Abstract
Chronic pain following surgery limits social activity, interferes with work and causes emotional suffering. A major component of such pain is is reported as “resting” or spontaneous pain with no apparent external stimulus. Although experimental animal models can simulate the stimulus-evoked chronic pain that occurs after surgery, there have been no studies of spontaneous chronic pain in such models. Here the Conditioned Place Preference (CPP) paradigm was used to reveal resting pain after experimental thoracotomy. Male Sprague-Dawley rats received a thoracotomy with 1 hour rib retraction, resulting in evoked tactile hypersensitivity, previously shown to last for at least 9 weeks. Intraperitoneal injections of morphine (2.5 mg/kg) or gabapentin (40mg/kg) gave equivalent 2-3h long relief of tactile hypersensitivity, when tested 12-14 days post-operative. In separate experiments, single trial CPP was conducted 1 week before thoracotomy and then 12 days (gabapentin) or 14 days (morphine) after surgery, followed the next day by one conditioning sesssion with morphine or gabapentin, both vs saline. The gabapentin-conditioned, but not the morphine-conditioned rats showed a significant preference for the analgesia-paired chamber, despite the two agents’ equivalent effect in relieving tactile allodynia. These results show that experimental thoracotomy in rats causes spontaneous pain, and that some analgesics, such as morphine, that reduce evoked pain do not also relieve resting pain, suggesting that pathophysiological mechanisms differ between these two aspects of long-term post-operative pain.
Keywords: chronic pain, spontaneous pain, morphine, conditioned place preference, gabapentin
INTRODUCTION
A broad variety of surgical procedures results in chronic post-operative pain that persists for at least 3 and sometimes 6 months or longer after surgery57,45,19,41. Among these procedures, thoracotomy ranks high with as many as half of patients reporting long-lasting pain2 that is characterized by movement-induced mechano-hypersensitivity (“evoked pain”) and by pain at rest that occurs without an apparent stimulus (“spontaneous pain”).46,27,80 Peripheral tissue damage, including intercostal nerve damage, contributes to initiating post-operative pain3,60, and continued afferent input is probably required for its maintenance28,16. However, the anatomical structures in spinal cord and brain, and their cellular mechanisms, which are modified by the initial and the ongoing afferent inputs, have not been unequivocally identified37,29.
Animal models of clinical pain syndromes have been useful for studying underlying mechanisms59,50, and post-thoracotomy pain has been modeled by a procedure in rats that has strong parallels to human surgery7. Pain-like behavior that is more elaborate than simple limb withdrawal or muscle twitches74,18 can be evoked after thoracotomy by tactile stimulation of the skin near and at a distance from the incision/retraction locus, respectively signalling primary and secondary hyperalgesia58. Such evoked responses are transiently reversed by systemic administration of morphine, implying that they are truly nocifensive indicators of a painful perception76. In contrast, spontaneous pain, which is a common clinical feature of post-thoracotomy and other chronic, neuropathic pain syndromes4, has not been demonstrated in this animal model. The purpose of this study was to do so.
METHODS
Animal Handling
Adult male Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA) and kept in groups of two in the animal housing facilities at Brigham and Women's Hospital, with controlled relative humidity (20%–30%), at an ambient temperature of 24 ± 1 °C and maintained under a normal light-dark cycle (12:12 hr). Pelleted rat chow and tap water were allowed ad libitum. All experimental procedures were approved by the Harvard Medical Area Standing Committee on Animals (Boston, MA), and are in keeping with published guidelines for the use of laboratory animals (National Research Council, Guide, 2011). The rats were handled for 5-7 days before the procedure to familiarize them with the experimental environment, so as to minimize stress-induced analgesia and to establish baseline behavioral parameters for each individual animal73. At the time of thoracotomy surgery, animals weighed 280-310 g.
Thoracotomy and Rib Retraction Surgery
Rats were briefly anesthetized with 4%–5% sevoflurane (Sevorane, Abbott Laboratory, North Chicago, IL, USA) before receiving intraperitoneal pentobarbital sodium (50 mg/kg; Nembutal , Akorn, Inc., Lake Forest, IL). Animals were then tracheally intubated by a method modified from Weksler et al.77. The endotracheal catheter was connected to a small animal pressure controlled ventilator (model TOPO220; Kent Scientific Corporation, Torrington, CN), which was set at a respiratory rate of 65-80/min. A CO2 analyzer (CapStar-100; IITC Inc., Woodland Hills, CA) was connected to the expiratory end to monitor end tidal CO2, which was maintained at 25-40 mm Hg for the entire surgical procedure.
The anesthetized rats were placed in the left decubitous position with a pillow under the contralateral armpit to elevate the surgical field. One 3 cm long incision was made in the skin of the right lateral chest wall along the fourth intercostal line, beginning from 1 cm lateral to the midline and 1 cm below the inferior angle of right scapula. The superficial and deep lateral thoracic muscles covering the ribs were incised and retracted to expose the intercostal muscles. A 1.0 cm incision was made through the intercostal muscle and pleura along the cranial border of the fifth rib. The blunt tines of a small retractor (Model 17003-03, Goldstein, 3x3 sharp teeth with depth 4.5 mm, teeth width 6.5 mm; FST, Inc., Foster City, CA) were placed under the fourth and fifth ribs. The retractor was opened to separate the ribs by 1 cm, and was left in place for 60 min, as previously described7. The open wound was covered with wet-dressing gauze kept moist with sterile phosphate buffered saline (PBS). After one hour the retractor was closed and removed and the fourth and fifth ribs were approximated and ligated tightly with 4-0 chromic gut sutures (Covidien, Mansfield, MA). Air was aspirated from the pleural cavity with a 5-mL syringe attached to the polyethylene tubing to restore normal intrapleural pressure. The superficial muscle covering the ribs was then apposed with 4-0 Vicryl sutures (MYCO Medical, Cary, NC), and the skin was closed with 3-0 silk sutures (Angiotech, Reading, PA). The animals were allowed to recover in separate cages, and the endotracheal catheter was removed once spontaneous breathing was re-established.
Sham operated rats underwent anesthesia and incision with pneumothorax, but without retraction of the ribs. Previous study results65 were confirmed here to show that tactile allodynia does not last longer than 1-2 days in such rats (data not shown).
Drugs and Injections
Morphine sulfate, USP grade, was purchased from West-Ward Pharmaceuticals (Eatontown, NJ) and a stock solution of 2.5 mg/mL made by dissolving in sterile saline. Different volumes of this stock solution were injected intraperitoneally with a 30g needle attached to an 1 cc insulin syringe to achieve a final dose of 2.5 mg/kg. No signs of ataxia, locomotion or imbalance were detected after this dosing, suggesting that no major motor deficits were present. Gabapentin was purchased from Sigma-Aldrich Co. and a stock solution of 10 mg/mL made by dissolving in sterile saline. Different volumes of this stock solution were injected intraperitoneally with a 30g needle attached to an 1 cc insulin syringe to achieve a final dose of 40 mg/kg.
