Abstract
Autoreactive CD8 T cells play a central role in the destruction of pancreatic islet β-cells that leads to type 1 diabetes, yet the key features of this immune-mediated process remain poorly defined. In this study, we combined high definition polychromatic flow cytometry with ultrasensitive peptide-human leukocyte antigen class I (pHLAI) tetramer staining to quantify and characterize β-cell-specific CD8 T cell populations in patients with recent onset type 1 diabetes and healthy controls. Remarkably, we found that β-cell-specific CD8 T cell frequencies in peripheral blood were similar between subject groups. In contrast to healthy controls, however, patients with newly diagnosed type 1 diabetes displayed hallmarks of antigen-driven expansion uniquely within the β-cell-specific CD8 T cell compartment. Molecular analysis of selected β-cell-specific CD8 T cell populations further revealed highly skewed oligoclonal T cell receptor (TCR) repertoires comprising exclusively private clonotypes. Collectively, these data identify novel and distinctive features of disease-relevant CD8 T cells that inform the immunopathogenesis of type 1 diabetes.
Type 1 diabetes is an autoimmune disease characterized by T cell-mediated destruction of insulin-producing β-cells in the islets of Langerhans (1; 2). Several lines of evidence implicate the CD8 T cell lineage in this process: (i) CD8 T cells predominate in islet-centred leukocytic infiltrates close to diagnosis (3; 4); (ii) autoreactive CD8 T cells with β-cell epitope specificities have been detected in such early infiltrates (3); (iii) CD8 T cell clones specific for preproinsulin-derived peptides can kill β-cells in vitro (5; 6); and (iv) large genetic association studies link disease susceptibility to the inheritance of specific human leukocyte antigen class I (HLAI) alleles (7). This mounting functional and epidemiological evidence, combined with the expanding array of reported HLAI-restricted β-cell epitopes (8; 9), provides a strong rationale for detailed studies of autoreactive CD8 T cells in type 1 diabetes.
Technological advances have facilitated the design of CD8-centric studies, enabling enhanced data retrieval from cell-limited samples to illuminate fundamental immunobiological processes. In particular, antigen-specific CD8 T cells can now be enumerated routinely by flow cytometry irrespective of functional outputs due to the advent of recombinant peptide-HLAI (pHLAI) proteins in various fluorochrome-tagged multimeric formats (10-13). Moreover, developments in instrumentation and fluorochrome technology continue to expand the horizons of polychromatic flow cytometry (14; 15), facilitating the identification of functionally distinct T cell subsets across a spectrum of phenotypic heterogenity (16). The collective application of such innovations has transformed our understanding of T cell ontogeny in response to infectious “foreign” antigens. However, it is unclear whether the emerging conceptual frameworks extend similarly to autoimmune processes.
Although β-cell epitope-specific CD8 T cell expansions have been identified in the peripheral blood of patients with type 1 diabetes (6; 17), the functional and phenotypic properties of these cells in the context of disease relevance remain largely uncharacterized. This is a significant knowledge gap for two important reasons. First, it cannot be assumed that autoreactive CD8 T cells will follow the rules of antigen engagement established in previous studies. Indeed, autoreactive T cell receptors (TCRs) characteristically display low affinity interactions with their cognate pHLA antigens (18; 19), presumably reflecting the effects of thymic culling to eliminate potentially dangerous self-specific clonotypes from the peripheral repertoire. Moreover, autoantigens are expressed continuously and guarded by peripheral tolerance mechanisms designed to limit cognate T cell expansion (1). Second, immune intervention strategies designed specifically to target either effector T cells or innate inflammatory pathways that could impact adaptive immune responses are currently being trialed in type 1 diabetes (20-23). The identification of T cell-related biomarkers could facilitate immune monitoring in this setting and delineate correlates of therapeutic efficacy. Accordingly, we undertook a multiparametric analysis of β-cell-specific CD8 T cell populations in patients with type 1 diabetes and healthy controls to identify the key cellular features associated with disease.
