Abstract
Purpose
Curcumin exhibits antioxidant properties potentially beneficial for human health; however, its use in clinical applications is limited by its poor solubility and relative instability. Nanoparticles exhibit interesting features for the efficient distribution and delivery of curcumin into cells, and could also increase curcumin stability in biological systems. There is a paucity of information regarding the evolution of the antioxidant properties of nanoparticle-encapsulated curcumin.
Method
We described a simple method of curcumin encapsulation in poly-lactic-co-glycolic acid (PLGA) nanoparticles without the use of detergent. We assessed, in epithelial cells and in an acellular model, the evolution of direct antioxidant and antinitrosant properties of free versus PLGA-encapsulated curcumin after storage under different conditions (light vs darkness, 4°C vs 25°C vs 37°C).
Results
In epithelial cells, endocytosis and efflux pump inhibitors showed that the increased antioxidant activity of PLGA-encapsulated curcumin relied on bypassing the efflux pump system. Acellular assays showed that the antioxidant effect of curcumin was greater when loaded in PLGA nanoparticles. Furthermore, we observed that light decreased, though heat restored, antioxidant activity of PLGA-encapsulated curcumin, probably by modulating the accessibility of curcumin to reactive oxygen species, an observation supported by results from quenching experiments. Moreover, we demonstrated a direct antinitrosant activity of curcumin, enhanced by PLGA encapsulation, which was increased by light exposure.
Conclusion
These results suggest that the antioxidant and antinitrosant activities of encapsulated curcumin are light sensitive and that nanoparticle modifications over time and with temperature may facilitate curcumin contact with reactive oxygen species. These results highlight the importance of understanding effects of nanoparticle maturation on an encapsulated drug’s activity.
Keywords: PLGA nanoparticles, antioxidant, curcumin, evolution, maturation
Background
Curcumin (Cur) is a natural polyphenolic compound extracted from Curcuma longa (turmeric). Long used in traditional medicine, it has more recently attracted considerable research attention since it exhibits a wide spectrum of biological activities: antioxidant, anti-inflammatory, antiviral, antimicrobial, and anticancer.1 Among its antioxidant activities, curcumin inhibits lipid peroxidation and scavenges superoxide anions, singlet oxygen, nitric oxide, and hydroxyl radicals.2,3 However, nonlinear dose–response curves for the antioxidant activity of curcumin have been described. Low doses of curcumin appeared protective for reactive oxygen species (ROS) induction or ROS-induced DNA damage, while higher doses were deleterious.4,5
Curcumin behaves as a universal anti-inflammatory drug but studies have revealed that one of the major problems with curcumin is its poor bioavailability in vivo due to its hydrophobic nature. Another drawback of curcumin is its stability, influenced by pH,6,7 temperature,8,9 light,10 and enzymatic modifications.11 Some of the resultant metabolites are biologically active and possess antioxidant properties.7,12–14 It follows that only traces of orally administered curcumin appear in blood plasma, while most is excreted after rapid metabolism in the intestine.15
Curcumin has been shown to interact with phospholipids,16,17 surfactants,18 or proteins.19 Hence, curcumin is usually taken orally as an oil emulsion. To enhance curcumin delivery, methods have been developed including incorporation into liposomes20 and lipid-based nanoparticles (NPs).21 An obvious alternative is the use of polymer-based NP,22 an approach that has been used to deliver natural products or synthetic drugs.23–25
Poly-lactic-co-glycolic acid NP (PLGA-NP), a biodegradable polymer, are well characterized and suitable for clinical trials.26,27 Curcumin-loaded PLGA-based NP (Cur-NP) have been observed to improve curcumin biological activity, particularly as an anticancer drug.28–31 However, little is known about the antioxidant activity of Cur-NP.32 In this paper, we evaluate the loading of curcumin into PLGA-NP <100 nm in size and study the mechanisms involved in their antioxidant activity, in both epithelial cells and acellular assays, as well as the light and temperature stability of Cur-NP with respect to this antioxidant activity. A direct antinitrosant activity of curcumin and Cur-NP is also described. Finally, we propose a model depicting the mechanisms involved in the evolution of the antioxidant activity of curcumin as Cur-NP concurrent with the maturation of the PLGA-NP.
Methods
Materials
RG503H Resomer® (PLGA), H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate), DAF-FM DA (diaminofluorescein-FM diacetate), TBHP (tert-butyl hydroperoxide), curcumin, filipin III, nystatin, phenylarsine oxide, and chlorpromazine were obtained from Sigma-Aldrich (St Louis, MO, USA). Elacridar was supplied by Santa-Cruz Biotechnology Inc. (Dallas, TX, USA) and PapaNONOate (1-hydroxy-2-oxo-3-(3-aminopropyl)-3-propyl-1-triazene) by Enzo Life Sciences (Villeurbanne, France). ATTO540Q® quencher was purchased from Atto-Tec Gmbh (Siegen, Ger-many). DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicar bocyanine, 4-chlorobenzenesulfonate salt), RPMI-1640, fetal calf serum, glutamine, and antibiotics were purchased from Life Technologies (Saint-Aubin, France).
Cell culture
A549 airway epithelial cells were maintained in RPMI-1640 supplemented with 10% fetal calf serum, 2 mM glutamine, and 1% penicillin-streptomycin in 5% CO2 at 37°C. Seeding and propagation were performed by trypsinization.
Synthesis and characterization of PLGA-NP and Cur-NP
PLGA-NP were produced by nanoprecipitation at room temperature.25,33 Resomer® (PLGA polymer) was dissolved at 10 mg/mL in an acetone/ethanol mixture (85:15) composing the organic phase. Dissolution was performed for 5 minutes under stirring at 150 rpm. Dissolved Resomer® was then injected into ultrapure water (aqueous phase) under stirring at 150 rpm. No surfactant was added at any step of the synthesis. Residual organic solvents were eliminated under vacuum evaporation at 27°C.
To produce Cur-NP, curcumin was added directly to the organic phase after the dissolution of the Resomer® and before injection into the aqueous phase. Curcumin was thus encapsulated in NP during nanoprecipitation. For fluorophore-labeled PLGA-NP, DiD (0.1% w/w) was also added to the organic phase after dissolution of Resomer® and before injection into the aqueous phase.
