Abstract
The potent trypanolytic properties of human apolipoprotein L1 (APOL1) can be neutralized by the trypanosome variant surface antigen gene product known as serum resistance-associated protein. However, two common APOL1 haplotypes present uniquely in individuals of West African ancestry each encode APOL1 variants resistant to serum resistance-associated protein, and each confers substantial resistance to human African sleeping sickness. In contrast to the dominantly inherited anti-trypanosomal activity of APOL1, recessive inheritance of these two trypanoprotective APOL1 alleles predisposes to kidney disease. Proposed mechanisms of APOL1 toxicity have included BH3 domain-dependent autophagy and/or ion channel activity. We probed these potential mechanisms by expressing APOL1 in Xenopus laevis oocytes. APOL1 expression in oocytes increased ion permeability and caused profound morphological deterioration (toxicity). Coexpression of BCL2 family members rescued APOL1-associated oocyte toxicity in the order MCL1 ∼ BCLW > BCLXL ∼ BCL2A1 ≫ BCL2. Deletion of nine nominal core BH3 domain residues abolished APOL1-associated toxicity, but missense substitution of the same residues abolished neither oocyte toxicity nor its rescue by coexpressed MCL1. The APOL1 BH3 domain was similarly dispensable for the ability of APOL1 to rescue intact mice from lethal trypanosome challenge. Replacement of most extracellular Na+ by K+ also reduced APOL1-associated oocyte toxicity, allowing demonstration of APOL1-associated increases in Ca2+ and Cl− fluxes and oocyte ion currents, which were similarly reduced by MCL1 coexpression. Thus APOL1 toxicity in Xenopus oocytes is BH3-independent, but can nonetheless be rescued by some BCL2 family proteins.
Keywords: apolipoprotein L1, Xenopus oocyte, trypanosome, two-electrode voltage clamp, hydrodynamic gene delivery
the prevalence of chronic kidney disease in African Americans is four- to fivefold higher than in Americans of European descent. This major health discrepancy was recently linked to genetic variants in the APOL1 gene encoding the serum HDL component apolipoprotein L1 (APOL1) (15, 16, 56). Expressed only in humans and a few higher primates (36, 45, 48, 53), APOL1 is the major trypanolytic factor of human serum (57) and a component of the innate immune system (39, 53). Trypanosoma brucei variants rhodesiense and gambiense cause African sleeping sickness in humans and have evolved mechanisms to evade lysis by APOL1. The agent of evasion in T. brucei rhodesiense is “serum resistance-associated” protein (SRA), a modified variant surface glycoprotein that directly binds and neutralizes APOL1, thus restoring pathogenicity (49, 53, 61). Two common APOL1 haplotypes uniquely found in Africans and Americans of African ancestry encode variants (G1 and G2) with reduced SRA-binding affinity, and each confers some resistance to T. b. rhodesiense-associated African sleeping sickness with a dominant inheritance pattern. However, homozygous or compound heterozygous inheritance of these APOL1 resistance alleles increases by 7- to 17-fold the risk of adult-onset focal segmental glomerulosclerosis (16, 28a), or the risk for human immunodeficiency virus-associated nephropathy by 13- to 90-fold (26, 28a). Risk is also increased to lesser degrees for a range of nondiabetic kidney disorders, any of which may progress to renal failure and the need for renal replacement therapy.
Available data suggest the renal disease associated with APOL1 risk variants reflects a pathological gain-of-function. Genetic absence of APOL1 is compatible with normal renal function (25), and APOL1 knockdown in human podocytes is without apparent effect (30), whereas overexpression of “wild-type” (WT) APOL1 or of APOL1 risk variants is toxic in human cell lines (30, 39, 60). These data have together suggested that APOL1-associated toxicity is normally held in check by an endogenous SRA-like factor yet to be identified (30).
The central portion of the APOL1 polypeptide was predicted to constitute a pore-forming domain, based on homology to E. coli colicin (44). Indeed, native APOL1 within the purified heteromeric serum complex, trypanolytic factor 1 (TLF1) (34), recombinant APOL1 pore-forming domain (44), and recombinant holo-APOL1 (52) each has been shown to confer increased ion permeability to liposomes and/or planar lipid bilayers. However, reported ion selectivities have differed, and secondary ion transport events may be triggered in intact cells (30, 34, 44, 52).
The identification of a putative BH3-like domain within the APOL1 sequence (58) suggested APOL1 as a novel, atypical BH3-only protein, promoting autophagic cell death (60) by a mechanism potentially similar to that of Beclin-1 (47). However, increased autophagy might equally be attributed to functions of a pore-forming domain (9, 10, 17, 28). The oocytes of Xenopus laevis have been extensively used to correlate the functions of transmembrane ion channels and transporters with cellular toxicity associated with transgene expression (2, 5, 8, 12, 13, 51, 55). We have used the Xenopus oocyte to study the importance of the putative BH3 domain of APOL1 in mediating the cell toxicity and ion transport activities associated with heterologous expression of APOL1.
METHODS
Materials.
Na36Cl was from ICN (Irvine, CA). 45CaCl2 was from PerkinElmer (Waltham, MA). Restriction enzymes and T4 DNA ligase were from New England Biolabs (Beverly, MA). EXPAND High-fidelity PCR System was from Roche (Indianapolis, IN). 4,4-Diisothiocyanostilbene-2,2-disulfonic acid (DIDS) was from Calbiochem (La Jolla, CA). 4,4-Dinitrostilbene-2,2-disulfonic acid (DNDS) was from Pfalz & Bauer (Waterbury, CT). ZVAD-FMK was from Tocris (R&D Systems, Minneapolis, MN). Trametinib and obatoclax were from LC Labs (Woburn, MA). MBCQ was from Santa Cruz (Dallas, TX). Necrostatin-1 was from ENZO (Farmington, NY). Spautin-1 was from Junying Yuan (Harvard Med. School). Other reagent-grade reagents were from Sigma-Aldrich (St. Louis, MO) or Fluka (Milwaukee, WI).
Solutions.
MBS consisted of (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, and 10 HEPES (pH 7.40 adjusted with NaOH). High K+ (HiK) MBS consisted of (in mM) 1.6 NaCl, 87.4 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, and 10 HEPES (pH 7.40 adjusted with KOH). ND-96 consisted of (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES free acid (adjusted to pH 7.40 or pH 8.50 with HCl). For all experiments at pH 5, HEPES was replaced by equimolar MES, and pH was adjusted accordingly. NMDG-97 consisted of (in mM) 97.3 N-methyl-d-glucamine, 2 KCl, 1.2 CaCl2, 1 MgCl2, and 5 HEPES free acid (pH 7.40 and pH 8.50). Cl− substitution was achieved by mole-for-mole replacement with Na cyclamate. Cl− salts of K+, Ca2+, and Mg2+ were substituted on an equimolar basis with the corresponding gluconate salts as needed.
Synthesis of cDNA and cRNA.
APOL1 WT cDNA (BC141823 encoding AAI4182; Open Biosystems, Huntsville, AL) was modified to retain the native Kozak sequence and 35 nt of native 3′-UTR and subcloned into transcription vector pTX7 (11), hereafter described as APOL1 WT. The amino acid sequence encoded by BC141823 (and this APOL1 WT cDNA) is identical to NP_003652 (APOL1 isoform a, also known as APOL1 G0) with the exceptions of known coding polymorphisms E150K, M228I, and R255K. APOL1 BH3 domain mutants were generated by four primer polymerase chain reaction (PCR). The upstream flanking primer oligonucleotide for all APOL1 mutagenesis experiments was T7 RNA polymerase binding site primer 5′-taatacgactcactataggg-3′. The downstream flanking primer was 5′-tgctctagaggcatatctctcctggtggctg-3′. Table 1, top, summarizes the additional oligonucleotide primers used to generate the engineered mutant forms of APOL1 studied in Xenopus laevis oocytes.
Table 1.
Mutagenic oligonucleotides
| APOL1 mutagenic oligonucleotides for oocyte experiments. | ||
| APOL1 mutant | Mutagenic oligonucleotides | |
| Starting from APOL1 “WT”: | ||
| APOL1.ΔBH3 | F 5′-ctccgtgcccttgcagctggggttcagaaggtc-3′ | |
| R 5′-gaccttctgaaccccagctgcaagggcacggag-3′ | ||
| APOL1.D163A | F 5′-ctccgtgcccttgcagctggggttcagaaggtc-3′ | |
| R 5′-gaccttctgaaccccagctgcaagggcacggag-3′ | ||
| Starting from APOL1.D163A: | ||
| APOL1.L158A/D163A/V165A | F 5′-gaagggcccgtgcacttgcagctggggctcagaaggtccacaaag-3′ | |
| R 5′-agccccagctgcaagtgcacgggcccttcttatgttatcctcaag-3′ | ||
| Starting from APOL1.L158A/D163A/V165A: | ||
| APOL1.9ALA | F 5′-cataagaagggccgctgcagctgcagctgcggctgcaaaggtccacaaag-3′ | |
| R 5′-ctttgtggaccttcgcagccgcacgtgcagctgcagcggcccttcttatg-3′ | ||
| BCL2 family member oligonucleotides for RP-PCR. | ||
| Human BCL2 Family Member | PCR oligonucleotides | |
| BCL2 | NM_000633 | F 5′-ccggaattcctctgggaaggatggcgcac-3′ |
| R 5′-tgctctagattggggcaggcatgttgacttc-3′ | ||
| BCLXL | NM_138578 | F 5′-ccggaattcgagactcagtgagtgagcagg-3′ |
| R 5′-tgctctagagggagggtagagtggatggtcag-3′ | ||
| BCLW | NM_004050 | F 5′-cacggaattcattaagagctgccatcccggctg-3′ |
| R 5′-tgctctagacctggccctggactttcacttgc-3′ | ||
| BCL2A1 | NM_004049 | F 5′-ccggaattctccaccaggcagaagatgacag-3′ |
| R 5′-tgctctagagtcctttctggtcaacagtattgc-3′ | ||
| MCL-1 | NM_021960 | F 5′-ccggaattcggcgactggcaatgtttggcctc-3′ |
| R 5′-tgctctagactaggaagttacagcttggagtcc-3′ | ||
| APOL1 mutagenic oligonucleotides for PRG977 mouse expression vector. | ||
| APOL1 mutant | Mutagenic oligonucleotides | |
| Starting from APOL1 “WT”: | ||
| APOL1.ΔBH3 | F 5′-gaggataacataagaaggaaggtccacaaaggcacc-3′ | |
| R 5′-ggtgcctttgtggaccttccttcttatgttatcctc-3′ | ||
| Starting from APOL1 “WT”: | ||
| APOL1.L158A | F 5′-gaggataacataagaagggcccgtgcccttgcagatg-3′ | |
| R 5′-catctgcaagggcacgggcccttcttatgttatcctc-3′ | ||
| Starting from APOL1 “WT”: | ||
| APOL1.R159A | F 5′-ataacataagaaggctcgctgcccttgcagatggg-3′ | |
| R 5′-ccccatctgcaagggcagcgagccttcttatgttat-3′ | ||
| Starting from APOL1 “WT”: | ||
| APOL1.D163A | F 5′-ccgtgcccttgcagctggggttcagaag-3′ | |
| F 5′-ccgtgcccttgcagctggggttcagaag-3′ | ||
| Starting from APOL1.D163A: | ||
| APOL1.L158A/R159A/D163A: | F 5′-tgaggataacataagaagggccgctgcccttgcagctgggg-3′ | |
| F 5′-tgaggataacataagaagggccgctgcccttgcagctgggg-3′ | ||
Full-length cDNAs encoding BCL2 family members were PCR-amplified from cDNA of human blood, kidney, and placenta, using the specific primers listed in Table 1, middle. Inclusion of Q Solution (Qiagen) in the reactions was required for successful amplification of MCL1 cDNA and for optimal amplification of BCL2 cDNA. Purified PCR products were digested with KpnI and XbaI and inserted into the Kpn1 and Spe1 sites of pXT7. Integrity of all PCR-generated cDNAs was ensured by complete sequencing of both strands.
cDNA plasmids were linearized with XbaI, BamHI or SalI as required. Capped cRNA was transcribed at 37°C from linearized plasmid cDNA template using the Megascript T7 kit (Life Technologies), purified with the RNeasy mini-kit (Qiagen, Valencia, CA), and quantitated by Nanodrop spectrometer (ThermoFisher, Waltham, MA). RNA integrity was verified by formaldehyde gel electrophoresis.
cRNA expression in Xenopus oocytes.