Behavioral Testing
The investigator conducting the behavioral testing was blinded as to the surgical condition of the animals (sham or TRR), but was aware of the drug (gabapentin, morphine or saline) that was injected. Tactile evoked responses. To test mechanical hyperalgesia, each rat was placed in a loose restraining cage (8cm × 9cm × 20cm) and allowed to rest there for 15 min. A series of calibrated von Frey filaments (VFH; Stoelting Co, Wood Dale, IL) with bending forces ranging from 0.4 to 15.0 g were applied perpendicularly to and 0.5 – 1cm away from the incision, starting from the lowest force, to calculate the threshold for a nocifensive response. Each VFH was probed twice, pressing with a 3-sec duration spaced 3-sec apart. The VFH force was increased progressively until a defined response occurred (see below) and then reduced and again increased to verify the threshold force. Rats responding to the lowest force filament that was used, 0.4 g, were assigned this as the threshold, and those not responding to the highest force were assigned a “ceiling” threshold of 15.1 g. Higher forces were avoided to minimize the tactile sensitization that occurs with these stiffer VFHs.
Behavioral evaluations were made over the 2 days before thoracotomy, and averaged as the baseline threshold, and then again at post-operative days (PODs) 10 and 14, the last in order to assess gabapentin's ability to relieve post-operative hypersensitivity. In this case the analgesic was given to the rats twice, first for the conditioning phase of the Place Preference protocol (see below), and second, 4 days later, to assay the effect on mechanically evoked pain.
Because of the effects that preceding morphine administration has on the phramacodynamics of subsequent injections48, morphine was only given once to each group, either to test its ability to relieve tactile hypersensitivity or for Conditioned Place Preference. Morphine's ability to relieve post-operative mechano-hypersensitivity was assessed in 3 groups of rats. In these experiments rats were either not operated on (naïve, n=11), had a sham operation consisting of an incision without retraction (n=5), or had the full thoracotomy and retraction (TRR, n=12). Sham and TRR-treated rats were injected with morphine 13 days after the respective surgery, and the threshold for nocifensive responses measured at 0.5, 1, 2, 3, 4 and 5 hrs.
Conditioned Place Preference
Single trial Conditioned Place Preference (CPP) was performed one week before and 10 day after thoracotomy surgery, following the method described by King et al.40,39. On POD10, conditioning sessions were started after verifying that the rats had develop tactile allodynia or not; rats without allodynia (threshold force >10g) were not tested further. The CPP apparatus contains a center chamber that can be opened or closed off to either of two end chambers (San Diego Instruments, San Diego, CA). During the preconditioning session, rats were placed in the middle chamber and familiarized with the environment with full access to all three chambers for 30 min/day for 2 days (POD10 and POD11). The two end chambers can be differentiated by texture of floor (rough vs smooth), wall pattern (gray wall vs horizontally striped wall) and odor (banana vs vanilla; Chapstik, Miller-Norton Company, Richmond VA). On the third day of CPP (POD12), the unconditioned bias of each rat was determined by monitoring its travel between all 3 chambers for 15 min. The time spent in each chamber and the frequency of entries to every chamber were monitored by the 4 × 16 photobeam arrays that detected position within each chamber. Rats that showed a pre-conditioning place preference “bias”, with time spent in any one chamber more than 12 min or less than 2 min during this test period, were excluded from the CPP analysis.
On the “conditioning” day (POD13), animals were restricted to a single chamber immediately following saline vehicle or drug injection, for 30 minutes (after morphine or saline) or 60 min (after gabapentin). The group of rats that received morphine (n=12) was different from the group that received gabapentin (n=10; one rat was excluded from the CPP analysis because the rat showed a pre-conditioning place preference bias). On that conditioning day, rats were first administrated vehicle control (saline, i.p.) in the morning, and placed in a randomly chosen chamber. In the afternoon, four hours after the vehicle injection, the analgesic (morphine or gabapentin) was injected and the rat placed in the other chamber. Chamber pairings were counterbalanced such that as many rats received drug and were placed in the striped wall chamber as those that were placed in the gray wall chamber after drug. On the following, “test” day (POD14), 20 hours after the afternoon pairing of the conditioning day, rats were placed in the middle chamber with all doors open, allowing them free access to all chambers.
Ambulation time, numbers of entries to each chamber and time spent in each chamber were monitored for 15 minutes for analysis of chamber preference. Difference scores were calculated as these parameters measured during the “test” period minus the parameters measured during the “preconditioning” period.
Statistical Analysis
Threshold forces are presented as mean ± SEM, and are compared for statistically significant differences among groups by multi-group ANOVA followed by Tukey's test. When compared to the pre-operative baseline value or to the post- operative + pre-morphine value, these analyses are corrected for repeated measures adjustments. For CPP experiments, difference scores from the drug-paired chamber and the saline-paired chamber were analyzed using Kruskal-Wallis with multiple comparisons followed by post hoc Dunn's test. The correlation between the degree of allodynia reversal after gabapentin and the change in the CPP were analyzed by linear regression analysis. All statistical tests were conducted using SAS version 9.3 software (SAS, Cary, N.C.). Unless specifically noted, significance occurred for P<0.05.
RESULTS
Consistent with previous publications, rats showed tactile allodynia by a drop in threshold for nocifensive responses (Figure 1A) that had reached a constant minimum by 10 days after TRR surgery7,65,76. These earlier studies had also shown that tactile thresholds remained at this low level with no sign of abating for at least several months.
