Abstract
Recent legislative successes allowing expanded access to recreational and medicinal cannabis have been associated with its increased use by the public, despite continued debates regarding its safety within the medical and scientific communities. Despite legislative changes, cannabis is most commonly used by smoking, although alternatives to inhalation have also emerged. Moreover, the composition of commercially available cannabis has dramatically changed in recent years. Therefore, developing sound scientific information regarding its impact on lung health is imperative, particularly because published data conducted prior to widespread legalization are conflicting and inconclusive. In this commentary, we delineate major observations of epidemiologic investigations examining cannabis use and the potential associated development of airways disease and lung cancer to highlight gaps in pulmonary knowledge. Additionally, we review major histopathologic alterations related to smoked cannabis and define specific areas in animal models and human clinical translational investigations that could benefit from additional development. Given that cannabis has an ongoing classification as a schedule I medication, federal funding to support investigations of modern cannabis use in terms of medicinal efficacy and safety profile on lung health have been elusive. It is clear, however, that the effects of inhaled cannabis on lung health remain uncertain and given increasing use patterns, are worthy of further investigation.
Over the past decade, US cannabis legislation has changed dramatically at the state level, enabling sales for medicinal and recreational use. Although not necessarily causal, paralleling these legislative changes, per capita cannabis consumption has also risen.1,2 In 2013, 650,000 Washington State residents (9% of the population), and 393,000 Colorado residents (7.3% of the population) reported using cannabis at least monthly. Among these regular cannabis users, 25% to 32% used cannabis daily, with an average of 3.5 to 3.9 joints smoked per day.1,2 More worrisome, legalization of medicinal marijuana has been associated with both decreased risk perceptions and increased use among Colorado youth compared with 34 states without medicinal laws.3 Because smoking remains the most prevalent route of cannabis consumption,4 its increasingly widespread use and social acceptability raise concerns regarding its future impact on lung health in the United States.
In this commentary, we highlight limitations in understanding the effects of inhaled cannabis on lung health based on epidemiologic investigations regarding the relationship of cannabis to airways diseases and lung cancer. We also focus on areas in animal model and human clinical and translational research where novel investigations can broaden the scope of current research knowledge.
Biology of the Cannabis Plant
To understand the potential clinical impact of inhaled cannabis use on lung health, it is important to appreciate the plant’s complexity and mechanisms of action.5 Cannabis contains 483 unique compounds, including 66 cannabinoids. Ten subclasses of cannabinoids have been characterized, including Δ-9-tetrahydrocannabinols (THCs) and seven subclasses of cannabidiols (CBDs).6 THCs are the primary psychoactive substances, with pharmacologic properties including euphoria and analgesia. In contrast, CBDs possess anxiolytic properties that counter effects of THC. Two cannabinoid receptors, G-protein-coupled transmembrane proteins negatively coupled to adenylyl cyclase termed CB1 and CB2, have been identified in humans.7 These receptors are activated by both endogenous endocannabinoids (a family of locally produced, phospholipid-derived substances8) and many exogenous cannabinoids. CB1 receptors are predominantly localized on neurons within the CNS where they mediate the psychogenic effects of cannabis and are found in the autonomic innervation of airway smooth muscle, whereas CB2 receptors are primarily localized on immune cells.
Epidemiologic Investigations Related to Cannabis and Lung Health
The focus of epidemiologic data published in the past 15 years has primarily been on understanding the relationship between cannabis use and airways diseases9‐15 (Table 1). Smoking cannabis has been associated with an increase in total lung capacity and FVC,9,10,13 potentially resulting from deep-breathing maneuvers of users.9,10 In the setting of heavy, prolonged cannabis use (typically > 20 joint-years),9,11,13,15 a dose-response association between development of airflow limitation (either decreased FEV1 or decreased FEV1/FVC) has also been reported, the latter possibly attributable to increased FVC. Symptoms referable to chronic bronchitis, including daily cough, phlegm production, and wheeze, have been noted in cannabis smokers after less-intense use.9,11,12,15 In terms of lung cancer, epidemiologic data suggesting an association between increased duration and quantity of cannabis exposure with higher odds of lung malignancy development16‐18 have not been consistent19 (Table 2). Together, these epidemiologic data imply that modest consumption of cannabis may have a minimal impact on lung health. Importantly, however, these data were largely collected prior to recent legislative changes; therefore, the relatively small percentage of heavy cannabis consumers surveyed may not reflect emerging trends in cannabis use.
TABLE 1 ] .
