Abstract
Background: Giant cell tumor of bone (GCT) is a potentially malignant tumor. CD147 is a multifunctional protein, which expresses itself in many tumors. In this study, we have investigated the correlation between CD147 and PCNA, VEGF, MMPs expression in giant cell tumor of bones. We have also explored the relationship between its clinical pathology and prognosis. Results: A significant difference of the expression level of CD147, MMPs was found in cases of GCT with Jaffe grading and prognosis (P<0.05). But, it was not in accordance with the patient’s age and sex. An expression of CD147 was positively correlated with MMP-9, VEGF, MVD, PCNA (r=0.271, P=0.025; r=0.411, P=0.000; r=0.872, P=0.000; r=0.394, P=0.001). Conclusion: The expression level of CD147 in giant cell tumors of bones is correlated with the development of cancers and relapse. There was a positive correlation between expressions of CD147 and MMP-9, VEGF, MVD, PCNA, and CD147. This is an important indicator in evaluating the malignant degree and prognosis of giant cell tumors of bone. It may be the new target for ensuring chemopreventive strategies.
Keywords: Giant cell tumor of bone, extracellular matrix metalloproteinases inducer, EMMPRIN, matrix metalloproteinases, MMP, vascular endothelial growth factor, immunohistochemistry
Introduction
Giant cell tumor of bone (GCT) is a potentially malignant tumor. The incidence of this tumor in China is 2-3 times more common than in the western countries. Local aggressive growth and post-operative recurrence scale is as high as 40%-50%. The pathological classification and image credits type of GCT cannot be precisely predicted with its clinical behavior. So, it’s very important to consider other factors, which relate with the GCT’s biological behavior and prognosis. EMMPRIN (CD147) is a member of immunoglobulin (Ig) super family, and a cell surface glycoprotein can be expressed through numerous cell types. It is involved in the origin and development of the tumor through several tumorigenic mechanisms [1,2]. It has the capacity to degrade extracellular basal membranes and stromal elements through angiogenesis, thereby stimulating MMPs production. In this study, we have examined the immune-histochemical staining patterns in a series of GCT patients, with the objective of elucidating their prognostic role and their correlation with CD147, MMPs, VEGF, PCNA, and CD34.
Materials and methods
Patients and tissue specimens
All specimens were handled according to ethical and legal standards. The identity of the samples was kept anonymous. 68 patients (32 males and 36 females, aging 12-58 years, average 34 years) with GCT were obtained from the Department of Orthopedics, Xi-jing Hospital of the Fourth Military University, Xi’an, China. Two senior pathologists performed pathological diagnosis on the GCT group. According to Jaffe staging system, 24 tumors were diagnosed as stage I. 37 tumors were diagnosed as stage II, and 7 tumors were diagnosed as stage III. All the specimens were fixed in 10% neutral buffered formalin. These were subsequently embedded in the paraffin. The paraffin-embedded tissues were cut at 4 μm.
Consent was obtained from all patients, and there is ethical approval from our institution for this procedure.
Immunohistochemical staining and analysis
The following antibodies were bought from Zhong-Shan Golden bridge Biotechnology, Beijing: rabbit antihuman CD147 polyclonal antibody, mouse antihuman CD34 monoclonal antibody, rabbit antihuman VEGF monoclonal antibody, mouse antihuman PCNA monoclonal antibody, rabbit antihuman MMP-2 polyclonal antibody, and rabbit antihuman MMP-9 polyclonal antibody. Sections (4 μm) were de-waxed in xylene, and rehydrated in graded alcohols. Antigen retrieval was performed by immersing it in citrate buffer for 10 min at 100°C in a water bath. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide in methanol for 20 min. Then, to block non-specific IgG binding, tissue or cell areas were incubated in normal goat serum for 30 min. Sliders were incubated overnight at 4°C in a wet chamber using primary antibodies. A biotinylated goat anti-rabbit or mouse IgG were incubated further, after subjecting it thrice to stringent washing in PBS. Then, a streptavidin-biotin complex system (ABC regents) with diaminobenzidene (DAB) was used as a chromogen for color development. Slides were finally counterstained with hematoxylin, and examined with the help of a light microscope.
