Abstract
The field of organocatalysis has blossomed over the past few decades, becoming an alternative to transition-metal catalysis or even replacing the realm of transition-metal catalysis. However, a truly powerful organocatalyst with a high turnover number (TON) and turnover frequency (TOF) while retaining high enantioselectivity is yet to be discovered. Similar to metal catalysis, extremely low catalyst loading (p.p.m. or p.p.b. levels) is the ultimate goal of the organocatalysis community. Herein we report a remarkable contribution in this context: 1 p.p.m. loading of a simple 1,1′-bi-2-naphthol-based organocatalyst was enough to achieve highly enantioselective silylation reactions of alcohols. The unprecedented TONs and excellent enantioselectivity are ascribed to the robustness of the catalyst and systematic cooperative hydrogen-bonding organocatalysis in a densely confined chiral space.
 Organocatalytic methods typically do not approach the extremely low loadings that can be achieved with some metal catalysed processes. Here, the authors report a remarkably powerful organocatalyst capable of performing asymmetric silylations with sub parts-per-million level loadings.
Organocatalytic methods typically do not approach the extremely low loadings that can be achieved with some metal catalysed processes. Here, the authors report a remarkably powerful organocatalyst capable of performing asymmetric silylations with sub parts-per-million level loadings.
Catalysis, in principle, should offer an infinite turnover number (TON) for a desired chemical transformation by regenerating the catalyst without altering its molecular-level structure and physicochemical properties. However, limitations arise from different deactivation mechanisms and catalyst poisoning by chemical impurities and/or side products, lowering the lifetime of catalytically active species1.
The current strong interest in asymmetric organocatalysis2,3 has pinpointed that small organic molecules are comparable to transition-metal catalysts owing to their noble activation modes and excellent selectivity. However, further application of organocatalysts in industry is often hampered by their relatively low turnover efficiency than those of the corresponding homogeneous or heterogeneous metal-catalysed reactions4. Despite many efforts4,5,6, the current limit of catalyst loading for asymmetric organocatalysis is usually in the range of 0.1–1 mol% (refs 6, 7) for overriding of the non-selective background pathway. Thus, high turnover organocatalysis (for example, TON>100,000) is considered as a formidable challenge8.
Our group has developed a new type of easily accessible organocatalysts, 1,1′-bi-2-naphthol (BINOL)-based polyether catalysts 1 (Fig. 1), that bears phenols and polyether units, for asymmetric cation-binding catalysis9,10,11. Its unprecedented activation mode and remarkable enantioselectivity are explained by multiple binding and hydrogen-bonding interactions of the polyether and phenols with the metal salt to generate reactive ‘chiral' anions. Strikingly, the robustness of catalyst 1 enabled the one-pot large-scale production of unnatural α-amino acids even under harsh reaction conditions (refluxing in 6 N HCl) and the subsequent quantitative recovery of the catalyst11. The structural simplicity and vast application potential of the catalyst stimulated us to explore other challenging catalytic asymmetric reactions.
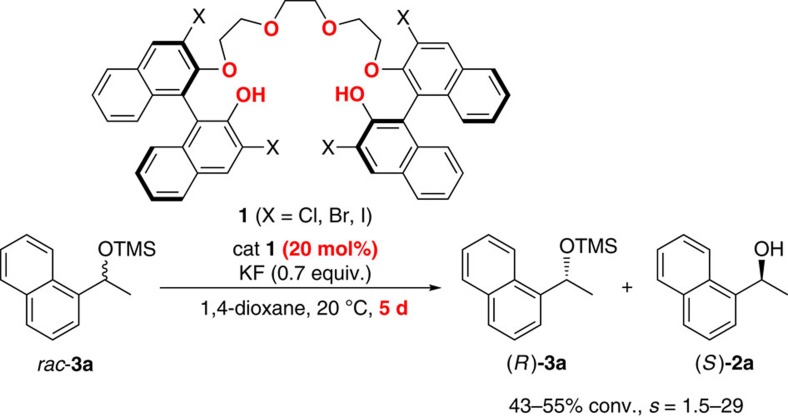
Figure 1. Structure of BINOL-based polyether catalyst 1 and our previous work: desilylative kinetic resolution of racemic alcohols.
Selectivity factor (s)=(rate of fast reacting enantiomer)/(rate of slow reacting enantiomer).
The preparation of enantiopure secondary alcohols is of significant interest in the pharmaceutical industry; therefore, diverse methodologies have been developed for preparing these synthons with high enantiomeric excess12,13,14,15. The kinetic resolution of racemic alcohols is an ideal approach to access both enantiomers by one enantioselective transformation, for example, acylative kinetic resolution reactions16. Although silylation of alcohols is one of the most common strategies in protecting alcohol functionalities, the asymmetric silylation reactions17,18,19,20,21,22,23,24,25,26,27,28 of functionally unbiased alcohols is still challenging17,18,21 due to the lack of additional functional groups needed to interact with a chiral catalyst. Until now, the substrate scope of the reported silylation reactions has been mostly limited to the substrates with pendant donors (for example, additional hydroxyl or pyridyl groups), which are necessary to achieve good selectivity by increasing the binding affinity towards catalysts. Snapper and Hoveyda demonstrated remarkable examples in this context by using amino-acid-derived bifunctional organocatalysts25,26,27,28. However, as a high catalyst loading (20–30 mol%) was necessary to obtain the desired products with high enantioselectivity, it may hamper the large-scale application of these organocatalysts. On the other hand, our group showed that catalysts such as 1 (X=Cl, Br or I) catalyse desilylative kinetic resolution of silyl-protected racemic alcohols in the presence of potassium fluoride. Although this approach provided a new synthetic route for chiral alcohols, a practical application was also far from satisfactory due to the high catalyst loading (20 mol%) and very low turnover frequency (TOF; 43–55% conversion after 5 days at 20 °C) (Fig. 1)10.