The mean diameter of NP was determined by laser light scattering with the ZetaSizer NanoZS analyzer (Malvern Instruments, Malvern, UK). The analyses were performed in ultrapure water. The sizes are expressed as Z-average. The zeta potential of NP, expressed in mV, was determined by electrophoretic migration in water (ZetaSizer NanoZS analyzer, Malvern Instruments).
Encapsulation efficiency of curcumin into Cur-NP
Cur-NP were purified by size-exclusion chromatography on a PD-10 column packed with Sephadex G25 following the manufacturer’s protocol (GE HealthCare LifeSciences, Velizy, France). Fractions were collected and the purified Cur-NP were lyophilized and resuspended in acetonitrile (encapsulated curcumin). In parallel, nonpurified Cur-NP were directly lyophilized and resuspended in acetonitrile (total curcumin). The amount of curcumin in NP was analyzed and quantified by high-performance liquid chromatography (HPLC) with an external calibration. Percent encapsulation efficiency (EE) was calculated as follows: % EE = (Curcumin encapsulated/Curcumin total) ×100. The chromatographic analyses were performed on a Waters 600 HPLC system equipped with a Waters 996 Photodiode Array detector (curcumin detection at OD =429 nm). The sample loop was 20 µL (Rheodyne 7125 injector). Chromatographic data were collected and processed on a computer running Millennium 2010 software, version 3.20. The analytical column (SunFire C18, 250×4.6 mm, internal diameter 5 µm) was purchased from Waters (Tokyo, Japan). The mobile phase consisted of acetonitrile/water (50:50, v/v). The flow rate was 0.6 Ml·min−1. All separations were carried out at 30°C. Acetonitrile of analytical grade was obtained from Merck (Nogentsur-Marne, France) and deionized water was obtained from Milli-Q system (Millipore, Molsheim, France).
ROS and RNS measurements
A549 epithelial cells were seeded on 96-well plates at a density of 3×104 cells per well for 72 hours. Cells were washed twice with PBS and a detector probe was added for 30 minutes at 37°C. Cells were again washed twice and the inducer was added, immediately followed by free or Cur-NP. For acellular experiments, the detector probe, inducer and free or NP-formulated curcumin were added to empty 96-well plates. For ROS measurements, the ROS detector probe was 10 µM H2DCF-DA in PBS and the inducer was 100 µM TBHP in PBS. For reactive nitrogen species (RNS) measurements, the RNS detector probe was 5 µM DAF-FM DA and the inducer was 10 µM PapaNONOate. Both of these detector probes are nonfluorescent compounds based on fluorescein that, upon oxidation by ROS or RNS, becomes fluorescent (λex: 495 nm, λem: 515 nm). The evolution of antioxidant activity of free curcumin and Cur-NP was measured in various storage conditions of light (natural light without direct sun exposure vs darkness) and temperature (4°C, room temperature of 22°C, and 37°C in a cell culture incubator).
Mechanism of entry of curcumin and NP into cells
The A549 epithelial cells were seeded for 72 hours in six-well plates at a density of 5×105 cells per well. After two washes with PBS, cells were treated with endocytosis or efflux pump inhibitors. After 5 minutes, free curcumin or DiD-labeled PLGA-NP (15 µg) were added for 40 minutes. Cells were then washed with PBS, detached with trypsin, harvested by centrifugation, and diluted in PBS before analysis by flow cytometry on a CyAn ADP Analyzer (Beckman Coulter, Brea, CA, USA). Cells were selected by their size and cellular complexity (side scatter and forward scatter). For each event (minimum 5,000 cells), fluorescence intensities (FITC) were reported and the mean fluorescence intensities were calculated. The inhibitors were used at the following concentrations: filipin III (10 µg/mL), nystatin (20 µg/mL), phenylarsine oxide (2 µg/mL), chlorpromazine (15 µg/mL), elacridar (55 µg/mL).
Quenching of free or formulated curcumin
Free or formulated curcumin was exposed to light for 0–2 days and then incubated for 1 day at 37°C. The ATTO540Q® quencher was dissolved in dimethyl sulfoxide (DMSO) at 1 mg/mL. Control (H2O), PLGA-NP, free cur-cumin, and Cur-NP were then added to a 96-well plate with H2O (control), DMSO, or ATTO540Q® and fluorescence of curcumin (λex: 485 nm/λem: 538 nm) was measured. Values for Cur-NP treated with DMSO represented unquenched curcumin, while those obtained after ATTO540Q® treatment represented quenched curcumin. The percentage of quenching was then calculated as follows: % Q = 100 - ([Quenched curcumin/Unquenched curcumin] ×100).
Statistics
Student’s t-tests or analysis of variance (ANOVA) were used to determine the significance of variations between groups using GraphPad Prism 5® software.
Results
Design and characterization of curcumin-loaded PLGA-NP
PLGA-NP were made by a simple, one-step, and surfactant-free nanoprecipitation method. PLGA was dissolved into organic solvents and injected into distilled water. Solvents were then eliminated by vacuum evaporation. PLGA-NP had a mean diameter of 96.36 nm and a poly-dispersity index of 0.18, indicating that the NP were monodispersed. Their zeta potential was -40.9 mV (Table 1).
Table 1.
Size, zeta potential, and encapsulation efficiency of PLGA-NP and Cur-NP
| Formulation | Size (SD), nm | pdi | Zeta potential (mV) |
|---|---|---|---|
| PLGA-NP | 96.36 (40.88) | 0.18 | −40.9 |
| Cur-NP | 88.39 (38.52) | 0.19 | −51.1 |
Notes: PLGA-NP and Cur-NP (0.75‰ w/w) were synthesized by nanoprecipitation in water. Size and zeta potential were analyzed by dynamic light scattering and electrophoretic mobility.
Abbreviations: PLGA, poly-lactic-co-glycolic acid; NP, nanoparticles; Cur-NP, curcumin-loaded PLGA-based nanoparticles; pdi, polydispersity index.
The size of Cur-NP was 88.39 nm and their poly-dispersity index 0.19, while their zeta potential was -51.1 mV (Table 1). Size was slightly lower than empty PLGA-NP while zeta potential was slightly higher.