Epidemiologic Studies of Cannabis Effects on Airways
| Study Type | Cohort Size, Location, Racial Features | Cannabis Users in Cohort, With and Without Tobacco | How Cannabis Use Assessed and Quantitateda | Approximate Cannabis Use Among Cannabis Users | Years of Cannabis Use | Main Findings and Observations | Reference |
| Longitudinal (8-y period) | 394 United States 100% white |
131 cannabis 112 cannabis + tobacco 65 tobacco, 86 nonsmokers |
Detailed drug questionnaires endorsed by ATS, NHLBI, and NIDA | 3-4 joints/d or 45-56 joint-y (first visit) |
1983-1993 | No ↓FEV1 over 8 y associated with cannabis smoking; no additive effect of cannabis and tobacco on FEV1; only 20 of 394 followed full 8 y | 14 |
| Longitudinal (data obtained from subjects at age 18, 21, and 26 y) | 900 New Zealand Race not reported |
50% occasional cannabis users 10% cannabis dependent (DSM-IV) Unspecified number of tobacco smokers |
No. occasions cannabis used in preceding 12 mo by survey | At age 18 y, 22 of 859 subjects (2.5%) cumulatively used cannabis > 300 d At age 26, 108 of 930 subjects (11.6%) had used > 300 d |
1990-1998 | Subjects at risk for ↓FEV1/FVC either used cannabis on > 900 occasions or smoked ≥ 20 cigarettes/d; additive effects from cannabis and tobacco use on ↓FEV1/FVC | 15 |
| Cross-sectional | 6,728 United States (NHANES III) 68% white |
94 cannabis 320 cannabis + tobacco 1,525 tobacco 4,789 nonsmokers |
Questionnaires on lifetime use of cannabis and past month use of cannabis | Cannabis smoked 10.2 d/mo; 16% near-daily cannabis users; 77% also smoked tobacco | 1988, 1994 | After controlling for tobacco, cannabis use was associated with chronic bronchitis, cough, phlegm production, and wheezing but not ↓FEV1/FVC < 70% | 12 |
| Cross-sectional | 339 New Zealand 90% white |
75 cannabis 91 cannabis + tobacco 92 tobacco 81 nonsmokers |
Questionnaires on lifetime cannabis use, joint-y | Average 46-54 joint-y among cannabis users; wide CIs | Before 2007 | Dose-response relationship of joint-y cannabis smoking and ↓FEV1/FVC, ↓sGaw, ↑TLC; one joint of cannabis had equal effects on ↓FEV1/FVC as 2.5-5 tobacco cigarettes | 9 |
| Longitudinal (data obtained from subjects at age 18, 21, 26, and 32 y) | 1,037 New Zealand European ethnicity |
248 cannabis 435 cannabis + tobacco 58 tobacco 226 nonsmokers |
Questionnaires on No. times cannabis used in past year in joint-y since age 17 y; tobacco smoked/d | 23% (222 of 967) with > 1 joint-y (110 of 222 [50%] with > 10 pack-y tobacco) 48% (461 of 967) with ≤ 1 joint-y (82 of 461 [18%] with > 10 pack-y tobacco) |
1980-2004 | Longitudinal analyses (adjusted) in cannabis users revealed ↑FVC; in tobacco users, ↓FEV1, FEV1/FVC; cannabis-only smokers had ↑TLC, Va, ↓sGaw; only 40 of 967 in cohort smoked > 1 joint-y and did not smoke cigarettes | 10 |
| Longitudinal (data obtained over a 20-y period) | 5,115 United States 48% white and 51% black |
795 cannabis 1,065 cannabis + tobacco 851 tobacco, 2,305 nonsmokers |
Questionnaires on lifetime exposure in joint-y; joints/d in past 30 d; tobacco in pack-y | 2-3 d of use in past 30 d; 0.9-1.5 joint-y | 1985-2005 | At ≥ 20 joint-y cannabis, ↓FEV1, ↑FVC; trend for ↓FEV1 ≥ 10 joint-y; tobacco smoking had stronger impact on airflow limitation than cannabis | 13 |
| Cross-sectional | 6,723 United States (NHANES III) 67% white and 12% black |
184 cannabis 671 cannabis + tobacco 2,975 tobacco 2,893 nonsmokers |
Questionnaires on lifetime exposure in joint-y; past 30-d cannabis use; tobacco in pack-y | Among current cannabis users (tobacco and nontobacco users), 15.8 joint-y 12.0 d of use/mo |
2007-2010 | In logistic regression analyses, > 20 joint-y history predicted ↓FEV1/FVC < 70%, as did tobacco use between 1 and 5 pack-y; current cannabis users with ↑respiratory symptoms associated with longer duration and more intense use | 11 |
ATS = American Thoracic Society; DSM-IV = Diagnostic and Statistical Manual, Fourth Edition; NHANES = National Health and Nutrition Examination Survey; NHLBI = National Heart, Lung, and Blood Institute; NIDA = National Institute on Drug Abuse; sGaw = specific airway conductance; TLC = total lung capacity; Va = alveolar volume.
