Abstract
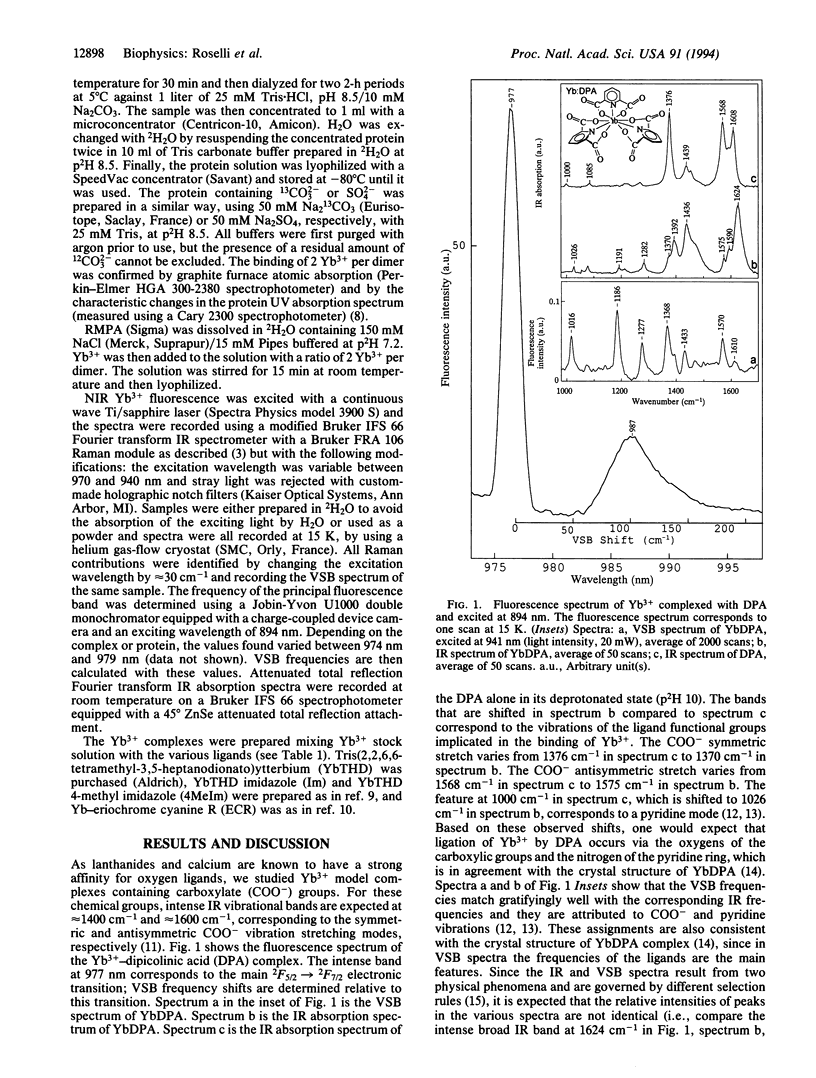
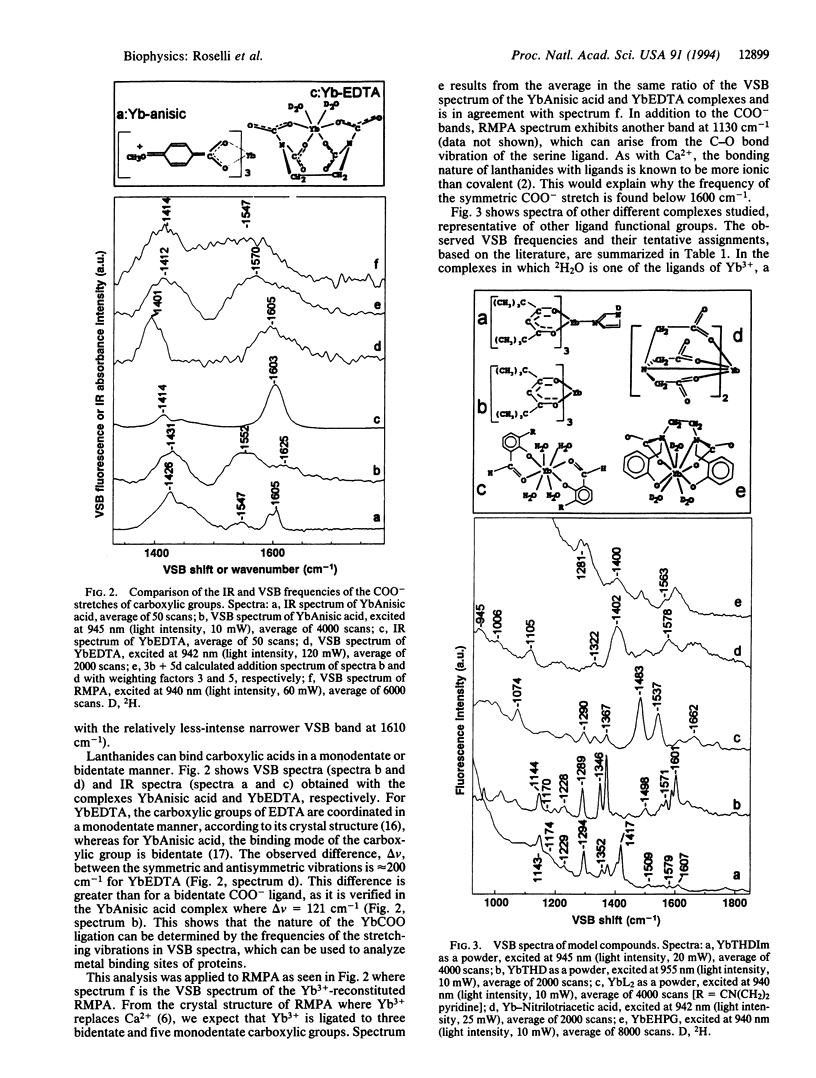
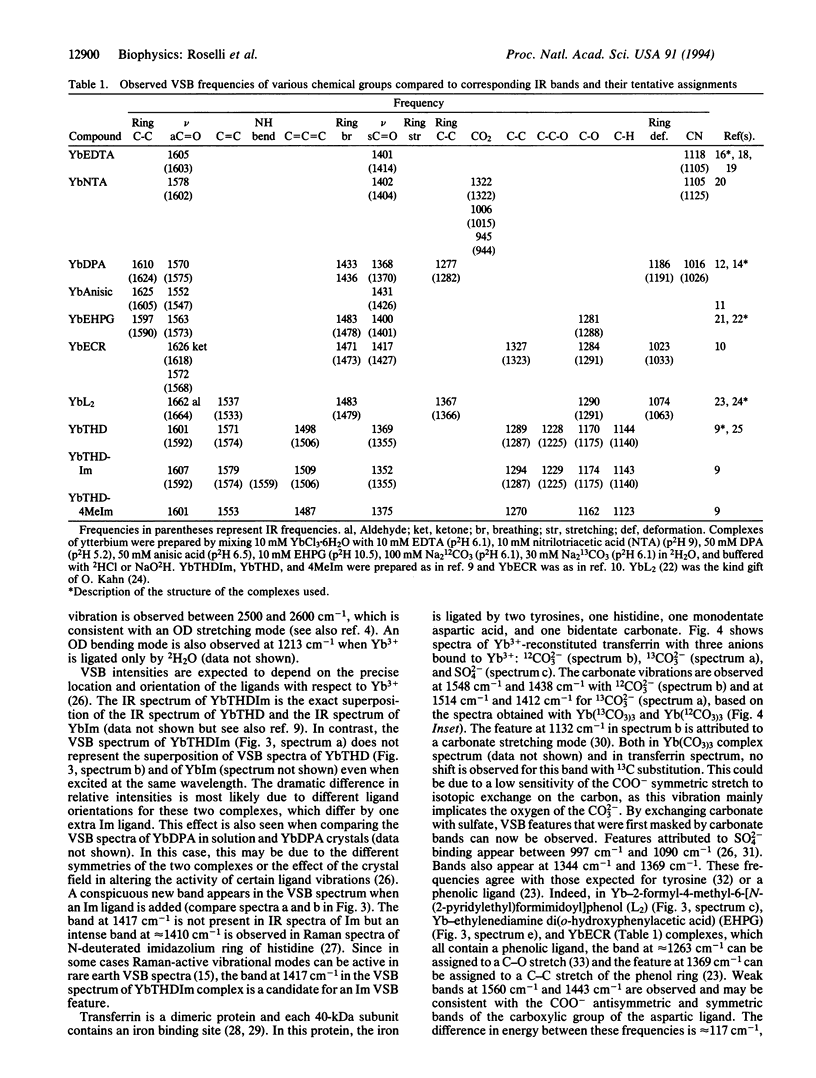
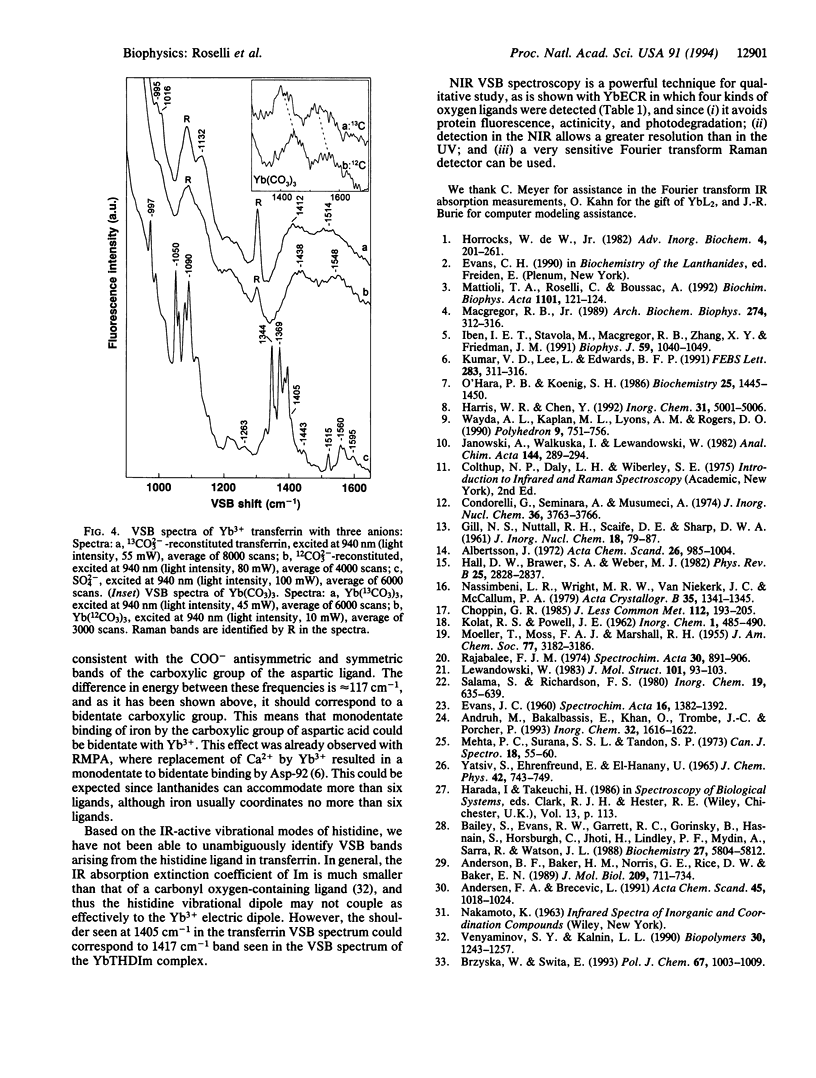
Near-infrared Yb3+ vibronic side band (VSB) spectroscopy is used to obtain structural information of metal binding sites in metalloproteins. This technique provides a selective "IR-like" vibrational spectrum of those ligands chelated to the Yb3+ ion. VSB spectra of various model complexes of Yb3+ representing different ligand types were studied to provide references for the VSB spectra of Yb(3+)-reconstituted metalloproteins. Ca2+ in the calcium-binding protein parvalbumin and Fe3+ in the iron-transporting protein transferrin were replaced with Yb3+. The fluorescence of Yb3+ reconstituted into these two proteins exhibits weak VSBs whose energy shifts, with respect to the main 2F5/2-->2F7/2 Yb3+ electronic transition, represent the vibrational frequencies of the Yb3+ ligands. The chemical nature of the ligands of the Yb3+ in these proteins, as deduced by the observed VSB