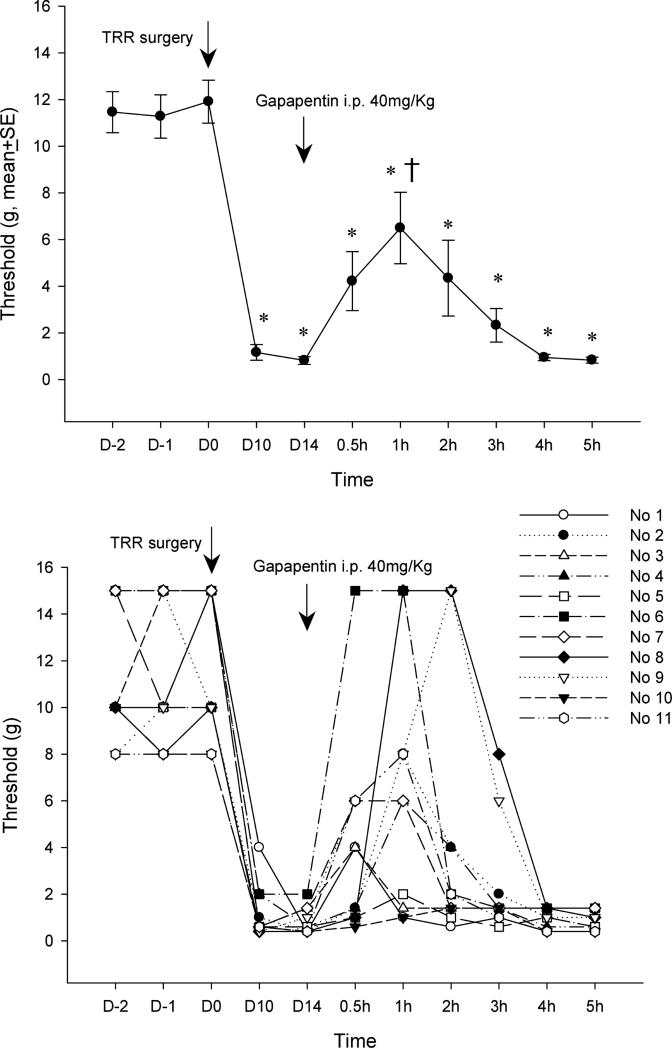
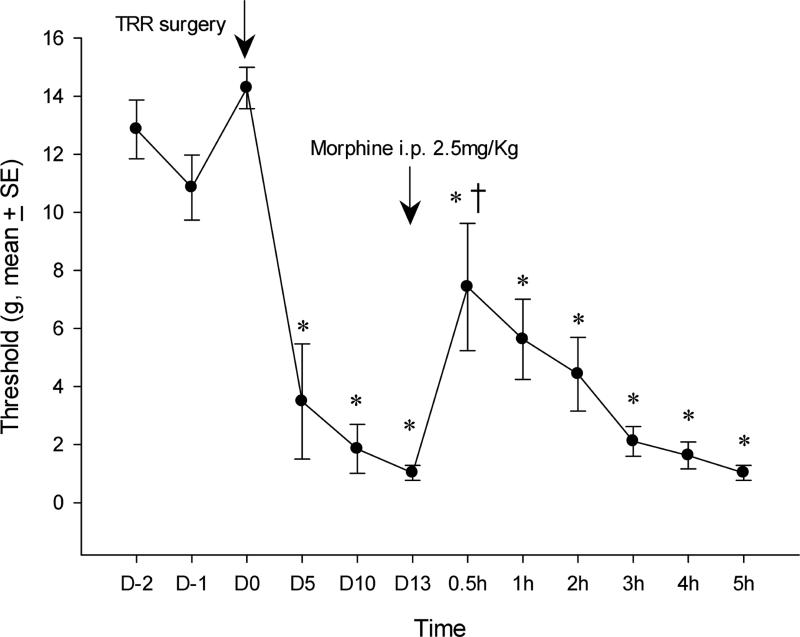
Figure 1.
(A) Systemic gabapentin injected on post-operative day 13 raises the mechanical threshold for nocifensive response back towards pre-operative levels. *P<0.05 compared to the pre-operative level (repeated measures ANOVA on responses after gabapentin) shows that the relief by gabapentin never reaches the pre-operative condition. † P<0.05 compared to the threshold at POD 13 (repeated measure ANOVA), just before gabapentin injection, shows that relief is only significant for the 1 h after gabapentin. (B) Responses to gabapentin of threshold in individual rats in the group that is averaged for Figure 1A. TRR, thoracotomy with rib retraction.
Systemic administration of gabapentin (40mg/kg, i.p.) partially reversed tactile hypersensitivity after TRR surgery. At 1h after gabapentin injection the average threshold was significantly higher than that of POD13 before i.p. gabapentin, but still below that of the pre-operative threshold of this cohort. Examination of the responses of individual animals showed the variation in the degree and time-course of threshold change after gabapentin, with three of eleven rats (Nos. 1, 6, 9) threshold's fully reversing to pre-operative levels and two (Nos. 5, 10) not reversing at all (Figure 1B). There was no correlation between the pre-operative thresholds and the post-operative response to gabapentin.
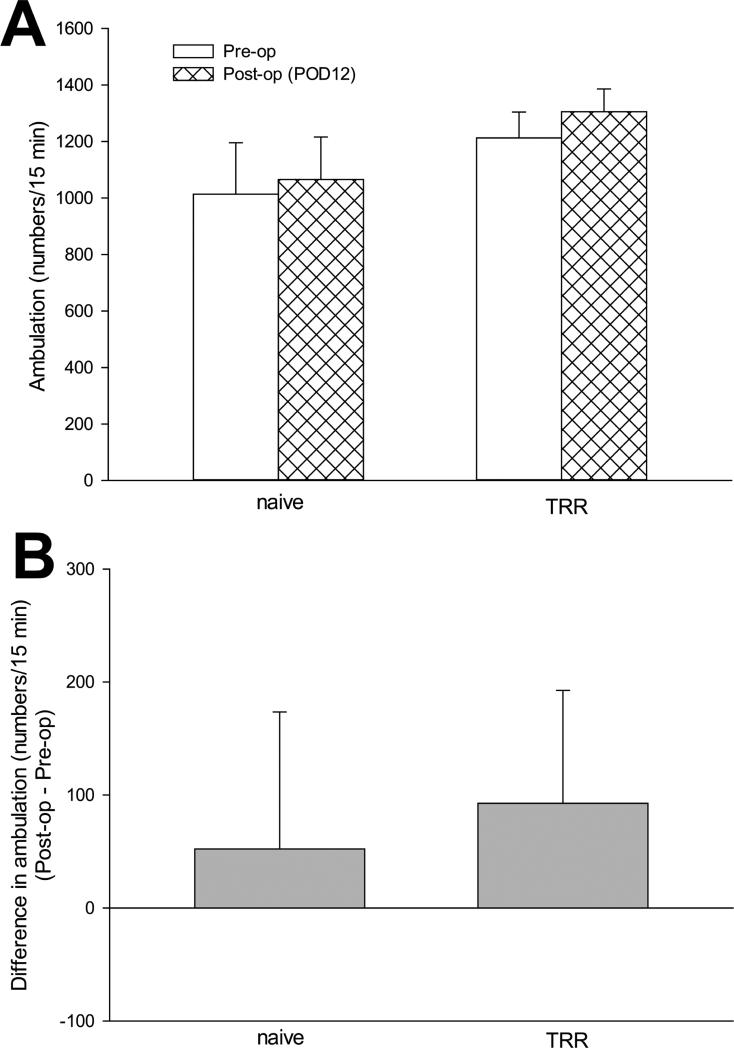
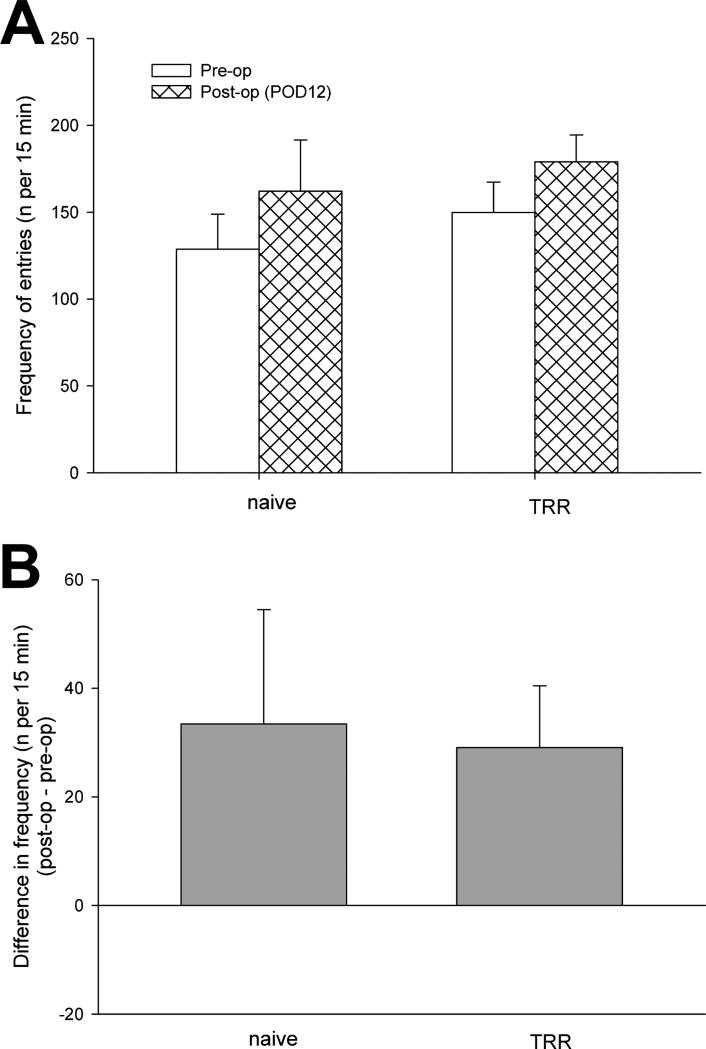
Spontaneous pain was assessed by Conditioned Place Preference. Among the conditioned place preference parameters, there were no significant differences in ambulation activity (Figure 2) and frequency of shuttling (Figure 3) between rats before and 12 days after TRR (during pre-conditioning testing), showing that surgery, and allodynia of the thoracic