RESEARCH DESIGN AND METHODS
Study subjects
The study cohort comprised 14 HLA-A*0201+ patients (mean age, 30 years +/− SD = 6.4) with newly diagnosed type 1 diabetes (mean disease duration, 4 months) and 14 HLA-A*0201+ healthy controls (mean age, 30 years +/− SD = 5.0). Autoantibodies against GAD65 and IA-2 were detected in 64% (9/14) and 71% (10/14) of patients in the type 1 diabetes group, respectively. Local research ethics committee approval (National Research Ethics Committee, Bromley NRES Committee, Ref 08/H0805/14) was granted at each participating center and written informed consent was obtained in all cases.
Blood samples
Fresh venous blood was collected into heparinized tubes and transported for processing within 3 h of collection. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Nycomed), washed twice in RPMI-1640 supplemented with 1% penicillin/streptomycin and 2% human AB serum (all Life Technologies), then resuspended in freezing medium comprising 90% heat-inactivated, filter-sterilized fetal bovine serum and 10% dimethyl sulfoxide (Sigma-Aldrich). Aliquots of 10-20 × 106 cells/mL per vial were cooled overnight at a controlled rate of −1°C/min to −80°C prior to storage in liquid nitrogen. All samples were analyzed within two years of cryopreservation.
Tetrameric pHLAI complexes
Soluble, fluorochrome-conjugated pHLA-A*0201 tetramers were produced as described previously (24). Incorporated peptides were synthesized at >95% purity (BioSynthesis). The corresponding epitopes are summarized in Table 1.
Table 1. HLA-A*0201-restricted CD8 T cell epitopes.
| Antigen | Epitope | Sequence | Subjects* |
|---|---|---|---|
| Preproinsulin | PPI15-24 | ALWGPDPAAA | 14/14 |
| Insulin B chain | InsB10-18 | HLVEALYLV | 10/13 |
| Islet-specific glucose-6-phosphatase catalytic subunit-related protein |
IGRP265-273 | VLFGLGFAI | 13/12 |
| Islet tyrosine phosphatase | IA-2797-805 | MVWESGCTV | 11/13 |
| Glutamic acid decarboxylase 65 | GAD65114-123 | VMNILLQYVV | 11/10 |
| Cytomegalovirus pp65 | CMV pp65495-503 | NLVPMVATV | 14/13 |
| Epstein-Barr virus BMLF1 | EBV BMLF1280-288 | GLCTLVAML | 13/12 |
Numbers of healthy controls/type 1 diabetes patients studied for each epitope specificity.
Polychromatic flow cytometry
Thawed PBMCs were pre-treated with 50 nM dasatinib before tetramer staining to enhance the detection of low avidity T cells as described previously (25). The following monoclonal antibodies (mAbs) were used for phenotypic analysis: (i) αCD3-H7APC, αCD4-V500, αCD45RO-PECy7, αCD57-FITC, αCD95-PE and αCCR7-PerCPCy5.5 (BD Biosciences); (ii) αCD8-QD705 (Life Technologies); and (iii) αCD27-PECy5 (Beckman Coulter). Dead cells were excluded from the analysis using the amine-reactive dye ViViD (Life Technologies); monocytes and B cells were eliminated in the same dump channel after staining with αCD14-PacBlue and αCD19-PacBlue, respectively (Life Technologies). Stained samples were acquired using an LSRFortessa™ flow cytometer (BD Biosciences) and analyzed with FlowJo version 9.4 (TreeStar Inc.). The gating strategy is illustrated in Supplementary Figure 1. For quality control purposes, tetramers were batch-tested prior to experimentation using specific CD8 T cell clones where available (5). Assay variability was monitored throughout the study using aliquots of cryopreserved PBMCs drawn from a single healthy donor at a single time point (Supplementary Figure 2).
T cell receptor clonotyping
Clonotypic analysis of antigen-specific CD8 T cell populations was performed as described previously with minor modifications (26). Briefly, 222-1,071 viable tetramer-labeled CD3+CD8+ T cells were sorted directly ex vivo into 1.5 mL microtubes (Sarstedt) containing 100 μL RNAlater (Applied Biosystems) using a custom-modified FACSAria™ II flow cytometer (BD Biosciences). Unbiased amplification of all expressed TRB gene products was conducted using a template-switch anchored reverse transcription-polymerase chain reaction (RT-PCR) with a 3′ constant region primer (5′-TGGCTCAAACAAGGAGACCT-3′). Amplicons were sub-cloned, sampled, sequenced and analyzed as described previously (24). The IMGT nomenclature is used in this report (27).