Antioxidant effect of Cur-NP in epithelial cells
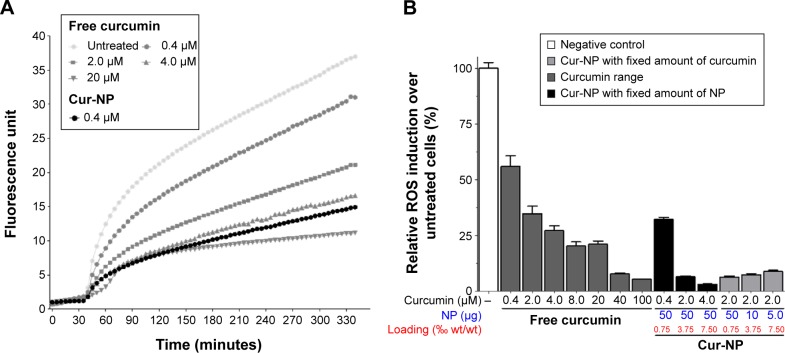
Cells were treated with free curcumin (from 0 to 20 µM) or Cur-NP, then ROS were induced after 30 minutes with TBHP treatment. Increasing doses of free curcumin lowered the ROS induction in a dose-dependent manner. Cur-NP (containing 0.4 µM of curcumin) showed a similar antioxidant effect to that of 4 µM of free curcumin as soon as TBHP was added (Figure 1A). This was not dependent on the PLGA-NP themselves which did not show any antioxidant or pro-oxidant activity (Figure S1). To determine the effect of the curcumin:NP ratio on antioxidant activity, dose–response curves of free and NP-encapsulated curcumin were obtained using two approaches: either with a fixed amount of PLGA-NP that encapsulated increasing doses of curcumin, or with a fixed dose of curcumin encapsulated in increasing amounts of PLGA-NP. This was done in order to discriminate between the antioxidant properties of curcumin observed when using NP highly concentrated in curcumin from those observed when using NP weakly concentrated in curcumin. A gradual increase of antioxidant activity was observed when the amount of free curcumin was increased. Increasing amounts of Cur-NP also exhibited a proportional antioxidant activity (Figure 1B). Interestingly, the dose of 4 µM of free curcumin had comparable antioxidant activity to 0.4 µM of Cur-NP, meaning that encapsulation into PLGA-NP led to a tenfold increase in antioxidant activity. For 2 µM and 4 µM of curcumin in Cur-NP, a 20- to 50-fold increase over the values for free curcumin was observed.
Figure 1.
NP-formulated curcumin is more potent antioxidant that free curcumin.
Notes: (A) A549 cells were loaded with H2DCF-DA then treated with TBHP and free (from 0 to 20 µM) or encapsulated (0.4 µM) curcumin. Fluorescence of H2DCF-DA was measured to quantify ROS induction. (B) A549 cells were treated as in (A). Various amounts of curcumin (by varying the load of curcumin into PLGA-NP) or various amounts of PLGA-NP (with the same of concentration of curcumin) were used to determine and compare the antioxidant activity of free and NP-formulated curcumin. Theoretical drug loading is indicated and results are expressed in mean ± SEM.
Abbreviations: Cur-NP, curcumin-loaded PLGA-based NP; NP, nanoparticles; H2DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; TBHP, tert-butyl hydroperoxide; PLGA, poly-lactic-co-glycolic acid; SEM, standard error of the mean; ROS, reactive oxygen species; wt, weight.
We also observed that decreasing the loading of curcumin per NP while keeping the curcumin concentration constant (2 µM) did not alter the antioxidant effects observed. This meant that the amount of NP was not deleterious for the entry of NP into the cells. Since equivalent antioxidant effects were achieved with 40–100 µM of free curcumin or 2 µM of Cur-NP, this last dose was considered as optimal. Finally, retaining the dose of 2 µM of curcumin, the curcumin loading was modulated by using various amounts of NP. Lower loading (using more NP to deliver the same curcumin dose) tended to produce a slight increase in the antioxidant effects of curcumin, although these differences were not statistically significant (Figure 1B). All subsequent experiments were therefore conducted with 2 µM of PLGA-encapsulated cur-cumin with a loading of 0.75‰. The EE of this antioxidant Cur-NP formulation was determined by HPLC as being 44.2%±1.01%.
Mechanism of free curcumin and NP entry into epithelial cells
To determine the mechanisms involved in the uptake of free curcumin and Cur-NP by epithelial cells and to investigate their possible effect upon the greater observed antioxidant activity of Cur-NP relative to free curcumin, endocytosis pathway and efflux pump inhibitors were used. Treatment of cells with curcumin or NP was preceded by inhibitor treatment. Cells were then analyzed by cytometry by exploiting the intrinsic fluorescence of curcumin.
For free curcumin, a slight increase in uptake by cells was observed using caveolae (filipin III and nystatin) and clathrin pathway inhibitors (phenylarsine oxide and chlorpromazine). However, a greater increase was shown with elacridar (Figure 2A), an inhibitor of the efflux pump system that targets P-gp/ABCG1 and BCRP/ABCG2. This suggested that free curcumin diffuses through the plasma membrane but is excluded from cells by the multidrug resistance system.
Figure 2.
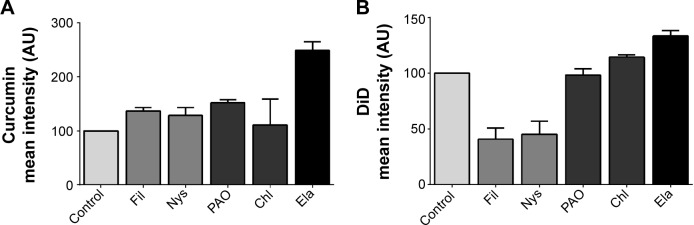
Mechanism of potentiation of antioxidant activity by PLGA-NP.
Notes: A549 cells were treated with endocytosis pathway (Fil, Nys, PAO, Chl) or efflux pump (Ela) inhibitors then loaded with free curcumin (A) or PLGA/DiD-NP (B). The fluorescence was analyzed by cytometry and results are expressed as mean fluorescence intensity ± SEM.
Abbreviations: PLGA, poly-lactic-co-glycolic acid; NP, nanoparticles; DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt; SEM, standard error of the mean; Fil, filipin III; Nys, nystatin; PAO, phenylarsine oxide; Chl, chlorpromazine; Ela, elacridar.