Herein we report that the same catalyst 1 can catalyse the enantioselective silylation of simple aryl alkanols, which do not contain any pendant donors, exhibiting unprecedented catalytic TONs (substrate to catalyst=up to 1,000,000) and excellent enantioselectivity. Extremely high TONs and excellent enantioselectivity were obtained by thorough catalyst structure analysis, detailed mechanistic studies and the identification of catalyst regeneration conditions.
Results
Reaction design
We commenced our study by selecting 1,1,1,3,3,3-hexamethyldisilazane (HMDS) as the silylating reagent (Supplementary Table 1). HMDS is a stable, commercially available and inexpensive reagent for the trimethylsilylation of alcohols, releasing ammonia as the sole by-product. However, the weak silylating ability of HMDS is the main drawback to its application29, particularly in asymmetric catalysis. Because HMDS is usually activated using an acidic catalyst29, chiral Brønsted acid catalysts were chosen first for investigation of the enantioselective silylation of alcohols with HMDS; however, these catalysts resulted in very low conversions and selectivities (Supplementary Fig. 1).
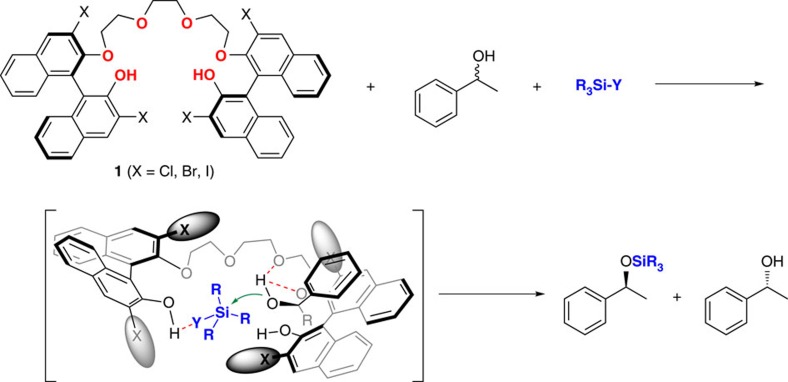
Given our previous studies on catalyst 1 (refs 10, 11), in spite of the lower acidity of phenolic protons than those of the common Brønsted acid catalysts30, we presumed that catalyst 1 would act as a catalyst for the silylative kinetic resolution of racemic alcohols; the interaction between the acidic phenolic protons of the catalyst and the leaving group of the silylating reagent (Y) by hydrogen bonding would enhance the electrophilicity of Si. Moreover, the ether moieties of the catalyst would function as a Brønsted base by the association with an alcohol substrate, enhancing alcohol reactivity (Fig. 2).
Figure 2. Proposed catalytic enantioselective silylation of racemic alcohols.
The proposed transition state for silylation of rac-alcohol is shown.
Catalyst screening
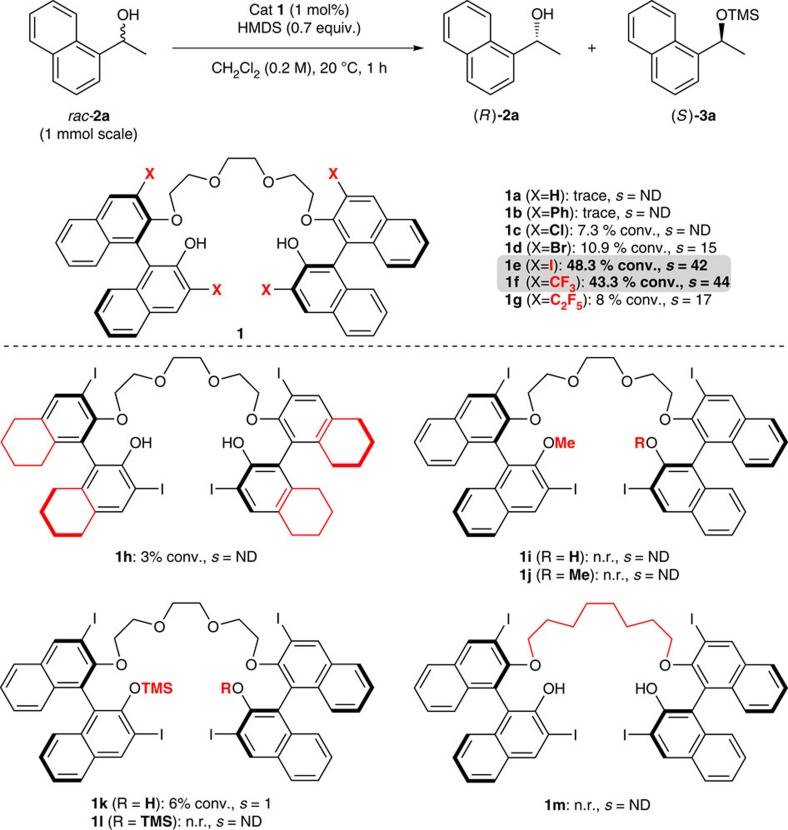
To prove this assumption, several chiral BINOL-based bis(hydroxy) polyethers 1a–1m (Fig. 3) were screened to shed light on the relationship between the catalyst's structure and the reaction outcome. Gratifyingly, after the optimization of the reaction conditions (Supplementary Tables 1–3), catalysts 1c, 1d, 1e, 1f and 1g bearing electron-withdrawing groups Cl, Br, I, CF3 and C2F5, respectively, on the 3,3′-positions showed some catalytic activity (Fig. 3). The catalytic performance of these catalysts depends significantly on the steric demand of the substituents. Thus, catalysts 1e and 1f show the best catalytic performance with s-factors of 42 and 44, respectively (Fig. 3). In contrast, other catalysts without electron-withdrawing substituents at the 3,3′-positions (catalysts 1a and 1b) were inactive under identical reaction conditions. In addition, the less acidic H8-BINOL-derived catalyst 1h showed negligible conversion, thus, indicating the importance of the Brønsted acidity of the phenols. To