Pack-y = 1 pack/d of tobacco cigarettes × 20 cigarettes/pack × 365 d/y; joint-y = 1 joint/day × 365 d/y.
TABLE 2 ] .
Epidemiologic Studies of Cannabis Association With Lung Cancer
| Study Type | Cohort Size, Location, Racial Features | Subjects With Cancer and Control Subjects | How Cannabis Use Assessed and Quantitateda | Approximate Cannabis Use Among Cannabis Smokers | Years of Cannabis Use | Main Findings and Observations | Reference |
| Case-control cohort | 403 New Zealand residents |
79 lung cancer 324 control |
Duration of cannabis use, joint-y, pack-y tobacco use | 12 of 79 cases with > 10.5 joint-y compared with four of 324 control subjects | 2001-2005 | After adjustment for family history and pack-y tobacco, relative risk for lung cancer was 1.08 (95% CI, 1.02-1.15) per joint-y cannabis smoking | 16 |
| Cross-sectional | 29,195 National surveys on drug use and health, United States Adjusted for race/ethnicity |
Lung cancer by duration of cannabis use: Never = 9 ≤ 1 y = 23 2-10 y = 23 ≥ 11 y = 67 |
Duration of cannabis use (y); daily tobacco use also ascertained for use in models | Not reported | 2005-2007 | After adjustment, cannabis use of ≥ 11 y associated with OR for lung cancer development of 7.87 (95% CI, 1.28-48.4) | 18 |
| Longitudinal evaluation over 40 y of single cohort | 49,321 Swedish men born in 1949-1951 conscripted in 1969-1970 for military service |
189 lung cancer 49,132 control |
Ever or never use of cannabis, lifetime frequency of cannabis use, No. tobacco cigarettes per day | 5,156 ever cannabis users 831 of 5,156 (16%) reported > 50 times of use 515 of 831 (62%) also smoked > 10 cigarettes/d |
1969-2009 | After adjusting for tobacco and alcohol consumption, smoking cannabis > 50 times was associated with a hazard ratio of 2.12 (95% CI, 1.08-4.14) for lung cancer development over 40 y; ever use was not associated with an increased hazard ratio for lung cancer | 17 |
| Analysis of pooled data from six case-control studies | 5,144 International Lung Cancer Consortium: United States, Europe, New Zealand 79% white, 11% black |
2,159 lung cancer 2,985 control |
Lifetime cannabis use, joint-y; 86% of habitual cigarette smokers also used cannabis | 10% of patients with lung cancer were habitual cannabis users 6% with ≥ 10 joint-y |
1999-2013 | Little or no association between intensity, duration, or cumulative use of cannabis and risk of lung cancer development; pooled OR for association between cannabis smoking (habitual vs nonhabitual) and lung cancer risk was 0.95 (95% CI, 0.66-1.38) | 19 |
Pack-y = 1 pack/d tobacco cigarettes × 20 cigarettes/pack × 365 d/y; joint-y = 1 joint/d × 365 d/y.
Inhaled Cannabis and Tobacco Smoking
Cannabis consumers in most epidemiologic studies and users in general rarely consume only cannabis and no tobacco, a fact that limits understanding cannabis-specific effects on lung health. Moreover, in certain populations, cannabis often is smoked simultaneously with tobacco. Reasons for this preference include the fact that the combustion of both cannabis and tobacco together increases the vaporization efficiency of THC per gram of cannabis and may contribute to enhanced psychogenic effects compared with smoking cannabis alone.20 This suggests that concurrent use of tobacco and cannabis could compound effects on airways pathology, and indeed, one study reported additive effects.15 Therefore, accurate histories regarding both cannabis and tobacco use along with additional information regarding methods of use are important clinically and in scientific investigations. Routine spirometric monitoring of heavy or long-term cannabis users also appears to be warranted given the prevalence of airflow limitation among the heaviest users11 and limited longitudinal data in heavy users.13 Finally, as with tobacco use, cannabis use is more prevalent in individuals who use and abuse other drugs, including alcohol.21 Identification of cannabis use in clinical or research settings should, therefore, prompt screening for unhealthy use of other drugs and alcohol.