Mature female Xenopus laevis (Dept. Systems Biology, Harvard Medical School) were subjected to partial ovariectomy under hypothermic tricaine anesthesia following protocols approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Stage VI oocytes were prepared by overnight incubation of ovarian fragments in MBS with 2 mg/ml collagenase B (Alfa Aesar, Ward Hills, MA), followed by a 20 min rinse in Ca2+-free MBS, with subsequent manual selection and defolliculation as needed. Oocytes were injected with cRNA on the day of isolation and maintained at 17.5°C in MBS or HiK MBS supplemented with gentamicin (10 μg/ml) for the times indicated.
Oocyte morphology assessment.
Preliminary experiments monitored changes in morphological degradation in groups of oocytes in 60 mm polystyrene dishes containing 10 ml of MBS, using a Nikon SMZ-1 microscope equipped with an Olympus D-510 2.5 MP digital camera (data not shown). Subsequent experiments (all those reported with statistical analysis) monitored the progression of morphological deterioration in individual oocytes in single wells of 96-well round bottom polystyrene plates (Thermo Scientific Nunc). Individual oocytes were imaged (×18 magnification) at 24, 48, and 72 h post-cRNA injection with a CPW1308C 8bit CCD digital camera (Scion) through an Olympus S7X7 microscope. Images saved as tif files were scored as “unaffected” (green), “discolored” (yellow), or blebbing/oozing (red) and similarly color-coded in Figs. 1–3 and 7. In each experiment, comparisons were restricted to groups of cRNA-injected and uninjected oocytes harvested from the same frog(s) and prepared at the same time, to minimize lot-to-lot variation of oocyte responses to heterologous expression of APOL1 and to BCL2 family members.
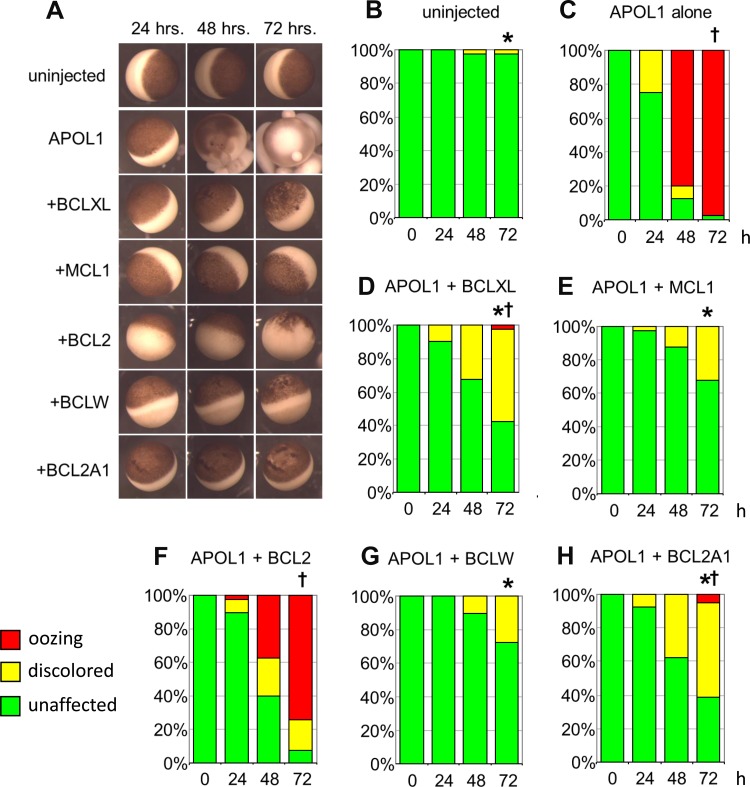
Fig. 1.
Toxic effects of apolipoprotein L1 (APOL1) expression in Xenopus oocytes are rescued or attenuated by coexpression of BCL2 family members. A: representative micrographs of oocytes recorded 24, 48, and 72 h post-injection of APOL1 cRNA (10 ng) without or with cRNA encoding the indicated individual BCL2 family polypeptides (50 ng), compared with uninjected oocyte controls (top row). Oocyte viability was graded with three toxicity categories: “unaffected” (resembling uninjected oocytes, i.e., complete rescue), “discolored” (with variably mottled or depigmented animal pole), and “oozing” or “blebbing” (with loss of physical integrity). The colored bars in the histograms correspond to the key in the bottom left: unaffected (green), discolored (yellow), and oozing (red). B and C: uninjected oocytes and oocytes injected with 10 ng APOL1 cRNA were imaged and graded at time t = 24, 48, and 72 h post-injection. C–H: oocytes coinjected with 10 ng APOL1 cRNA and 50 ng cRNA encoding the indicated BCL2 family polypeptides were imaged and graded at t = 24, 48, and 72 h post-injection. n = 40 in each group, made up of 10 oocytes from each of 4 frogs. Pairwise statistical differences between groups at 72 h post-injection were determined by ANOVA of ranks with a Tukey post hoc test. *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes.
Fig. 3.
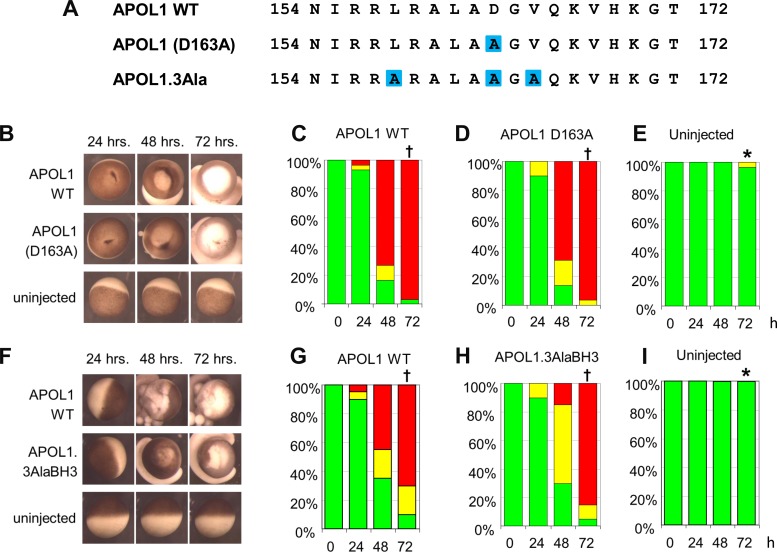
Conserved residues of the APOL1 BH3 domain are not required for APOL1-associated oocyte toxicity. A: aligned BH3 domain sequences of WT APOL1 and of BH3 mutants, bracketed by amino acid sequence numbers. Mutated residues are highlighted in blue. B: representative oocytes imaged at the indicated times post-injection with cRNA (10 ng) encoding APOL1 WT or APOL1 D163A, compared with uninjected oocytes (bottom row). C–E: oocyte morphology category histograms at 24, 48, and 72 h post-injection of cRNA encoding APOL1 (C) or APOL1 D163A (D), compared with uninjected oocytes (E). n = 30 in each group (10 oocytes from each of 3 frogs). F: representative oocytes imaged at the indicated times post-injection with cRNA (10 ng) encoding APOL1 WT or APOL1 triple mutant L158A/D163A/V165A (3AlaBH3 compared with uninjected oocytes; bottom). G–I: oocyte morphology histograms at 24, 48, and 72 h post-injection of cRNA encoding the indicated cRNAs. n = 20 in each group (10 oocytes from each of 2 frogs). Statistical differences between groups at 72 h was determined as described in Fig. 1. *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes.
Fig. 7.
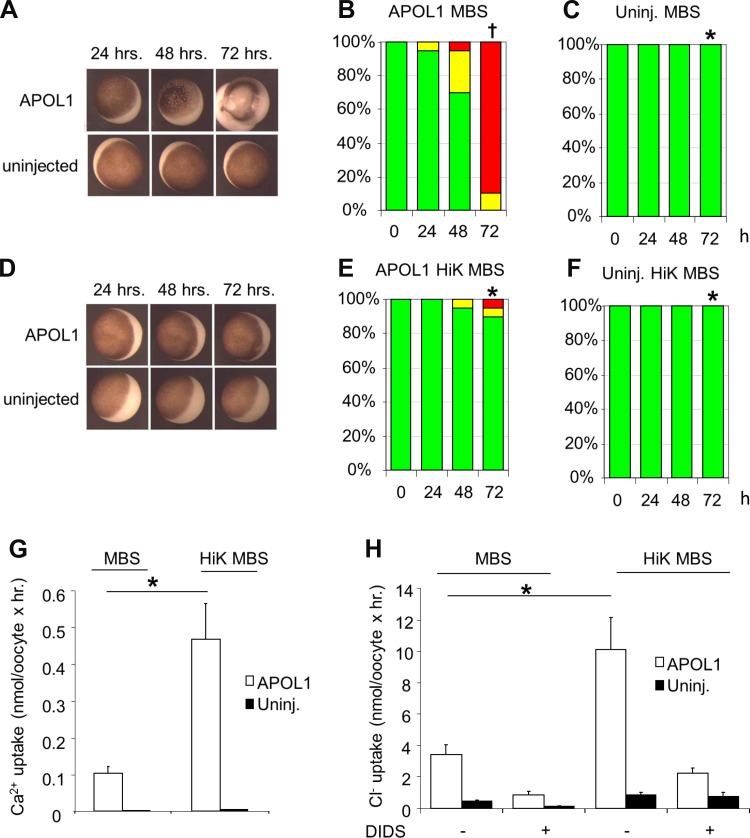
Oocyte exposure to high extracellular [K+] for 3 days post-injection of APOL1 cRNA attenuates morphological toxicity and increases ion fluxes in surviving oocytes. A: representative uninjected oocytes and oocytes injected with 10 ng APOL1 cRNA and maintained in MBS for the indicated times. B: oocyte morphology histograms at 24, 48, and 72 h post-injection of APOL1 cRNA with subsequent maintenance in MBS. C: oocyte morphology histograms for uninjected oocytes maintained in MBS for 24, 48, and 72 h. D: representative uninjected oocytes and oocytes injected with 10 ng APOL1 cRNA after maintenance in high K+ (HiK) MBS for the indicated times. E: oocyte morphology histograms at 24, 48, and 72 h post-injection of APOL1 cRNA, with maintenance in HiK MBS. F: oocyte morphology histograms for uninjected oocytes maintained in HiK MBS for 24, 48, and 72 h. n = 10 oocytes in each group in E and F. *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes. One of six similar experiments is shown. G and H: oocytes injected with APOL1 cRNA 72 h previously with subsequent maintenance in HiK MBS exhibit higher 45Ca2+ uptake (G) and higher DIDS-sensitive 36Cl− uptake (H) than oocytes maintained in MBS. Solid bars, uninjected oocytes. Values are means ± SE; n = 10 oocytes in each group. *P < 0.05.
Isotopic influx experiments.
