Abstract
IMPORTANCE
Randomized clinical trials demonstrate no benefit for epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in unselected patients with head and neck squamous cell carcinoma (HNSCC). However, a patient with stage IVA HNSCC received 13 days of neoadjuvant erlotinib and experienced a near-complete histologic response.
OBJECTIVE
To determine a mechanism of exceptional response to erlotinib therapy in HNSCC.
DESIGN, SETTING, AND PARTICIPANTS
Single patient with locally advanced HNSCC who received erlotinib monotherapy in a window-of-opportunity clinical trial (patients scheduled to undergo primary cancer surgery are treated briefly with an investigational agent). Whole-exome sequencing of pretreatment tumor and germline patient samples was performed at a quaternary care academic medical center, and a candidate somatic variant was experimentally investigated for mediating erlotinib response.
INTERVENTION
A brief course of erlotinib monotherapy followed by surgical resection.
MAIN OUTCOMES AND MEASURES
Identification of pretreatment tumor somatic alterations that may contribute to the exceptional response to erlotinib. Hypotheses were formulated regarding enhanced erlotinib response in preclinical models harboring the patient tumor somatic variant MAPK1 E322K following the identification of tumor somatic variants.
RESULTS
No EGFR alterations were observed in the pretreatment tumor DNA. Paradoxically, the tumor harbored an activating MAPK1 E322K mutation (allelic fraction 0.13), which predicts ERK activation and erlotinib resistance in EGFR-mutant lung cancer. The HNSCC cells with MAPK1 E322K exhibited enhanced EGFR phosphorylation and erlotinib sensitivity compared with wild-type MAPK1 cells.
CONCLUSIONS AND RELEVANCE
Selective erlotinib use in HNSCC may be informed by precision oncology approaches.
The discovery of activating mutations in the epidermal growth factor receptor (EGFR) gene accelerated the clinical deployment of small-molecule tyrosine kinase inhibitors (TKIs) that effectively target the altered protein,1 yielding clinical benefit in many patients with EGFR-mutant lung adenocarcinoma.2 In unselected patients with head and neck squamous cell carcinoma (HNSCC), phase 1/2 trials suggested that EGFR TKIs might be clinically active.3–7 However, randomized phase 2 and 3 trials of EGFR TKIs for locally advanced or recurrent and/or metastatic HNSCC failed to show clinical benefit when added to standard-of-care regimens in unselected populations.8,9
Complete responses to EGFR TKIs in solid tumors are extremely rare, poorly understood,10 and have been observed in EGFR wild-type settings.11 Genomic correlates of extraordinary response to targeted therapeutics have been demonstrated in other contexts,12–16 raising the possibility that a rare extreme response to erlotinib hydrochloride may result from somatic alterations in a patient’s tumor.
A man with locally advanced HNSCC received neoadjuvant erlotinib for 13 days in a window-of-opportunity clinical trial in which patients scheduled to undergo primary cancer surgery were treated briefly with an investigational agent. Unexpectedly, this patient experienced a near-complete histologic response without recurrence more than 2 years after therapy. Whole-exome sequencing of his pretreatment tumor and germline was performed to investigate molecular profiles permissive of this response.
Methods
Study Oversight
The patient provided written informed consent for an institutional review board–approved protocol to perform genomic profiling on tumor and germline DNA.
Pathologic Analysis and Sequencing
Tumor samples from pretreatment and surgical specimens were reviewed by an HNSCC pathologist (S.C.). Clinical human papillomavirus in situ hybridization testing detecting types 6, 11, 16, 18, 30, 31, 33, 35, 45, 51, and 52 was performed. DNA was extracted from tumor and matched germline, followed by whole-exome sequencing and analysis (eMethods in the Supplement).18
Experimental Analysis
MAPK1E322K (HSC-6), wild-type MAPK1(CAL-33), and MAPK1-deleted (FaDu) HNSCC cells were identified using published resources.19,20 MAPK1-deleted cells were transfected with vector, wild-type MAPK1 or MAPK1 E322K expression constructs. Western blotting was performed for EGFR and MAPK pathway members as described previously.21 Viability and senescence following erlotinib treatment were evaluated in engineered FaDu cells and in HSC-6 cells transfected with MAPK1-targeting or control small interfering RNA (eMethods in the Supplement).