Alternative Devices to Inhale Cannabis
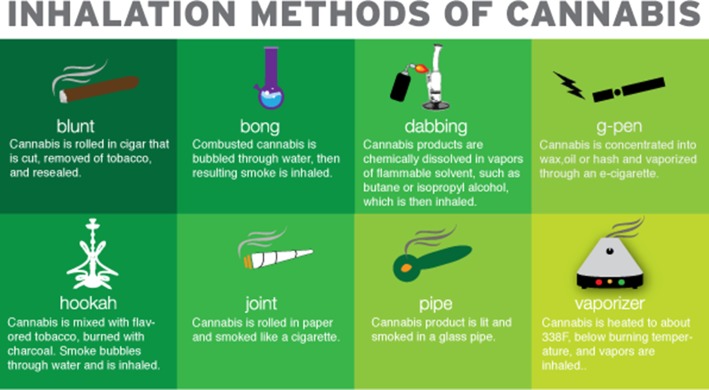
Epidemiologic studies have indicated that smoking remains the exceedingly most common route of cannabis use. The preference for smoking compared with oral or topical use may result from superior bioavailability and relatively quick onset of effects.22 Numerous methods to smoke cannabis currently exist (Fig 1); additionally, a variety of mechanisms for noncombusted cannabis inhalation have also increased in popularity, such as vaporizer devices that aerosolize cannabinoids by heating them to below burning temperature (Table 3). Vaporized use of cannabis has received increased interest both in the medical literature and by medicinal cannabis supporters in hopes to achieve the therapeutic benefits of cannabis without the potentially adverse effects from by-products of cannabis combustion. The use of portable, battery-operated vaporization devices has also been increasing, particularly among youth.23 Clinicians caring for patients reporting the use of vaporized or other noncombusted cannabis products need to be aware that these devices are touted as eliminating carcinogenic combustion products common to both smoked cannabis and tobacco (eg, tar, acrolein). However, neither carcinogenic nor other downstream effects of vaporized cannabis on airways have been rigorously tested. Additionally, effects of potentially increased THC delivery from vaporizers or other devices on pulmonary immune function, particularly alveolar macrophage function, remain unknown. Investigations outside the United States with commercially available vaporization devices are presently establishing methods to test pharmacologic effects of vaporized CBD and THC24; however, future investigations to delineate safety of vaporization or other routes of use on lung health in the United States will remain limited given the schedule I controlled status of cannabis.
Figure 1 –
Although many methods to inhale cannabis are well known, in recent years some older methods have gained increasing popularity (eg, hookah), and a number of new methods to inhale cannabis have emerged (eg, dabbing). These methods involve either inhaling combusted product or inhaling vaporized, noncombusted products (eg, G-pen). Rationales for an individual choice are highlighted in Table 3, along with potential harms and disadvantages associated with the method.
TABLE 3 ] .
Rationale and Disadvantages of Various Inhaled Cannabis Methods
| Mode of Inhaled Cannabis | Advantages | Disadvantages |
| Blunt | Inexpensive, effects of given quantity cannabis may be enhanced relative to consumption by other methods | Made from tobacco or cigar paper, smoke harsh in quality, difficult to roll |
| Bong | Water can trap some of the more harmful products of combustion | Expensive, easily broken, not as portable |
| Dabbing | Condensed product is easy to conceal, potent psychogenic effects | Burn injury common related to volatile agents used to solubilize condensed cannabis products |
| G-pen | More discreet, no smoke odor or visible smoke, no products of combustion | Little regulation of ingredients, use rising among youth |
| Hookah | Can smoke with multiple people, higher volume of smoke, flavors available | Product mixed with tobacco, combustion products potentially more damaging to the lungs |
| Joint | Small and convenient | Fragile, difficult to roll |
| Pipe | Product can be inhaled directly, producing more rapid psychogenic effects | Glass pipes can break, possible to inhale harmful resin |
| Vaporizer | No smoke odor, no products of combustion | Device relatively expensive, not portable, little regulation of ingredients |
See also Figure 1.
Lack of Objective Methods to Quantify Cannabis Use
All epidemiologic studies mentioned quantitated cannabis use by self-report using validated surveys and, therefore, are subject to recall bias. For occasional cannabis users, such bias would not likely be problematic, but in daily users, cognitive impairment from cannabis has the potential to influence data accuracy. Additionally, subject candor and accuracy may have suffered from the illegal nature of cannabis during the time when or in the place where data were collected. Therefore, development of biomarkers enabling an accurate assessment of current and past cannabis use are urgently needed to objectively determine the dose of cannabis that may compromise lung health. Assays of urine (less commonly saliva or blood) are widely available and reliably confirm cannabis exposure.25 However, their accuracy may be influenced by the time elapsed since last cannabis use; furthermore, they do not provide a quantitative estimate of use. Accurate quantitation can be performed through liquid chromatography-tandem mass spectrometry, although this method is cumbersome for routine clinical applications.26 To address these limitations, portable and sensitive real-time methods, including the application of an electronic nose, are currently undergoing clinical testing to detect subtle body odors related to metabolic changes on the human skin surface that indicate cannabis consumption.27 Understanding the relationship of pulmonary pathophysiology and symptoms to changes in biomarkers quantifying cannabis exposure may help to establish a threshold for harmful cannabis use and help to set policies regulating drug sales.