Unidirectional 45Ca2+ influx studies were carried out for periods of 30 min in 148 μL ND-96 and 2 μL 45CaCl2 (∼2 μCi; final bath [Ca2+] 1.8 mM, where brackets denote concentration). Unidirectional 36Cl− influx studies were carried out for periods of 30 min in 148 μl ND-96 and 2 μl Na36Cl (∼0.2 μCi; final bath [Cl−] 107 mM). Bath solutions for all 36Cl− influx experiments were supplemented with 10 μM bumetanide to block Cl− influx by native oocyte Na-2Cl cotransporter-1. Influx experiments were terminated with three washes in ice-cold isotonic Na cyclamate solution. Washed oocytes were individually lysed in 150 μl 2% sodium dodecyl sulfate (SDS). Triplicate 10 μl aliquots of influx bath solution were used to calculate specific activity of radiolabeled substrate ions. Oocyte ion uptakes were calculated from cpm values of cold-washed oocytes and from bath specific activity.
Two-electrode voltage clamp measurements.
Microelectrodes from borosilicate glass made with a Sutter P-87 puller (Sutter Instruments, Novato, CA) were filled with 3 M KCl and had resistances of 2–3 MΩ. Oocytes previously injected with cRNA were placed in a 1 ml chamber (model RC-11, Warner Instruments, Hamden, CT) on the stage of a dissecting microscope and impaled with microelectrodes under direct view. Steady-state currents achieved within 2–5 min following bath change were measured with a Geneclamp 500 amplifier (Molecular Devices, Burlingame, CA) interfaced to a Dell computer with a Digidata 1322A digitizer (Molecular Devices).
Data acquisition and analysis utilized pCLAMP 8.0 software (Molecular Devices). The voltage pulse protocol generated with the Clampex subroutine consisted of 20 mV steps between −100 mV and +40 mV, with durations of 738 ms, and separated by 30 ms at the holding potential of −30 mV. Bath resistance was minimized by 3 M KCl agar bridges. A virtual ground circuit clamped bath potential to zero.
Raising anti-APOL1 antibody.
Synthetic peptide NH2-(C)PSGTDTGDPQSKPLGDW-CONH encoding aa 39–55 of the 398 aa isoform of human APOL1 with an N-terminal Cys-tag was HPLC purified (>85% purity), sequence validated by mass spectroscopy, and haptenized by Cys-coupling with m-maleimidobenzoyl-N-hydroxysuccinimide ester. Haptenized peptide was used to raise polyclonal antiserum in rabbits, and antisera were affinity-purified (21st Century Biochemicals, Marlboro, MA). Additional anti-APOL1 antibodies used in this paper were from Sigma (HPA01885) and from ProteinTech (11486-2-AP).
Confocal immunofluorescence microscopy.
cRNA-injected oocytes and uninjected oocytes were incubated for 3 days in at 17.5°C in HiK MBS containing gentamicin (10 μg/ml). Ten to twelve oocytes in each experimental group were fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at room temperature, washed three times with PBS-Tween (PBST), permeablized with 1% SDS in PBS supplemented with 0.02% Na azide (PBS-azide) for 1–2 min, and washed again three times with PBST.
Fixed, permeabilized oocytes were incubated overnight at 4°C in 0.5 ml PBST containing affinity-purified polyclonal rabbit anti-APOL1 (1:500), rinsed in PBST, and stained with Cy3-conjugated donkey-anti-rabbit secondary Ig (1:1,000) for 90 min at 20°C. Stained oocytes were washed 3× in PBST, 2× in PBS-azide, and postfixed in paraformaldehyde for 10 min. Fixed oocytes were washed 2× in PBST, extensively washed with PBS-azide, and stored in PBS-azide at 4°C until imaged. Cy3-labeled oocytes aligned in uniform orientation along a Plexiglas groove were sequentially imaged through the ×10 objective of a Zeiss LSM510 laser scanning confocal microscope using the 543 nm laser line at 512 × 512 resolution at a uniform setting of 80% intensity, pinhole 54 (1.0 Airy units), detector gain 650, Amp gain 1, zero amp offset.
Polypeptide abundance at or near the oocyte surface was estimated by quantitation of specific fluorescence intensity (FI) at the circumference of one quadrant of an equatorial focal plane image of the oocyte (Image J v. 1.38, National Institutes of Health). Background correction was performed by subtraction from FI of each cRNA-injected oocyte the mean FI value of an equatorial plane quadrant of water-injected oocytes.
Oocyte lysate immunoblots.
Twenty to twenty-five oocytes previously injected with cRNA (10 ng APOL1 and/or 50 ng MCL1) were placed in MBS in a 1.5 ml centrifuge tube. After 24, 48, or 72 h incubation, MBS was aspirated and replaced with RIPA buffer containing Complete Protease inhibitor (6 μl per oocyte), then vortexed vigorously and immediately stored at −80°C for at least 2 h. The thawed mixture was again vortexed and then centrifuged 20–30 min at 4°C at maximal microfuge speed. The fairly clear infranatant layer between the pellet and foamy debris was withdrawn and subjected to two more cycles of vortexing, 4°C centrifugation, and infranatant harvest. The final, clarified protein extracts were assayed by BCA for protein and stored at −80°C until use. Twenty micrograms of protein were brought up to 10 μl volume with RIPA buffer containing protease inhibitors, with further addition of 4 μl SDS load buffer plus β-mercaptoethanol. The mixture was heated to 97°C for 5 min, cooled, loaded on a 10% polyacrylamide Tris-acetate gel (BIO-RAD), and subjected to SDS-PAGE. Protein was transferred to PVDF membrane (iBlot 2, Life Technologies), washed in TBST, and blocked 1 h with TBST plus 5% powdered milk. The blocked membrane was washed with TBST and incubated overnight at 4°C with rabbit anti-APOL1 (Sigma HPA018885) diluted 1:300 in TBST/5% BSA and then further washed and incubated 1 h with horseradish peroxidase-coupled secondary goat anti-rabbit Ig (Thermo Scientific no. 31460; diluted 1:8,000 in TBST/5% milk). Signal was developed (Supersignal West DURA kit, Life Technologies) and imaged (FluorChem E, Bio-Techne). To control for loading, each membrane was probed with horseradish peroxidase-coupled anti-GAPDH (Genetex, GTX-627408-01 diluted 1:10,000) either simultaneously with the secondary antibody or afterwards, as indicated in the figure legends.
Trypanosome infection of mice transiently expressing APOL1 or BH3 domain missense mutants of APOL1 following hydrodynamic gene delivery.
Mouse experiments were approved by the Institutional Animal Care and Use Committee of Hunter College.
APOL1 expression vectors used in mice were constructed in plasmid pRG997, containing a mammalian ubiquitin promoter, 5′ intronic splicing sequences from rabbit β-globin, and a 3′ SV40 polyadenylation signal. APOL1 missense and deletion mutants were generated by the Stratagene mutagenesis kit (La Jolla, CA), using primers listed in Table 1, bottom. All plasmids injected into mice were purified with the Qiagen Endotoxin-free Maxiprep Kit.
Approximately 20 g female outbred Swiss Webster mice (Taconic Biosciences, Hudson, NY) underwent tail vein injection (27 g needle) with 2–3 ml saline (10% body weight) containing 50 μg (14.5 pmol) of expression plasmid pRG997-APOL1 (5.2 kb). Two days after this hydrodynamic DNA injection, mice were subjected to intraperitoneal injection of 5 × 103 Trypanosoma brucei brucei 427 parasites in 200 μl DMEM. Twenty-microliter tail vein blood samples were taken from infected mice on day 3 postinfection and twice per week thereafter to monitor parasitemia. Mice were euthanized at ∼1 × 109 parasites/ml (29, 53), and postinfection survival times were recorded.
Immunoblot of mouse serum and HDL.
Two days after hydrodynamic DNA injection, mouse serum was prepared from a tail bleed sample (BD serum separators no. 365956, 6,000 × g × 90 s).
Murine HDL was purified from whole serum collected immediately prior to parasite injection, as previously described (35). HDL or serum was diluted 1:40 in Laemmli running buffer and subjected to SDS-PAGE on 10% precast gels (Bio-Rad). Overnight protein transfer to PVDF membrane (4°C, 30 V) was followed by immunodetection using anti-human APOL1 (ProteinTech 11486-2-AP, 1:10,000), anti-mouse APOA1 (Abcam ab20453, 1:10,000), and secondary antibody (anti-rabbit TrueBlot-HRP; Rockland 18-8816-33, 1:2,500).
Statistics.
Ordinal data scoring oocyte morphological toxicity [with integral values of 0 (unaffected), 1 (discolored), and 2 (oozing/blebbing)] as previously described (6, 12, 51), were subjected to the Mann-Whitney U-test with a Bonferroni correction (www.socstatistics.com). Continuous data reported as means ± SE (ion flux, ion current, FI) were compared by Student's paired or unpaired two-tailed t-tests (Microsoft Excel), or by ANOVA with Tukey post hoc analysis. P < 0.05 was interpreted as statistically significant.
RESULTS
Toxic effects of APOL1 expression in Xenopus oocytes are rescued or attenuated by coexpression of BCL2 family members.
Expression of certain gene products in Xenopus oocytes elicits toxic morphological changes associated with varied mechanisms of cell death (2, 5, 6, 8, 12, 13, 51, 55). Xenopus oocytes were therefore chosen for semiquantitative assessment of the toxic effects of APOL1 expression.
Individual oocytes previously injected with 10 ng APOL1 WT cRNA were photographed at 24, 48, and 72 h postinjection and compared with uninjected oocytes from the same harvest (Fig. 1A, top 2 rows). Oocytes injected with APOL1 WT cRNA exhibited profound morphological changes grossly resembling degenerative changes previously reported in oocytes expressing toxic proteins with ion transport or channel activity (6, 12, 51). Oocytes were classified as unaffected (green), discolored (yellow), or oozing/blebbing (red). In the histograms of Figs. 1–3 and 7, morphological categories of green, yellow, and red were given respective ordinal scores of 0, 1, and 2 for statistical analysis. Comparison of APOL1-injected oocytes with uninjected oocytes demonstrated APOL1-associated toxic morphological changes (Fig. 1, A–C).
As a putative BH3-only protein (58, 60, 63), APOL1-induced toxicity might be expected to be rescued or attenuated by coexpression of an anti-apoptotic BCL2 family member (38, 43, 62). Expression of the pro-apoptotic protein BCL-XS was previously shown to be toxic to Xenopus oocytes, but coexpression of the anti-apoptotic BCL-XL rescued viability (5). In view of the varied BH3-binding specificities of different BCL2-family members (7), additional anti-apoptotic BCL2 family members were individually coexpressed with APOL1 and assessed for ability to attenuate APOL1-associated morphological toxicity (Fig. 1A).
Oocytes were coinjected with 10 ng APOL1 cRNA and 50 ng cRNA encoding the indicated anti-apoptotic BCL2 family proteins (Fig. 1, A, D–H). The degree of rescue of APOL1-associated toxicity fell into three distinct groups. BCL2 alone failed to rescue APOL1-associated toxicity or had minimal effect (compare Fig. 1, B and C, with F). Coexpression with APOL1 of BCLXL or BCL2A1 resulted in morphological changes of intermediate severity that significantly differed from those of APOL1 alone as well as from those of uninjected oocytes (Fig. 1, D and H). Coexpression of MCL1 or BCLW almost completely rescued APOL1-associated toxicity and differed from oocytes expressing APOL1 alone but not from uninjected oocytes (Fig. 1, E and G). The observed rescue or amelioration of APOL1-induced oocyte toxicity by coexpression of selected BCL2 family proteins is consistent with the hypothesis that the putative BH3 domain of APOL1 might itself be the effector of cell death associated with APOL1 expression. However, coexpressed human MCL1 rescued APOL1 toxicity even in the presence of the BH3 domain-dependent MCL1 inhibitor, obatoclax (1 μM, extra- and intracellular; not shown), suggesting other possible mechanisms of MCL1 action.