further confirm the importance of an acidic phenol moiety for catalysis, we synthesized the catalysts 1i–1l. A total loss in catalytic activity was observed when one or both phenol groups were replaced by OMe (1i and 1j). Silylated catalysts (1k and 1l) also showed no activity, excluding the possibility of a silyl-transfer reaction from the silylated catalyst to the starting alcohol to afford the desired product. The importance of the polyether chain was confirmed unambiguously by replacing the polyether with the alkyl chain (catalyst 1m), indicating that the polyether backbone is crucial in achieving the observed catalytic activity.
Figure 3. Effect of variations in catalyst structure on the silylative kinetic resolution of substrate 2a.
Calculated conversion, c=ee(R)-2a/(ee(R)-2a+ee(S)-3a). Selectivity factor (s)=ln((1−c)(1−ee(R)−2a))/ln((1−c)(1+ee(R)−2a))=ln(1−c(1+ee(S)-3a))/ln(1−c(1−ee(S)−3a)).
The complexation of an alcohol substrate with the polyether moiety of the catalyst was clearly verified by observing the decreased 13C spin–lattice relaxation time (T1) on complexation of catalyst 1e with racemic alcohol 2a (Supplementary Table 4). This significant decrease in the T1 value of ether moiety of 1e indicates that the complexation of the alcohol with catalyst 1e significantly reduces the mobility of the ether units. Moreover, the fast-reacting (S)-configured substrate ((S)-2a) exhibited a more decreased T1 value of ether moiety than that of the slow-reacting substrate, (R)-2a, directly indicating a selective association of the catalyst with the fast-reacting (S)-2a (ref. 31). On the other hand, no change in T1 values was observed when alcohol 2a was mixed with the alkyl chain-replaced catalyst 1m, verifying the crucial role of the polyether backbone for the interaction with alcohols (Supplementary Table 5). 29Si nuclear magnetic resonance (NMR) experiments were also used to establish the interaction between the phenolic proton of the catalyst and HMDS (Supplementary Table 6). As expected, the 29Si NMR data of HMDS in the presence of diverse phenols exhibited 29Si chemical shifts that are shifted downfield relative to the uncoordinated HMDS, dependent on phenol acidity. This result clearly indicates that the acidic phenolic proton interacts with HMDS, increasing the electrophilic character of the silicon atom. The results from NMR spectroscopy and other experimental results (Fig. 3) strongly support our proposed transition-state model shown in Fig. 2 (Y=NHSiMe3).
Optimization of the reaction conditions
More importantly, during the optimization studies, we also realized that the major deactivation pathway of the catalyst is the silylation of the phenol moieties under the reaction conditions. In a separated set of experiments, we verified that the steric demand of the 3,3′-substituents of the catalyst is crucial to this deactivation pathway (Supplementary Table 7). As mentioned earlier, the silylated catalysts (1k and 1l) are inactive towards the kinetic resolution reaction (Fig. 3).