A strong decrease in endocytosis of NP was observed with caveolae inhibitor treatment (Figure 2B). Contrary to what was observed with free curcumin, elacridar treatment had no effect on NP endocytosis.
Taken together, these results demonstrate that curcumin and NP entered the cells by diffusion and endocytosis, respectively. They also suggest that the increase of the antioxidant activity of Cur-NP was probably due to an increase of the delivery by the NP into vesicles bypassing the multidrug resistance efflux pumps.
Acellular system to measure antioxidant activity of curcumin
An in vitro, acellular system was developed to analyze the antioxidant activity of Cur-NP under conditions where no delay in endocytosis or degradation of the NP could occur. Free or Cur-NP was directly added to a ROS inducer (TBHP) with a ROS detector probe (H2DCF-DA). In the absence of curcumin, a progressive increase in ROS was detected and attained a 6.5-fold induction after 1 hour, while addition of free curcumin reduced this to a maximum threefold induction; Cur-NP was even more efficient than free curcumin and completely prevented ROS induction (Figure 3). This showed that curcumin encapsulated in NP has stronger antioxidant activity compared with free curcumin, even in the acellular system. This system further enabled us to follow the evolution of the antioxidant properties of PLGA-encapsulated curcumin when the formulations were exposed to light or heat.
Figure 3.
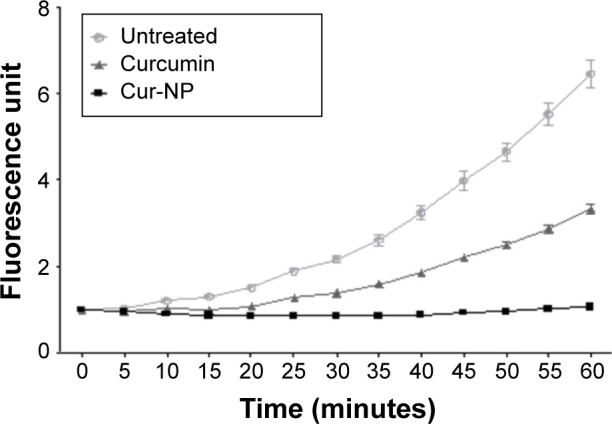
Development of an in vitro acellular method for measuring the antioxidant activity of Cur-NP.
Notes: The oxidation probe H2DCF-DA and the ROS donor THBP were put into an empty 96-well plate. Then free or formulated curcumin (2 µM) was added and fluorescence of the probe was followed for 60 minutes. The Cur-NP was more antioxidant than free curcumin. Results are expressed in mean ± SEM.
Abbreviations: H2DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen species; THBP, tert-butyl hydroperoxide; PLGA, poly-lactic-co-glycolic acid; Cur-NP, curcumin-loaded PLGA-based nanoparticles; SEM, standard error of the mean; NP, nanoparticles.
Light and temperature effects on antioxidant properties of curcumin
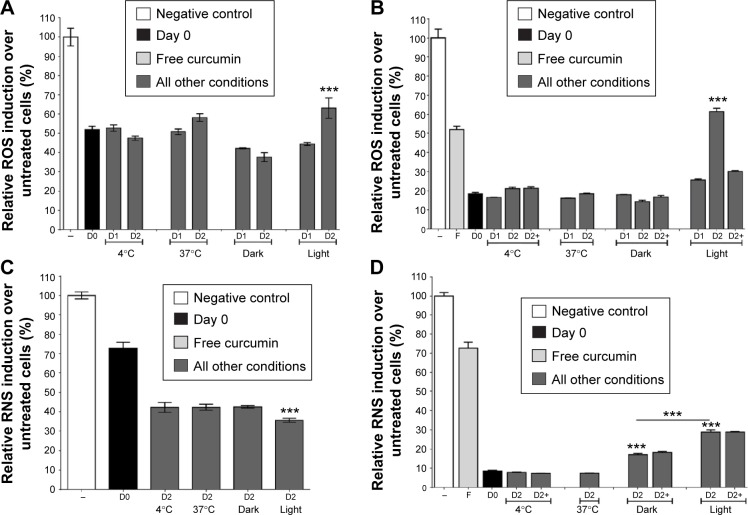
The stability of free or Cur-NP antioxidant activity was then studied in the acellular system described earlier. Free curcumin was stored for 2 days in the dark at 4°C, 37°C, or 25°C, and under natural light at 25°C. The antioxidant effect of free curcumin reduced the amount of ROS by about 50% compared with the control. This antioxidant activity did not change over time or as a function of storage temperature. However, an increased in ROS was observed when curcumin was stored for 2 days under conditions of natural light, meaning that curcumin lost some of its antioxidant activity under these storage conditions (Figure 4A). Cur-NP was also examined under the same conditions. Compared with D0, which had an antioxidant effect of over 80%, no difference was observed when Cur-NP formulations were stored in the dark for 2 days at 4°C, 37°C, or 25°C. Nonetheless, as for free curcumin, more ROS were observed with Cur-NP stored at 25°C under light for 2 days, meaning that the antioxidant properties of Cur-NP had decreased. All Cur-NP (except those initially stored at 37°C) were then kept for an additional day in the dark at 37°C (D2+), to determine whether temperature modulates PLGA porosity and degradation, and hence curcumin accessibility. No difference was observed for 4°C or 25°C (darkness) conditions; however, formulations first exposed to light and then 37°C demonstrated renewed antioxidant activity (Figure 4B). These data suggest that the antioxidant activity of Cur-NP that had been reduced by light exposure could be subsequently restored by exposure to moderate heat (37°C).
Figure 4.
Light and heat effects on Cur-NP antioxidant and antinitrosant activities.