Needs in Future Research
Excellent reviews have been published describing the respiratory tract effects of cannabis, including its influence on inflammation and immunity that may contribute to the development of airways disease or lung cancer.7,28 Notable effects of smoked cannabis on airways include enhanced mucous secretion, airway hyperemia, basal and goblet cell hyperplasia, and squamous metaplasia.29‐31 Additionally, impaired alveolar macrophage phagocytosis and increased apoptosis have been consistently demonstrated in long-term cannabis smokers (Fig 2).32‐35 Nevertheless, changes in the composition of cannabis for purchase coupled with increasing consumption indicate that further research is warranted. As cannabis sales become increasingly commercialized, growers have successfully manipulated cannabis plant characteristics through methods such as indoor hydroponics technologies36 and alterations in electrical lighting power,37 resulting in the generation of cultivars with higher THC and CBD content. As a result, the quantity of THC in cannabis products has steadily increased from 3% in the 1980s to 12% in 2012.38,39 Moreover, Internet sales of seeds for high-THC cultivars have increased the reach of high-THC products,5 suggesting an unforeseen impact on lung health.
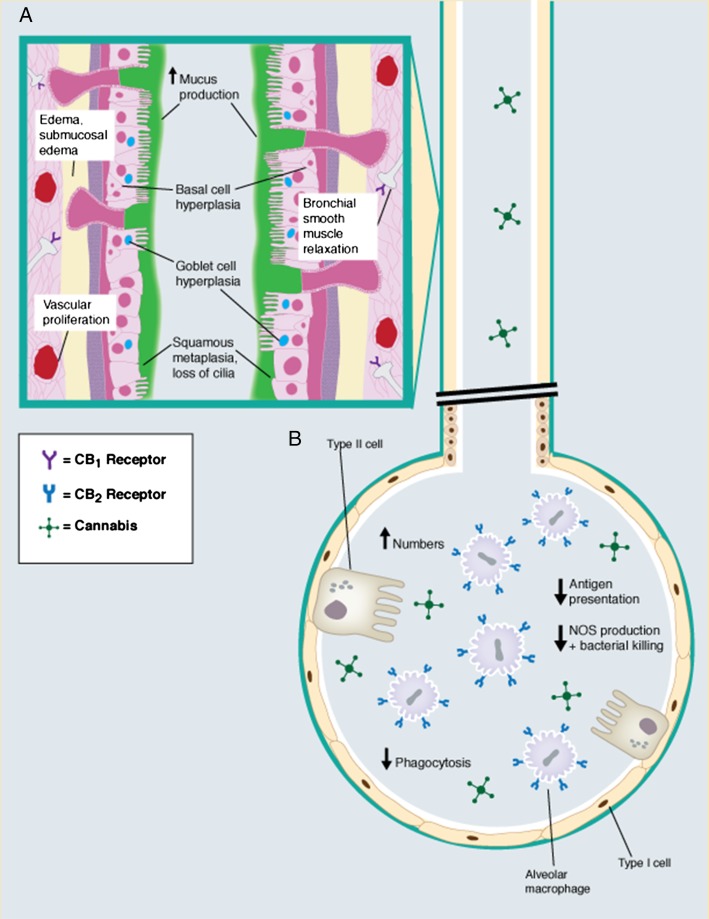
Figure 2 –
A, B, Laboratory investigations have historically ascribed a number of effects associated with habitual use of cannabis, particularly when it is used daily or for many years. A, Effects of inhaled cannabis on the upper airway feature increased mucous production and impaired mucociliary clearance that symptomatically correlate with increased cough and phlegm production reported by cannabis smokers.30,31 The presence of CB1 receptors on terminal axons that innervate airways smooth muscle are believed to be responsible for the acute bronchodilatory effects associated with inhaled cannabis.32 Premalignant lesions described with cannabis use include squamous metaplasia, an increased nuclear-to-cytoplasmic ratio, increased Ki-67 antigen, and increased epidermal growth factor receptor expression.29 B, In the alveoli, inhaled cannabis has been associated with increased numbers of alveolar macrophages. Moreover, the normal function of these cells may be impaired due to cannabis-CB2 receptor interactions,33‐35 further contributing to symptoms of chronic bronchitis reported by long-term cannabis users. However, the literature does not unequivocally support an increased incidence of pneumonia among long-term cannabis users. Airway lymphocytes (not shown) also possess CB2 receptors, but cannabinoid receptors have not been described on neutrophils. CB = cannabinoid; NOS = nitric oxide synthase.