Recognizing that the rate of desilylation of trimethylsilyl (TMS)-protected phenols using KF in oligoethylene glycol32 is much faster than those of TMS-protected secondary alcohols, we speculated that the addition of KF and an appropriate proton source would regenerate the catalyst in situ (Table 1a). As predicted, the desilylation reaction of the silylated catalysts (1k and 1l) and subsequent protonation of the K salt of catalysts clearly improved TON and TOF (Table 1b). As shown in entries 1 and 7 of Table 1b, with catalyst loading of 0.01 mol% (100 p.p.m.), almost no catalytic activity was observed without the additives. However, the addition of KF and a proton source such as water, t-BuOH and Amberlite CG 50 (a weakly acidic cation-exchange resin)33 was able to restore catalysis (entries 2–5). In particular, a combination of KF and Amberlite CG 50 provided the best result (entry 5). Even with 100 p.p.m. of catalyst loading, 52% conversion was obtained in 12 h with excellent selectivity (s=57). This can be ascribed to the adequate acidity of Amberlite CG 50 for rapid protonation of the K salt of 1e, releasing the insoluble polymeric K salt of Amberlite CG 50 as a by-product without interfering with the catalysis. Moreover, Amberlite CG 50 alone was completely inactive towards catalysis. In addition, we also confirmed that, under the reaction condition where KF and Amberlite CG 50 were used as additives (condition in entry 5), the unwanted desilylation process that takes place with a TMS-protected secondary alcohol such as (R)-3a did not occur (Supplementary Fig. 2) and that the relative catalytic activity of 1a–1f is very similar with that shown in Fig. 3 (Supplementary Table 8).
Table 1. Proposed mechanism of catalyst reactivation (a) and effects of KF and proton source additives on turnover numbers (b).
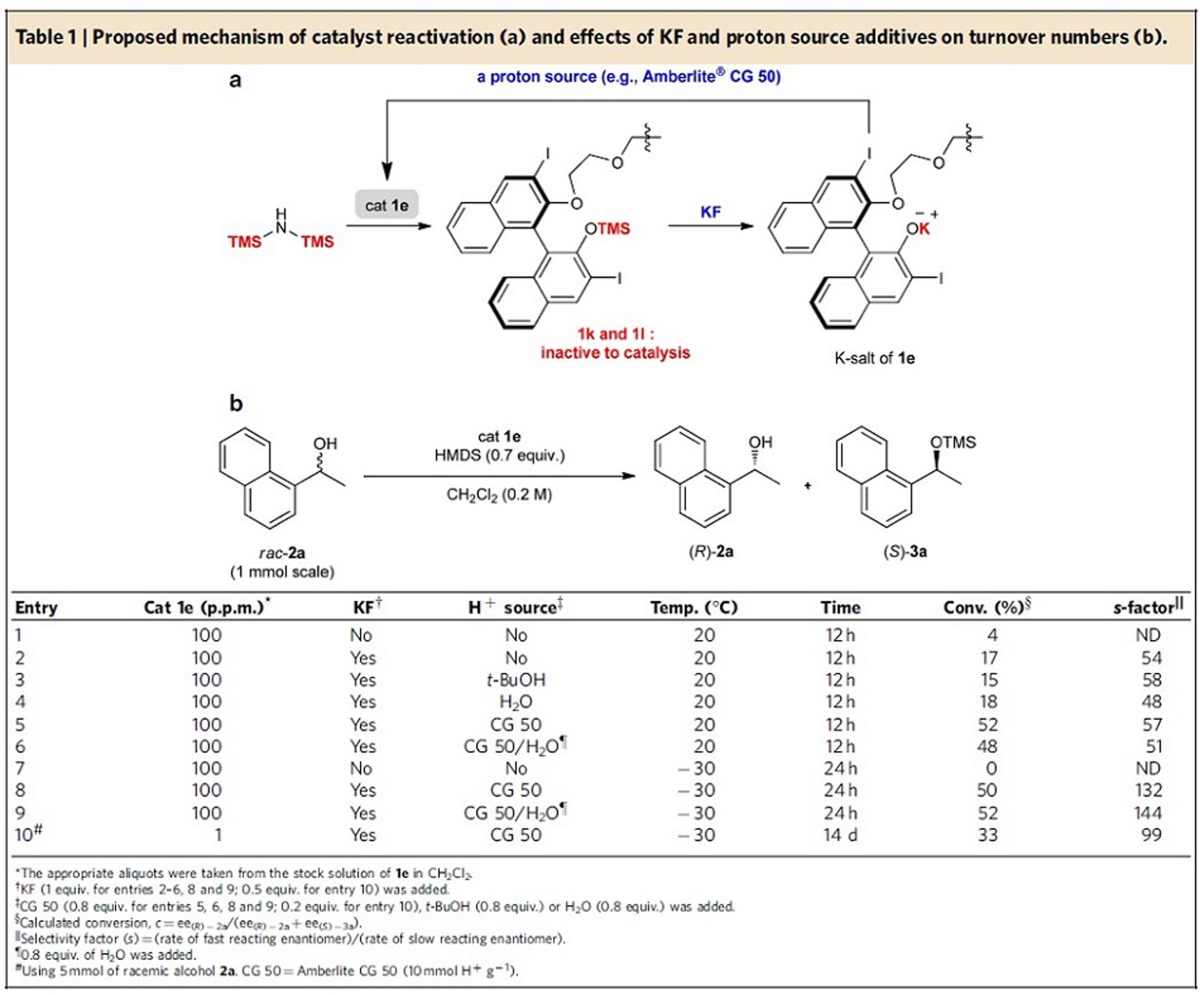
Further improvement of selectivity was achieved by lowering the reaction temperature to −30 °C (s=132) (entry 8). More surprisingly, the catalyst is active even at 1 p.p.m. catalyst loading (0.0001, mol%) with excellent s-factor (s=99 in Table 1, entry 10). The reaction proceeds with p.p.b.-level catalyst loading (100 p.p.b.); however, a significant decrease in selectivity (s=17) was observed (Supplementary Fig. 3). To the best of our knowledge, this is the lowest catalyst loading for metal-free organocatalysis reported to date, with excellent enantioselectivities and high TOF (TOF ∼1,000 h−1, entry 11) also being achieved. The high turnover efficiency can be attributed to the stability of the catalyst, its insensitivity to impurities and rapid reactivation under the reaction conditions. Notably, the reactions do not require exclusion of air and moisture, and thus solvent purification is not needed. As shown from the results of entries 6 and 9, the presence of water does not affect catalytic results, which is highly beneficial for further large-scale applications.