Notes: Antioxidant (A, B) and antinitrosant (C, D) activities of free (A, C) or NP-formulated (B, D) curcumin were analyzed in vitro. Free or NP-formulated curcumin were stored in various conditions of light and temperature (darkness: 4°C, 25°C, 37°C; light: 25°C exposed to light) for 0 (D0), 1 (D1), or 2 (D2) days. An extra-day at 37°C (D2+) was included for some conditions to study PLGA-NP maturation and its effect on curcumin release and availability. (A) Light exposure of free curcumin decreased its antioxidant activity. ***P<0.005 versus D1 light. (B) Light exposure of NP-formulated curcumin decreased its antioxidant activity, but this was restored by a 1-day incubation at 37°C. ***P<0.005 versus D0, D1, and other D2. (C) Light exposure of free curcumin increased its antinitrosant activity. ***P<0.005 versus other D2. (D) Incubation at 25°C of NP-formulated curcumin decreased its antinitrosant activity; this was not restored by a 1-day incubation at 37°C. Light exposure exacerbated the decrease in antinitrosant activity of NP-formulated curcumin. ***P<0.005 versus D0 and other D2. Results are expressed in mean ± SEM.
Abbreviations: Cur-NP, curcumin-loaded PLGA-based nanoparticles; F, free curcumin; PLGA, poly-lactic-co-glycolic acid; NP, nanoparticles; SEM, standard error of the mean; ROS, reactive oxygen species; RNS, reactive nitrogen species.
Light and temperature effects on antinitrosant properties of curcumin
To gain more insight into the properties of curcumin, antinitrosant activity tests targeting RNS were designed, studying the same storage conditions as mentioned earlier, and using our acellular system as before. As RNS induction decreased, the antinitrosant activity of free curcumin increased with time and was not affected by temperature. However, exposure to light increased the antinitrosant activity of curcumin (Figure 4C). The antinitrosant activity of Cur-NP was higher than free curcumin at D0. This activity seemed maximal and did not vary when Cur-NP were stored in the dark for 2 days at 4°C or 37°C. However, decreased antinitrosant activity was shown when NP formulations were stored at 25°C, and this was exacerbated with light exposure. An additional day of storage at 37°C did not renew the antinitrosant activity of Cur-NP in the same way as observed for their antioxidant activity (Figure 4D).
Evolution of curcumin accessibility in PLGA-NP with light exposure
In an attempt to explain the evolution of antioxidant and antinitrosant activities of Cur-NP when exposed to light, quenching experiments were performed. A quencher dye, (ATTO540Q®) able to absorb the emission wavelengths of curcumin upon contact, was used. Free curcumin fluorescence was easily quenched (more than 80%) in this system (Figure 5A), since it was freely accessible. For NP-formulated curcumin, quenching was about 40%, meaning that curcumin was only partially accessible to the quencher dye. This quenching further decreased after 2 days storage under natural light (Figure 5B), suggesting that PLGA-NP formulated curcumin was less accessible after light exposure, and perhaps explaining the reduced antioxidant activity seen in the ROS experiment earlier. Subsequent incubation of the PLGA-curcumin formulation at 37°C returned quenching measurements to their initial value, suggesting an increase in the porosity of the NP or an intra-particle redistribution of curcumin upon exposure to heat.
Figure 5.
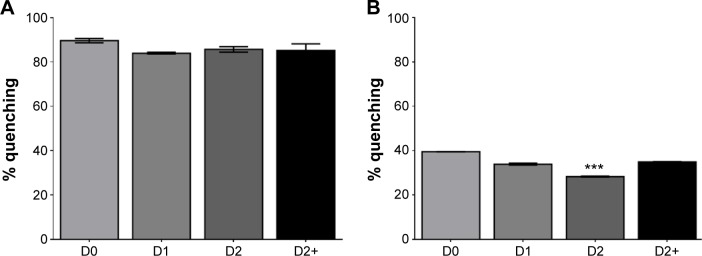
Quenching of free or NP-formulated curcumin fluorescence.
Notes: Free or NP-formulated curcumin were stored at 25°C under light and analyzed after 0 (D0), 1 (D1), or 2 days (D2). An extra-day at 37°C (D2+) was included to study PLGA-NP maturation and its effect on curcumin availability. Free or NP-formulated curcumin were loaded in a 96-well plate with ATTO540Q quencher to inhibit the autofluorescence of curcumin. Results are expressed in percentage of quenching compared to unquenched conditions. (A) Free curcumin fluorescence is quenched by ATTO540Q and no variation was observed. (B) Partial quenching of NP-formulated curcumin fluorescence was observed. After 2 days of incubation, quenching decreased indicating that curcumin was less accessible to the quencher. Quenching was restored by an additional 1-day incubation at 37°C showing that curcumin accessibility was renewed. ***P<0.005 versus all conditions. Results are expressed in mean ± SEM.
Abbreviations: NP, nanoparticles; PLGA, poly-lactic-co-glycolic acid; SEM, standard error of the mean.
Discussion
The PLGA is a copolymer approved by the US Food and Drug Administration, and the European Medicines Agency, for use as a drug delivery system by parenteral administration. It is widely studied as a vector for a range of drugs – hydrophilic or hydrophobic, small molecules, or macromolecules.34 NP are potentially interesting to better target the delivery of drugs to cells, and to specifically deliver the drugs within the cells and thereby increase their efficacy.
In this study, we used a fabrication process without detergent permitting us to prepare PLGA-NP <100 nm in size. The incorporation of curcumin within the NP did not significantly modify their size (Table 1). Compared to unloaded PLGA-NP, the slight decrease in size of Cur-NP observed may be due to the higher charge of the formulation reinforcing the intra-particular ionic interactions and leading to a shrinkage of the NP (as has been demonstrated for other organic NP).35
Numerous studies on PLGA particles as drug vectors have been performed using microparticles of a size greater than 200 nm;36–38 however, this is not optimal for efficient endocytosis.39–41 In this airway epithelial cell model, the anionic Cur-NPs entered the cells via the caveolae pathway, a pathway that has been observed for NP <100 nm in size.42,43 Interestingly, another study in our laboratory showed that cationic NP of a similar size (<100 nm) enter cells via the clathrin pathway,44 suggesting that surface charge has a strong influence on NP endocytosis.
Studies have underlined the link between ROS and bronchial asthma, and the need for an efficient delivery of antioxidants to the epithelial airway barrier.45 In airway epithelial cells, the Cur-NP possessed antioxidant activity 20–50 times greater than that observed with free curcumin. This is probably due to a more efficient entry of curcumin into airway epithelial cells, mediated by NP endocytosis and the Cur-NP’s bypassing of the cells’ efflux pump (Figure 3B).