Leica light microscope was used for evaluating the findings in this study. All immunostained sections were blindly evaluated by two independent investigators. The scoring of expression was based on both intensity and extensity. The percentage of positive tumor cells was determined semiquantitatively by assessing the whole tumor section, and each sample was scored on a scale of 0 to 4:0, negative; 1, positive staining in 1-25% cells; 2, 25-50% cells; 3, 50-75% cells; and 4, 75-100% cells. The intensity of staining was expressed as follows: grade 1 for light yellow staining; 2 for dark yellow staining; and 3 for brown yellow staining. The sum of these grades was termed as negative for scores between 0 and 1 (-), weak for 2 and 3 (+), mild for 4 and 5 (++), and strong for >6 (+++) (Table 3).
Table 3.
Immunohistochemistry Stage
| Percentage (%) | Score | Intensity (Color) | Score |
|---|---|---|---|
| 0 | 0 | Negative | 0 |
| 1~25 | 1 | Weak (Light yellow) | 1 |
| 26~50 | 2 | Mild (Dark yellow) | 2 |
| 51~75 | 3 | Strong (Brown yellow) | 3 |
CD34 was used in the present study to visualize the microvessels in the tumor specimen. For counting the microvessels, the entire section was systematically scanned at ×100 magnification and the area containing the greatest number of CD34 antigen positive cells was classified as a ‘hot spot’. In order to be defined as a ‘hot spot’, the density of antigen positive cells and cell clusters needed to be greater relative to adjacent areas. A vessel was considered positive if there was staining for a single endothelial cell, endothelial cell cluster, or microvessel that was clearly separated from adjacent microvessels. The magnification was changed to ×200 then ×400, and the slide was repositioned over the area of the ‘hot spot’ with the most vessels per field. For each section, the ‘hottest’ spots were assessed and the mean count of all independent readings was calculated, defined as the mean count of microvessels per ×400 field area.
Statistical analysis
Statistical analysis was performed using the software of SPSS version 13.0 for Windows. The association between CD147, VEGF, MMPs expression, and various clinic-pathological characteristics was analyzed with the help of nonparametric test. The correlation between CD147, VEGF, MMPs, CD34, and PCNA expression was analyzed using Spearmen’s rank correlation analysis. When P was less than 0.05, the differences were considered as being statistically significant.
Results
Expression and location of CD147, MMPs, VEGF, CD34 and PCNA in GCT tissues
In 68 GCT specimens, CD147 staining was positive in 61 samples (89.70%), and highly expressed in 42 GCT patients (61.76%). Microscopic observations indicated that the signals concentrated primarily within the cytoplasm of different types of cells, that was, spindle-shaped stromal cells (CD147, Figure 1A), osteoclast-type giant cells (MMP-2, MMP-9, Figure 1B, 1C), cytoplasm of spindle-shaped stromal cells, osteoclast-type giant cells (VEGF, Figure 1D), cytoplasm of interstitial small vessels, microvascular endothelial cells (CD34, Figure 1E), and nucleus of spindle-shaped stromal cells (PCNA, Figure 1F).
Figure 1.