At a Glance.
The presence of MAPK1 E322K predicts resistance to erlotinib therapy in preclinical models but was identified in the tumor of an extraordinary responder.
Engineered MAPK1 E322K cells exhibit enhanced erlotinib sensitivity compared with MAPK1 wild-type cells.
MAPK1 E322K induces EGFR activation in head and neck squamous cell carcinoma (HNSCC) in vitro models.
MAPK1 E322K is present at low frequencies in HNSCC and cervical cancers.
Report of a Case
A 32-year-old man presented with a painful lesion on the right side of the oral tongue. The patient drank 6 beers daily and had a 28.5 pack-year smoking history. Biopsy of the lesion revealed invasive squamous cell carcinoma (Figure 1B and C). The tissue was negative for human papillomavirus and p16. Following biopsy, the primary ventral tongue tumor measured 1.9 cm in diameter. Physical examination was notable for palpable right cervical adenopathy (level Ib). A contrast-enhanced computed tomographic scan showed soft-tissue asymmetry of the right side of the tongue, bilateral lymphadenopathy, and no evidence of distant metastatic disease. The patient’s disease was clinically staged as T1N2cM0 oral cavity squamous cell carcinoma (stage IVA).
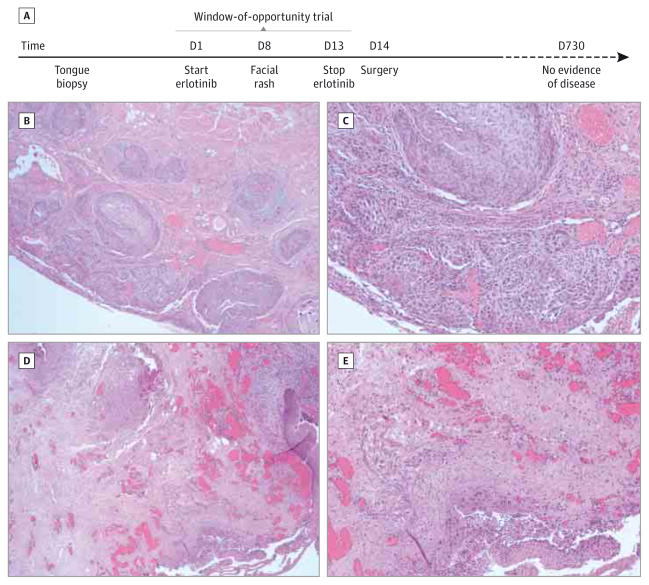
Figure 1. Clinical Course and Histologic Findings.
A, Time course for the patient’s clinical experience, highlighting the number of days receiving erlotinib and the time elapsed since surgery. D indicates day. B and C, Representative histologic analysis images of the pretreatment tumor biopsy confirm squamous cell carcinoma (hematoxylin-eosin; B, original magnification ×40; C, original magnification ×100). D and E, representative histology images from surgical specimens taken after 13 days of erlotinib therapy (hematoxylin-eosin; D, original magnification ×40; E, original magnification ×100).
The patient was enrolled in a randomized, placebo-controlled window-of-opportunity clinical trial studying blockade of EGFR and/or Src pathways in HNSCC (NCT00779389). He was randomized to receive erlotinib monotherapy at 150 mg daily for 13 days (Figure 1A). On day 8, the patient developed a facial rash, which has been associated with erlotinib response.17 On day 14, the patient underwent right partial glossectomy and bilateral modified neck dissection (levels IA-IV). Clinically, the 1.9-cm primary tongue tumor had resolved. Histologic evaluation revealed 2 residual foci (approximately 2 mm each) of invasive, moderately differentiated squamous cell carcinoma within the tongue resection specimen (Figure 1D and E). There was no evidence of lymph node metastasis (0 of 36 nodes in the right neck; 0 of 36 nodes in the left neck). The treated pathologic stage was ypT1N0. The patient received no adjuvant therapy and had no evidence of disease recurrence 24 months postoperatively.