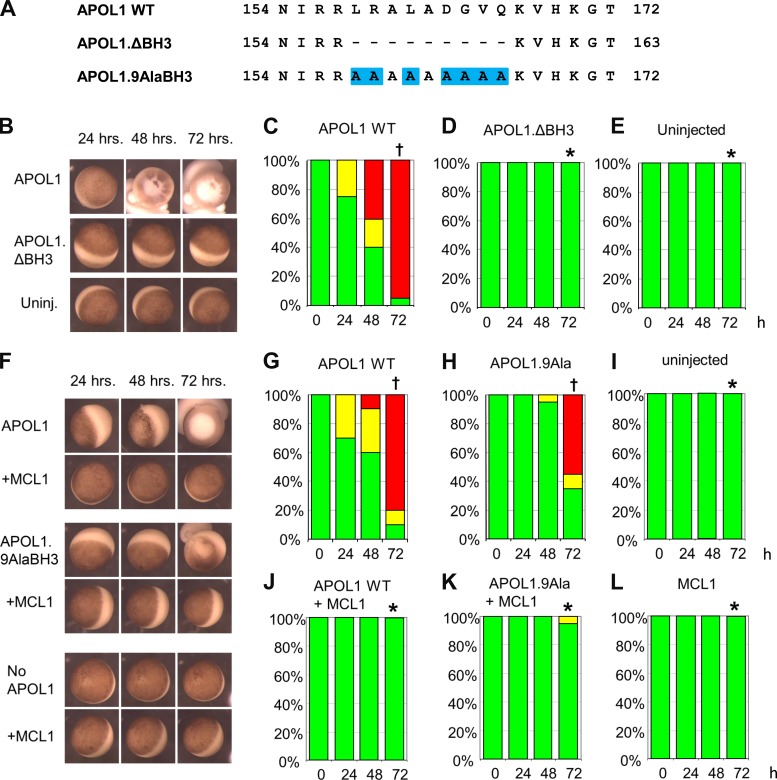
APOL1 BH3 domain deletion prevents, whereas Ala(9) substitution of BH3 minimally reduces, oocyte toxicity without preventing rescue by MCL1.
If the BH3 domain serves an important role in either the toxicity of APOL1 or the interaction with anti-apoptotic BCL2 family proteins, then mutation of the BH3 domain should result either in loss of APOL1-associated toxicity, or in loss of rescue from this toxicity by anti-apoptotic BCL2 family proteins. To test these hypotheses, mutants targeting the nine core amino acid residues of the putative BH3 domain (58, 60, 63) of APOL1 were constructed (Fig. 2A). Xenopus oocytes injected with deletion mutant APOL1.ΔBH3 cRNA or with unmutated APOL1 (APOL1 WT) were compared with uninjected oocytes, as described in Fig. 1. Oocytes expressing APOL1.ΔBH3 indeed exhibited no APOL1-associated morphological toxicity (Fig. 2, B–E). Initially, these results appeared to support the previously suggested hypothesis (60) that the APOL1 BH3 domain is the effector of cell death. However, deletion of core BH3 domain residues from the APOL1 pore-forming domain may destabilize larger regions of the polypeptide. Therefore, all nine BH3 residues of APOL1 were substituted with helix-preserving Ala residues [APOL1.9AlaBH3 (Fig. 2A)]. Indeed, APOL1 toxicity was not eliminated by APOL1.9AlaBH3, but only minimally attenuated (Fig. 2, F–I). If the function of the nominal BH3 domain of APOL1 is to interact with BCL2 family proteins, then the APOL1.9AlaBH3 mutant should not be rescued by MCL1 coexpression. However, MCL1 coinjection rescued APOL1.9AlaBH3 as completely as it did APOL1 WT (Fig. 2, F, J–L).
Fig. 2.
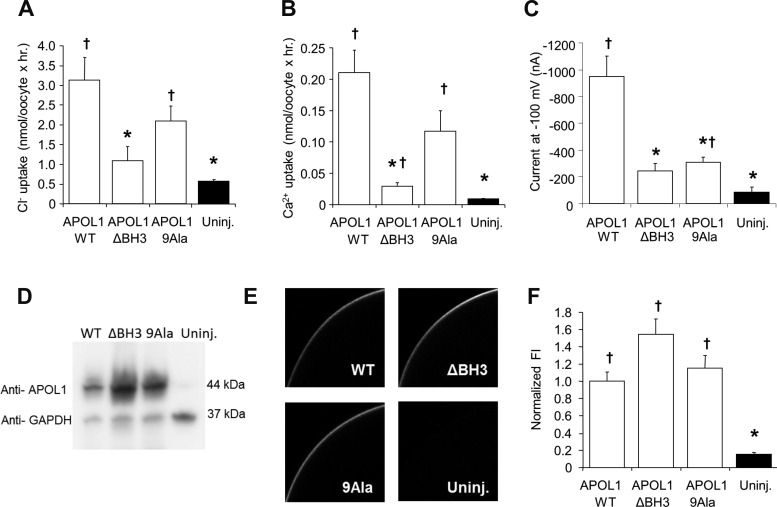
APOL1 BH3 domain deletion (APOL1.ΔBH3) prevents APOL1-associated oocyte toxicity, whereas BH3 Ala(9) substitution (APOL1.9AlaBH3) only minimally reduces oocyte toxicity but does not inhibit rescue of toxicity by MCL1. A: aligned BH3 domain sequences of wild-type (WT) APOL1 and of BH3 mutants, bracketed by amino acid sequence positions (numbers). Mutated residues are highlighted in blue. B: representative oocytes imaged at the indicated times post-injection with cRNA (10 ng) encoding APOL1 or APOL1 ΔBH3, compared with uninjected oocytes (bottom row). C–E: oocyte morphology category histograms at 24, 48, and 72 h post-injection of cRNA (10 ng) encoding APOL1 (C) or APOL1.ΔBH3 (D), compared with uninjected oocytes (E). n = 20 in each group (10 oocytes from each of 2 frogs). F: representative oocytes imaged at the indicated times post-injection with cRNA (10 ng) encoding APOL1 WT or APOL1.9AlaBH3, without or with coinjected MCL1 cRNA (50 ng), and compared with uninjected oocytes or oocytes injected with MCL1 alone (bottom). G–L: oocyte morphology histograms at 24, 48, and 72 h post-injection of the indicated cRNAs. n = 20 in each group (10 oocytes from each of 2 frogs). Statistical differences among groups at 72 h was determined as described for Fig. 1. *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes.
Conserved core residues of the APOL1 putative BH3 domain are not required for APOL1-associated oocyte toxicity.
The toxicity associated with oocyte expression of APOL1.9AlaBH3 failed to support the hypothesis that the putative BH3 domain is the effector of APOL1-associated cell death. The role of the APOL1 BH3 domain was further examined by investigating less extensive missense substitutions in more highly conserved BH3 domain residues (Fig. 3A). APOL1 residue D163 was proposed as an important contributor to APOL1-associated cellular toxicity (60), but oocyte expression of APOL1 D163A produced morphological toxicity indistinguishable from that associated with WT APOL1 expression (Fig. 3, B–E). Combined Ala substitution of three highly conserved BH3 domain residues (L158, D163, and V165) (14) in mutant APOL1.3Ala slightly reduced morphological toxicity but remained nearly indistinguishable from that of WT APOL1 (Fig. 3, F–I).
As found for APOL1 WT, the morphological toxicity associated with expression of APOL1 D163A and with APOL1.3Ala was completely rescued by coexpression of MCL1 (data not shown). However, attempts in lysates of cotransfected human embryonic kidney (HEK)-293 cells to demonstrate physical interaction of APOL1 WT with epitope-tagged MCL1 were unsuccessful under the conditions tested (not shown). Thus the putative BH3 domain of APOL1 is not an essential contributor to the toxic morphological effects of APOL1 expression in Xenopus oocytes. Initial tests of multiple pharmacological inhibitors failed to implicate defined particular cell death pathways in APOL1-expressing oocytes.1
Integrity of the putative BH3 domain does not contribute to APOL1 protection of mice from lethal infection by trypanosoma brucei brucei.
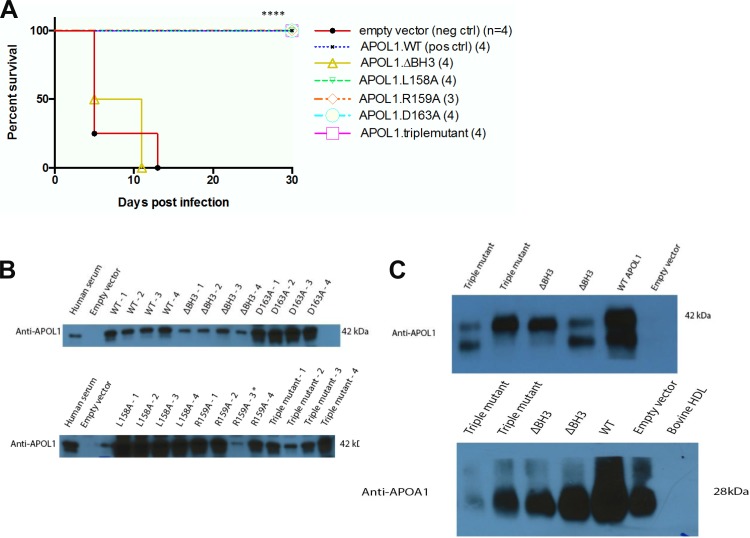
Since APOL1 is found only in the genomes of humans and a small group of higher primates, tests of APOL1 function in experimental animals require heterologous gene expression. Hydrodynamic gene delivery by intravenous injection of naked expression plasmid DNA has proven to be a powerful tool for transient APOL1 expression in mice (29, 53). As shown in Fig. 4A, T. b. brucei-infected mice were completely protected from death during the 30 day observation period by hydrodynamic gene delivery of APOL1 WT (G0) cDNA. Whereas hydrodynamic injection with APOL1.ΔBH3 cDNA led to death of all mice by postinfection day 11, mice injected with cDNAs encoding less extensive missense substitutions of the BH3 domain of APOL1, including mutants L158A, R159A, D163A, and triple mutant L158A/R159A/D163A, were completely protected against the lethal effects of trypanosomal infection. These results were indistinguishable from those with WT APOL1 (Fig. 4A). Heterologous human APOL1 expression was confirmed by immunoblot of serum from each mouse subjected to hydrodynamic gene delivery (Fig. 4B). The lack of protection by injected APOL1.ΔBH3 did not reflect diminished or absent association with HDL. As shown in Fig. 4C, immunoblot detection of APOL1 and APOA1 in purified HDL fractions from serum (Fig. 4C) was consistent with normal in vivo assembly of TLF1. Thus, the inability of APOL1.ΔBH3 to kill Xenopus oocytes, African trypanosomes, and cancer cell lines (60) reflects loss of the lytic activity of the deletion mutant, likely due to APOL1 misfolding. However, neither the trypanolytic activity of APOL1 nor the Xenopus oocyte toxicity associated with APOL1 expression requires integrity of APOL1's nominal BH3 domain sequence itself.
Fig. 4.