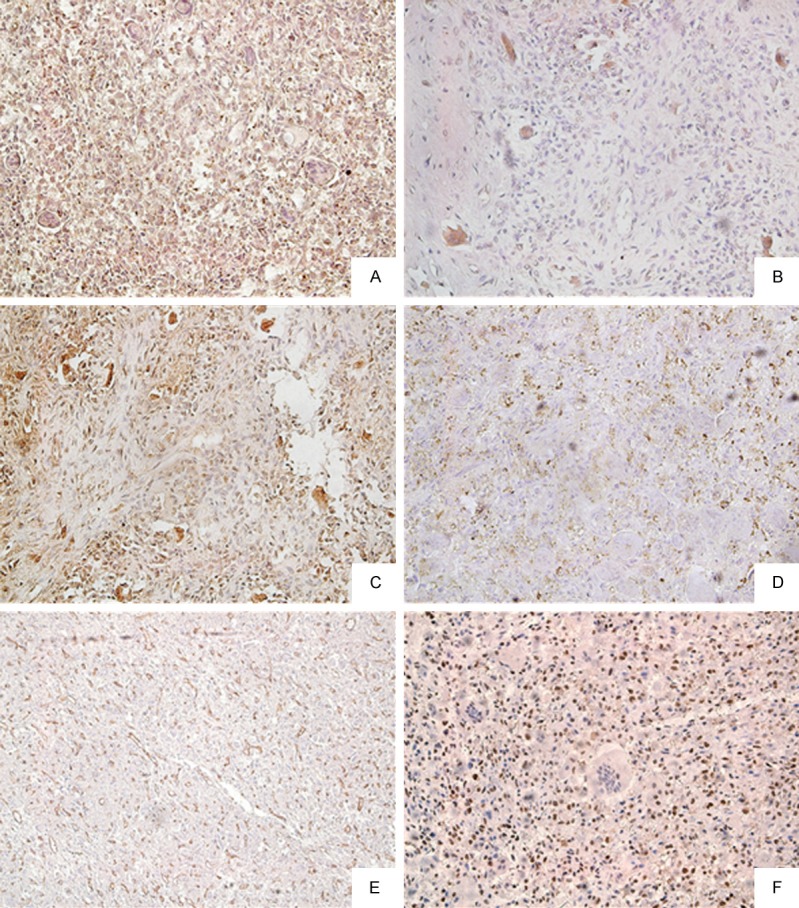
Immunohistochemical staining for CD147, MMp-2, VEGF, CD34 and PCNA expression in GCT. A: CD147; B: MMP-2; C: MMP-9; D: VEGF; E: CD34; F: PCNA. (Original magnification ×200).
Correlation of CD147, MMPs, and VEGF expression with the clinic-pathological features of GCT
Relationships between CD147, MMPs, and VEGF expression and the clinic-pathological features of patients with GCT were shown in Table 1. Statistically significant differences were found between CD147, MMP-2 expression, and Jaffe staging system (P-0.045, P-0.032, respectively). The differences between CD147, MMP-2, MMP-9 expression, and their prognosis were also statistically significant (P-0.008, P-0.024, P-0.034, respectively). Although other clinic-pathological features were not associated with CD147, MMPs, and VEGF expression, further large studies are still needed to investigate the correlation between CD147 expression and these parameters.
Table 1.
Correlation of CD147, VEGF, MMP-2 and MMP-9 expression with clinical features in GCT
| Clinical features | No. | CD147 | P | VEGF | P | MMP-2 | P | MMP-9 | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||||
| - | + | ++ | +++ | + | ++ | +++ | + | ++ | +++ | + | ++ | +++ | ||||||
| Gender | ||||||||||||||||||
| Male | 32 | 3 | 8 | 16 | 5 | 0.886 | 8 | 19 | 5 | 1.000 | 12 | 14 | 6 | 1.000 | 6 | 12 | 14 | 0.700 |
| Female | 36 | 4 | 11 | 14 | 7 | 15 | 19 | 2 | 9 | 15 | 11 | 9 | 12 | 15 | ||||
| Age(years) | ||||||||||||||||||
| ≤20 | 11 | 0 | 4 | 5 | 2 | 0.115 | 3 | 6 | 2 | 0.285 | 3 | 5 | 3 | 0.156 | 4 | 3 | 4 | 0.126 |
| 21~ | 36 | 7 | 9 | 16 | 4 | 13 | 20 | 3 | 16 | 10 | 10 | 8 | 10 | 18 | ||||
| 41~ | 21 | 0 | 6 | 9 | 6 | 7 | 12 | 2 | 2 | 14 | 5 | 3 | 11 | 7 | ||||
| Jaffe stage | ||||||||||||||||||
| I | 24 | 4 | 7 | 8 | 5 | 0.045* | 13 | 9 | 2 | 0.21 | 8 | 10 | 5 | 0.032* | 5 | 9 | 10 | 0.050 |
| II | 37 | 3 | 10 | 18 | 6 | 7 | 27 | 3 | 12 | 16 | 10 | 8 | 13 | 16 | ||||
| III | 7 | 0 | 2 | 4 | 1 | 3 | 2 | 2 | 1 | 4 | 2 | 2 | 2 | 3 | ||||
| Prognosis | ||||||||||||||||||
| Normal | 50 | 5 | 15 | 22 | 8 | 0.008* | 15 | 30 | 5 | 0.052 | 16 | 24 | 11 | 0.024* | 13 | 16 | 21 | 0.034* |
| Canceration | 3 | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | ||||
| Recurrence | 14 | 2 | 3 | 6 | 3 | 6 | 7 | 1 | 3 | 6 | 5 | 1 | 7 | 6 | ||||
| Transfer | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ||||
P<0.05.