frequencies, is entirely in agreement with their known crystal structures. For transferrin, replacement of the 12CO3(2-) metal counterion with 13CO3(2-) yielded the expected isotopic shift for the VSBs corresponding to the carbonate vibrational modes. This technique demonstrates enormous potential in elucidating the localized structure of metal binding sites in proteins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Iben I. E., Stavola M., Macgregor R. B., Zhang X. Y., Friedman J. M. Gd3+ vibronic side band spectroscopy. New optical probe of Ca2+ binding sites applied to biological macromolecules. Biophys J. 1991 May;59(5):1040–1049. doi: 10.1016/S0006-3495(91)82319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. D., Lee L., Edwards B. F. Refined crystal structure of ytterbium-substituted carp parvalbumin 4.25 at 1.5 A, and its comparison with the native and cadmium-substituted structures. FEBS Lett. 1991 Jun 3;283(2):311–316. doi: 10.1016/0014-5793(91)80616-b. [DOI] [PubMed] [Google Scholar]
- MacGregor R. B., Jr Vibrational spectroscopy in the ultraviolet via Gd3+ fluorescence: application to biological systems. Arch Biochem Biophys. 1989 Oct;274(1):312–316. doi: 10.1016/0003-9861(89)90444-x. [DOI] [PubMed] [Google Scholar]
- Mattioli T. A., Roselli C., Boussac A. Near-infrared Yb(3+) vibronic sideband spectroscopy: application to Ca(2+)-binding proteins. Biochim Biophys Acta. 1992 Jul 6;1101(1):121–124. doi: 10.1016/0167-4838(92)90475-s. [DOI] [PubMed] [Google Scholar]
- O'Hara P. B., Koenig S. H. Electron spin resonance and magnetic relaxation studies of gadolinium(III) complexes with human transferrin. Biochemistry. 1986 Mar 25;25(6):1445–1450. doi: 10.1021/bi00354a038. [DOI] [PubMed] [Google Scholar]
- Trudnowski R. J., Mehta M. P., Rucinski M. Evaluation of the mutagenic potential of enflurane and isoflurane by sister chromatid exchange. J Med. 1987;18(1):55–60. [PubMed] [Google Scholar]
- Venyaminov SYu, Kalnin N. N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers. 1990;30(13-14):1243–1257. doi: 10.1002/bip.360301309. [DOI] [PubMed] [Google Scholar]