Statistical analysis
Single experimental variables were analyzed using the Mann-Whitney U-test or the Wilcoxon signed-rank test in GraphPad Prism 5 (GraphPad Software). Multivariate analyses of flow cytometric data were performed using the probability binning algorithm in FlowJo version 9.7.2 (TreeStar Inc.).
RESULTS
Ex vivo identification of β-cell-specific CD8 T cells
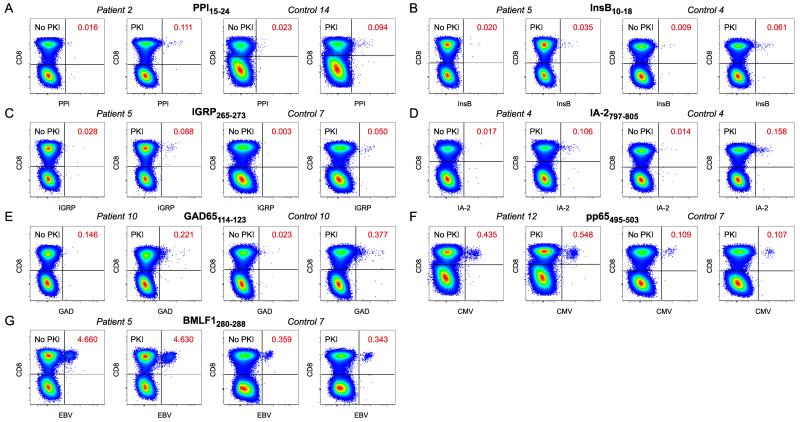
To ensure the optimal detection of autoreactive β-cell-specific CD8 T cell populations directly ex vivo, we conducted extensive pilot experiments with dasatinib, a reversible protein kinase inhibitor (PKI) that lowers the TCR affinity threshold required for tetramer binding at the cell surface (25). This approach enables the visualization of low avidity CD8 T cells that would otherwise remain undetectable and enhances the intensity of tetramer staining via active inhibition of TCR downregulation. In line with our previous findings, we observed greater frequencies of β-cell-specific CD8 T cells in all test subjects after sample pre-treatment with dasatinib (Figure 1 A-E) and clearer separation of tetramer-labeled events across all specificities (Figure 1 A-G).
Figure 1.
Identification of antigen-specific CD8 T cell populations. A-G: Thawed PBMCs were stained with pHLA-A*0201 tetramers representing PPI15-24 (A), InsB10-18 (B), IGRP265-273 (C), IA-2797-805 (D), GAD65114-123 (E), CMV pp65495-503 (F) and EBV BMLF1280-288 (G). Gates were set serially on singlets, live CD3+CD14−CD19− cells and lymphocytes prior to Boolean exclusion of dye aggregates and subsequent analysis in bivariate CD8 versus tetramer plots (Supplementary Figure 1 A). Representative paired data in the absence or presence of dasatinib are shown for type 1 diabetes patients and healthy controls. Tetramer-binding CD8 T cell frequencies are indicated.