The PLGA is a biodegradable and biocompatible polymer, but its biodegradability (resulting from the hydrolysis of ester bonds to give lactic and glycolytic acids) may be problematic with respect to the shelf life of the formulation. Therefore, the study of drug release and drug availability from inside the polymer as a function of storage time, temperature, and light exposure was necessary for further drug development.46–48
The Cur-NP activity is directly related to endocytosis, cell cycle and differentiation,49–51 and long-term effect (over 24 hours) of curcumin could occur if drug persists in the cells (eg, target gene transcription or epigenetic effects).52,53 To improve our understanding of the mechanisms involved in Cur-NP maturation, we therefore developed an acellular system to allow rapid and direct measurements of antioxidant and antinitrosant activities of free or NP-formulated curcumin in the absence of cellular compensation. In this acellular model, curcumin and Cur-NP retained their antioxidant activities precluding the necessity of curcumin to be inside the cells to act. Our observations appear to have corroborated the previously reported scavenger ability of curcumin54 and validated this acellular model for the investigation of the direct antioxidant effects of this drug. Interestingly, we also observed a direct antinitrosant activity of curcumin and Cur-NP in our acellular system (Figure 4C and D). This may be due to the ability of curcumin to scavenge RNS.55,56 The use of an acellular system also precluded any confounding by indirect antinitrosant activity through iNOS inhibition.55,57
At the same dose, NP-formulated curcumin was more antioxidant and antinitrosant than free curcumin (Figure 3 and data not shown). It has been reported that curcumin can exist in crystalline or amorphous state. Some evidence showed that curcumin encapsulated in PLGA-NP was in amorphous state, leading to a greater solubility and potential increased efficiency.58–60 However, at the doses used in our experiments (<1 µg/mL), curcumin is soluble, and the curcumin solution was limpid and filterable (data not shown). This also supported that curcumin could be homogeneously entrapped in PLGA-NP and potentially be released as a soluble drug. It is also possible that PLGA-NP create a nano-environment61 that concentrates and facilitates interactions of the curcumin with ROS and RNS, and hence augments the antioxidant and antinitrosant activities of curcumin. This further emphasizes that PLGA-NP–encapsulated curcumin has stronger antioxidant activities compared to free curcumin.
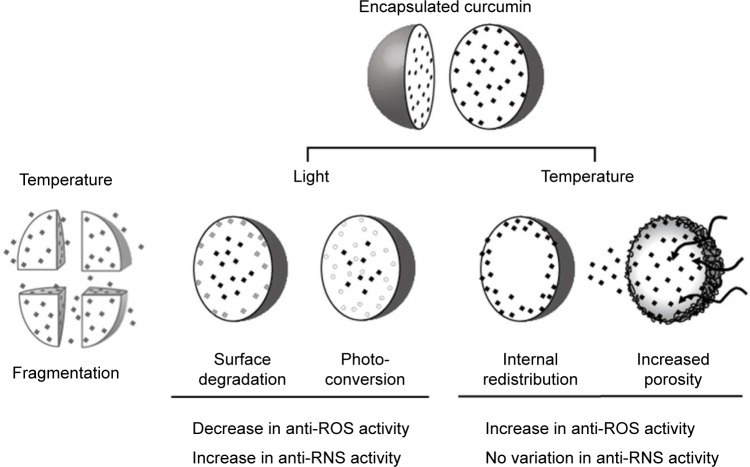
We also observed that NP-formulated curcumin was light sensitive, and that temperature could restore light degradation of the antioxidant activity of Cur-NP. Conversely, light exposure increased antinitrosant activity but this was not modulated by subsequent exposure to heat (Figure 4). Several hypotheses to explain these observations are summarized in Figure 6. Time and temperature exposure are known to degrade PLGA-NP, notably because of the autohydrolysis of the copolymer in aqueous solution.62 This could cause NP fragmentation; however, we observed no change in particle size (data not shown) precluding this hypothesis.
Figure 6.
Hypothesis of curcumin release from nanoparticles.
Notes: Time, light, and temperature exert various effects on nanoparticles, curcumin, and NP-formulated curcumin. Nanoparticle degradation may lead to the fragmentation of nanoparticles or their increased porosity, though no change in NP size was observed indicating fragmentation was negligible (data not shown). Increased NP porosity, induced by heat, could increase the availability of curcumin to its environment, either facilitating the entry of oxidant molecules into the NP, or by redistributing curcumin previously localized in the core of the nanoparticle. Light exposure modifies antioxidant and antinitrosant properties of curcumin by degradation or conversion. Our results suggest a combination of these hypotheses regarding the antioxidant properties of encapsulated curcumin.
Abbreviations: NP, nanoparticles; ROS, reactive oxygen species; RNS, reactive nitrogen species.
Light exposure led to the photodegradation of curcumin,10 and it was noteworthy that some curcumin degradation products showed biological effects.7,12–14 In the case of NP-formulated curcumin, light exposure may result in the degradation of curcumin on the surface of the NP, while curcumin loaded in the NPs’ core should be protected.18,63 Free curcumin also exhibited direct antinitrosant activities and light exposure increased this property (Figure 4C). However, NP-formulated curcumin was less antinitrosant when exposed to light than free curcumin (Figure 4D). These results suggest that freely diffusible antinitrosant degradation products could be generated by light exposure, leading to enhanced antinitrosant activity of free, but not NP-formulated, curcumin; they also support the hypothesis that photodegradation of curcumin took place only at the surface of the NP.
Restoration of the antioxidant properties of light-exposed Cur-NP formulations was achieved by further incubation at 37°C under darkness (Figure 4B). This could imply that heating modifies either the NP porosity (so that ROS can enter the NP and come into contact with nonmodified curcumin), or the distribution of curcumin inside the NP (so that curcumin could diffuse onto the surface of NP). Quenching experiments on light-exposed free or NP-formulated curcumin were performed to investigate this hypothesis. The quencher is a small molecule that could easily mimic a ROS to contact curcumin and is used for collisional energy transfer, thereby decreasing curcumin fluorescence. We showed that curcumin was less accessible to quencher when NP formulations were exposed to light for 2 days, and that subsequent heating restored the accessibility of curcumin to quencher (Figure 5). Together, these results prompted our hypothesis that heat modulates the permeability of NP, renewing the interactions of curcumin with the milieu.