Association of CD147 expression with MMPs, VEGF, CD34, PCNA expression in GCT tissues
PCNA has been demonstrated to be a useful tool in evaluating cell proliferation. MMP is considered as an important contributor to tumor invasion and metastasis. To determine the proliferation and metastasis index of tumor cells, the section were immunehistochemically stained with PCNA and MMP antibody. CD34 is a well-known endothelial cell marker. Immunoreaction for CD34 was cytoplasmic and endothelial cells were especially strongly positive. In our study, CD34 was used to visualize the microvessels in GCT tissues to calculate MVD. According to Spearmen’s rank correlation analysis, there was a significantly positive correlation between CD147 and MMP-9, VEGF, CD34, PCNA (r=0.271, P=0.025; r=0.411, P=0.000; r=0.872, P=0.000; r=0.394, P=0.001) (Table 2).
Table 2.
Correlation of CD147 expression with MMPs, VEGF, CD34, PCNA expression
| CD147 | MMP-2 | MMP-9 | VEGF | CD34 | PCNA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||||||
| + | ++ | +++ | r | P | + | ++ | +++ | r | P | + | ++ | +++ | r | P | r | P | r | P | |
| - | 5 | 0 | 2 | 0.227 | 0.063 | 2 | 2 | 3 | 0.271 | 0.025* | 4 | 3 | 0 | 0.411* | 0.000 | 0.872 | 0.000* | 0.394 | 0.001* |
| + | 10 | 5 | 4 | 7 | 8 | 4 | 9 | 10 | 0 | ||||||||||
| ++ | 4 | 17 | 9 | 5 | 8 | 17 | 8 | 9 | 13 | ||||||||||
| +++ | 3 | 7 | 2 | 0 | 6 | 6 | 0 | 10 | 2 | ||||||||||
P<0.05.
Discussion
Extracellular MMP inducer (EMMPRIN, CD147), also referred to as basigin, is produced by tumor cells [3-5]. CD147 is a multifunctional protein, which plays an important role in the development, reproductive physiology [6], immune regulation [7], and induction of MMP production. However, the latter is probably most relevant to neoplastic setting, because an increased expression of CD147 has been recorded in a variety of tumor types. It has been shown in vivo and in vitro that we need to correlate with the up-regulation of various MMP, including MMP2 and MMP9 [3-5]. Furthermore, CD147 secretion by tumor cells can stimulate CD147 synthesis of fibroblasts. Thus, it potentially increases the activation of MMP in the tumor-associated stroma. EMMPRIN in tumor invasiveness has been confirmed immunohistochemically in several types of cancer cells, including astrocytomas, myeloma, and melanomas [8].
PCNA, an auxiliary protein of DNA polymerase, is a proliferation associated marker. Its maximal expression peak is in the late G1-phase and S-phase of the cell cycle. PCNA has been used as a proliferation marker in different neoplasms. Matrix metalloproteinases (MMPs) comprises of more than 20 enzymes, which degrade the basement membrane and extra-cellular matrix (ECM) components in numerous physiological and pathological situations [9-11]. An increased MMP activity is considered as an important contributor to tumor invasion and metastasis, which destroys ECM components. These potentiate tumor cell formation, migration, intravasation, and extravasation [12-14]. Matrix metalloproteinase-9 (MMP-9) acts as an important oncogene, thereby improving the invasiveness of cancer cells [15,16]. Most research studies have indicated that a high level of MMP-9 confers a poor prognosis in various cancers [17]. On the other hand, in the context of neoplasia, MMP2 (gelatinase A) has attracted particular interest, as it degrades type IV collagen, a major component of basement membrane undergoing destruction at an early stage of the invasive process [18]. However, MMP2 has a broader substrate specificity, which includes other collagen subtypes, elastin, and fibronectin. It also activates other members of the MMP family. Indeed, it is now clear that MMP activity in neoplasia has not been restricted to the physical degradation of ECM elements, but it includes influences on cellular proliferation, survival, and angiogenesis [19-21]. An increased expression of various MMP has been described in numerous human tumors and animal models of malignancy. In general, an over-expression has been associated with adverse clinico-pathological factors and increased metastatic potential.