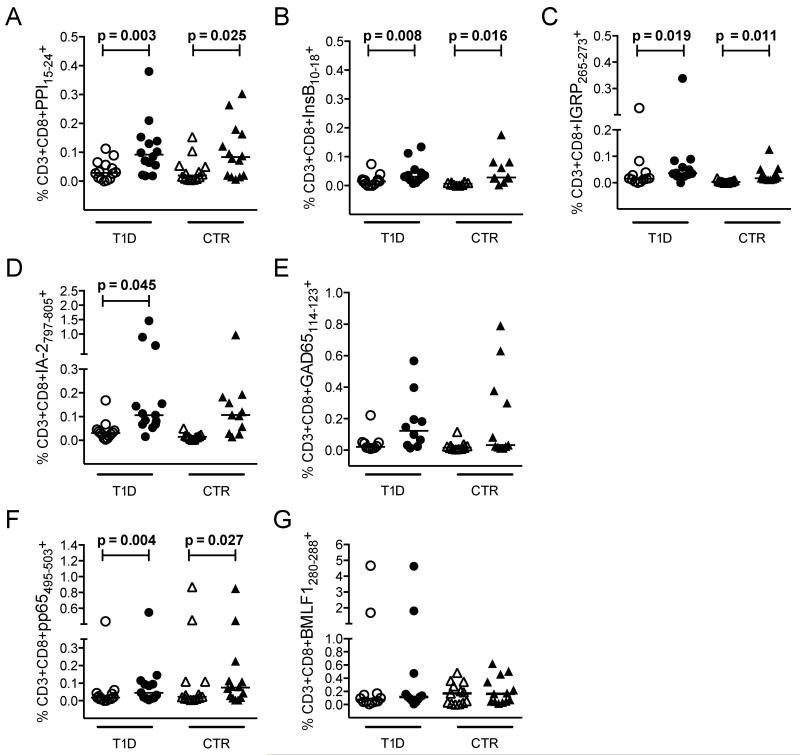
Next, we used this approach to compare β-cell-specific CD8 T cell frequencies in newly diagnosed type 1 diabetes patients and healthy non-diabetic controls across five distinct HLA-A*0201-restricted specificities (PPI15-24, InsB10-18, IGRP265-273, IA-2797-805 and GAD114-123). Two pHLA-A*0201 tetramers representing immunodominant epitopes from common persistent herpesviruses (CMV pp65495-503 and EBV BMLF1280-288) were also included for control purposes (Table 1). Again, increased β-cell-specific CD8 T cell frequencies were observed for the majority of subjects in the presence of dasatinib, reaching statistical significance for most specificities in each subject group (Figure 2). Although less striking, we noted a similar frequency enhancement for CMV pp65495-503 tetramer-binding CD8 T cells. However, this most likely reflects improved visualization of non-amplified precursors close to the technical limit of detection rather than a biologically relevant phenomenon within the antigen-experienced pool. Consistent with this interpretation, no such differences were observed for EBV BMLF1280-288 tetramer-binding CD8 T cells, which are driven to expand in the majority of HLA-A*0201+ donors as a consequence of high viral prevalence. More importantly, there were no significant differences between type 1 diabetes patients and healthy controls with respect to CD8 T cell frequencies across any of the seven incorporated specificities, either in the presence or absence of dasatinib. Accordingly, we hypothesized that disease-related differences in autoreactive CD8 T cell populations may reflect an antigen-driven inflammatory process that does not manifest in simple numerical terms, at least within the peripheral circulation.
Figure 2.
Summary of antigen-specific CD8 T cell frequencies. A-G: Thawed PBMCs were stained with pHLA-A*0201 tetramers representing PPI15-24 (A), InsB10-18 (B), IGRP265-273 (C), IA-2797-805 (D), GAD65114-123 (E), CMV pp65495-503 (F) and EBV BMLF1280-288 (G). Graphs show tetramer-binding CD8 T cell frequencies in the absence (empty symbols) or presence (filled symbols) of dasatinib for type 1 diabetes patients (T1D) and healthy controls (CTR). No significant differences across identical comparisons were observed between subject groups. Bars represent median values. Statistical analyses were performed using the Wilcoxon signed-rank test; p values ≤ 0.05 are shown.