Scope of the reaction
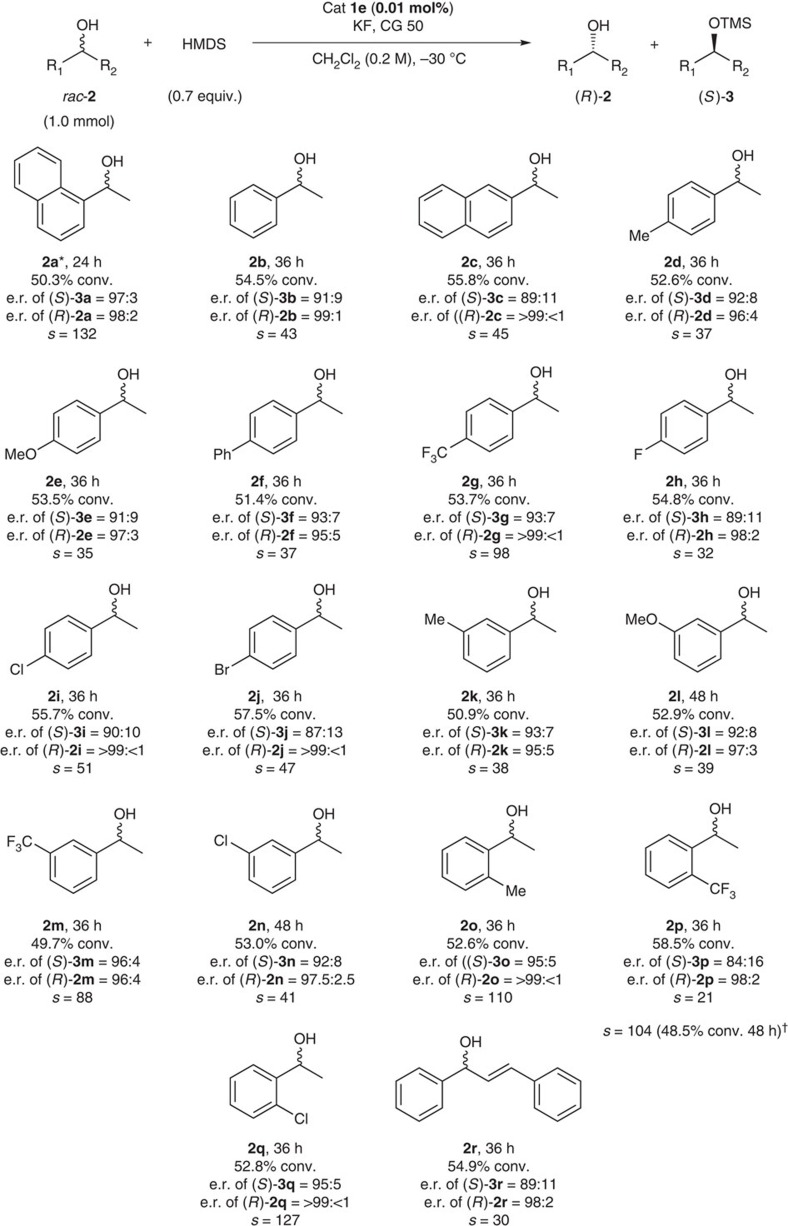
With the optimized reaction conditions in hand, the substrate scope of our protocol was investigated, and the results are shown in Fig. 4. Various simple acyclic 1-arylalkanols 2a–2r without any secondary binding functionality were successfully resolved with excellent selectivity factors. Both the electron-withdrawing and electron-donating substituents on the aromatic ring were tolerated under the reaction conditions. Sterically demanding ortho-substituted substrates were also smoothly and selectively converted to TMS-protected alcohols with excellent s-factors. Notably, the s-factors for the silylation of the sterically bulky alcohol 2p increased from 21 to 104 by changing the catalyst 1e (X=I) to 1d (X=Br), a sterically less demanding catalyst, indicating that stereoselectivity can be controlled by tuning the cage size of the catalyst34,35. However, 1-cyclohexylethanol, which does not contain an aromatic moiety, exhibited a poor selectivity factor (s=1.4, Supplementary Fig. 4), indicating that the π–π interaction between catalyst and substrate might play an important role in obtaining high stereoselectivity, as shown in Fig. 2.
