Abstract
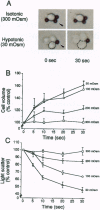
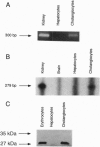
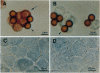
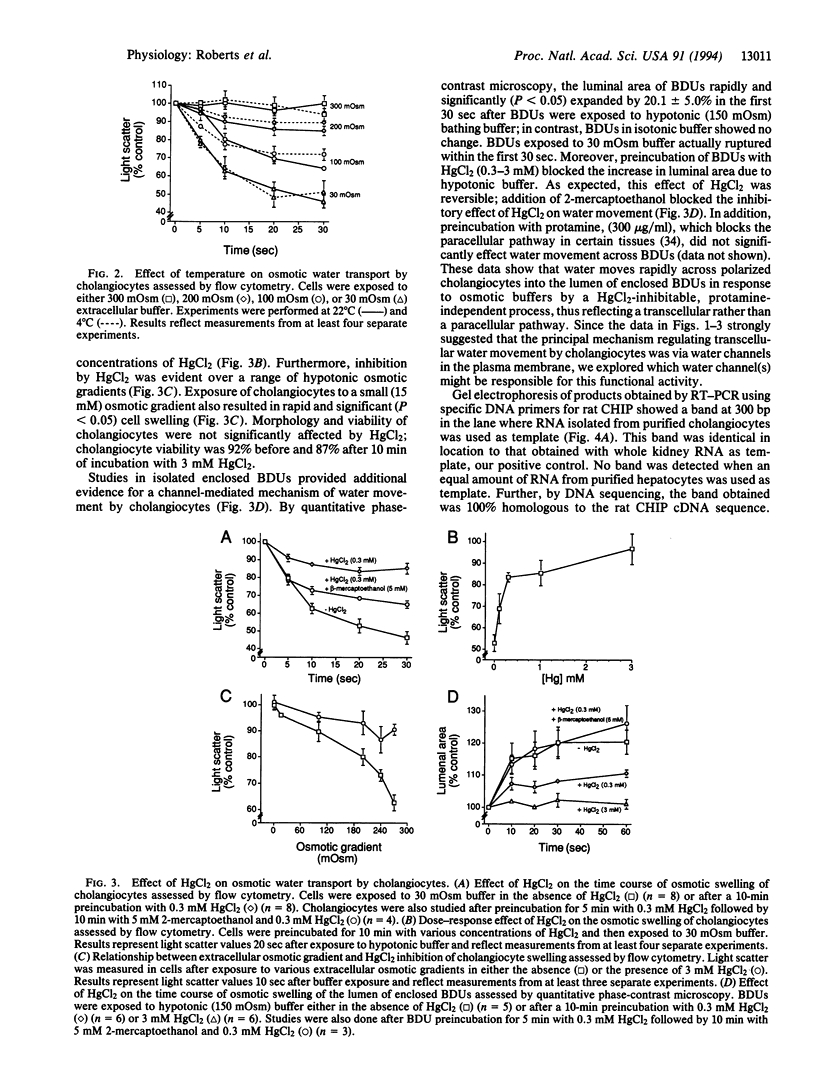
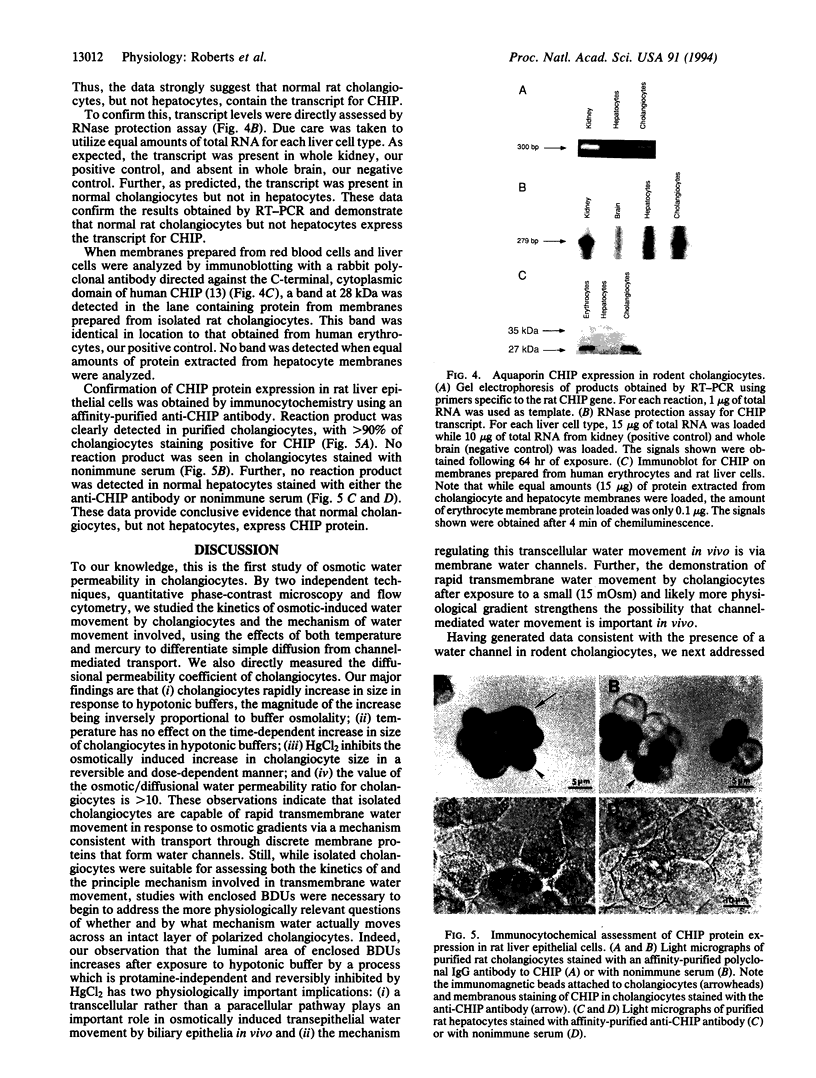
Cholangiocytes line the intrahepatic bile ducts and regulate salt and water secretion during bile formation, but the mechanism(s) regulating ductal water movement remains obscure. A water-selective channel, the aquaporin CHIP, was recently described in several epithelia, so we tested the hypothesis that osmotic water movement by cholangiocytes is mediated by CHIP. Isolated rodent cholangiocytes showed a rapid increase in volume in the presence of hypotonic extracellular buffers; the ratio of osmotic to diffusional permeability coefficients was > 10. The osmotically induced increase in cholangiocyte volume was inversely proportional to buffer osmolality, independent of temperature, and reversibly blocked by HgCl2. Also, the luminal area of isolated, enclosed bile duct units increased after exposure to hypotonic buffer and was reversibly inhibited by HgCl2. RNase protection assays, anti-CHIP immunoblots, and immunocytochemistry confirmed that CHIP transcript and protein were present in isolated cholangiocytes but not in hepatocytes. These results demonstrate that (i) isolated cholangiocytes and intact, polarized bile duct units manifest rapid, mercury-sensitive increases in cell size and luminal area, respectively, in response to osmotic gradients and (ii) isolated cholangiocytes express aquaporin CHIP at both the mRNA and the protein level. The data implicate aquaporin water channels in the transcellular movement of water across cholangiocytes lining intrahepatic bile ducts and provide a plausible molecular explanation for ductal water secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Alpini G., Ulrich C. D., 2nd, Phillips J. O., Pham L. D., Miller L. J., LaRusso N. F. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol. 1994 May;266(5 Pt 1):G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- Alvaro D., Cho W. K., Mennone A., Boyer J. L. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993 Sep;92(3):1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Bondy C., Chin E., Smith B. L., Preston G. M., Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Verbavatz J. M., Valenti G., Lui B., Sabolić I. Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur J Cell Biol. 1993 Aug;61(2):264–273. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Dempster J. A., Wieringa B., Van Os C. H. Isolation of a cDNA for rat CHIP28 water channel: high mRNA expression in kidney cortex and inner medulla. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1267–1273. doi: 10.1016/0006-291x(92)91368-z. [DOI] [PubMed] [Google Scholar]