β-cell-specific CD8 T cells are more differentiated in type 1 diabetes patients
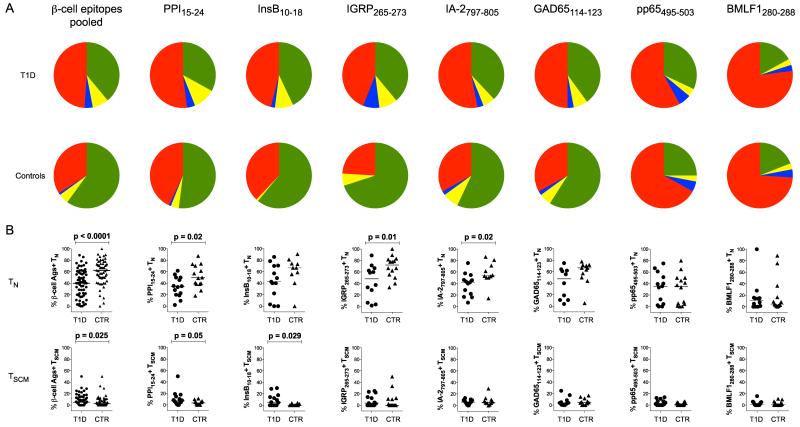
To examine the phenotypic characteristics of β-cell-specific CD8 T cells, we constructed a polychromatic flow cytometry panel designed to exclude irrelevant events (ViViD, CD14 and CD19), assign lineage (CD3, CD4 and CD8) and define differentiation status (CD27, CD45RO, CD57, CD95 and CCR7). Across all pooled β-cell specificities, we found that the percentage of autoreactive CD8 T cells with a naïve phenotype (TN - CD27+CD45RO−CD57−CD95−CCR7+) was significantly lower in type 1 diabetes patients compared to healthy controls (p<0.0001; Figure 3). This pattern held within individual specificities, reaching significance for PPI15-24 (p=0.02), IGRP265-273 (p=0.01) and IA-2797-805 (p=0.02). Importantly, no such differences were detected between groups for either of the virus-derived specificities. Moreover, total naïve CD8 T cell frequencies were similar in type 1 diabetes patients and healthy controls (Supplementary Figure 3). Of note, a large proportion of CD8 T cells specific for the CMV pp65495-503 epitope displayed a classical naïve phenotype. In contrast, very few CD8 T cells specific for the EBV BMLF1280-288 epitope were naïve. These observations substantiate the interpretation above that dasatinib-mediated frequency amplification within the CMV specificity reflects enhanced precursor detection in seronegative individuals.
Figure 3.
Phenotypic subset analysis of antigen-specific CD8 T cells. A: Pie chart representations of mean subset percentages for pooled β-cell-specific CD8 T cells and individual specificities in type 1 diabetes patients (top row) and healthy controls (bottom row). Subsets are defined and color-coded as follows: green, TN (CD27+CD45RO−CD57−CD95−CCR7+); yellow, TSCM (CD27+CD45RO−CD95+CCR7+); blue, TEFF (CD27−CD45RO−CD95+CCR7−); red, all remaining memory cells. B: Column plots showing TN and TSCM subset percentages for pooled β-cell-specific CD8 T cells and individual specificities in type 1 diabetes patients (T1D) and healthy controls (CTR). Representative data are shown in Supplementary Figure 1 B&C. Almost identical results were obtained with the TN subset defined in the absence of CD57 (CD27+CD45RO−CD95−CCR7+). Bars represent median values. Statistical analyses were performed using the Mann-Whitney U-test; p values ≤ 0.05 are shown.
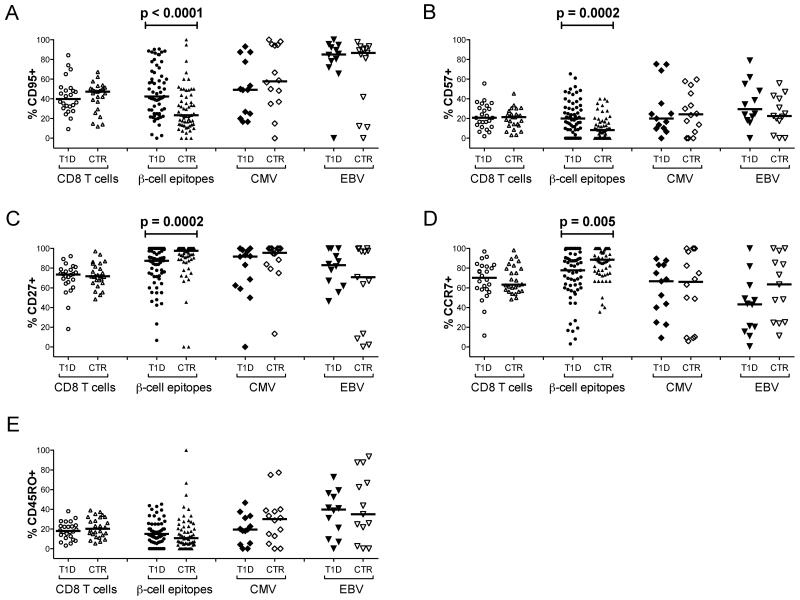
Collectively, these data suggest that recent onset type 1 diabetes is characterized by antigen-driven expansion of β-cell-specific CD8 T cells into more differentiated compartments, likely facilitated by tissue-specific inflammatory processes. Consistent with this notion, greater proportions of CD8 T cells with a stem cell memory phenotype (TSCM - CD27+CD45RO−CD95+CCR7+) (28) were present in type 1 diabetes patients compared to healthy controls across all pooled β-cell specificities (p=0.025), as well as individually within the autoreactive populations specific for PPI15-24 (p=0.05) and InsB10-18 (p=0.029). Moreover, single marker analyses revealed that β-cell-specific CD8 T cells expressed higher frequencies of CD57 (p=0.0002) and CD95 (p<0.0001) in type 1 diabetes patients compared to healthy controls (Figure 4). These surface proteins demarcate terminal differentiation and memory status, respectively (16; 29). Conversely, β-cell-specific CD8 T cells less frequently expressed CD27 and CCR7 in type 1 diabetes patients compared to healthy controls (p=0.0002 and p=0.005, respectively). These markers classically delineate naïve and early memory T cells (30; 31). No single marker differences between subject groups were observed for either of the virus-derived specificities or CD8 T cells as a whole.