Results
Genetic Analysis of the Pretreatment Tumor
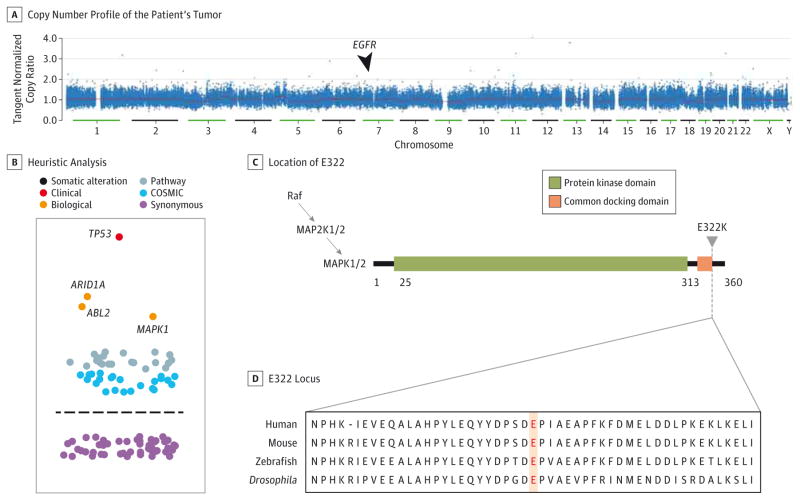
The mean target coverage across the exome was 85X in the tumor sample and 183X in the germline sample. There were 39 missense mutations, 3 nonsense mutations, and 2 frame shift deletions, for a nonsilent mutation rate of 2.00 mutations/Mb (eTables 1 and 2 in the Supplement). No somatic mutations, short insertions or deletions, or copy number alterations were observed in EGFR (Figure 2A and eTables 3 and 4 in the Supplement), PTEN, PIK3CA, or HRAS.22–24 Heuristic analysis identified somatic alterations in 4 clinically or biologically relevant cancer genes (Figure 2B): TP53,25,26 ABL2, ARID1A, and MAPK1.
Figure 2. Whole-Exome Sequencing of the Pretreatment Tumor.
A, There is no evidence of EGFR amplification. B, Heuristic analysis of the somatic mutations, short insertions and deletions, and copy number alterations across the exome identifies 4 mutations for additional evaluation: TP53, ARID1A, ABL2, and MAPK1. The horizontal line denotes the separation between nonsynonymous and synonymous variants, and COSMIC indicates the Catalogue of Somatic Mutations in Cancer database. C, The location of E322 is near the terminal end of the MAPK1 protein, in the common docking domain. Numbers indicate the color-coded regions of the protein. D, The E322 locus is highly conserved across species.
The MAPK1 alteration (allelic fraction, 0.13; median, 0.15) was particularly unexpected in this patient. MAPK1 codes for ERK2, a mitogen-activated signaling (MAPK) pathway member downstream of Raf and MEK (Figure 2C), and the E322 locus is highly conserved across species (Figure 2D).27 The E322K mutation occurs in approximately 1% of HNSCC28,29 and 8% of cervical squamous cell carcinomas.30 MAPK1 E322K causes constitutive activation of ERK2.20,31 MAPK1 amplification, which may also activate ERK signaling, confers EGFR TKI resistance in lung adenocarcinoma pre-clinical models.32 Thus, the presence of MAPK1 E322K would be predicted to result in ERK signaling activation and intrinsic resistance, rather than exquisite sensitivity, to EGFR-directed therapies.