lumbar region did not affect locomotion. After surgery, times spent in the saline- or gabapentin-paired chambers during pre-conditioning were statistically equivalent across all groups and, therefore, all data were pooled across groups for assessing pre-conditioning behavior (n=22).
Figure 2.
(A) The change of ambulation activity of rats in conditioned place preference apparatus before and after TRR surgery. Data were obtained from the preconditioning test on POD12 or before surgery, from a recording of the number of times any of the motion-sensing light beams was broken during a 15 min test period. (B) Difference in ambulation (post-op – pre-op) verified no significant ambulation activity change after TRR surgery. Data are expressed as means ± S.E of 11 rats per group.
Figure 3.
(A) The change of shuttling activity (frequency of entries to every chamber) of rats in conditioned place preference apparatus before and after TRR surgery. Data were obtained from the preconditioning test on POD12 or before surgery of recording for 15 min. (B) Difference in frequency of shuttling (post-op – pre-op) showed no significant shuttling change after TRR surgery. Data are expressed as means ± S.E (n = 11 for each group).
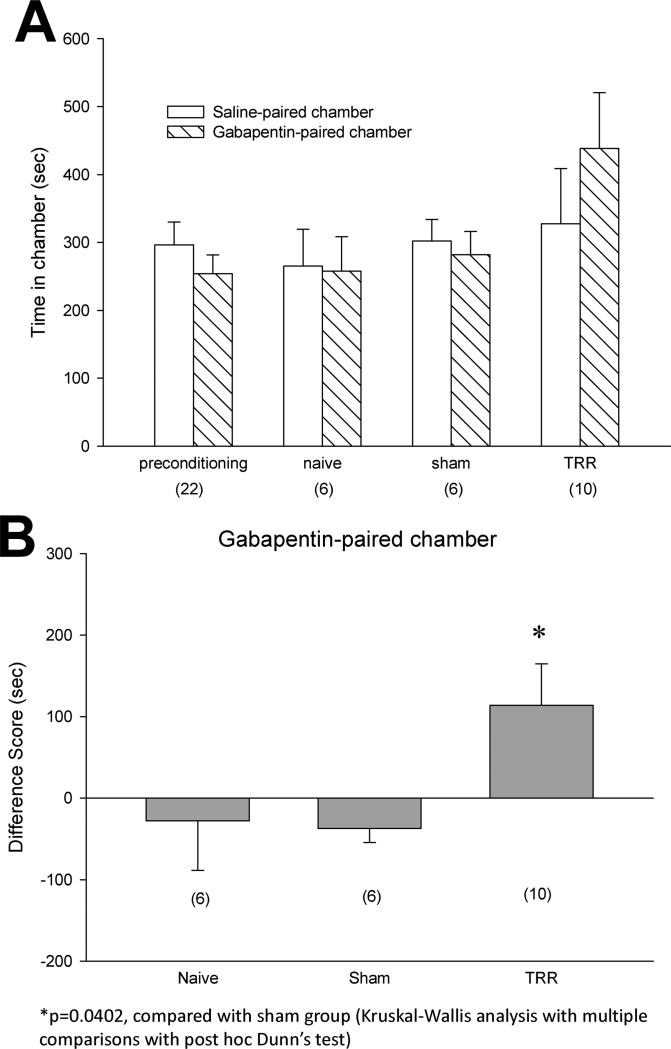
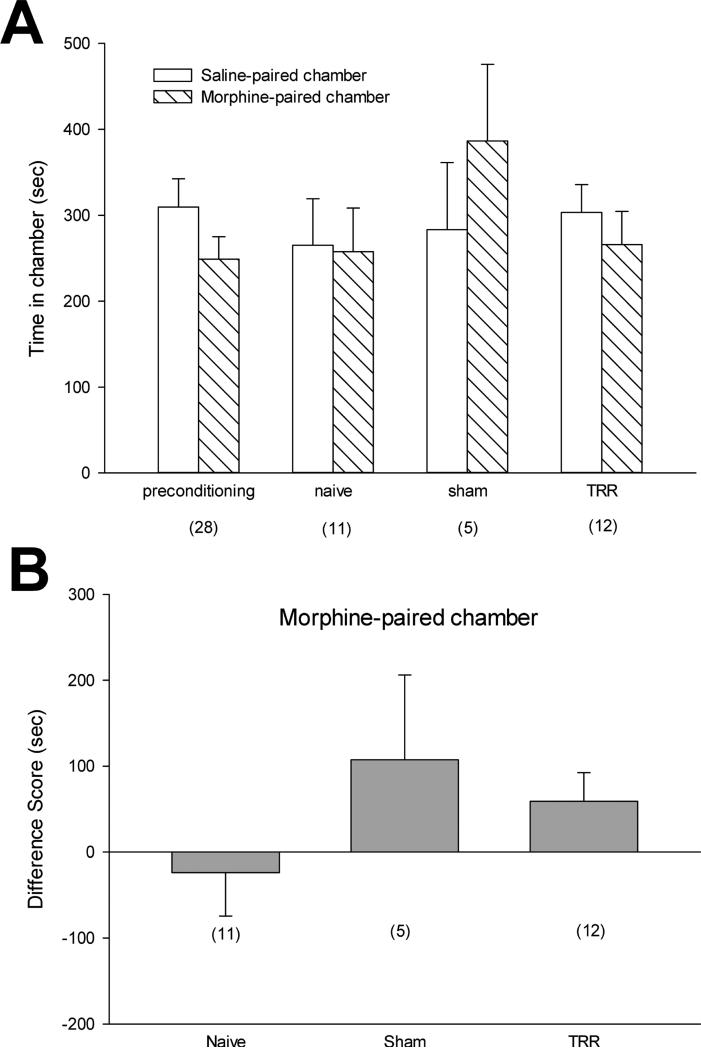
After conditioning with gabapentin, TRR rats spent more time in the gabapentin-paired chamber than in the saline-paired chamber (Figure 4A). In contrast, naïve and sham rats showed no preference between the saline- or gabapentin-paired chamber, as rats spent equal amounts of post-conditioning times in both chambers. The difference scores from the individual rats confirmed that systemic gabapentin administration altered TRR-induced spontaneous pain because only TRR rats showed significantly increased time spent in the gabapentin-paired chamber (Figure 4B).