Figure 4.
Single marker analysis of antigen-specific CD8 T cells. A-E: Percent expression of CD95 (A), CD57 (B), CD27 (C), CCR7 (D) and CD45RO (E) is shown for type 1 diabetes patients (T1D) and healthy controls (CTR) across all CD8 T cells and the indicated specificities. Corresponding data for individual β-cell-derived epitope-specific CD8 T cell populations are shown in Supplementary Figure 4. Bars represent median values. Statistical analyses were performed using the Mann-Whitney U-test; p values ≤ 0.05 are shown.
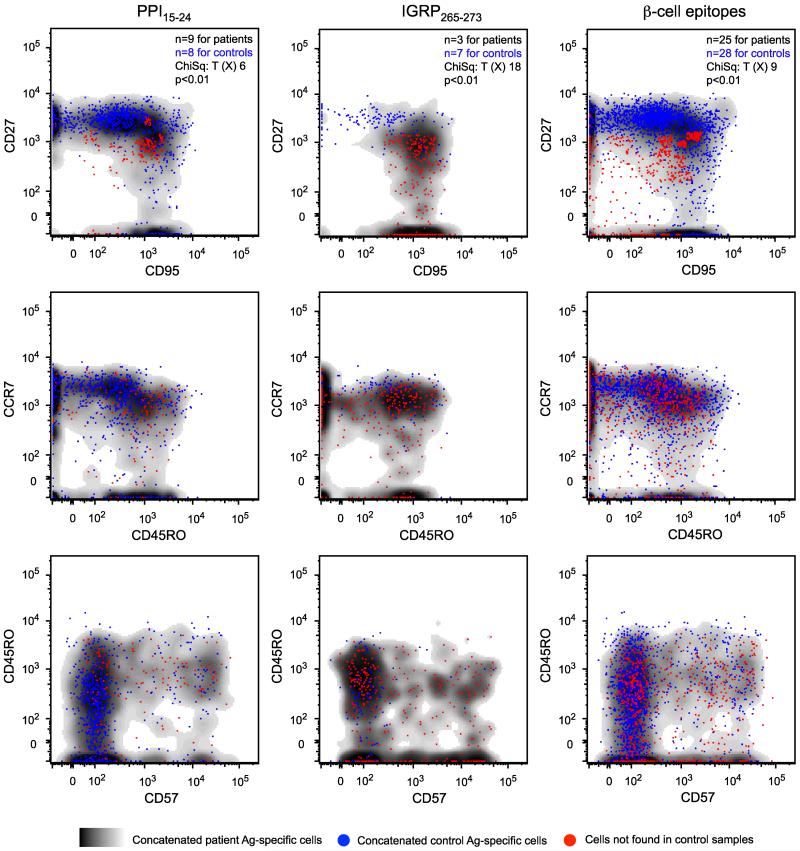
To extend these findings, we used frequency difference gating and probability binning to conduct multivariate analyses across concatenated datasets (32; 33). A phenotypically distinct CD27intermediateCD95+ population of CD8 T cells was identified in type 1 diabetes patients at significantly higher frequencies compared to healthy controls for the PPI15-24 (p<0.01), IGRP265-273 (p<0.01) and pooled β-cell (p<0.01) specificities (Figure 5). The majority of these cells within the PPI15-24 specificity coexpressed CD45RO and CCR7, whereas greater variability was apparent for the IGRP265-273 and pooled β-cell specificities. Expression of CD57 was heterogeneous in all cases. These data confirm the preceding observations and define a β-cell-specific early memory phenotype associated with type 1 diabetes.