MAPK1 E322K Effects on EGFR Activation and Erlotinib Sensitivity
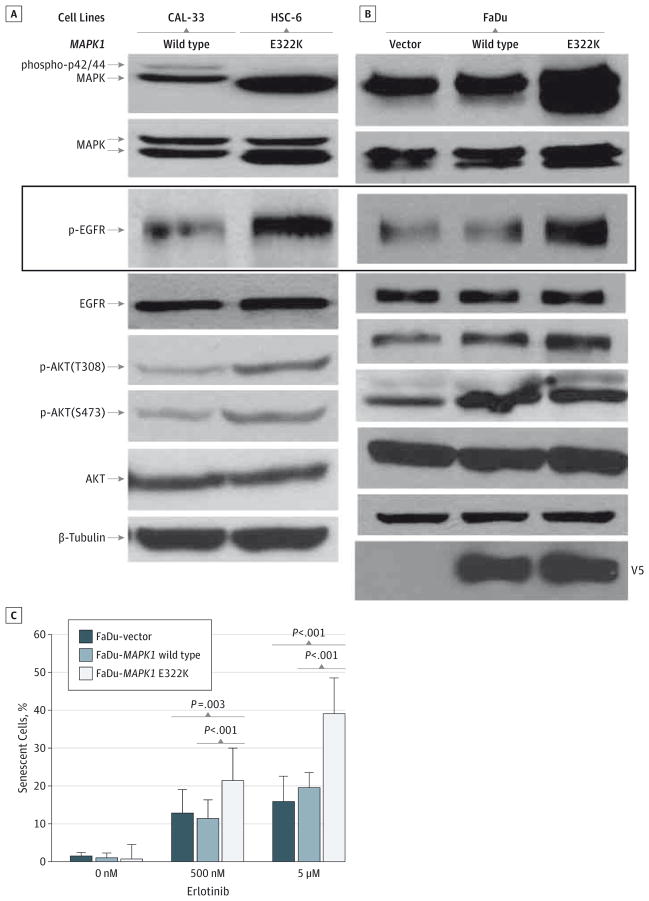
To explore the possibility that the MAPK1 E322K mutation may have paradoxically conferred exquisite dependency on aberrant EGFR signaling in this patient, wild-type MAPK1 (CAL-33) and MAPK1 E322K (HSC-6) HNSCC cells were analyzed for relative EGFR protein and pathway activation (Figure 3A). The HNSCC cells harboring endogenous MAPK1 E322K expressed higher basal levels of PY1068 EGFR, a surrogate for activated EGFR, and downstream AKT signaling compared with wild-type MAPK1 cells.
Figure 3. MAPK1 E322K Mutations and EGFR Signaling.
A, HSC-6 cells that harbor an endogenous MAPK1 E322K mutation express higher basal levels of phospho-p42/44 MAPK, p-EGFR(Y1068), and pAKT(S473) when compared with CAL-33 cells that endogenously express MAPK1 wild type. B, The head and neck squamous cell carcinoma cells with deleted MAPK1 (FaDu) were engineered to express increased levels of mutant E322K and demonstrate upregulation of phospho-p42/44 MAPK, p-EGFR(Y1068), and pAKT(S473) levels compared with vector-control transfectants. Similar results were observed at least 3 times. C, FaDu-MAPK1 E322K cells demonstrated significantly increased senescence compared with FaDu-vector and FaDu-wild-type MAPK1 following 48-hour treatment with erlotinib. β-Galactosidase activity at pH 6 was detected in senescent cells by means of light microscopy (original magnification ×100) following staining using the senescence staining kit (Cell Signaling Technology). Number of senescent cells and total number of cells per field were analyzed for at least 5 fields for each cell type and treatment condition. Mean (SD) percent of senescent cells from a representative of 3 independent experiments is presented.