Figure 4.
Systemic gabapentin administration alleviated TRR-induced spontaneous pain. (A) preconditioning times spent in the saline- or gabapentin-paired chambers were equivalent across all groups and all data were pooled across groups for preconditioning graphical representation (mean ±SE, n=22). After conditioning, TRR rats spent more time in the gabapentin-paired chamber than in the saline-paired chamber. Naïve and sham rats showed no preference for the saline- or gabapentin-paired chamber. (B) TRR rats showed significantly increased of difference scores in the gabapentin-paired chamber. *P<0.05 compared with sham group (Kruskal-Wallis analysis with multiple comparisons with post hoc Dunn's test).
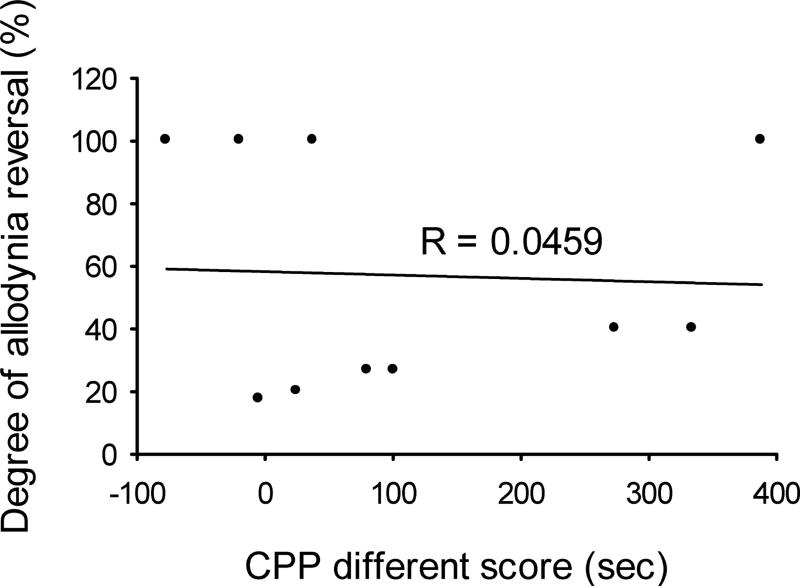
We questioned whether the degree of threshold reversal afforded by gabapentin treatment was predictive of the ability of this analgesic to cause place preference. Figure 5 compares the degree of allodynia reversal (% of change of threshold back to the pre-op value) after gabapentin with the difference scores of time spent in the gabapentin-paired chamber, for each individual rat. The lack of correlation between these parameters, shown by the very low linear correlation coefficient (R=0.0459; P>0.5) reveals the absence of any relationship between actions of gabapentin on the non-evoked, resting pain and the mechanical stimulus-evoked pain.
Figure 5.
The correlation between the degree of allodynia reversal (change of threshold back to pre-op value) after gabapentin and the difference scores in the gabapentin-paired chamber for each individual rat.
Another lack of correlation between relief of evoked pain and place preference occurred in the morphine-treated rats. The reversal of the average post-operative fall in threshold by systemic morphine was quite similar to that from gabapentin (compare Figs. 6 and 1A). However, morphine failed to cause a preference for the paired chamber in TRR-treated rats (Figure 7). If anything, any morphine-conditioned preference was suggested in the sham population, although the difference did not reach significance. Correlations of these two parameters among the individual rats was not possible since different animals were tested for relief of evoked pain and conditioned place preference.
Figure 6.
Systemic morphine injected on post-operative day 13 partially reversed tactile allodynia after TRR. *P<0.05 compared with the pre-operative level (repeated measures ANOVA). † P<0.05 compared to the threshold at POD 13 (repeated measure ANOVA), just before morphine injection, shows that relief is only significant for the 0.5 h after morphine. Data are expressed as means ± S.E of 7 rats.
Figure 7.
Systemic morphine administration failed to alter TRR-induced spontaneous pain. (A) preconditioning times spent in the saline- or morphine-paired chambers were equivalent across all groups and all data were pooled across groups for preconditioning graphical representation (mean ±SE, n=28). (B) After conditioning, the rats in all groups showed no preference for the saline- or morphine-paired chamber.
DISCUSSION
Chronic post-operative pain remains a significant obstacle to healthy recovery from surgery. Thoracotomy results in chronic pain in as many as 50% of patients2, with evidence of a neuropathic pain component from nerve injury in many thoracotomy patients29,46,69. Nerve injury has also been documented in the animal model used here7,76. Animal models may be useful in the development of treatments to prevent chronic pain and, the more problematical situation, to effect its reversal once it occurs. Most studies so far have focused on the hyper-responsiveness to tactile or thermal stimulation as a measure of brief or prolonged post-operative pain6,20,12,18,24,76 and have not dealt with the prevalent problem of chronic spontaneous pain, a hallmark of clinical post-operative and neuropathic pain but more difficult to evaluate in animals47.