Figure 5.
Frequency difference gating analysis of antigen-specific CD8 T cells. Overlays of concatenated data from type 1 diabetes patients (cloud plots) and healthy controls (blue dots) are shown for the indicated β-cell specificities across bivariate phenotypic profiles. Populations of CD27intermediateCD95+ CD8 T cells, present at significantly higher frequencies in type 1 diabetes patients compared to healthy controls, are displayed as red dots.
Ex vivo repertoire analysis of β-cell-specific CD8 T cells
To complement our flow cytometric studies, we examined the TCR repertoire of β-cell-specific CD8 T cell populations using a template-switch anchored RT-PCR to amplify all expressed TRB gene rearrangements in an unbiased and quantitative manner (24; 26). The PPI15-24 specificity was considered representative for this purpose on the basis of consistent phenotypic differences between subject groups across all analyses. Sufficient numbers of cognate tetramer-binding CD8 T cells were isolated by ultrapure flow cytometric sorting from three type 1 diabetes patients and two healthy controls to enable this analysis. In all cases, the ex vivo repertoires were oligoclonal and highly skewed towards one or more dominant clonotypes (Table 2). No consistent amino acid patterning or length bias was apparent across the third complementarity-determining region (CDR3) and TRB gene usage was heterogeneous. Thus, the repertoire of CD8 T cells specific for PPI15-24 is unpredictable and private (34).
Table 2. Clonotypic analysis of CD8 T cells specific for the PPI15-24 epitope.
| Subject | TRBV | CDR3 | TRBJ | Frequency (%) |
|---|---|---|---|---|
| Control 2 | 25-1 | CASSDLQAGQPQH | 1-5 | 81.48 |
| 20-1 | CSASVAGEQF | 2-1 | 16.67 | |
| 25-1 | CASSDGQAGQPQH | 1-5 | 1.85 | |
| Control 28 | 10-1 | CASSEFRRWNYGYT | 1-2 | 71.74 |
| 30 | CAWSVNFYINEQF | 2-1 | 28.26 | |
| Patient 25 | 20-1 | CSARDLLWTSGEETQY | 2-5 | 75.29 |
| 2 | CASRPGTGGINEQF | 2-1 | 7.06 | |
| 9 | CASSDYGRGANVLT | 2-6 | 4.71 | |
| 29-1 | CSVQGTGAYEQY | 2-7 | 3.53 | |
| 4-3 | CASSHDADGYT | 1-2 | 3.53 | |
| 14 | CASSPTDRGRGNTEAF | 1-1 | 2.35 | |
| 9 | CASSDFQGAGNTIY | 1-3 | 2.35 | |
| 10-3 | CAISWDRRTYEQY | 2-7 | 1.18 | |
| Patient 26 | 9 | CASSGGWREQF | 2-1 | 71.60 |
| 10-3 | CAISDGDNSHGYT | 1-2 | 16.05 | |
| 27 | CASSLTGTSSYEQY | 2-7 | 8.64 | |
| 7-7 | CASSTYRGRVSLDEQF | 2-1 | 2.47 | |
| 7-7 | CASSTYRGRVSLEEQF | 2-1 | 1.23 | |
| Patient 4 | 27 | CASSPTPSTYNEQF | 2-1 | 20.83 |
| 5-1 | CASSFRTGESYEQY | 2-7 | 16.67 | |
| 5-6 | CASSLGVFGTPSYEQY | 2-7 | 10.42 | |
| 12-3/12-4 | CASSPYGGRNGELF | 2-2 | 10.42 | |
| 30 | CAWALFGSAYEQY | 2-7 | 6.25 | |
| 29-1 | CSVDAPWSSSTDTQY | 2-3 | 4.17 | |
| 7-2 | CASSFYVTGNTEAF | 1-1 | 4.17 | |
| 30 | CAWSVGAGNGYT | 1-2 | 4.17 | |
| 18 | CASSIEVGYEQY | 2-7 | 4.17 | |
| 7-2 | CASSGTGGSYEQY | 2-7 | 4.17 | |
| 5-1 | CASSLAGQGANYGYT | 1-2 | 2.08 | |
| 12-3/12-4 | CASSSRDRVTDTQY | 2-3 | 2.08 | |
| 18 | CASSPSGVRQPQH | 1-5 | 2.08 | |
| 7-2 | CASSLAIGNTEAF | 1-1 | 2.08 | |
| 5-1 | CASSWDRVYNEQF | 2-1 | 2.08 | |
| 2 | CAIPGTALNEQF | 2-1 | 2.08 | |
| 12-3/12-4 | CASSTTDTQY | 2-3 | 2.08 |
Individual clonotypes are represented in order of relative frequency. Gene usage and CDR3 amino acid sequence are shown in each case.