Protection of mice against lethal challenge with Trypanosoma brucei brucei by hydrodynamic APOL1 gene delivery does not require integrity of the BH3 domain in APOL1. A: survival curves of Swiss Webster mice infected with T. brucei brucei 2 days after hydrodynamic DNA injection with plasmids encoding APOL1 WT or the indicated missense mutants (see methods). Four mice were injected with each construct. (One mouse from the R159A group was removed from the analysis due to lack of transgene expression.) Mice expressing WT APOL1 and variants L158A, R159A, D163A, and the triple mutant L158A/R159A/D163A developed no detectable parasitemia (not shown) and exhibited 100% survival during the 30-day infection period (top-most trace), differing significantly from the shortened survival of mice expressing APOL1.ΔBH3 or injected with empty vector (****P < 0.0001; log rank test). B: APOL1 immunoblots of serum from DNA-injected mice collected 2 days post-injection, showing expression of the indicated APOL1 variants. C: immunoblots of APOL1 (top) and APOA1 (bottom) detected in HDL purified from representative individual mice previously subjected to hydrodynamic injection of the indicated APOL1 expression constructs. Purified bovine HDL serves as a negative control for both the anti-human APOL1 and anti-murine APOA1 antibodies.
APOL1-induced oocyte toxicity is accompanied by, but not caused by, elevated oocyte Cl− flux.
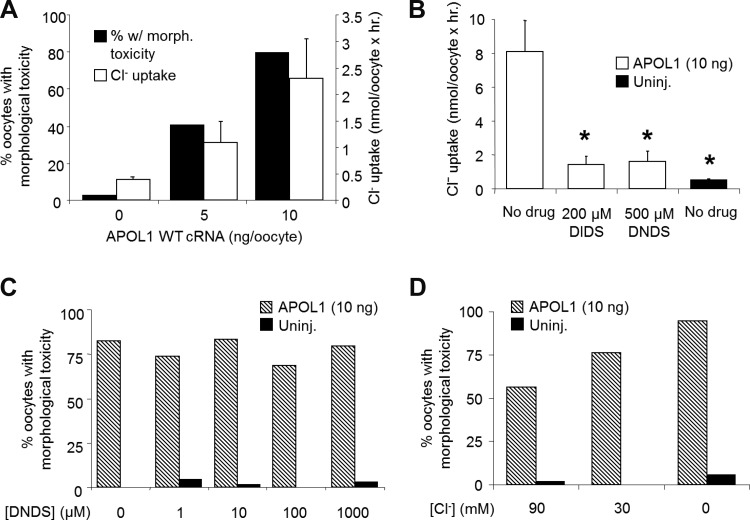
The reported association of APOL1′s trypanocidal effects with elevated trypanosomal Cl− flux (44) prompted examination of APOL1′s effects on oocyte Cl− flux. Only morphologically intact oocytes (see Fig. 1) were selected for these initial studies, to minimize nonspecific plasma membrane “leak”. The magnitude of Cl− flux in morphologically intact oocytes correlated with the injected amount of APOL1 cRNA, as well as with the proportion of oocytes exhibiting morphological toxicity (Fig. 5A). Blockade of oocyte Cl− flux did not reflect nonspecific “leak”, since fluxes were blocked by the Cl− transport inhibitors DIDS (30, 44) and its noncovalently reactive analog (21) DNDS at 200 μM or 500 μM (Fig. 5B), and (not shown) by the Ca2+-activated Cl− channel inhibitor, tannic acid (50 μM). However, inhibition of Cl− flux by the nontoxic, reversible Cl− transport antagonists DNDS (Fig. 5C) and tannic acid (not shown) did not rescue APOL1-associated morphological toxicity measured after 72 h. APOL1-mediated trypanolysis was previously reported to be rescued by substitution of extracellular Cl− with gluconate during periods of 1–2 h (34, 44). However, prolonged exposure of APOL1 cRNA-injected oocytes to extracellular solutions containing reduced or (nominally) zero [Cl−] failed to rescue morphological toxicity of oocytes (Fig. 5D).
Fig. 5.
APOL1-induced oocyte toxicity is accompanied by, but not caused by, elevated Cl− flux. A: correlation between oocyte survival 72 h post-injection of 10 ng APOL1 cRNA (solid bars representing percentage of 37–39 originally injected oocytes later discarded due to morphological changes) and 36Cl− influx into oocytes that remained morphologically intact at 72 h post-injection. Open bars, means ± SE; n = 9–10 oocytes/group. B: 36Cl− influx into oocytes injected 72 h earlier with 10 ng APOL1 cRNA was sensitive to inhibition by 4,4-diisothiocyanostilbene-2,2-disulfonic acid (DIDS; 200 μM) and to 4,4-dinitrostilbene-2,2-disulfonic acid (DNDS; 500 μM). Open bars, means ± SE; n = 9–10 oocytes. *P < 0.05. C: oocyte toxicity is not prevented by pharmacological inhibition of 36Cl− transport. Percentage of oocytes unsuitable for isotopic flux assay (with morphological toxicity) were either injected with 10 ng APOL1 cRNA (hatched bars) or were uninjected oocytes (solid bars) after 72 h incubation in MBS in the absence or presence of DNDS (200 or 500 μM). D: oocyte toxicity is not prevented by prolonged reduction of extracellular Cl− concentration ([Cl−]). Percentage of oocytes with morphological toxicity previously were injected with 10 ng APOL1 cRNA (hatched bars) or were uninjected oocytes (solid bars) after 72 h incubation in MBS or in MBS modified to contain either 30 mM Cl− or 0 mM Cl−.
APOL1 expression in oocytes elicits Ca2+ flux and ion currents.
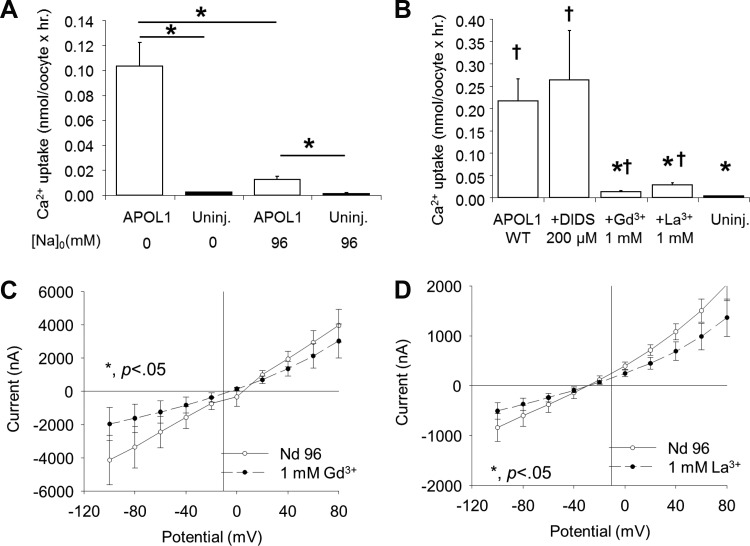
APOL1-induced increases in cation permeability have been described previously (34, 52). Xenopus oocytes expressing APOL1 exhibited increased uptake of 45Ca2+ in the presence of extracellular Na+ that was further increased upon Na+ substitution with the impermeant cation N-methyl-d-aspartate (Fig. 6A). The influx of 45Ca2+ was inhibited by extracellular La3+ and by Gd3+ (both at 1 mM), but not by DIDS (Fig. 6B), or by the low-specificity transient receptor potential vanilloid 4 cation channel inhibitor, ruthenium red (100 nM, not shown). These data are consistent with a nonspecific cation flux pathway activated by APOL1 expression, accompanied by secondarily activated oocyte Cl− permeability pathways.
Fig. 6.
APOL1 expression in oocytes elicits Ca2+ flux and currents. A: oocytes injected 72 h previously with APOL1 cRNA (10 ng, open bars) or uninjected oocytes (solid bars) were subjected to measurements of 45Ca2+ influx in the absence or presence of 96 mM bath Na+ (*P < 0.05). B: 45Ca2+ uptake by APOL1-expressing oocytes (open bars) is inhibited by 1 mM Gd3+ or La3+, but not by DIDS (200 μM). *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes. C: oocytes injected 72 h previously with APOL1 cRNA (5 ng) were subjected to two-electrode voltage clamp measurements of whole oocyte currents in the absence (ND 96, open circles, n = 5) and subsequent presence of 1 mM Gd3+ (solid circles, n = 5). D: a different group of oocytes injected 72 h previously with APOL1 cRNA (5 ng) was subjected to two-electrode voltage clamp measurements of whole oocyte currents in the absence (ND 96, open circles, n = 5) and subsequent presence of 1 mM La3+ (solid circles). *P < 0.05. Values are means ± SE.
Oocytes previously injected with 5 ng APOL1 cRNA and lacking morphological toxicity (as in shown in Fig. 1A) were subjected to two-electrode voltage clamp (TEVC) measurements of whole cell currents in the absence and subsequent presence of inhibitors. Both La3+ and Gd3+ significantly inhibited the APOL1-associated currents at holding potentials of −100 mV (Fig. 6, C and D). In addition, TEVC currents were significantly inhibited by tannic acid (50 μM) and by DIDS (200 μM) and activated by bath addition of 10 mM Ca2+ (data not shown). The wide spectrum of modest pharmacological inhibition, variably elevated current magnitudes, and variably depolarized reversal potentials together suggest that APOL1 expression in oocytes induces a mix of cation and anion currents.
Elevated extracellular [K+] attenuates APOL1-associated morphological toxicity while increasing ion fluxes in morphologically intact oocytes.
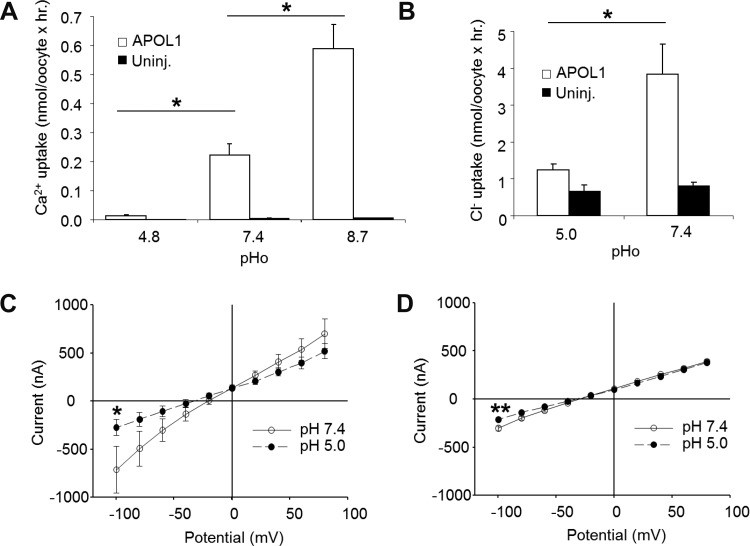
Mammalian cell susceptibility to bacterial ionophore toxins can be minimized or prevented by exposure to elevated extracellular [K+] (17). We therefore tested the effect of substituting extracellular Na+ with K+ during the 72 h post-cRNA injection (17, 34, 52). Not only was the treatment (HiK MBS) well tolerated by uninjected oocytes, but APOL1-associated morphological toxicity in oocytes was greatly reduced by incubation in HiK MBS (Fig. 7, A–F). In contrast, 72 h incubations of APOL1-expressing oocytes in bath solutions of either reduced Ca2+ or reduced Na+ (NMDG substitution) were without comparable protective effects (not shown). APOL1-expressing oocytes incubated in HiK MBS also exhibited significantly higher uptakes of 45Ca2+ (Fig. 7G) and of 36Cl− uptake (Fig. 7H) compared with APOL1-expressing oocytes maintained in MBS without added K+.
Coexpression of MCL1 inhibits APOL1-associated Ca2+ uptake and currents.