VEGF is the most extensively studied angiogenic factor. It has been related with tumor progression, metastasis, and prognosis. Konno et al reported that an over expression of VEGF in colorectal cancer, when compared with that of the normal tissue. It also propounded that it had a strong correlation with the size of tumor [22]. VEGF and its receptor targeted the biological therapy in tumor, as it had a relatively high tolerance effect [23].
Our studies suggest that there was no significant difference between CD147, MMPs, VEGF, gender, and age of GCT patients. This suggests that it cannot be indicator of invasion and recurrence. The differences between CD147, MMP-2 expression and Jaffe staging system were statistically significant (P-0.045, P-0.032, respectively). Moreover, the differences between CD147, MMP-2, MMP-9 expression, and prognosis were also statistically significant (P-0.008, P-0.024, P-0.034, respectively). This suggests that CD147 and MMPs were correlated with the invasion, migration, and prognosis of GCT. Recently, quite a few research studies have confirmed that the histological appearance of tumor was not in accordance with the biological behavior. However, Jaffe and Enneking staging system can estimate the local invasion of tumor. They cannot predict the possibility of recurrence and migration. In recent times, research in P53 and C-myc suggests that the mutation of P53 significantly correlates with the local recurrence of GCT. An over expression of C-myc has been correlated with the migration of GCT [24,25]. According to our study, CD147 and MMPs expression correlated with the stage and prognosis of GCT, thereby suggesting that CD147 and MMPs is an important indicator in the prognosis of GCT.
In the present study, we have presented evidence that there is a significantly positive correlation between CD147 and MMP-9, VEGF, CD34, PCNA (r=0.271, P=0.025; r=0.411, P=0.000; r=0.872, P=0.000; r=0.394, P=0.001 respectively). These findings confirm the existence of cervical cancer [26], which suggests that CD147 plays an important role in the formation and migration of GCT by stimulating it with MMPs and VEGF. Kanekura et al was of the view that CD147 primarily stimulates the fibroblast around tumor syntheses MMPs, and releases some factors of angiogenesis feedback circuits, including VEGF [27]. Furthermore, Tang et al propounded that CD147 VEGF expression was unregulated through the signal pathway of PI3K-Akt [28]. MMPs have played an important role in the invasion and migration of tumors. They block the activity of MMPs through a method which restricts the development of tumors. However, artificial MMPs blockers cannot achieve the desired effects. In another method, we avoid the generation of excessive MMPs in the tumor tissues. CD147 is an important factor stimulating MMPs syntheses. In addition, CD147 facilitates the formation of vascular tumors through tumor cell interactions. So, it is immensely useful from the point of view of research. Quite a few tools block the activity of CD147, including functional block peptide [29], antibody, antisense express structure [30], and CD147 siRNA. Monoclonal antibody inhibits CD147 activity. This has been effectively confirmed by restraining the invasion and migration of tumors in the laboratory. So, it is important to improve the prognosis of GCT patients, and investigate the expression and mechanism of CD147 in tumors. This will help us in finding a new way for GCT treatment by developing inhibitors of CD147.