Conclusion
To conclude, we observed that curcumin possesses antioxidant and direct antinitrosant activities. These effects were greater when curcumin was formulated in PLGA-NP, probably owing to the formation of a nanoenvironment conducive to interactions of curcumin with ROS/RNS. Light appeared to convert curcumin to byproducts exhibiting antinitrosant activity, but with reduced antioxidant properties. Subsequent heating modulated NP porosity, renewing the accessibility of curcumin to ROS, either by facilitating penetration of ROS into the NP or by the redistribution of curcumin in the NPs’ core toward the surface. Together, our results highlight a complex evolution of curcumin in PLGA-NP and a combination of these mechanisms probably occurred (Figure 6). This study underlines the necessity to carefully study the evolution of NP-drug formulations during storage before using them for further studies.
Supplementary materials
PLGA-NP do not interfere with ROS induction.
Notes: (A) A549 cells were loaded with H2DCF-DA and treated with PLGA nanoparticles (150 µg/cm2). Fluorescence of H2DCF-DA was measured to quantify ROS induction. Positive control was pyocyanin. Cells without H2DCF-DA served as specificity test for fluorescence. (B) A549 cells loaded with H2DCF-DA and treated with THBP, a ROS donor, with or without PLGA-NP (30 or 150 µg/cm2). Fluorescence of H2DCF-DA was measured to quantify ROS induction.
Abbreviations: PLGA, poly-lactic-co-glycolic acid; NP, nanoparticles; H2DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen species; THBP, tert-butyl hydroperoxide.
Acknowledgments
The authors would like to thank the DigestScience Foundation for their support.
Footnotes
Author contributions
The manuscript was written through contributions of all the authors. All the authors have given approval to the final version of the manuscript. The design and conception of experiments were contributed by RC and DB. Interpretation of data was done by RC and DB. Acquisition of data was performed by RC and EL. All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tsang CK, Kelly TL, Sailor MJ, Li YY. Highly stable porous silicon-carbon composites as label-free optical biosensors. ACS Nano. 2012;6(12):10546–10554. doi: 10.1021/nn304131d. [DOI] [PubMed] [Google Scholar]
- 2.Jovanovic SV, Boone CW, Steenken S, Trinoga M, Kaskey RB. How curcumin works preferentially with water soluble antioxidants. J Am Chem Soc. 2001;123(13):3064–3068. doi: 10.1021/ja003823x. [DOI] [PubMed] [Google Scholar]
- 3.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kelly MR, Xu J, Alexander KE, Loo G. Disparate effects of similar phenolic phytochemicals as inhibitors of oxidative damage to cellular DNA. Mutat Res. 2001;485(4):309–318. doi: 10.1016/s0921-8777(01)00066-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanwar V, Sachdeva J, Kishore K, et al. Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: a biochemical, histopathological, and electron microscopic evidence. Cell Biochem Func. 2010;28(1):74–82. doi: 10.1002/cbf.1623. [DOI] [PubMed] [Google Scholar]
- 6.Tonnesen HH, Karlsen J. Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z Lebensm Unters Forsch. 1985;180(5):402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 7.Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 8.Liu KN, Lai CM, Lee YT, et al. Curcumin’s pre-incubation temperature affects its inhibitory potency toward amyloid fibrillation and fibril-induced cytotoxicity of lysozyme. Biochim Biophys Acta. 2012;1820(11):1774–1786. doi: 10.1016/j.bbagen.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Niu Y, Ke D, Yang Q, et al. Temperature-dependent stability and DPPH scavenging activity of liposomal curcumin at pH 7.0. Food Chem. 2012;135(3):1377–1382. doi: 10.1016/j.foodchem.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Tonnesen HH, Karlsen J, van Henegouwen GB. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z Lebensm Unters Forsch. 1986;183(2):116–122. doi: 10.1007/BF01041928. [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Qiu F. Curcuminoid metabolism and its contribution to the pharmacological effects. Curr Drug Metab. 2013;14(7):791–806. doi: 10.2174/13892002113149990102. [DOI] [PubMed] [Google Scholar]
- 12.Lirdprapamongkol K, Sakurai H, Kawasaki N, et al. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25(1):57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Maurya DK, Devasagayam TP. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol. 2010;48(12):3369–3373. doi: 10.1016/j.fct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810(2):170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]
- 16.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1 –2):155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60(2):171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 18.Tonnesen HH. Solubility, chemical and photochemical stability of cur-cumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Pharmazie. 2002;57(12):820–824. [PubMed] [Google Scholar]
- 19.Kumar V, Lewis SA, Mutalik S, Shenoy DB, Venkatesh, Udupa N. Biodegradable microspheres of curcumin for treatment of inflammation. Indian J Physiol Pharmaco. 2002;46(2):209–217. [PubMed] [Google Scholar]
- 20.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 21.Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352(1–2):287–293. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 23.Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MN. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm. 2007;67(2):361–369. doi: 10.1016/j.ejpb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MN. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J Drug Target. 2006;14(1):27–34. doi: 10.1080/10611860600565987. [DOI] [PubMed] [Google Scholar]
- 25.Le Broc-Ryckewaert D, Carpentier R, Lipka E, et al. Development of innovative paclitaxel-loaded small PLGA nanoparticles: study of their antiproliferative activity and their molecular interactions on prostatic cancer cells. Int J Pharm. 2013;454(2):712–719. doi: 10.1016/j.ijpharm.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Jain AK, Das M, Swarnakar NK, Jain S. Engineered PLGA nanoparticles: an emerging delivery tool in cancer therapeutics. Crit Rev Ther Drug Carrier Syst. 2011;28(1):1–45. doi: 10.1615/critrevtherdrugcarriersyst.v28.i1.10. [DOI] [PubMed] [Google Scholar]