Because CAL-33 and HSC-6 cells have additional non-overlapping alterations, we also engineered FaDu cells, which have a preexisting heterozygous MAPK1 deletion and are wild type for PIK3CA, PTEN, NRAS, HRAS, and AKT,19 to express vector, wild-type MAPK1 or the MAPK1-E322K mutant. The resulting engineered MAPK1 E322K cells expressed higher basal levels of PY1068 EGFR (Figure 3B) and demonstrated significantly increased senescence (Figure 3C and eFigure 1 in the Supplement) and significantly increased cell death (eFigure 2 in the Supplement) following erlotinib treatment compared with cells expressing wild-type MAPK1 or vector control cells. Finally, knockdown of ERK2 protein levels in MAPK1 E322K cells (HSC-6) resulted in reduced cell death following erlotinib treatment compared with parental and nontargeting small interfering RNA transfected cells (eFigure 3 in the Supplement). In aggregate, these data are consistent with the clinical observation that the presence of MAPK1 E322K can contribute to erlotinib sensitivity in HNSCC.
Discussion
We identified a patient with stage IVA HNSCC who experienced an exceptional response to a 13-day course of neoadjuvant erlotinib. This response occurred in the context of an activating somatic MAPK1 E322K mutation and thus was particularly noteworthy given that MAPK1 amplification or activation predicts erlotinib resistance (rather than sensitivity) in preclinical cancer models. We then demonstrated that EGFR and downstream pathway members show increased activation in HNSCC cells harboring MAPK1 E322K compared with wild-type MAPK1 cells. We speculate that EGFR dependency may have resulted from an activated Erk–mediated increase in EGFR ligand production in this patient’s tumor. Increased levels of amphiregulin as a result of Erk activation have been reported for bronchial epithelial cells,33 and high levels of amphiregulin have been associated with response to EGFR TKIs.34
Although only observed in a single patient, this finding may have important clinical implications. This type of genomic event would not occur at sufficient frequency to influence the results of erlotinib-oriented phase 2/3 studies in unselected patients with HNSCC. Instead, this case highlights the potential contribution of the “long tail” of clinically relevant cancer genes that may prove actionable in some patients.18
This study also demonstrates how different activating alterations in the same gene may lead to clinically distinct responses to the same therapy; the effect of 2 different ERK-activating mechanisms (point mutation or amplification) may not be clinically equivalent. This result indicates the promise of more expansive precision oncology efforts linking genomics with clinically annotated cases, so that phenotype-to-genotype relationships can be rapidly identified and studied. Toward this end, it is unknown whether this MAPK1 E322K mutation may contribute to similar sensitivity to erlotinib therapy in other tumor types (eg, cervical cancer30), or with antibody-mediated EGFR inhibition (eg, cetuximab). This will need to be assessed in additional studies.
There are limitations to this study. First, whereas the degree of response to brief erlotinib therapy was impressive, the relative contributions of erlotinib or surgery for the duration of response cannot be definitively determined. Furthermore, whereas forced overexpression of MAPK1 E322K did increase erlotinib sensitivity in MAPK1-deleted HNSCC cells, the MAPK1 E332K mutation’s effect on erlotinib sensitivity was modest. The preclinical models examined here were not derived from this patient’s tumor; thus, they may have arisen through different carcinogenic paths or harbor additional alterations that confound the study of erlotinib sensitivity. Conversely, the patient’s exceptional response may have been enhanced by other somatic alterations. Importantly, although experimental studies did not fully phenocopy the exquisite erlotinib sensitivity of the patient tumor, the results tracked with the patient’s response and countered the expectation that MAPK1 E332K would confer erlotinib resistance.
Conclusions
Broadly, these results support the notion that outlier genomics (eg, the National Cancer Institute’s Exceptional Responders Initiative) may yield unexpected insights into cancer biology and clinical management for both approved and investigational therapies.