Acknowledgements
This work was supported by the Ministry of Science and Technology of China (2011CB964703) and National Natural Science Foundation of China (81472043), and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13051). No benefits in any form have been or will be received from a commercial party directly or indirectly by the authors of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Stewart CJ, Crook ML. CD147 (EMMPRIN) and matrix metalloproteinase-2 expression in uterine endometrioid adenocarcinoma. Pathol Res Pract. 2011;207:30–36. doi: 10.1016/j.prp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011;20:13–19. doi: 10.1016/j.suronc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H, Tanaka H, Nagaike K, Uchiyama S, Itoh H. Role of cancer cell-stroma interaction in invasive growth of cancer cells. Hum Cell. 2003;16:1–14. doi: 10.1111/j.1749-0774.2003.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 5.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–317. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igakura T, Kadomatsu K, Taguchi O, Muramatsu H, Kaname T, Miyauchi T, Yamamura K, Arimura K, Muramatsu T. Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem Biophys Res Commun. 1996;224:33–36. doi: 10.1006/bbrc.1996.0980. [DOI] [PubMed] [Google Scholar]
- 8.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 9.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 10.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 11.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 12.Lichtinghagen R, Helmbrecht T, Arndt B, Boker KH. Expression pattern of matrix metalloproteinases in human liver. Eur J Clin Chem Clin Biochem. 1995;33:65–71. doi: 10.1515/cclm.1995.33.2.65. [DOI] [PubMed] [Google Scholar]
- 13.Westerlund A, Hujanen E, Puistola U, Turpeenniemi-Hujanen T. Fibroblasts stimulate human ovarian cancer cell invasion and expression of 72-kDa gelatinase A (MMP-2) Gynecol Oncol. 1997;67:76–82. doi: 10.1006/gyno.1997.4808. [DOI] [PubMed] [Google Scholar]
- 14.Parsons SL, Watson SA, Collins HM, Griffin NR, Clarke PA, Steele RJ. Gelatinase (MMP-2 and -9) expression in gastrointestinal malignancy. Br J Cancer. 1998;78:1495–1502. doi: 10.1038/bjc.1998.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhul AA, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signalling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 17.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 18.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 19.Konno H, Tanaka T, Baba M, Kanai T, Matsumoto K, Kamiya K, Nakamura S, Baba S. Quantitative analysis of vascular endothelial growth factor in colon cancer. Clinical and experimental. Eur Surg Res. 1998;30:273–278. doi: 10.1159/000008587. [DOI] [PubMed] [Google Scholar]
- 20.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 21.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 22.Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci (Landmark Ed) 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 23.Papanastassiou I, Ioannou M, Papagelopoulos PJ, Arealis G, Mihas C, Iakovidou I, Demertzis N. P53 expression as a prognostic marker in giant cell tumor of bone: a pilot study. Orthopedics. 2010:33. doi: 10.3928/01477447-20100329-15. [DOI] [PubMed] [Google Scholar]
- 24.Gamberi G, Benassi MS, Bohling T, Ragazzini P, Molendini L, Sollazzo MR, Merli M, Ferrari C, Magagnoli G, Bertoni F, Picci P. Prognostic relevance of C-myc gene expression in giant-cell tumor of bone. J Orthop Res. 1998;16:1–7. doi: 10.1002/jor.1100160102. [DOI] [PubMed] [Google Scholar]
- 25.Yu W, Liu J, Xiong X, Ai Y, Wang H. Expression of MMP9 and CD147 in invasive squamous cell carcinoma of the uterine cervix and their implication. Pathol Res Pract. 2009;205:709–715. doi: 10.1016/j.prp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res. 2006;4:371–377. doi: 10.1158/1541-7786.MCR-06-0042. [DOI] [PubMed] [Google Scholar]
- 28.Nabeshima K, Suzumiya J, Nagano M, Ohshima K, Toole BP, Tamura K, Iwasaki H, Kikuchi M. Emmprin, a cell surface inducer of matrix metalloproteinases (MMPs), is expressed in T-cell lymphomas. J Pathol. 2004;202:341–351. doi: 10.1002/path.1518. [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 2008;65:2933–2942. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo JH, Cheng HP, Yu L, Zhao S. Natural antisense transcripts of Alzheimer’s disease associated genes. DNA Seq. 2006;17:170–173. doi: 10.1080/10425170600609165. [DOI] [PubMed] [Google Scholar]