- 27.Lu JM, Wang X, Marin-Muller C, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int. 2014;2014:394264. doi: 10.1155/2014/394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmaco Sin. 2012;33(6):823–831. doi: 10.1038/aps.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi M, Chauhan SC. Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metabol. 2012;13(1):120–128. doi: 10.2174/138920012798356952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yallapu MM, Khan S, Maher DM, et al. Anti-cancer activity of cur-cumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35(30):8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew A, Fukuda T, Nagaoka Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PloS One. 2012;7(3):e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang J, Paillard A, Passirani C, et al. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29(6):1495–1505. doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]
- 34.Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Leon T, Carvalho EL, Seijo B, Ortega-Vinuesa JL, Bastos-Gonzalez D. Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behavior. J Colloid Interface Sci. 2005;283(2):344–351. doi: 10.1016/j.jcis.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 36.Bilati U, Allemann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24(1):67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Kim JC, Sul D, et al. Nanoparticle formulation for controlled release of capsaicin. J Nanosci Nanotechnol. 2011;11(5):4586–4591. doi: 10.1166/jnn.2011.3636. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi G, Nokhodchi A, Barzegar-Jalali M, et al. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces. 2011;88(1):39–44. doi: 10.1016/j.colsurfb.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri A, Battaglia G, Golestanian R. The effect of interactions on the cellular uptake of nanoparticles. Phys Biol. 2011;8(4):046002. doi: 10.1088/1478-3975/8/4/046002. [DOI] [PubMed] [Google Scholar]
- 40.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-dependent endocytosis of nanoparticles. Adv Mater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic. 2007;8(8):970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 43.Jana NR. Design and development of quantum dots and other nanoparticles based cellular imaging probe. Phys Chem Chem Phys. 2011;13(2):385–396. doi: 10.1039/c0cp00726a. [DOI] [PubMed] [Google Scholar]
- 44.Dombu CY, Kroubi M, Zibouche R, Matran R, Betbeder D. Characterization of endocytosis and exocytosis of cationic nanoparticles in airway epithelium cells. Nanotechnology. 2010;21(35):355102. doi: 10.1088/0957-4484/21/35/355102. [DOI] [PubMed] [Google Scholar]
- 45.Sugiura H, Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxid Redox Signal. 2008;10(4):785–797. doi: 10.1089/ars.2007.1937. [DOI] [PubMed] [Google Scholar]
- 46.Gopinathan N, Yang B, Lowe JP, Edler KJ, Rigby SP. NMR cryoporometry characterisation studies of the relation between drug release profile and pore structural evolution of polymeric nanoparticles. Int J Pharm. 2014;469(1):146–158. doi: 10.1016/j.ijpharm.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grottkau BE, Cai X, Wang J, Yang X, Lin Y. Polymeric nanoparticles for a drug delivery system. Curr Drug Metab. 2013;14(8):840–846. doi: 10.2174/138920021131400105. [DOI] [PubMed] [Google Scholar]
- 48.Rescignano N, Amelia M, Credi A, Kenny JM, Armentano I. Morphological and thermal behavior of porous biopolymeric nanoparticles. Eur Polym J. 2012;48(7):1152–1159. [Google Scholar]
- 49.Fielding AB, Willox AK, Okeke E, Royle SJ. Clathrin-mediated endocytosis is inhibited during mitosis. Proc Natl Acad Sci. 2012;109(17):6572–6577. doi: 10.1073/pnas.1117401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foerg C, Ziegler U, Fernandez-Carneado J, Giralt E, Merkle HP. Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases. Pharm Res. 2007;24(4):628–642. doi: 10.1007/s11095-006-9212-1. [DOI] [PubMed] [Google Scholar]
- 51.Lee KD, Nir S, Papahadjopoulos D. Quantitative analysis of liposome-cell interactions in vitro: rate constants of binding and endocytosis with suspension and adherent J774 cells and human monocytes. Biochemistry. 1993;32(3):889–899. doi: 10.1021/bi00054a021. [DOI] [PubMed] [Google Scholar]
- 52.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6(2):93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Reyes S, Guzman-Beltran S, Medina-Campos ON, Pedraza-Chaverri J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid Med Cell Longev. 2013;2013:801418. doi: 10.1155/2013/801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PloS One. 2011;6(10):e26012. doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Parmacol. 1997;49(1):105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 56.Onoda M, Inano H. Effect of curcumin on the production of nitric oxide by cultured rat mammary gland. Nitric Oxide. 2000;4(5):505–515. doi: 10.1006/niox.2000.0305. [DOI] [PubMed] [Google Scholar]
- 57.Johnston BD, DeMaster EG. Suppression of nitric oxide oxidation to nitrite by curcumin is due to the sequestration of the reaction intermediate nitrogen dioxide, not nitric oxide. Nitric Oxide. 2003;8(4):231–234. doi: 10.1016/s1089-8603(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 58.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3875. [PubMed] [Google Scholar]
- 59.Pawar YB, Shete G, Popat D, Bansal AK. Phase behavior and oral bioavailability of amorphous Curcumin. Eur J Pharm Sci. 2012;47(1):56–64. doi: 10.1016/j.ejps.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Al-Rohaimi AH. Comparative anti-inflammatory potential of crystalline and amorphous nano curcumin in topical drug delivery. J Oleo Sci. 2015;64(1):27–40. doi: 10.5650/jos.ess14175. [DOI] [PubMed] [Google Scholar]
- 61.Pfeiffer C, Rehbock C, Huhn D, et al. Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J R Soc, Interface. 2014;11(96):20130931. doi: 10.1098/rsif.2013.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suwannateep N, Wanichwecharungruang S, Haag SF, et al. Encapsulated curcumin results in prolonged curcumin activity in vitro and radical scavenging activity ex vivo on skin after UVB-irradiation. Eur J Pharm Biopharm. 2012;82(3):485–490. doi: 10.1016/j.ejpb.2012.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PLGA-NP do not interfere with ROS induction.
Notes: (A) A549 cells were loaded with H2DCF-DA and treated with PLGA nanoparticles (150 µg/cm2). Fluorescence of H2DCF-DA was measured to quantify ROS induction. Positive control was pyocyanin. Cells without H2DCF-DA served as specificity test for fluorescence. (B) A549 cells loaded with H2DCF-DA and treated with THBP, a ROS donor, with or without PLGA-NP (30 or 150 µg/cm2). Fluorescence of H2DCF-DA was measured to quantify ROS induction.
Abbreviations: PLGA, poly-lactic-co-glycolic acid; NP, nanoparticles; H2DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen species; THBP, tert-butyl hydroperoxide.