Both peripheral and central mechanisms contribute to the enduring postoperative hyperalgesia, reflecting the neuroplasticity of primary afferent fiber excitability and central synaptic processing and connectivity61. Pharmacological efficacy in animal models studying evoked pain is modified by these neuro-plastic changes, as shown by the very different ability of drugs, such as resolvins31 and inhibitors of P-p38 MAPkinase78,32, to effectively prevent development of hyperalgesia when given pre-operatively yet have very limited capacity to reverse such hyperalgesia once it has developed.
With regard to acute clinical pain, inhibition of afferent input into the CNS during the immediate post-operative period is an effective anti-hyperalgesic strategy53,5,79,1, as is inhibition of NMDA receptor activity in the spinal cord during this period64,10,68. These clinical actions are paralleled by results from animal models that show diminished evoked pain as the response to local and spinal anesthetic blockade of, respectively, afferent discharge81,55 and its spinal input84,25,65,32.
Spontaneous pain has been virtually unexamined in animal studies of post-operative pain. Since, judging from their different pharmacological susceptibilities78,31,32, the cellular pathways in spinal cord that subserve the induction of post-operative pain differ from those involved in the maintenance of that pain, it is also possible that the mechanisms underlying chronic evoked hyperalgesia differ from those of chronic spontaneous pain. In the present paper we show the presence of spontaneous pain in a rat post-thoracotomy model, using the conditioned place preference paradigm with gabapentin. Unexpectedly, we discovered that morphine, which was as effective as gabapentin in temporarily relieving evoked tactile hypersensitivity, in agreement with the original study of Buvanendran et al.7 and with our previous that reported changes in the quality of the nocifensive response during morphine's relief of post-thoracotomy pain (Wang et al. 2013), was apparently ineffective in relieving spontaneous pain. Furthermore, although gabapentin was effective in temporarily relieving both tactile hypersensitivity and spontaneous pain, measured as averaged responses of a population, the relief of the evoked pain in individual rats showed no correlation to the relief from spontaneous pain in those same individuals. Mechanically evoked post-thoracotomy pain thus does not appear to be tightly coupled to spontaneous pain in this experimental model.
Systemic gabapentin is a broadly effective analgesic, relieving experimental evoked tactile hyperalgesia from spinal nerve ligation (SNL;43,75), peripheral inflammation21, diabetic neuropathy (DN,22) and a rodent model of herniorrhaphy that involves no peripheral nerve damage24. Gabapentin purportedly acts to reduce the pre-synaptic entry of Ca+2, through N-type calcium channels, that is essential for release of neurotransmitters involved in pain transmission51,42,83,11, with targets located in spinal cord and brain71,72. Nerve injury leads to an increased expression and functional contribution from these N-type channels49,82 and, importantly, in the amounts of these channels’ α-2 δ sub-units that are the putative binding site for gabapentin26. This change in expression may account for gabapentin's suppression of hyperalgesic conditions without having any anti-nociceptive activity at the same doses.
Peripheral nerve hyperexcitability accompanying DN is partially reversed by daily administration of gabapentin (50mg/kg/day), which also lowers the DN-elevated TTX-sensitive Na+ channel Nav1.7 and the activated p-ERK1,2 levels of isolated dorsal root ganglia back towards control levels85. Whether these actions of gabapentin are indirect results of N-type Ca+2 channel blockade or due to direct actions on Na+ channels is not known.
The broad distribution of N-type channels in the nervous system parallels the many and varied effects of gabapentin on emotional and neurological activities, including anxiety. Rats given gabapentin i.p., at doses one-half and lower than the one used here, have significantly less anxiety as expressed by their willingness to perform in elevated maze and forced swimming tests (Kilic et al. 2014). Although this anxiolytic action presents a potential confound in interpreting the post-operative pain behavior described here, we suspect that changes in overall ambulation and frequency of entry would occur, in addition to a preference for the place where anxiety was reduced, and we did not detect such changes.
Pain-related activity in the CNS is also affected by gabapentin. Both electrically- and mechanically-stimulated responses of spinal dorsal horn (DH) neurons, which are elevated after SNL8, are suppressed by systemic gabapentin (10-100 mg/kg s.c.;9). Relevant to the current study, SNL also increases spontaneous firing of wide dynamic range neurons located in the deep DH (LV-VI)8, firing that is sensitive to systemic (s.c.) gabapentin and spinal (i.t.) morphine, yet is unaffected by systemic (i.v.) morphine71, a finding of potential relevance to the current work. The correlation between the pharmacological profile of “spontaneous” DH neurophysiological activity and that of spontaneous pain suggests that this spontaneous firing of these neurons is tightly coupled to spontaneous pain. Such “spontaneous” firing of WDR neurons might be truly ectopic, independent of other inputs and capable of excitation of more rostral pain processing loci in the brain, or it might be a substrate for the maintenance of hyperexcitability in these other pain encoding regions of the CNS. In turn, “spontaneous” activity in DH neurons might be a spinal manifestation of unstimulated, ectopic activity detected in peripheral afferent fibers after nerve injury (partial sciatic nerve ligation;63). Both the tactile allodynia in this partial ligation model and the ectopic activity are suppressed by systemic gabapentin56, as noted above, gabapentin suppresses the expression of Nav1.7 Na+ channels known to be critical for peripheral pain coding (cit, Nav1.7).
Changes in “spontaneous” activity in the DH can also be manifestations of changes in brain activity. Regardless of their mechanistic origins, peripheral and spinal hyperactivity after nerve injury leads to changes in brain and, notably, in the descending modulatory system of the brainstem. The brainstem sends both facilitatory and inhibitory projections to the spinal cord, that, respectively, positively and negatively modulate the throughput of nociceptive signaling to the brain, and intense acute pain usually results in activation of the brainstem's inhibitory projections23,30. After nerve injury leading to hyperalgesia, however, the overall activity in the brain is actually reduced33 although stimulus-evoked activity in the brainstem is relatively increased. Strikingly, systemic gabapentin blocks this general deactivation of the brain and also suppresses the brainstem's stimulus-evoked activation33. Perhaps these two effects of gabapentin respectively account for its ability to suppress spontaneous pain and evoked hyperalgesia. It would be interesting to examine the ability of systemic morphine to effect these changes in brain activity as a validation of the proposed mechanisms for spontaneous chronic post-operative pain.
If the experimental findings of the current study also hold in the clinical domain one would conclude that drugs that are effective in suppressing evoked, e.g. movement-related post-thoracotomy pain, such as that caused by stretching or coughing, might be ineffective in dealing with spontaneous pain. Furthermore, if one psycho-physiological logical consequence of spontaneous pain is an elevated guarding reflex, then the tonic, unprovoked resting pain could result in heightened vigilance for and responsiveness to evoked pain, even when that evoked pain had been suppressed by directed peripheral treatments.
Perspective.