Expanding Preclinical Investigations Using Novel Technologies
Given the suppressive effect of THC on immune cell function,7,28 investigations establishing the impact of high-concentration THC products on lung health and systemic immunity seem indicated and may be facilitated by developments in novel technologies and thoughtful study design. Parenteral administration of THC, a common research strategy in animal models, has been associated with marked differences in pharmacokinetic clearance and physiologic effects as the same dose of THC administered through inhalation. Because animal studies with inhaled cannabis more accurately reflect human use, investigations using experimental vaporized cannabis may provide more relevant data and are currently being testing in animals.40 Future animal investigations clarifying how both THC and non-THC cannabinoids alter immune function will also be critical to understanding the interplay between immune cell function in the lung and other organs. To facilitate cannabis research of specific cannabinoids, the National Institute on Drug Abuse contracts with academically based cannabis growers whose produce may be used with appropriate regulatory oversight in laboratory and clinical research investigations.41 Cannabis produced through this mechanism includes both smoked products and purified cannabinoids. Investigations using specific cannabinoids in known quantities with emerging technologies will be particularly crucial to understanding the immunologic ramifications of cannabis use. Highlighting one recent example, chromatin immunoprecipitation sequencing technology was used to define the influence of cannabis on T-cell gene expression and demonstrated that epigenetic regulation involving histone modifications can modulate the response in murine immune cells subjected to treatment with THC.42
Moreover, independent of cannabinoid effects, clarifying effects of combustion products related to smoking cannabis on airway epithelium deserves attention. Prior investigations have reported that mainstream cannabis smoke contains substantially higher quantities of ammonia, hydrogen cyanide, nitric oxide, and nitrogen oxides than comparable cigarette smoke,43 implying a more toxic effect from cannabis. Further support for the harm of cannabis smoke comes from toxicogenomic analyses comparing effects of cannabis and tobacco smoke extract on toxicologic pathways in cultured primary lung cells. Cannabis smoke extract elicits perturbations in oxidative stress, apoptosis, and inflammation pathways more potently than tobacco smoke and in a dose-dependent fashion.44 Other investigations with novel technologies have demonstrated a dose-dependent formation of acetaldehyde-DNA adducts, potential carcinogens, associated with cannabis smoke exposure.45 Finally, neuroscientists have begun using proteomics technologies evaluating brain tissue in the context of illicit drug use to demonstrate that cannabis use is associated with oxidative stress and alterations in signal transduction.46 However, use of proteomics and other omics technologies to establish mechanistic effects of cannabis on lung remain virtually unexplored.
Developmental Areas in Human Clinical Translational Research
Translational investigations supporting pathologic alterations in the lung related to cannabis smoking are limited by small sample sizes and data collection under restrictive cannabis legislation,31,47 calling into question the accuracy of cannabis reporting in both subjects and control subjects. Moreover, human investigations examining whether higher-concentration THC and CBD products or noncombusted inhaled cannabis alternatives influence lung pathology or systemic immunity are virtually nonexistent in the medical literature.
An area particularly deserving of further research regarding cannabis use is asthma. As with other epidemiologic investigations, associations between cannabis use and asthma symptoms are equivocal. A substantial number of people with asthma regularly use cannabis (19.3% of current cannabis users in the study by Kempker et al11). Interestingly, however, duration of cannabis use has not been associated with an increased odds for developing asthma.18 Moreover, both inhaled and oral THC have been found to induce bronchodilation in patients with asthma and healthy subjects, with a systematic review of 12 studies suggesting an association between short-term challenge cannabis exposure and bronchodilation.48 Until recently, however, mechanisms underpinning the potential bronchodilatory effects of cannabis were unknown. A translational investigation in 88 normal human bronchi demonstrated that prejunctional CB1 receptors, when activated by either natural (including THC) or synthetic cannabinoids, mediated inhibition of electrical field stimulation-induced cholinergic contraction of smooth muscle.32 This observation raises the possibility of examining specific cannabinoids in well-designed and controlled translational experiments for their efficacy as adjunctive bronchodilator agents in asthma and other reactive airway conditions. However, given the similarities in toxic and carcinogenic substances with smoked cigarettes, practitioners should caution patients against using smoked cannabis to treat asthma and be aware that although noncombusted cannabis alternatives may prove to be useful in asthma, the optimal routes and doses remain to be determined.
Virtually all clinical studies examining cannabis use in the context of airways disease have reported alterations in spirometric parameters related to the use of smoked cannabis as major outcome variables, although such results may not fully characterize the underlying pathology in this setting. Advances in radiologic imaging, including quantitative airway imaging through CT scanning, have emerged in recent years, enabling more-detailed characterization of airways diseases, particularly COPD. These techniques allow objective assessment of lung pathology in correlation with subjective clinical symptoms, such as dyspnea, along with lung physiologic data. To this end, radiologic imaging data may complement spirometry in delineating anatomic changes elicited by use of inhaled cannabis products even before physiology is altered. To date, only one study examining chest CT scans of cannabis users has been published9; it demonstrated associations between long-term cannabis smoking and an increased percentage of low-density lung tissue, whereas long-term tobacco smoking was associated with macroscopic emphysema. Moreover, a dose-response relationship between cannabis smoking and airflow obstruction, impaired large airways function, and hyperinflation was also present. Another investigation in a cohort of 947 subjects (49% with COPD) suggested that both the quantity of emphysematous changes and the airway wall thickness on chest CT scan correlate with mortality.49 Therefore, as imaging techniques are validated for their utility in airways disease prognosis and the number of cannabis smokers continues to climb, investigations regarding the utility of quantitative chest CT imaging may prove worthwhile in patients who smoke cannabis.