- Eckloff B. W., Podzorski R. P., Kline B. C., Cockerill F. R., 3rd A comparison of 16S ribosomal DNA sequences from five isolates of Helicobacter pylori. Int J Syst Bacteriol. 1994 Apr;44(2):320–323. doi: 10.1099/00207713-44-2-320. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Basavappa S., McGill J., Melhus O., Cohn J. A. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993 Jan;91(1):319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Palant C. E., Bentzel C. J., Hegel U. Protamine reversibly decreases paracellular cation permeability in Necturus gallbladder. J Membr Biol. 1985;87(2):141–150. doi: 10.1007/BF01870660. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Strange K., Zeidel M. L. Current understanding of the cellular biology and molecular structure of the antidiuretic hormone-stimulated water transport pathway. J Clin Invest. 1991 Jul;88(1):1–8. doi: 10.1172/JCI115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Lian S. C., Finkbeiner W. E., Verkman A. S. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol. 1994 Apr;266(4 Pt 1):C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Kato A., Gores G. J., LaRusso N. F. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992 Aug 5;267(22):15523–15529. [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Roberts S. K., Kuntz S. M., Gores G. J., LaRusso N. F. Regulation of bicarbonate-dependent ductular bile secretion assessed by lumenal micropuncture of isolated rodent intrahepatic bile ducts. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9080–9084. doi: 10.1073/pnas.90.19.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Boulpaep E. L. Mechanisms of water transport by epithelial cells. Q J Exp Physiol. 1989 Jul;74(4):385–417. doi: 10.1113/expphysiol.1989.sp003288. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Water channels in cell membranes. Annu Rev Physiol. 1992;54:97–108. doi: 10.1146/annurev.ph.54.030192.000525. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zhang R. B., Logee K. A., Verkman A. S. Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J Biol Chem. 1990 Sep 15;265(26):15375–15378. [PubMed] [Google Scholar]