Spontaneous pain, a hallmark of chronic post-operative pain, is here demonstrated in a rat model of experimental post-thoracotomy pain, further validating use of this model for development of analgesics to treat such symptoms. Although stimulus-evoked pain was sensitive to systemic morphine, spontaneous pain was not, suggesting different mechanistic underpinnings.
Highlights.
● Experimental thoracotomy with rib retraction in the rat causes tactile allodynia.
● Systemic delivery of gabapentin or morphine can temporarily relieve this allodynia.
● Gabapentin but not morphine conditions a Place Preference response.
● Thus, spontaneous pain is present at 1-2 weeks after thoracotomy.
● The mechanisms underlying allodynia differ from those for spontaneous pain.
ACKNOWLEDGMENTS
The authors are thankful for the advice and assistance of Dr. Tamara King, University of New England (Biddeford ME), in setting up the Conditioned Place device and learning the methodology, and for reading a preliminary version of this paper. Mr. James Bell deserves much credit for refining the figures, and Ms. Fannie Polcari for secretarial assistance; both of these folks are in the Department of Anesthesiology, Perioperative and Pain Medicine, Brigham & Women's Hospital.
Partial funding for this research was provided by USPHS grants (to GS) NIH/NCI 080153 and NIH.NINDS NS078173.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors attest that they have no conflicts of interest related to any of the material presented in this article.
REFERENCES
- 1.Barreveld AM, Witte J, Chahal H, Durieux ME, Strichartz G. Preventive analgesia by local anesthetics:The reduction of post-operative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116:1141–61. doi: 10.1213/ANE.0b013e318277a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta-analysis. JPain. 2014;15:887–97. doi: 10.1016/j.jpain.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Amanzio M, Casadio C, Filosso PL, Molinatti M, Oliaro A, Pischedda F, Maggi G. Postoperative pain and superficial abdominal reflexes after posterolateral thoracotomy. Ann Thorac Surg. 1997;64:207–10. doi: 10.1016/s0003-4975(97)82829-9. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012 Oct;13(10):921–9. doi: 10.1016/j.jpain.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Bong CL, Samuel M, Ng JM, Ip-Yam C. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2005;19:786–93. doi: 10.1053/j.jvca.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996 Mar;64(3):493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 7.Buvanendran A, Kroin JS, Kerns JM, Nagalla SN, Tuman KJ. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg. 2004;99:1453–60. doi: 10.1213/01.ANE.0000134806.61887.0D. [DOI] [PubMed] [Google Scholar]
- 8.Chapman V, Suzuki R, Chamarette HLC, Rygh L, Dickenson A. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998:261–272. doi: 10.1016/s0304-3959(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 9.Chapman V, Suzuki R, Dickenson A. Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. J Physiol. 1998 1998 Mar 15;507(Pt 3):881–94. doi: 10.1111/j.1469-7793.1998.881bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Liao Z, Li H. Reducing the prevalence of chronic post-thoracotomy pain syndrome: is total intravenous anaesthesia superior to inhalation anaesthesia? Eur J Cardiothorac Surg Mar. 2013;43(3):659. doi: 10.1093/ejcts/ezs503. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JK, Chiou LC. Mechanisms of the antinociceptive actions of gabapentin. J Pharmacol Sci. 2006;100:471–86. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JK, Pan HL, Eisenach JC. Anti-allodynic effect of intrathecal gabapentin and its interaction with clonidine in a rat model of postoperative pain. Anesthesiology. 2000;92:1126–31. doi: 10.1097/00000542-200004000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci. 1997;20:404–19. doi: 10.1017/s0140525x97251484. [DOI] [PubMed] [Google Scholar]
- 14.Dajczman E, Gordon A, Dreisman H, Wolkove N. Long-term post-thoracotomy pain. Chest. 1991;99:270. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]
- 15.Decosterd I, Allchorne A, Woolf C. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth Analg. 2004;99:457–63. doi: 10.1213/01.ANE.0000131967.69309.4F. [DOI] [PubMed] [Google Scholar]
- 16.Devor M. Ectopic discharge in A-Beta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–28. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 17.Dirks J, Moiniche S, HIlsted KL, Dahl JB. Mechanisms of postoperative pain: Clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97:1591–96. doi: 10.1097/00000542-200212000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of post-incisional allodynia by subcutaneous bupivacaine: Findings with a new model in the hairy skin of the rat. Anesthesiol. 2005;103:113–25. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, McDermott MP, Raja SN. Preventing chronic postsurgical pain: how much of a difference makes a difference? Anesthesiology. 2010;112:516–18. doi: 10.1097/ALN.0b013e3181cf4253. [DOI] [PubMed] [Google Scholar]
- 20.Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther. 1997;282:1242–1246. [PubMed] [Google Scholar]
- 21.Field MJ, Oles RJ, Lewis AS, McClearly S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- 23.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 24.Flatters SJL. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Neurosci Lett. 2010;14(477):43–7. doi: 10.1016/j.neulet.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008;437:180–83. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 27.Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin J Pain. 2008;24:708–16. doi: 10.1097/AJP.0b013e318174badd. [DOI] [PubMed] [Google Scholar]
- 28.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–94. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 29.Guastella V, Mick G, Soriano C, Vallet L, Escande G, Dubray C, Eschalier A. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011;152:74–81. doi: 10.1016/j.pain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Wang C-F, Serhan CN, Strichartz G. Enduring prevention and transient reduction of post-operative pain by intrathecal Resolvin D1. Pain. 2011A;152:557–65. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang L, Gao Y-J, Wang J, Strichartz G. Shifts in cell-type expression accompany a diminishing role of spinal p38-MAPKinase activation over time during prolonged postoperative pain. Anesthesiology. 2011;115:1281–90. doi: 10.1097/ALN.0b013e31823499cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A. 2005;102:18195–200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology. 2009;111:640–48. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]
- 35.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 38.Kilic F, Ismailoglu S, Kaygisiz B, Oner S. Effects of single and combined gabapentin use in elevated plus maze and forced swimming tests. Acta Neuropsychiatr. 2014;26:307–14. doi: 10.1017/neu.2014.17. [DOI] [PubMed] [Google Scholar]