Supplementary Material
Acknowledgments
Funding/Support: The study was supported by the American Cancer Society (Drs Van Allen and Grandis), the Patricia L. Knebel Fund of the Pittsburgh Foundation (Dr Lui), the National Cancer Institute K07 CA137140 (Dr Egloff), the Sheikh Khalifa bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (Drs Zhang and Mills), the Slim Initiative for Genomic Medicine, a joint US-Mexico project funded by the Carlos Slim Health Institute (Drs Golub and Garraway), the National Cancer Institute Head and Neck Cancer Specialized Program of Research Excellence P50 CA097190 (Dr Grandis), OSI Pharmaceuticals (Dr Grandis), and Bristol-Myers Squibb (Dr Grandis). This work was supported in part by the US Department of Veterans Affairs Career Development Award, Biomedical Laboratory Research and Development (Dr Duvvuri).
Footnotes
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: This work does not represent the views of the US government or the Department of Veterans Affairs.
Additional Contributions: We appreciate the patient’s participation in this study. In addition, we thank Lauren Ambrogio, BS, Broad Institute of Massachusetts Institute of Technology and Harvard University, for project management contributions. She was not compensated for her contribution to the study.
Author Contributions: Dr Grandis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Van Allen, Lui, Egloff, Garraway, and Grandis contributed equally to this work.
Study concept and design: Van Allen, Lui, Johnson, Wagle, Golub, Mills, Grandis.
Acquisition, analysis, or interpretation of data: Van Allen, Lui, Egloff, Goetz, Li, Duvvuri, Bauman, Stransky, Zeng, Gilbert, Pendleton, Wang, Chiosea, Sougnez, Wagle, Zhang, Du, Close, Johnston, McKenna, Carter, Getz, Mills, Garraway, Grandis.
Drafting of the manuscript: Van Allen, Bauman, Stransky, Gilbert, Zhang, Carter, Getz, Mills, Grandis.
Critical revision of the manuscript for important intellectual content: Van Allen, Lui, Egloff, Goetz, Li, Johnson, Duvvuri, Bauman, Zeng, Pendleton, Wang, Chiosea, Sougnez, Wagle, Du, Close, Johnston, McKenna, Golub, Mills, Garraway, Grandis.
Statistical analysis: Van Allen, Lui, Stransky, Zeng, Gilbert, Pendleton, Zhang, Close, McKenna, Carter, Grandis.
Obtained funding: Golub, Mills, Garraway, Grandis.
Administrative, technical, or material support: Egloff, Li, Johnson, Duvvuri, Zeng, Wang, Chiosea, Sougnez, Du, Johnston, Mills, Grandis.
Study supervision: Van Allen, Egloff, Johnson, Carter, Golub, Mills, Garraway, Grandis.
Conflict of Interest Disclosures: Dr Garraway is a consultant and equity holder in Foundation Medicine. Dr Grandis receives research support from Novartis and Bristol-Myers Squibb. No other disclosures are reported.