Conclusions
Increasing prevalence of cannabis consumption in the United States and continued predominant use through an inhaled route suggest an immediate and far-reaching impact on lung health. As the composition of and methods to use cannabis expand, additional research capitalizing on new technologies in cell lines and animal models along with careful consideration addressing deficiencies in prior human clinical investigations will be necessary to establish the effects of cannabis on lung health. Clinicians should systematically and objectively address inhaled cannabis use in their patients, thereby facilitating the delivery of candid information regarding harm from smoked products and the dearth of quality data regarding newer formulations, particularly among regular users.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge Alicia McNally, BA, for assistance with the manuscript.
ABBREVIATIONS
- CBD
cannabidiol
- THC
Δ-9-tetrahydrocannabinol
Footnotes
FUNDING/SUPPORT: Funding support was provided through National Institutes of Health [Grant T32HL007085 to Dr Biehl].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Kilmer B, Caulkins JP, Midgette G, et al. Before the grand opening: measuring Washington State’s marijuana market in the last year before legalized commercial sales. RAND Corporation website. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR466/RAND_RR466.pdf. Published 2013. Accessed April 15, 2015.
- 2.Light MK, Orens A, Lewandowski B, et al. Market size and demand for marijuana in Colorado. Marijuana Policy Group, Colorado Department of Revenue. State of Colorado website. https://www.colorado.gov/pacific/sites/default/files/Market%20Size%20and%20Demand%20Study,%20July%209,%202014%5B1%5D.pdf. Published 2014. Accessed April 15, 2015.
- 3.Schuermeyer J, Salomonsen-Sautel S, Price RK, et al. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003-11. Drug Alcohol Depend. 2014;140:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10(3):239-247. [DOI] [PubMed] [Google Scholar]
- 5.Brenneisen R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In: ElSohly MA, ed. Forensic Science and Medicine: Marijuana and the Cannabinoids. Totowa, NJ: Humana Press; 2011:17-49. [Google Scholar]
- 6.ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539-548. [DOI] [PubMed] [Google Scholar]
- 7.Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166(1-2):3-18. [DOI] [PubMed] [Google Scholar]
- 8.Després JP. The endocannabinoid system: a new target for the regulation of energy balance and metabolism. Crit Pathw Cardiol. 2007;6(2):46-50. [DOI] [PubMed] [Google Scholar]
- 9.Aldington S, Williams M, Nowitz M, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007;62(12):1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancox RJ, Poulton R, Ely M, et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010;35(1):42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempker JA, Honig EG, Martin GS. The effects of marijuana exposure on expiratory airflow. A study of adults who participated in the U.S. National Health and Nutrition Examination Study. Ann Am Thorac Soc. 2015;12(2):135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BA, Augustson EM, Moser RP, Budney AJ. Respiratory effects of marijuana and tobacco use in a US sample. J Gen Intern Med. 2005;20(1):33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307(2):173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashkin DP, Simmons MS, Sherrill DL, Coulson AH. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. Am J Respir Crit Care Med. 1997;155(1):141-148. [DOI] [PubMed] [Google Scholar]
- 15.Taylor DR, Fergusson DM, Milne BJ, et al. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97(8):1055-1061. [DOI] [PubMed] [Google Scholar]
- 16.Aldington S, Harwood M, Cox B, et al. ; Cannabis and Respiratory Disease Research Group. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J. 2008;31(2):280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 2013;24(10):1811-1820. [DOI] [PubMed] [Google Scholar]
- 18.Han B, Gfroerer JC, Colliver JD. Associations between duration of illicit drug use and health conditions: results from the 2005-2007 national surveys on drug use and health. Ann Epidemiol. 2010;20(4):289-297. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LR, Morgenstern H, Greenland S, et al. ; Cannabis and Respiratory Disease Research Group of New Zealand. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136(4):894-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Kooy F, Pomahacova B, Verpoorte R. Cannabis smoke condensate II: influence of tobacco on tetrahydrocannabinol levels. Inhal Toxicol. 2009;21(2):87-90. [DOI] [PubMed] [Google Scholar]
- 21.Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001;64(3):319-327. [DOI] [PubMed] [Google Scholar]
- 22.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327-360. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JE. Students find way to secretly smoke marijuana in class. CBS Denver website. http://denver.cbslocal.com/2014/02/05/students-find-way-to-secretly-smoke-marijuana-in-class. Published February 2, 2014. Accessed April 15, 2015.