- 39.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kissin I, Gelman S. Chronic postsurgical pain: still a neglected topic? Journal of Pain Research. 2012;5:473–89. doi: 10.2147/JPR.S35145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labuda CJ, Little PJ. Pharmacological evaluation of the selective spinal nerve ligation model of neuropathic pain in the rat. J Neu Meth. 2005;144:175–181. doi: 10.1016/j.jneumeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Landau R, Kraft JC, Flint LY, Carvalho B, Richebe P, Cardoso M, Lavand'homme P, Granot M, Yarnitsky D, Cahana A. An experimental paradigm for the prediction of Post-Operative Pain (PPOP). J Vis Exp. 2010 Jan;27(35):1671. doi: 10.3791/1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 46.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–5. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012 Nov;33(11):568–73. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 49.Matthews EA, Dickenson AH. Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92:235–46. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 50.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Nara T, Saito S, Obata H, Goto F. A rat model of post-thoracotomy pain: behavioural and spinal cord NK-1 receptor assessment. Can J Anaesth. 2001;48:665–76. doi: 10.1007/BF03016201. [DOI] [PubMed] [Google Scholar]
- 52.National Research Council of the National Academies, the National Academies Press Guide for the Care and Use of Laboratory Animals 8th Edition. 2011 [Google Scholar]
- 53.Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth. 1999;46:1127–32. doi: 10.1007/BF03015520. [DOI] [PubMed] [Google Scholar]
- 54.Ochroch EA, Gottschalk A, Troxel AB, Farrar JT. Women suffer more short and long-term pain than men after major thoracotomy. Clin J Pain. 2006;22:491–98. doi: 10.1097/01.ajp.0000208246.18251.f2. [DOI] [PubMed] [Google Scholar]
- 55.Ohri R, Wang J, Blaskovich PD, Khodorova A, Pham LN, Costa DS, Nichols GA, Hildebrand WP, Scarborough NL, Herman CJ, Strichartz GR. Inhibition by local bupivacaine-releasing microspheres of acute postoperative pain from hairy skin incision. Anesth Analg. 2013;117:717–730. doi: 10.1213/ANE.0b013e3182a00851. [DOI] [PubMed] [Google Scholar]
- 56.Pan H-L, Eisenach JC, Chen S-R. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. JPET. 1999;288:1026–1030. [PubMed] [Google Scholar]
- 57.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 58.Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain. 1984;107:1179–88. doi: 10.1093/brain/107.4.1179. [DOI] [PubMed] [Google Scholar]
- 59.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–47. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Rogers ML, Henderson L, Mahajan RP, Duffy JP. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002;21:298–301. doi: 10.1016/s1010-7940(01)01104-6. [DOI] [PubMed] [Google Scholar]
- 61.Schaible HG. Peripheral and central mechanisms of pain generation. Handb Exp Pharmacol. 2007;177:3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt B, Ohri R, Wang JC-F, Blaskovich P, Kesselring A, Scarborough N, Herman C, Strichartz G. Local pathology and systemic serum bupivacaine after subcutaneous delivery of slow-releasing bupivacaine microspheres. Anesth and Analg. 2014;120:36–44. doi: 10.1213/ANE.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 63.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. Nov. 1990;43(2):205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 64.Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, Dilege S, Pembeci K. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94:11–15. doi: 10.1213/00000539-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Shin JW, Pancaro C, Wang CF, Gerner P. Low-dose systemic bupivacaine prevents the development of allodynia after thoracotomy in rats. Anesth Analg. 2008;107:1587–91. doi: 10.1213/ane.0b013e31818200aa. [DOI] [PubMed] [Google Scholar]
- 66.Shin JW, Pancaro C, Wang CF, Gerner P. The effects of resiniferatoxin in an experimental rat thoracotomy model. Anesth Analg. 2010;110:228–32. doi: 10.1213/ANE.0b013e3181c5c89a. [DOI] [PubMed] [Google Scholar]
- 67.Sihoe AD, Lee TW, Wan IY, Thung KH, Kim AP. The use of gabapentin for post-operative and post-traumatic pain in thoiracic surgery patients. Eur J Cardiothorac Surg. 2006;29:795–9. doi: 10.1016/j.ejcts.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 68.Song JG, Shin JW, Lee EH, Choi DK, Bang JY, Chin JH, Choi IC. Incidence of post-thoracotomy pain: a comparison between total intravenous anaesthesia and inhalation anaesthesia. Eur J Cardiothorac Surg. 2012;41:1078–82. doi: 10.1093/ejcts/ezr133. [DOI] [PubMed] [Google Scholar]
- 69.Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9:955–61. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki R, Chapman V, Dickenson AH. The effectiveness of spinal and systemic morphine on rat dorsal horn neuronal responses in the spinal nerve ligation model of neuropathic pain. Pain. 1999;80:215–228. doi: 10.1016/s0304-3959(98)00208-5. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006 Mar;198(1):72–80. doi: 10.1016/j.expneurol.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 72.Tanabe M, Takasu K, Takeuchi Y, Ono H. Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. J Neurosci Res. 2008;86:3258–64. doi: 10.1002/jnr.21786. [DOI] [PubMed] [Google Scholar]
- 73.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurological evaluation of the rat nerve block with lidocaine. Anesthesiology. 1995 Apr;82(4):1013–25. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 74.Theriault E, Diamond J. Nociceptive cutaneous stimuli evoke localized contractions in a skeletal muscle. J Neurophysiol. 1988;60:446–62. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- 75.Urban MO, Ren K, Park KT, Campbell B, Anker N, Stearns B, Aiyar J, Belley M, Cohen C, Bristow L. Comparison of the antinociceptive profiles of gabapentin and 3-methylgabapentin in rat models of acute and persistent pain: implications for mechanism of action. J Pharmacol Exp Ther. 2005;313:1209–1216. doi: 10.1124/jpet.104.081778. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Hung C-H, Gerner P, Ji R-R, Strichartz GR. The Qualitative Hyperalgesia Profile: A New Metric to Assess Chronic Post-Thoracotomy Pain. Open Pain J. 2013;6:190–8. doi: 10.2174/1876386301306010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weksler B, Ng B, Lenert J, Burt M. A simplified method for endotracheal intubation in the rat. J Appl Physiol. 1994;76:1823–25. doi: 10.1152/jappl.1994.76.4.1823. [DOI] [PubMed] [Google Scholar]
- 78.Wen Y-R, Suter MR, Ji R-R, Yeh G-C, Wu Y-S, Wang K-C, Kohno T, Sun W-Z, Wang C-C. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–65. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 79.Wheatley GH, 3rd, Rosenbaum DH, Paul MC, Dine AP, Wait MA, Meyer DM, Jessen ME, Ring WS, DiMaio JM. Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg. 2005;130:464–68. doi: 10.1016/j.jtcvs.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 80.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009;36:170–80. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–56. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N- and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998;794:329–32. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- 83.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 84.Zahn PK, Sluka KA, Brennan TJ. Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain. 2002;100:65–76. doi: 10.1016/s0304-3959(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 85.Zhang JL, Yang JP, Zhang JR, Li RQ, Wang J, Jan JJ, Zhuang Q. Gabapentin reduces allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing expression level of Nav1.7 and p-ERK1/2 in DRG neurons. Brain Res. 2013 Feb;1493:13–8. doi: 10.1016/j.brainres.2012.11.032. 1. [DOI] [PubMed] [Google Scholar]
- 86.Zhao C, Tall JM, Meyer RA, MS, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–11. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]