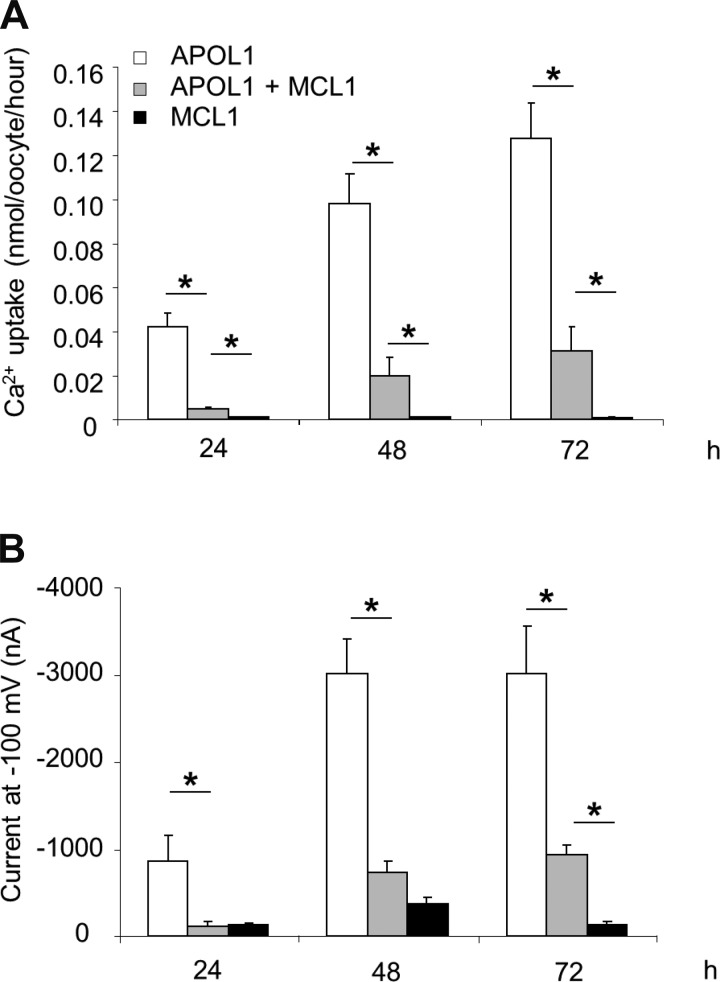
Since MCL1 coexpression rescued APOL1-associated oocyte toxicity, the effect of MCL1 coexpression on APOL1-associated ion transport was examined. As shown in Fig. 8, expression of MCL1 alone had little or no effect on 45Ca2+ uptake or on whole oocyte current measured by two microelectrode voltage clamp. The large increase in both 45Ca2+ uptake and whole oocyte current associated with APOL1 expression was almost completely attenuated by MCL1 coexpression after only 24 h in HiK MBS post-cRNA injection, and greatly reduced after 48 and 72 h. Thus the time-dependent increase in 45Ca2+ flux in APOL1-expressing oocytes maintained in HiK MBS parallels the increased morphological toxicity observed in APOL1-expressing oocytes maintained in MBS (Figs. 1, 3, and 7). Moreover, the attenuation of 45Ca2+ flux and whole-oocyte current by MCL1 coexpression mirrors the rescue by MCL1 of APOL1-associated morphological toxicity. Coexpression with APOL1 of BCL-XL similarly attenuated 45Ca2+ flux and whole cell currents in oocytes (data not shown).
Fig. 8.
Coexpression of MCL1 inhibits APOL1-associated Ca2+ uptake and currents. A: injection of 50 ng MCL1 cRNA together with 10 ng APOL1 cRNA greatly reduces APOL1-associated 45Ca2+ uptake at the indicated times postinjection. n = 20 in each group (10 oocytes from each of 2 frogs). B: injection of 50 ng MCL1 cRNA together with 10 ng APOL1 cRNA also greatly reduces APOL1-associated two-electrode voltage clamp current at the indicated times post-injection. Values are means ± SE. *P < 0.05.
Integrity of the APOL1 BH3 domain is not required for APOL1-associated increases in oocyte uptakes of Ca2+ and Cl−.
If APOL1-induced increases in ion flux reflect or contribute to APOL1-associated oocyte toxicity, then APOL1 BH3 mutants characterized by reduced or absent toxicity should elicit comparable changes in ion flux. Compared with the increased uptakes of 36Cl− and 45Ca2+ and whole cell ion currents in oocytes expressing APOL1 WT, increases in ion fluxes and whole cell currents in oocytes expressing APOL1.ΔBH3 were nearly abolished (Fig. 9), corresponding to lack of morphological toxicity in this group of oocytes (Fig. 2, B and D). However, oocytes expressing APOL1.9Ala exhibited intermediate levels of Ca2+ and Cl− influx (Fig. 9, A and B), together with low values of whole cell current (Fig. 9C), consistent with greatly reduced but not absent morphological toxicity (Fig. 2, F and H). These differences in ion transport activity appeared unrelated to changes in total APOL1 expression or in APOL1 surface expression, since expression of neither APOL1.ΔBH3 nor of APOL.9Ala was associated with any reduction in abundance of APOL1 polypeptide in the oocyte lysate (Fig. 9D), or at or near the oocyte surface as determined by immunocytochemistry (Fig. 9, E and F).
Fig. 9.
APOL1-associated oocyte uptakes of Ca2+ and Cl− are nearly abolished by BH3 domain deletion, but not by Ala substitution. Oocytes maintained 72 h in HiK MBS following injection with 5 ng cRNA encoding APOL1 WT, APOL1 ΔBH3, or APOL1 9Ala were maintained compared with uninjected oocytes in assays of 45Ca2+ uptake (n = 20 from 2 frogs; A), 36Cl− uptake (n = 20 from 2 frogs; B), and current at −100 mV as measured by two-electrode voltage clamp (n = 10 oocytes per group; C). D: immunoblot of detergent lysates (20 mg protein per lane) from oocytes expressing APOL1.WT, APOL1.ΔBH3, or APOL1.9Ala compared with lysate from uninjected oocytes. Simultaneous GAPDH probing of the same blot confirmed equal loading of lanes. One of two similar experiments with indistinguishable results is shown. E: representative median intensity images of whole mount confocal immunofluorescence micrographs of oocytes injected 72 h previously with the same WT and mutant APOL1 cRNAs, then maintained in HiK MBS prior to fixation and immunostaining with anti-APOL1 antibody. F: normalized mean fluorescence intensity values from oocytes treated as in D. Values are means ± SE; n = 20 (10 oocytes from each of 2 frogs). *P < 0.05 vs. oocytes expressing APOL1 alone. †P < 0.05 vs. uninjected oocytes.
MCL1 coexpression reduces APOL1 at or near the oocyte surface without reducing total APOL1 accumulation.
Accumulation of total APOL1 polypeptide in Xenopus oocytes plateaued after 24 h and was not detectably altered by coexpression of MCL1 (Fig. 10A). In contrast, MCL1 coexpression reduced by ∼50% the FI of immunostained APOL1 at or near the oocyte surface (Fig. 10, B and C). These data suggest that MCL1 regulates APOL1 surface expression either by decreasing APOL1 trafficking to the surface or increasing its endocytic retrieval from the surface.
Fig. 10.
MCL1 coexpression with APOL1 is associated with reduced steady-state levels of APOL1 at or near the oocyte surface. A: immunoblot of APOL1 in detergent lysates (20 mg protein per lane) from oocytes expressing APOL1 in the absence or presence of coexpressed MCL1. GAPDH reprobing of the same blot confirmed equal loading of lanes, a result supported by Ponceau red stain of the blot pretransfer (not shown). One of three similar experiments with indistinguishable results is shown. B: representative median intensity images of whole mount confocal immunofluorescence micrographs of uninjected oocytes or oocytes injected 72 h previously with cRNAs encoding APOL1, MCL1, or both, as indicated. C: normalized fluorescence intensity of APOL1 in 26–27 oocytes expressing the indicated cRNAs. Values are means ± SE. *P < 0.05 vs. oocytes expressing APOL1 alone; ANOVA with Tukey's t-test.
APOL1-associated ion fluxes and whole cell current are inhibited by acidic extracellular pH.
APOL1 function has been reported to be differentially regulated by pH change. The trypanolytic function of APOL1 requires endomembrane compartment acidification (31), and APOL1 residues situated between the putative pore forming domain and the C-terminal SRA binding domain were proposed to contribute to pH-sensing (44). Activity of the cation conductance mediated by purified holo-APOL1 incorporated into lipid bilayers required priming by cis-acidification. However, full activation of cation conductance required subsequent alkalinizing restoration to cis-neutral pH (52).
Extracellular pH (pHo) sensitivity of APOL1-associated ion transport activity was tested in Xenopus oocytes maintained 72 h in HiK MBS. Acid pHo dramatically suppressed APOL1-associated 45Ca2+ influx, whereas alkaline pHo activated it (Fig. 11A). 36Cl− influx was also substantially inhibited by acid pHo (Fig. 11B). APOL1-associated TEVC current was similarly inhibited by acid pHo (Fig. 11, C and D). The effect of acid pHo could not be tested on long-term morphological toxicity of APOL1, since prolonged exposure to acid HiK MBS itself was toxic to both uninjected and APOL1-expressing oocytes (not shown).
Fig. 11.
APOL1-associated ion flux and current are inhibited by acidic extracellular pH. A and B: extracellular protons inhibit 45Ca2+ uptake (n = 20; A) and 36Cl− uptake (n = 10–20; B) by oocytes injected 72 h earlier with 10 ng APOL1 cRNA and subsequently maintained in HiK MBS. *P < 0.05. C and D: extracellular protons inhibit the increased two-electrode voltage clamp current in oocytes maintained 72 h in HiK MBS after injection with 10 ng APOL1 cRNA (n = 11; C), but inhibit only minimally the smaller currents recorded in uninjected oocytes (n = 7; D). Values are means ± SE. *P < 0.05 by Mann-Whitney test. **P < 0.001 by t-test.
DISCUSSION
The mechanisms by which APOL1 influences innate immunity and by which APOL1 variants increase risk of renal disease remain little understood. The restricted presence of APOL1 in humans and a few higher primates requires its study in heterologous expression systems. We have shown here that expression of human APOL1 in Xenopus oocytes led to morphological toxicity (physical deterioration) of oocytes representing progression to or toward cell death during the 72 h after cRNA injection. Oocyte morphological toxicity was rescued by coexpression of individual, anti-apoptotic, BCL2 family proteins, with the notable exception of BCL2 itself. APOL1-associated oocyte morphological toxicity was also rescued by maintenance of APOL1-expressing oocytes in bath solutions in which extracellular Na+ was replaced by K+. Although APOL1-associated cell toxicity and death has been considered a reflection of APOL1′s activity as an atypical BH3-only protein, our findings demonstrate that sequence integrity of the BH3 domain is required neither for the APOL1-associated toxic morphological phenotype in oocytes, nor for rescue of that phenotype by coexpression of human MCL1 or BCL-XL. Integrity of the BH3 domain of APOL1 is similarly dispensable in a mouse model of APOL1-mediated protection against lethal trypanosome infection. Thus we conclude that, although deletion of nine amino acids from the BH3-like region of the pore-forming domain abrogates APOL1 activity, integrity of that BH3-like sequence is not required for APOL1 activity.
APOL1-associated oocyte morphological toxicity was accompanied by increased 36Cl− permeability blocked by stilbene disulfonates, and increased 45Ca2+ permeability inhibited by Na+, Gd3+, and La3+. These increased permeabilities of APOL1-expressing oocytes were accompanied by increased TEVC ion currents accompanied by variable depolarization. Fluxes and currents measured in APOL1-expressing oocytes maintained in high extracellular [K+] were of higher magnitude, possibly reflecting higher proportions of intact oocytes able to survive the mechanical stress of the ion transport assays. Coexpression of MCL1 reduces the magnitude of these fluxes and currents, which are also inhibited by acidic pHo. Integrity of the BH3 domain is required neither for APOL1-associated elevation of ion fluxes and current, nor for APOL1 expression at or near the oocyte surface.
