest positive study not only included remote monitoring, as is typical of modern ICU telemedicine installations, but also remote screening for evidence-based practice (8). Our findings suggest that at least part of the benefit achieved in that study was potentially due to the remote screening component of their intervention. Based on these findings, ICU telemedicine programs should consider including remote screening as part of their activities in an effort to maximize program effectiveness.
The intervention we describe could also be considered a low-cost alternative to telemedicine. We estimate the costs of our intervention to be approximately $380,000 at start-up and approximately $900,000 annually, including hardware, staffing and administrative costs. These costs are substantially less than the costs of a full telemedicine program, which can exceed $3 million annually (23). Given the reductions in duration of mechanical ventilation we observed, the intervention is likely to have resulted in substantial cost savings (13), making this intervention budget neutral even under conservative assumptions. We note that these projected cost savings rely on multiple assumptions about hospital case-mix and cost structures, and therefore are not assured. At the same time, the costs of the program are assured, making the overall financial benefit of this program at other hospitals uncertain.
Although we observed some benefits, not all targeted care processes were affected. For example, one of the primary roles of the our screening team was to evaluate for the appropriateness of urinary and central venous catheters, and prompt the bedside team if these catheters might no longer be necessary. However, these actions appeared not to reduce the incidence of infections associated with these devices. One possibility is that many of these practices were separately targeted by independent infection control nurses, which both screen for infections and feed back this information to the individual ICUs on a monthly basis. Indeed, infection control nurses are a model for the concept of external review and prompting (24), although at our hospital this screening is not preformed remotely. Another possibility is the intervention itself failed to effectively target these care practices, either through ineffective screening practices, ineffective communication, or through lack of buy-in on the part of bedside providers. For example, by giving prompts to the bedside nurse we relied upon that nurse to communicate the prompts to the multidisciplinary care team. To the degree that this communication was ineffective, the intervention itself would be ineffective.
A third possibility for this finding is that the remote screening team communicated primarily with the bedside nurse rather than physicians. Thus key information may not have been transmitted into actionable orders to remove catheters. Moreover, although the intervention was associated with shortened duration of mechanical ventilation, the incidence of ventilator-associated pneumonia was unchanged. This finding may indicate either that prolonged mechanical ventilation is not a major risk factor for pneumonia in our hospital, or that we lacked statistical power to detect a difference in already low pneumonia rates between study periods.
Our study has several limitations. First, although our intervention targeted multiple care processes, we only had valid data on two. Others were not reliably collected in the same manner before and after the intervention, and thus could not be validly studied. Thus we are unable to examine the direct impact of remote screening on all care processes of interest. Nonetheless, for the two care processes we did measure the effect of remote screening was dramatic. Second, we do not have data on the frequency with which the remote screening team contacted the target ICUs. Therefore it is impossible to say with certainty whether the activities of the remote screening team were frequent enough to cause the observed effect. Third, we performed a before-after study that lacked concurrent controls. Despite our efforts to examine potential temporal trends and co-incident interventions, as in all before-after studies we cannot rule out the possibility that our results are independent of the intervention. Fourth, we used a de novo severity of illness measure that has not been externally validated. Finally, we evaluated a quality improvement intervention in single, academic medical center. Our results may not generalize to other institutions.
CONCLUSIONS
As hospitals struggle to improve ICU quality in the setting of staffing and resource constraints, our findings suggest that remote screening and prompting for evidence-based practices is a viable and potentially effective way to support the implementation of evidence-based practice. More research is needed to understand the mechanism of the potential effect, determine the acceptability of the intervention to bedside providers, and determine the optimal way to integrate remote screening with traditional bedside care. Nonetheless, hospitals should consider this intervention, either by itself or in conjunction with other types of remote care enabled by telemedicine, as a useful approach to quality improvement in the ICU.
Supplementary Material
Acknowledgments
Funding: None
Footnotes
Disclosures: Dr. Kahn consulted for the U.S. Department of Veterans Affairs. Dr. Kahn’s institution received grant support from the U.S. Department of Health and Human Services. Dr. Gunn consulted for ICU Medical and Nestle Nutrition, served as an independent consultant for MedMal, and has Pending and active grants from the National Institutes of Health and HRSA on the topic of ICU telemedicine.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Prasad M, Christie JD, Bellamy SL, et al. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25:610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb BW, Wheeler AP, Ely EW. New ways to reduce unnecessary variation and improve outcomes in the intensive care unit. Curr Opin Crit Care. 2001;7:304–311. doi: 10.1097/00075198-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Baker AM, Dunagan DP. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. … England Journal of …. 1996 doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 4.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 5.Resar R, Pronovost P, Haraden C, et al. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005;31:243–248. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 6.LeBlanc JM, Kane-Gill SL, Pohlman AS, et al. Multiprofessional survey of protocol use in the intensive care unit. J Crit Care. 2012;27:738 e9–17. doi: 10.1016/j.jcrc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Hillestad R, Bigelow J, Bower A, et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff (Millwood) 2005;24:1103–1117. doi: 10.1377/hlthaff.24.5.1103. [DOI] [PubMed] [Google Scholar]
- 8.Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175–2183. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 10.Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23:126–137. doi: 10.1016/j.jcrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Rubenfeld GD, Rohrbach J, et al. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Medical care. 2008;46:1226–1233. doi: 10.1097/MLR.0b013e31817d9342. [DOI] [PubMed] [Google Scholar]
- 14.Liu V, Turk BJ, Ragins AI, et al. An electronic Simplified Acute Physiology Score-based risk adjustment score for critical illness in an integrated healthcare system. Crit Care Med. 2013;41:41–48. doi: 10.1097/CCM.0b013e318267636e. [DOI] [PubMed] [Google Scholar]
- 15.Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasilevskis EE, Kuzniewicz MW, Cason BA, et al. Mortality probability model III and simplified acute physiology score II: assessing their value in predicting length of stay and comparison to APACHE IV. Chest. 2009;136:89–101. doi: 10.1378/chest.08-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumley T, Diehr P, Emerson S, et al. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 18.Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177:170–177. doi: 10.1164/rccm.200706-893OC. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JM, Rubenfeld GD. Translating evidence into practice in the intensive care unit: the need for a systems-based approach. J Crit Care. 2005;20:204–206. doi: 10.1016/j.jcrc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med. 2011;184:680–686. doi: 10.1164/rccm.201101-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox ME, Adhikari NK. The effect of telemedicine in critically ill patients: systematic review and meta-analysis. Crit Care. 2012;16:R127. doi: 10.1186/cc11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn JM, Hill NS, Lilly CM, et al. The research agenda in ICU telemedicine: a statement from the Critical Care Societies Collaborative. Chest. 2011;140:230–238. doi: 10.1378/chest.11-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar G, Falk DM, Bonello RS, et al. The costs of critical care telemedicine programs: a systematic review and analysis. Chest. 2012 doi: 10.1378/chest.11-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook E, Marchaim D, Kaye KS. Building a successful infection prevention program: key components, processes, and economics. Infect Dis Clin North Am. 2011;25:1–19. doi: 10.1016/j.idc.2010.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.