Figure 4. Substrate scope of the silylative kinetic resolution of racemic alcohol 2 catalysed by 1e.
The reactions were performed in the presence of 1 equiv of KF and 0.8 equiv of Amberlite CG 50 (CG 50). *Using catalyst 1c (X=Cl, 16.8% conv., s=20), catalyst 1d (X=Br, 36.7% conv., s=48), catalyst 1f (X=CF3, 50.8% conv., s=50) and catalyst 1g (X=C2F5, 34.1% conv., s=30). †Using catalyst 1d (X=Br). e.r., enantiomeric ratio.
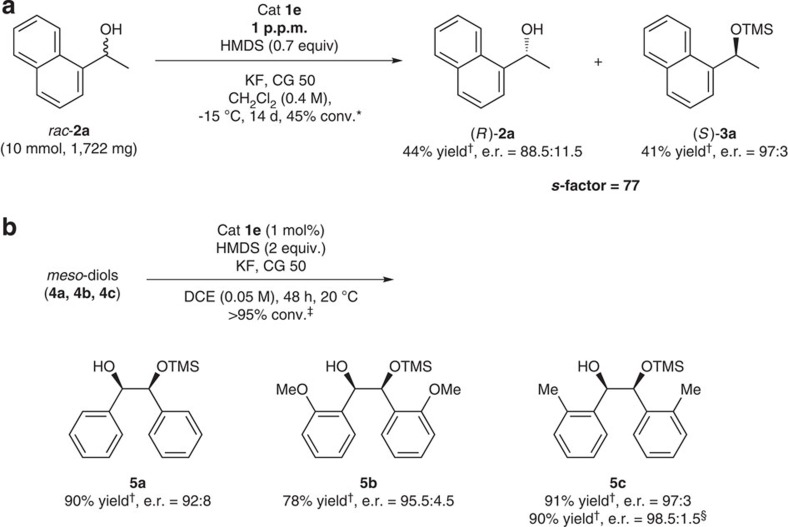
To demonstrate the synthetic utility and potential large-scale applications of this protocol, 1.722 g (10 mmol) rac-2a was resolved at −15 °C by using only 0.012 mg of the catalyst (1 p.p.m. to rac-2a), with a high TOF (1,309 h−1) and an excellent s-factor of 77 (Fig. 5a), affording TMS-ether (S)-3a (41% yield) and (R)-2a (44% yield) after simple filtration of insoluble additives (KF and CG 50) and column chromatography. Finally, to demonstrate the generality of our silylation protocol, the silylative desymmetrization reactions of meso-hydrobenzoins 4 were conducted using 1 mol% of catalyst 1e at room temperature. As shown in Fig. 5b, monosilylated alcohols 5a–5c were obtained in high yields and with excellent enantiomeric excesses (ee) (up to 97% ee). Furthermore, an essentially linear relationship between the optical purity of the catalyst and enantioselectivity of the major product 5a was observed, indicating that the reaction involves only one catalyst in the enantio-determining step, supporting our proposed model shown in Fig. 2 (Supplementary Fig. 5).
Figure 5. Applications of the silylation reaction protocol.
(a) Gram-scale kinetic resolution of secondary alcohol 2a using 1 p.p.m. of catalyst: The reactions were performed in the presence of 0.5 equiv of KF and 0.2 equiv of Amberlite CG 50 (CG 50). *Calculated conversion, c=ee(R)−2a/(ee(R)−2a+ee(S)−3a). †Isolated yields. (b) Catalytic enantioselective silylation of meso-diols 4 (0.1 mmol scale): The reactions were performed in the presence of 1 equiv of KF and 0.6 equiv of Amberlite CG 50 (CG 50). ‡The conversion was determined by 1H-NMR. †Isolated yields. §The reaction was performed at 0 °C. DCE, 1,2-dichloroethane; e.r., enantiomeric ratio.
Discussion
In summary, a p.p.m.-level loading organocatalytic enantioselective silylation of simple alcohols was developed using the easily accessible BINOL-based polyether catalyst with the commercially available HMDS as the silylating reagent. This unprecedented catalyst turnover was achieved by adding KF and a weakly acidic cation-exchange resin, thus regenerating the active form of the catalyst. Using this protocol, diverse racemic secondary alcohols were resolved with excellent selectivity factors under mild reaction conditions and the reaction was conducted without protecting moisture and without the use of an inert gas atmosphere. Moreover, the utility of this silylation protocol was further extended to the enantioselective silylation of meso-diols. The features of very low catalyst loading, high enantioselectivity, broad substrate scope and mild reaction conditions can make this protocol easily adaptable to the practical synthesis of diverse biologically and pharmaceutically important chiral alcohols. The extremely high turnover efficiency and selectivity achieved can be ascribed to the robustness of the catalyst and systematic cooperative hydrogen-bonding catalysis in a densely confined chiral space34,35, which mimics the action of enzymes. In a confined space, active sites activate both the reactants simultaneously and keep them in close proximity, thus enhancing reactivity and efficiently transferring the stereochemical information. Our results may contribute to the development of an ‘ideal' catalyst system based on weak interactions (hydrogen bondings, charge–charge interactions and π-interactions) such those of enzymes.