References
- 1.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6(6):352–366. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(23):8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 4.Kirby AM, A’Hern RP, D’Ambrosio C, et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94(5):631–636. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21(10):1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Choe MS, Chen Z, Klass CM, Zhang X, Shin DM. Enhancement of docetaxel-induced cytotoxicity by blocking epidermal growth factor receptor and cyclooxygenase-2 pathways in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(10):3015–3023. doi: 10.1158/1078-0432.CCR-06-2959. [DOI] [PubMed] [Google Scholar]
- 7.Klass CM, Choe MS, Hurwitz SJ, et al. Sequence dependence of cell growth inhibition by EGFR-tyrosine kinase inhibitor ZD1839, docetaxel, and cisplatin in head and neck cancer. Head Neck. 2009;31(10):1263–1273. doi: 10.1002/hed.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2013;31(11):1405–1414. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31(11):1415–1421. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- 10.Weber B, Sorensen BS, Knap MM, Madsen HH, Nexo E, Meldgaard P. Complete pathologic response in lung tumors in two patients with metastatic non-small cell lung cancer treated with erlotinib. J Thorac Oncol. 2011;6(11):1946–1949. doi: 10.1097/JTO.0b013e31822e71f2. [DOI] [PubMed] [Google Scholar]
- 11.Mody K, Strauss E, Lincer R, Frank RC. Complete response in gallbladder cancer to erlotinib plus gemcitabine does not require mutation of the epidermal growth factor receptor gene: a case report. BMC Cancer. 2010;10:570. doi: 10.1186/1471-2407-10-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagle N, Grabiner BC, Van Allen EM, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–1433. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagle N, Grabiner BC, Van Allen EM, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 2014;4(5):546–553. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imielinski M, Greulich H, Kaplan B, et al. Oncogenic and sorafenib-sensitive ARAF mutations in lung adenocarcinoma. J Clin Invest. 2014;124(4):1582–1586. doi: 10.1172/JCI72763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ahmadie H, Iyer G, Hohl M, et al. Synthetic lethality in ATM-deficient RAD50-mutant tumors underlies outlier response to cancer therapy. Cancer Discov. 2014;4(9):1014–1021. doi: 10.1158/2159-8290.CD-14-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78(1):8–15. doi: 10.1016/j.lungcan.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Van Allen EM, Wagle N, Stojanov P, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Med. 2014;20(6):682–688. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvind R, Shimamoto H, Momose F, Amagasa T, Omura K, Tsuchida N. A mutation in the common docking domain of ERK2 in a human cancer cell line, which was associated with its constitutive phosphorylation. Int J Oncol. 2005;27(6):1499–1504. [PubMed] [Google Scholar]
- 21.Klein JD, Christopoulos A, Ahn SM, Gooding WE, Grandis JR, Kim S. Antitumor effect of vandetanib through EGFR inhibition in head and neck squamous cell carcinoma. Head Neck. 2012;34(9):1269–1276. doi: 10.1002/hed.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psyrri A, Lee JW, Pectasides E, et al. Prognostic biomarkers in phase II trial of cetuximab-containing induction and chemoradiation in resectable HNSCC: Eastern Cooperative Oncology Group E2303. Clin Cancer Res. 2014;20(11):3023–3032. doi: 10.1158/1078-0432.CCR-14-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hah JH, Zhao M, Pickering CR, et al. HRAS mutations and resistance to the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in head and neck squamous cell carcinoma cells. Head Neck. 2014;36(11):1547–1554. doi: 10.1002/hed.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young NR, Liu J, Pierce C, et al. Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition. Mol Oncol. 2013;7(3):359–368. doi: 10.1016/j.molonc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreszer TR, Karolchik D, Zweig AS, et al. The UCSC Genome Browser database: extensions and updates 2011. Nucleic Acids Res. 2012;40:D918–D923. doi: 10.1093/nar/gkr1055. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valiathan GM, Thenumgal SJ, Jayaraman B, et al. Common docking domain mutation E322K of the ERK2 gene is infrequent in oral squamous cell carcinomas. Asian Pac J Cancer Prev. 2012;13(12):6155–6157. doi: 10.7314/apjcp.2012.13.12.6155. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506(7488):371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahalingam M, Arvind R, Ida H, Murugan AK, Yamaguchi M, Tsuchida N. ERK2 CD domain mutation from a human cancer cell line enhanced anchorage-independent cell growth and abnormality in Drosophila. Oncol Rep. 2008;20(4):957–962. [PubMed] [Google Scholar]
- 32.Ercan D, Xu C, Yanagita M, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2(10):934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchet S, Ramgolam K, Baulig A, Marano F, Baeza-Squiban A. Fine particulate matter induces amphiregulin secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2004;30(4):421–427. doi: 10.1165/rcmb.2003-0281RC. [DOI] [PubMed] [Google Scholar]
- 34.Yonesaka K, Zejnullahu K, Lindeman N, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14(21):6963–6973. doi: 10.1158/1078-0432.CCR-08-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.