- 24.Solowij N, Broyd SJ, van Hell HH, Hazekamp A. A protocol for the delivery of cannabidiol (CBD) and combined CBD and Δ9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol Toxicol. 2014;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toennes SW, Kauert GF, Steinmeyer S, Moeller MR. Driving under the influence of drugs — evaluation of analytical data of drugs in oral fluid, serum and urine, and correlation with impairment symptoms. Forensic Sci Int. 2005;152(2-3):149-155. [DOI] [PubMed] [Google Scholar]
- 26.Mercolini L, Mandrioli R, Protti M, Conti M, Serpelloni G, Raggi MA. Monitoring of chronic cannabis abuse: an LC-MS/MS method for hair analysis. J Pharm Biomed Anal. 2013;76:119-125. [DOI] [PubMed] [Google Scholar]
- 27.Voss A, Witt K, Kaschowitz T, et al. Detecting cannabis use on the human skin surface via an electronic nose system. Sensors (Basel). 2014;14(7):13256-13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81. [DOI] [PubMed] [Google Scholar]
- 29.Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst. 1998;90(16):1198-1205. [DOI] [PubMed] [Google Scholar]
- 30.Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112(2):319-326. [DOI] [PubMed] [Google Scholar]
- 31.Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med. 1998;157(3):928-937. [DOI] [PubMed] [Google Scholar]
- 32.Grassin-Delyle S, Naline E, Buenestado A, et al. Cannabinoids inhibit cholinergic contraction in human airways through prejunctional CB1 receptors. Br J Pharmacol. 2014;171(11):2767-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156(5):1606-1613. [DOI] [PubMed] [Google Scholar]
- 34.Barbers RG, Gong H, Jr, Tashkin DP, Oishi J, Wallace JM. Differential examination of bronchoalveolar lavage cells in tobacco cigarette and marijuana smokers. Am Rev Respir Dis. 1987;135(6):1271-1275. [DOI] [PubMed] [Google Scholar]
- 35.Sherman MP, Campbell LA, Gong H, Jr, Roth MD, Tashkin DP. Antimicrobial and respiratory burst characteristics of pulmonary alveolar macrophages recovered from smokers of marijuana alone, smokers of tobacco alone, smokers of marijuana and tobacco, and nonsmokers. Am Rev Respir Dis. 1991;144(6):1351-1356. [DOI] [PubMed] [Google Scholar]
- 36.Knight G, Hansen S, Connor M, Poulsen H, McGovern C, Stacey J. The results of an experimental indoor hydroponic cannabis growing study, using the ‘Screen of Green’ (ScrOG) method-Yield, tetrahydrocannabinol (THC) and DNA analysis. Forensic Sci Int. 2010;202(1-3):36-44. [DOI] [PubMed] [Google Scholar]
- 37.Potter DJ, Duncombe P. The effect of electrical lighting power and irradiance on indoor-grown cannabis potency and yield. J Forensic Sci. 2012;57(3):618-622. [DOI] [PubMed] [Google Scholar]
- 38.ElSohly MA. Potency Monitoring Program Quarterly Report, 9/16/2013 to 12/15/2013. National Center for Natural Products Research. Oxford, MS: University of Mississippi; 2014. [Google Scholar]
- 39.Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117(1):59-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods. 2014;70(1):112-119. [DOI] [PubMed] [Google Scholar]
- 41.NIDA’s role in providing marijuana for research. National Institute on Drug Abuse website. http://www.drugabuse.gov/drugs-abuse/marijuana/nidas-role-in-providing-marijuana-research. Published March 2015. Accessed April 15, 2015.
- 42.Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone modifications are associated with Δ9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem. 2014;289(27):18707-18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494-502. [DOI] [PubMed] [Google Scholar]
- 44.Maertens RM, White PA, Williams A, Yauk CL. A global toxicogenomic analysis investigating the mechanistic differences between tobacco and marijuana smoke condensates in vitro. Toxicology. 2013;308:60-73. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Sandhu J, Kaur B, et al. Evaluation of the DNA damaging potential of cannabis cigarette smoke by the determination of acetaldehyde derived N2-ethyl-2′-deoxyguanosine adducts. Chem Res Toxicol. 2009;22(6):1181-1188. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol. 2011;44(3):269-286. [DOI] [PubMed] [Google Scholar]
- 47.Roth MD, Whittaker K, Salehi K, Tashkin DP, Baldwin GC. Mechanisms for impaired effector function in alveolar macrophages from marijuana and cocaine smokers. J Neuroimmunol. 2004;147(1-2):82-86. [DOI] [PubMed] [Google Scholar]
- 48.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167(3):221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602-608. [DOI] [PubMed] [Google Scholar]