Methods
General procedure for catalytic asymmetric silylation of secondary alcohols
To a solution of chiral catalyst 1e (0.12 mg, 0.01 mol%) and secondary alcohols 2 (1.0 mmol) in distilled CH2Cl2 (5.0 ml), spray-dried KF (58.1 mg, 100 mol%) and Amberlite CG 50 (80 mg, 0.8 equiv.) were added in one portion. The reaction mixture was stirred at −30 °C for 10 min, followed by addition of HMDS (0.15 ml, 0.7 equiv.). After stirring for 24–48 h at −30 °C, the reaction mixture was filtered and the filtrate was concentrated in vacuo to afford a colourless oil. The residue thus obtained was purified by short silica gel chromatography (EA/hexanes=1/5). Silyl ethers 3a–3f, 3i–3j, 3m–3o and 3r are known compounds in the literature and their spectroscopic data were consistent with previously reported values (Supplementary Methods and Supplementary References).
The enantiomeric excesses of 2 and 3 were determined by chiral high-performance liquid chromatography (HPLC) analysis. The enatiomeric excess of TMS-ether products 3 was determined after converting them to their corresponding alcohols 2 by the deprotection of its trimethylsilyl group with TBAF. Thus, the absolute configurations of 3 were determined by comparison of the retention time of HPLC with the literature data of the corresponding alcohols 2a–2r (Supplementary Methods and Supplementary References).
For NMR and HPLC analysis of the compounds in this article, see Supplementary Fig. 6–20.
Additional information
How to cite this article: Park, S. Y. et al. Parts-per-million level loading organocatalysed enantioselective silylation of alcohols. Nat. Commun. 6:7512 doi: 10.1038/ncomms8512 (2015).
Supplementary Material
Supplementary Figures 1-20, Supplementary Tables 1-8, Supplementary Methods and Supplementary References
Acknowledgments
The authors are grateful for the financial support provided by the Ministry of Science, ICT and Future Planning (NRF-2014R1A2A1A01005794). In addition, we thank Prof. S.-G. Lee and Dr. M.T. Oliveira for their helpful dicsussions.
Footnotes
Author contributions C.E.S. designed and developed the strategy and the mechanistic concepts. S.Y.P. and J.W.L. performed the experiments. J.W.L. and C.E.S. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
References
- Crabtree R. H. Deactivation in homogeneous transition metal catalysis: causes, avoidance, and cure. Chem. Rev. 115, 127–150 (2015) . [DOI] [PubMed] [Google Scholar]
- List B. & Yang J. W. The organic approach to asymmetric catalysis. Science 313, 1584–1586 (2006) . [DOI] [PubMed] [Google Scholar]
- MacMillan D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008) . [DOI] [PubMed] [Google Scholar]
- Lee J. W. et al. Organotextile catalysis. Science 341, 1225–1229 (2013) . [DOI] [PubMed] [Google Scholar]
- Cozzi F. Immobilization of organic catalysts: when, why, and how. Adv. Synth. Catal. 348, 1367–1390 (2006) . [Google Scholar]
- Giacalone F., Gruttadauria M., Agrigento P. & Noto R. Low-loading asymmetric organocatalysis. Chem. Soc. Rev. 41, 2406–2447 (2012) . [DOI] [PubMed] [Google Scholar]
- Ingle G. K., Mormino M. G., Wojtas L. & Antilla J. C. Chiral phosphoric acid-catalyzed addition of thiols to N-acyl imines: access to chiral N,S-acetals. Org. Lett. 13, 4822–4825 (2011) . [DOI] [PubMed] [Google Scholar]
- Ratjen L., van Gemmeren M., Pesciaioli F. & List B. Towards high-performance Lewis acid organocatalysis. Angew. Chem. Int. Ed. 53, 8765–8769 (2014) . [DOI] [PubMed] [Google Scholar]
- Brak K. & Jacobsen E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. et al. A chiral–anion generator: application to catalytic desilylative kinetic resolution of silyl-protected secondary alcohols. Angew. Chem. Int. Ed. 49, 8915–8917 (2010) . [DOI] [PubMed] [Google Scholar]
- Yan H., Oh J. S., Lee J. W. & Song C. E. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat. Commun. 3, 1212 (2012) . [DOI] [PubMed] [Google Scholar]
- Reeves J. T., Senanayake C. H., Song J. J. & Farina V. Asymmetric synthesis of active pharmaceutical ingredients. Chem. Rev. 106, 2734–2793 (2006) . [DOI] [PubMed] [Google Scholar]
- Cobley C. J. & Holt-Tiffin K. E. Asymmetric routes to chiral secondary alcohols. Pharmaceutical Technology 34, 6–13 (2010) . [Google Scholar]
- Huisman G. W., Liang J. & Krebber A. Practical chiral alcohol manufacture using ketoreductases. Curr. Opin. Chem. Biol. 14, 122–129 (2010) . [DOI] [PubMed] [Google Scholar]