The status of APOL1 expressed in Xenopus oocytes as a BH3-only protein.
APOL1 homology with BCL2 family proteins was first noted by Perez-Morga et al. (44), leading soon thereafter to the suggestion that APOL1 is an atypical BH3-only protein with pore-forming properties (58). The BH3 designation was tested experimentally by Wan et al. (60), who showed that APOL1 expression induced macro-autophagic cell death in several tumor cell lines, by a mechanism proposed to resemble that mediated by the BH3-only protein Beclin-1 (46). Cell death was reduced or abrogated by deletion of nine core amino acid residues from the putative BH3 domain of APOL1, just as deletion of core BH3 residues from APOL6 had been previously reported to inhibit APOL6-mediated apoptosis in colon cancer cells (33). However, the related putative BH3-only protein APOL2 failed to mediate or increase susceptibility to several inducers of cell death, or to regulate autophagy in the several cell types tested (14).
We have demonstrated here that APOL1 expression greatly accelerates the morphological deterioration of Xenopus oocytes during ex vivo incubation. Deletion of putative BH3 domain core residues from APOL1, as previously tested in cancer cell lines (60), prevented toxic morphological deterioration of oocytes (Fig. 2) without altering APOL1 abundance at or near the oocyte surface (Fig. 9). However, missense substitutions in highly conserved residues of the putative core BH3 domain failed to abolish either the toxic morphological effects of APOL1 expression in Xenopus oocytes (Figs. 1–3) or the protection conferred by APOL1 against lethal trypanosome infection in mice (Fig. 4). Thus APOL1 toxicity and morphological degeneration do not require the conserved core sequence of the putative BH3 domain. APOL1-induced autophagic changes observed in murine embryonic fibroblasts were absent from embyronic fibroblasts lacking ATG5 or ATG7 (60). However, APOL1-associated morphological toxicity in Xenopus oocytes was unattenuated by multiple inhibitors of autophagy, by a caspase inhibitor, or by inhibitors of necroptosis or autosis. Thus the death pathway(s) activated by APOL1 in the Xenopus oocyte remains undefined. Cytotoxicity of APOL1 G0 transiently expressed in HEK-293T cells was similarly unaffected by inhibitors of caspase, of lysosomal function, and of clathrin-mediated endocytosis, and was independent of APOL1-associated impairment of autophagosomal maturation (27).
Rescue of APOL1 morphological toxicity by anti-apoptotic BCL2 proteins.
Despite the apparent lack of importance of the putative BH3 domain to oocyte morphological toxicity, coexpression of some human anti-apoptotic BCL2 family members rescued APOL1-associated toxicity in an apparently BH3-independent manner. Rescue was exhibited by MCL-1, BCL-XL, BCLW, and BCL2A1, but not by BCL2 itself. The distinct behavior of BCL2 overexpression might reflect the lack of intrinsic anti-apoptotic activity in oocytes [recombinant xBcl2 fails to regulate in vitro caspase activity and poly(ADP-ribose)polymerase activation in egg lysates], or the lack of endogenous xBcl2 expression in egg and ovary of X. laevis (54). In contrast, anti-apoptotic xMcl-1, xBcl-XL, xBcl-W, and xBcl-B/xBcl2A10 were easily detected in Xenopus egg and ovary. However, xMcl-1 could not be coimmunoprecipitated with its functional pro-apoptotic partner, xBid (54), consistent with our unsuccessful attempts to coimmunoprecipitate MCL1 with APOL1. Steady-state expression of APOL1 at or near the oocyte surface was decreased by MCL1 coexpression, without change in total APOL1 content of the oocyte (Fig. 10). Oocyte morphological rescue from APOL1-associated toxicity by the anti-apoptotic BCL2 family members might occur by direct interaction of insufficient affinity for detection by pulldown, or by induction of an alternate prosurvival pathway. Thus coexpressed BCL2 family members may decrease APOL1 surface delivery or increase its endocytosis from the surface, either specifically or by enhanced cell wound repair pathways (1).
APOL1 expression induces increased Cl− and Ca2+ fluxes, accompanied by increased whole oocyte currents.
APOL1-associated morphological toxicity in Xenopus oocytes was accompanied by increased Cl− and Ca2+ permeabilities, and by increased ion currents (Figs. 5–7). Stilbene disulfonates inhibited elevated Cl− flux (Fig. 5). La3+ and Gd3+ cations inhibited elevated Ca2+ flux, but DIDS did not (Fig. 6). The APOL1-associated increase in oocyte TEVC current was partially blocked by each of these inhibitors (Fig. 7 and not shown).
Rescue of the morphological toxicity by coexpression of MCL1 or BCL-XL (not shown) was paralleled by reduction in oocyte ion fluxes and currents (Figs. 8 and 9). In contrast, morphological rescue of APOL1-expressing oocytes by high extracellular [K+] was accompanied by substantially increased Ca2+ and Cl− fluxes (Fig. 7). The mechanistic relationship between oocyte morphological rescue by MCL-1 coexpression and morphological rescue by oocyte maintenance in HiK medium remains unclear. Apoptosis in cultured cells has been associated with elevated K+ efflux and cell shrinkage (4), although reduction in intracellular [K+] is not required for apoptosis (3). The protective effect of high extracellular [K+] on APOL1-associated morphological toxicity may be related to inhibition of excess Na+ entry, similar to the protective effect of bath K+ replacement during staurosporine-induced caspase activation and induction of non-inactivating Na+ channel activity in the Xenopus oocyte (13). Elevated extracellular [K+] also prolonged survival of primary neurons in culture (41) and rescued autophagy triggered by the ionophore toxin proaerolysin (17).
Chronic exposure to high extracellular [K+] should maintain or elevate cytosolic [K+] while preventing potentially pathological elevation of cytosolic [Na+]. However, substitution of extracellular Na+ or Cl− with impermeant cations or anions over 24–72 h failed to rescue the APOL1 morphological phenotype in Xenopus oocytes. In the absence of prominent voltage-gated Ca2+ channels in the oocyte, depolarization may serve to minimize electrogenic Ca2+ entry through nonspecific cation channels, preventing elevation of cytosolic Ca2+ and, secondarily, Ca2+-induced Ca2+ release from intracellular stores, or Ca2+ release controlled by other signals. Previously defined interactions of BCL2 family proteins with intracellular Ca2+ release pathways (22, 23, 42, 50) do not explain the BH3-independent rescue by BCL2 family members of APOL1-induced changes in oocyte morphology and Ca2+ flux. Further experiments will be needed to explore these pathways in APOL1-expressing oocytes and other cell types.
APOL1-associated oocyte permeabilities and the intrinsic ion channel activity of APOL1.
Recombinant APOL1 WT was expressed at or near the surface of Xenopus oocytes (Fig. 9), associated with DIDS-sensitive Cl− fluxes (Fig. 5), trivalent cation-sensitive Ca2+ fluxes, and increased TEVC currents partially blocked by DIDS and by trivalent ions, likely representing mixed anion and cation currents (Figs. 6–9, 11). The data are consistent with APOL1-induced Cl− permeability being secondary to primary elevation of Ca2+ or cation permeability in the oocyte. However, the relationship between these oocyte cation and anion permeabilities and the previously reported pore-forming activities of APOL1 remains incompletely defined.
Different preparations of recombinant APOL1 have yielded distinct types of complex ion currents upon reconstitution into planar lipid bilayers. Recombinant His-tagged APOL1 fragment aa 60–235 (encompassing the colicin-like domain and the putative BH3 domain) was expressed in E. coli and purified in the dialyzable detergent CHAPS. Fusion of recombinant protein into asolectin bilayers separating cis- and trans-solutions of symmetric pH 6 exhibited complex currents of 3.2:1 anion-to-cation selectivity. However, this construct lacking the APOL1 C-terminal putative coiled-coil domain itself lacked trypanolytic activity (44).
In contrast, purified hetero-oligomeric human TLF1 contained, in addition to APOL1, pore-forming cathelicidin (18–20), haptoglobin-related protein, and at least five additional protein components. Fusion of TLF1 into bilayers of whole trypanosome lipids separating solutions of cis pH 5.5 and trans pH 7.4, yielded complex currents with cation-to-anion selectivity of 4.8:1 (34). Most recently, recombinant His-tagged human APOL1 holoprotein was extracted from E. coli particulate fraction in Zwittergent 3–14, gel filtration-purified in dodecylmaltoside to apparent homogeneity, then fused into planar bilayers of asolectin depleted of nonpolar lipids. The resulting channels were ideally cation-selective, but required priming by cis compartment acid pH prior to dramatic activation by alkalinizing restoration of neutral pH (52). Interestingly, the acidic priming itself induced small currents similar in magnitude to those observed in the TLF1 experiments recorded at cis-acidic pH (34), but the restoration of neutral or alkaline pH produced currents of ∼100-fold greater magnitude (52).
The Cl− and Ca2+ fluxes and whole cell currents of APOL1-expressing oocytes measured at neutral or alkaline pH were similarly reduced at more acid pH values. The regulatory patterns of APOL1-associated function in whole oocytes complement those of recombinant APOL1 subdomains and intact proteins, and of APOL1-containing TLF1 studied in lipid bilayers. The ion transport manifestations of APOL1 expression in Xenopus oocytes likely integrate, in ways yet to be determined, intrinsic APOL1 activities with APOL1′s secondary effects on endogenous ion channels and/or transporters.
APOL1 phenotypes in Xenopus oocytes and APOL1-associated kidney disease.
Elevated renal disease risk is associated with homozygosity or compound heterozygosity for the G1 or G2 missense variants of APOL1. The genetic absence of APOL1 discovered in a single patient (59) has not been associated with evident renal insufficiency (25). Our studies of the toxic effects of APOL1 expression in Xenopus oocytes were performed with APOL1 G0 transcript 1 (39) (referred to as APOL1 WT). Under the conditions tested, oocyte expression of APOL1 disease risk variants G1 or G2 led to morphological deterioration indistinguishable from that of G0, as well as to comparably complete rescue by coexpressed MCL1 and BCL-XL (data not shown). This variant-independent toxicity of APOL1 observed in Xenopus oocytes under the conditions tested here differs from the increased cell death observed in HEK-293 cells transiently transfected with APOL1 risk variants G1 and G2 (39) or in immortalized human podocytes transduced by lentviral G1 or G2 (30), in which these risk variants sensitized cell responses to a diverse range of pharmacological and viral stressors.
Thus the Xenopus oocyte is useful for study of APOL1 toxicity, but conditions remain to be defined for which the renal disease-associated G1 and G2 risk variants are reliably more toxic than the G0 variant. We speculate that Xenopus oocytes may lack protein(s) that confer on cultured mammalian kidney cells discrimination of the G1 and G2 variants of APOL1 from G0. In this context, oocyte studies may provide both an important control and a useful contrast for studies in cultured cells.
GRANTS
This work was supported by National Institute on Minority Health and Health Disparities (NIMHD) Grants MD-007092 and MD-007898 and the NephCure Foundation (M. R. Pollak, D. J. Friedman, S. L. Alper) and MD-007599 (J. Raper) and by National Science Foundation (NSF) BREAD Award IOS-1249166 (J. Raper).
DISCLAIMER
The contents of this paper are solely the responsibility of the authors, and do not represent the official views of the NIMHD, the NIH, or the NSF.