- Noyori R. & Ohkuma T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 40, 40–73 (2001) . [PubMed] [Google Scholar]
- Müller C. E. & Schreiner P. R. Organocatalytic enantioselective acyl transfer onto racemic as well as meso alcohols, amines, and thiols. Angew. Chem. Int. Ed. 50, 6012–6042 (2011) . [DOI] [PubMed] [Google Scholar]
- Isobe T., Fukuda K., Araki Y. & Ishikawa T. Modified guanidines as chiral superbases: The first example of asymmetric silylation of secondary alcohols. Chem. Commun. 3, 243–244 (2001) . [Google Scholar]
- Sheppard C. I., Taylor J. L. & Wiskur S. L. Silylation-based kinetic resolution of monofunctional secondary alcohols. Org. Lett. 13, 3794–3797 (2011) . [DOI] [PubMed] [Google Scholar]
- Rendler S., Auer G. & Oestreich M. Kinetic resolution of chiral secondary alcohols by dehydrogenative coupling with recyclable silicon-streogenic silanes. Angew. Chem. Int. Ed. 44, 7620–7624 (2005) . [DOI] [PubMed] [Google Scholar]
- Klare H. F. T. & Oestreich M. Chiral recognition with silicon-stereogenic silanes: remarkable selectivity factors in the kinetic resolution of donor-functionalized alcohols. Angew. Chem. Int. Ed. 46, 9335–9338 (2007) . [DOI] [PubMed] [Google Scholar]
- Karatas B., Rendler S., Fröhlich R. & Oestreich M. Kinetic resolution of donor-functionalised tertiary alcohols by Cu-H-catalysed stereoselective silylation using a strained silicon-stereogenic silane. Org. Biomol. Chem. 6, 1435–1440 (2008) . [DOI] [PubMed] [Google Scholar]
- Rendler S. & Oestreich M. Kinetic resolution and desymmetrization by stereoselective silylation of alcohols. Angew. Chem. Int. Ed. 47, 248–250 (2008) . [DOI] [PubMed] [Google Scholar]
- Steves A. & Oestreich M. Facile preparation of CF3-substituted carbinols with an azine donor and subsequent kinetic resolution through stereoselective Si-O coupling. Org. Biomol. Chem. 7, 4464–4469 (2009) . [DOI] [PubMed] [Google Scholar]
- Weickgenannt A., Mewald M. & Oestreich M. Asymmetric Si-O coupling of alcohols. Org. Biomol. Chem. 8, 1497–1504 (2010) . [DOI] [PubMed] [Google Scholar]
- Zhao Y., Rodrigo J., Hoveyda A. H. & Snapper M. L. Enantioselective silyl protection of alcohols catalysed by an amino-acid-based small molecule. Nature 443, 67–70 (2006) . [DOI] [PubMed] [Google Scholar]
- Zhao Y., Mitra A. W., Hoveyda A. H. & Snapper M. L. Kinetic resolution of 1,2-diols through highly site- and enantioselective catalytic silylation. Angew. Chem. Int. Ed. 46, 8471–8474 (2007) . [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hoveyda A. H. & Snapper M. L. Catalytic enantioselective silylation of acyclic and cyclic triols: application to total syntheses of cleroindicins D, F, and C. Angew. Chem. Int. Ed. 48, 547–550 (2009) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manville N. et al. Enantioselective silyl protection of alcohols promoted by a combination of chiral and achiral Lewis basic catalysts. Nat. Chem. 5, 768–774 (2013) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruynes C. A. & Jurriens T. K. Catalysts for silylations with 1,1,1,3,3,3-hexamethyldisilazane. J. Org. Chem. 47, 3966–3969 (1982) . [Google Scholar]
- Christ P. et al. pKa Values of chiral Brønsted acid catalysts: phosphoric acids/amides, sulfonyl/sulfuryl imides, and perfluorinated TADDOLs (TEFDDOLs). Chem. Eur. J. 17, 8524–8528 (2011) . [DOI] [PubMed] [Google Scholar]
- Li Y. & Echegoyen L. Enatiomeric recognition between chiral triazole-18-crown-6 ligands and organic ammonium cations assessed by 13C and 1H NMR relaxation times. J. Org. Chem. 59, 6539–6542 (1994) . [Google Scholar]
- Yan H., Oh J. S. & Song C. E. A mild and efficient method for the selective deprotection of silyl ethers using KF in the presence of tetraethylene glycol. Org. Biomol. Chem. 9, 8119–8121 (2011) . [DOI] [PubMed] [Google Scholar]
- Müller S., Webber M. J. & List B. The catalytic asymmetric Fischer indolization. J. Am. Chem. Soc. 133, 18534–18537 (2011) . [DOI] [PubMed] [Google Scholar]
- Coric I. & List B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315–319 (2012) . [DOI] [PubMed] [Google Scholar]
- Shenoy S. R., Crisóstomo F. R. P., Iwasawa T. & Rebek J. Jr. Organocatalysis in a synthetic receptor with an inwardly directed carboxylic acid. J. Am. Chem. Soc. 130, 5658–5659 (2008) . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-20, Supplementary Tables 1-8, Supplementary Methods and Supplementary References