DISCLOSURES
M. R. Pollak and D. J. Friedman have filed patent applications related to APOL1.
AUTHOR CONTRIBUTIONS
Author contributions: J.F.H., D.H.V., J.A.G., J.R., D.J.F., M.R.P., and S.L.A. conception and design of research; J.F.H., D.H.V., B.E.S., and J.A.G. performed experiments; J.F.H., D.H.V., B.E.S., J.A.G., J.R., and S.L.A. analyzed data; J.F.H., D.H.V., J.A.G., J.R., D.J.F., M.R.P., and S.L.A. interpreted results of experiments; J.F.H., D.H.V., and J.A.G. prepared figures; J.F.H. and S.L.A. drafted manuscript; J.F.H., D.H.V., B.E.S., J.A.G., J.R., D.J.F., M.R.P., and S.L.A. edited and revised manuscript; J.F.H., D.H.V., B.E.S., J.A.G., J.R., D.J.F., M.R.P., and S.L.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Drs. Bryce MacIver, David B. Doroquez, Jiayue Zhang, and Opeyemi Olabisi for helpful discussion.
Footnotes
Since APOL1 overexpression was reported to lead to autophagic cell death in several mammalian epithelial cell lines of different tissue origins (60), Xenopus oocytes were treated with autophagy inhibitors by injection and bath exposure (or both), and assessed for attenuation of APOL1-associated morphological toxicity. Exposure to phosphatidyl-inositol 3-kinase inhibitors wortmannin (at 1, 100, and 1,000 nM) and to 3-methyladenine (5 mM) did not reduce APOL1-associated morphological toxicity in oocytes, despite their partial inhibition of APOL1-induced cell death and their apparently reduced formation of presumptive autophagic vacuoles in colon carcinoma cells (60). Lysosomal inhibitors choroquine (at 10, 100, and 500 μM) and H+-ATPase inhibitor bafilomycin (10 μM) had little or no effect on APOL1-associated morphological toxicity in oocytes, despite chloroquine's protective effect against a morphological index of lysosomal leakiness in cultured human podocytes overexpressing lentivirus-driven APOL1 (30). Caspase inhibitor Z-VAD-FMK (at 20 and 100 μM) had no effect on APOL1-associated morphological toxicity, consistent with resistance to exogenous cytochrome C-mediated caspase activation in intact Xenopus oocytes compared with unfertilized eggs (2, 24). Extracellular pyruvate (2.5 mM) did not reduce APOL1-associated morphological toxicity, consistent with pyruvate's lack of effect on caspase-dependent in vitro “apoptosis” in Xenopus egg extracts (40). Similarly, without effect on morphological toxicity were the autosis inhibitor ouabain (32) and the MEK inhibitor, trametinib (200 nM). [MEK inhibition prevents cMOS-dependent germinal vesicle breakdown in Xenopus oocytes (37).] Additional agents without consistent effect on APOL1-associated morphological toxicity were autophagy inhibitors spautin-1 (10 μM) and 4-[[3,4-(methy-lenedioxy)benzyl]amino]-6-chloroquinazoline (MBCQ) (10 μM) and Receptor-interacting protein 1 kinase inhibitor necrostatin-1 (50 μM) (data not shown). Thus pharmacological inhibitors of apoptosis, autophagy, and necroptosis tested under these experimental conditions did not reproducibly attenuate the morphological toxicity associated with APOL1 expression in Xenopus oocytes, or phenocopy the rescue of that toxicity by coexpressed MCL1. Note, however, that trametinib, spautin-1, MBCQ, and necrostatin-1 have not been established as active against the Xenopus laevis homologs of the drugs' known targets among the toxic and death signaling pathways of mammalian cells.
REFERENCES
- 1.Andrews NW, Almeida PE, Corrotte M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol 24: 734–742, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhuyan AK, Varshney A, Mathew MK. Resting membrane potential as a marker of apoptosis: studies on Xenopus oocytes microinjected with cytochrome c. Cell Death Diff 8: 63–69, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Borjesson SI, Englund UH, Asif MH, Willander M, Elinder F. Intracellular K+ concentration decrease is not obligatory for apoptosis. J Biol Chem 286: 39823–39828, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortner CD, Sifre MI, Cidlowski JA. Cationic gradient reversal and cytoskeleton-independent volume regulatory pathways define an early stage of apoptosis. J Biol Chem 283: 7219–7229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R. Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Molecular Cancer Res 1: 186–194, 2003. [PubMed] [Google Scholar]
- 6.Bunse S, Schmidt M, Prochnow N, Zoidl G, Dermietzel R. Intracellular cysteine 346 is essentially involved in regulating Panx1 channel activity. J Biol Chem 285: 38444–38452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Coll O, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Neutral sphingomyelinase-induced ceramide triggers germinal vesicle breakdown and oxidant-dependent apoptosis in Xenopus laevis oocytes. J Lipid Res 48: 1924–1935, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Crawford SE, Hyser JM, Utama B, Estes MK. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-beta signaling is required for rotavirus replication. Proc Natl Acad Sci U S A 109: E3405–E3413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Venanzio G, Stepanenko TM, Garcia Vescovi E. Serratia marcescens ShlA pore-forming toxin is responsible for early induction of autophagy in host cells and is transcriptionally regulated by RcsB. Infect Immun 82: 3542–3554, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci U S A 92: 8498–8502, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhardt K, Schmidt M, Tenbusch M, Dermietzel R. Effects on channel properties and induction of cell death induced by C-terminal truncations of pannexin1 depend on domain length. J Membr Biol 248: 285–294, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Englund UH, Gertow J, Kagedal K, Elinder F. A voltage dependent non-inactivating Na+ channel activated during apoptosis in Xenopus oocytes. PloS One 9: e88381, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galindo-Moreno J, Iurlaro R, El Mjiyad N, Diez-Perez J, Gabaldon T, Munoz-Pinedo C. Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein. Cell Death Dis 5: e1275, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genovese G, Friedman DJ, Pollak MR. APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol 9: 240–244, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol 13: 1026–1043, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci 65: 493–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutsmann T, Hagge SO, Larrick JW, Seydel U, Wiese A. Interaction of CAP18-derived peptides with membranes made from endotoxins or phospholipids. Biophys J 80: 2935–2945, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutsmann T, Larrick JW, Seydel U, Wiese A. Molecular mechanisms of interaction of rabbit CAP18 with outer membranes of gram-negative bacteria. Biochemistry 38: 13643–13653, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL. Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298: C1363–C1375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Hu X, Eno CO, Zhao G, Li C, White C. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem 288: 19870–19881, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Shah K, Bradbury NA, Li C, White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5: e1482, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CE, Freel CD, Kornbluth S. Features of programmed cell death in intact Xenopus oocytes and early embryos revealed by near-infrared fluorescence and real-time monitoring. Cell Death Diff 17: 170–179, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone DB, Shegokar V, Nihalani D, Rathore YS, Mallik L, Ashish Zare V, Ikizler HO, Powar R, Holzman LB. APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PloS One 7: e51546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, Limou S, Sezgin E, Nelson GW, Fogo AB, Goetsch S, Kopp JB, Winkler CA, Naicker S. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatua AK, Cheatham AM, Kruzel ED, Singhal PC, Skorecki K, Popik W. Exon 4-encoded sequence is a major determinant of cytotoxicity of apolipoprotein L1. Am J Physiol Cell Physiol 309: C22–C37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M. Pro-autophagic signal induction by bacterial pore-forming toxins. Med Microbiol Immunol (Berl) 199: 299–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacsics D, Raper J. Transient expression of proteins by hydrodynamic gene delivery in mice. J Vis Exp 2014: 87, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecordier L, Uzureau P, Tebabi P, Perez-Morga D, Nolan D, Schumann Burkard G, Roditi I, Pays E. Identification of Trypanosoma brucei components involved in trypanolysis by normal human serum. Mol Microbiol 94: 625–636, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Shoji-Kawata S, Sumpter RM Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PG, Puyal J, Levine B. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci U S A 110: 20364–20371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Lu H, Jiang Z, Pastuszyn A, Hu CA. Apolipoprotein l6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res 3: 21–31, 2005. [PubMed] [Google Scholar]
- 34.Molina-Portela MP, Lugli EB, Recio-Pinto E, Raper J. Trypanosome lytic factor, a subclass of high-density lipoprotein, forms cation-selective pores in membranes. Mol Biochem Parasitol 144: 218–226, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Molina-Portela MP, Samanovic M, Raper J. Distinct roles of apolipoprotein components within the trypanosome lytic factor complex revealed in a novel transgenic mouse model. J Exp Med 205: 1721–1728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJ. The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics 79: 539–546, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Mood K, Bong YS, Lee HS, Ishimura A, Daar IO. Contribution of JNK, Mek, Mos and PI-3K signaling to GVBD in Xenopus oocytes. Cell Signal 16: 631–642, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D'Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell 123: 89–103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park IS, Kim JE. Potassium efflux during apoptosis. J Biochem Mol Biol 35: 41–46, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Parys JB. The IP3 receptor as a hub for Bcl-2 family proteins in cell death control and beyond. Sci Signal 7: pe4, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homble F, Vanhamme L, Tebabi P, Pays A, Poelvoorde P, Jacquet A, Brasseur R, Pays E. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 309: 469–472, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Poelvoorde P, Vanhamme L, Van Den Abbeele J, Switzer WM, Pays E. Distribution of apolipoprotein L-I and trypanosome lytic activity among primate sera. Mol Biochem Parasitol 134: 155–157, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy 4: 989–997, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene 27, Suppl 1: S137–S148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith EE, Malik HS. The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res 19: 850–858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens NA, Hajduk SL. Endosomal localization of the serum resistance-associated protein in African trypanosomes confers human infectivity. Eukaryot Cell 10: 1023–1033, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung PJ, Tsai FD, Vais H, Court H, Yang J, Fehrenbacher N, Foskett JK, Philips MR. Phosphorylated K-Ras limits cell survival by blocking Bcl-xL sensitization of inositol trisphosphate receptors. Proc Natl Acad Sci U S A 110: 20593–20598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang CY, Chen YW, Jow GM, Chou CJ, Jeng CJ. Beauvericin activates Ca2+-activated Cl− currents and induces cell deaths in Xenopus oocytes via influx of extracellular Ca2+. Chem Res Toxicol 18: 825–833, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Thomson R, Finkelstein A. Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: relevance to trypanosome lysis. Proc Natl Acad Sci U S A 112: 2894–2899, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, Winkler CA, Kopp J, Rotimi C, Adeyemo A, Doumatey A, Ayodo G, Alper SL, Pollak MR, Friedman DJ, Raper J. Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A 111: E2130–E2139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuchiya Y, Yamashita S. Anti-apoptotic activity and proteasome-mediated degradation of Xenopus Mcl-1 protein in egg extracts. J Biol Chem 286: 15806–15814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tucker SJ, Pessia M, Moorhouse AJ, Gribble F, Ashcroft FM, Maylie J, Adelman JP. Heteromeric channel formation and Ca(2+)-free media reduce the toxic effect of the weaver Kir 3.2 allele. FEBS Lett 390: 253–257, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, Pays A, Tebabi P, Van Xong H, Jacquet A, Moguilevsky N, Dieu M, Kane JP, De Baetselier P, Brasseur R, Pays E. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422: 83–87, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Vanhollebeke B, Pays E. The function of apolipoproteins L. Cell Mol Life Sci 63: 1937–1944, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanhollebeke B, Truc P, Poelvoorde P, Pays A, Joshi PP, Katti R, Jannin JG, Pays E. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N Engl J Med 355: 2752–2756, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 283: 21540–21549, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xong HV, Vanhamme L, Chamekh M, Chimfwembe CE, Van Den Abbeele J, Pays A, Van Meirvenne N, Hamers R, De Baetselier P, Pays E. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 95: 839–846, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy 4: 1079–1082, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]