DISCUSSION
In this study, we combined high definition polychromatic flow cytometry with ultrasensitive pHLAI tetramer staining to define the magnitude and differentiation status of β-cell-specific CD8 T cell populations in type 1 diabetes patients and healthy controls. Moreover, we achieved sufficient resolution with this approach to enable direct ex vivo analysis of the autoreactive TCR repertoire in a subset of individuals. In contrast to healthy controls, patients with newly diagnosed type 1 diabetes displayed hallmarks of antigen-driven expansion within the β-cell-specific CD8 T cell compartment. No such differences were observed between subject groups for persistent viral specificities or CD8 T cells globally. Collectively, these data identify phenotypic biomarkers of disease-relevant CD8 T cell-mediated autoimmunity in type 1 diabetes.
Remarkably, we found that β-cell-specific CD8 T cell frequencies in peripheral blood were similar between type 1 diabetes patients and healthy controls. These findings are consistent with some previous studies of presumed autoimmune conditions (35), but discrepant with other reports in the field (6; 17), most likely due to differences in detection sensitivity and cohort composition. Of particular note, the use of dasatinib to enhance tetramer staining thresholds revealed autoreactive CD8 T cells in the present study that were not visible with standard protocols. The reliable identification of naïve precursors in addition to antigen-experienced subsets readily explains the equivalent frequencies of β-cell-specific CD8 T cells between subject groups. It is also important to recognize that peripheral CD8 T cell frequencies do not necessarily reflect the tissue-localized immune cell environment. Indeed, histopathological evaluations of β-cell-specific CD8 T cell populations in the insulitic lesions of patients who died close to the time of diagnosis have demonstrated pronounced variability in antigen targeting between islets and individuals (3; 36). Accordingly, it is difficult to equate quantitative measures of immune autoreactivity in the periphery with CD8 T cell-mediated events in the pancreas.
In contrast to the lack of simple numerical correlates, we found clear phenotypic signatures of functionally relevant β-cell-derived antigen exposure in type 1 diabetes patients. Conventional subset analyses revealed fewer TN cells and greater numbers of TSCM cells across all pooled β-cell specificities within the CD8 compartment of patients with newly diagnosed type 1 diabetes compared to healthy controls. Single marker evaluations confirmed this overall pattern, with reduced expression of CD27 and CCR7 and elevated expression of CD57 and CD95. The increased prevalence of antigen-specific TSCM cells, which serve as a reservoir to replenish more differentiated memory subsets (16; 37), could perpetuate the underlying autoimmune process of β-cell destruction. It is also notable that granzymes and perforin are strongly coexpressed with CD57, which acts accordingly as a surrogate marker of cytolytic activity (38; 39). Such highly differentiated cells may therefore associate with more severe disease manifestations (40-42). Multivariate analyses further identified a phenotypically distinct CD27intermediateCD95+ population of β-cell-specific CD8 T cells in type 1 diabetes patients that was significantly less frequent in healthy controls, again consistent with an antigen-driven disease process as reported previously in a viral system (43). Importantly, all three analytical approaches yielded significant differences between subject groups solely within the β-cell-specific CD8 T cell compartment. Thus, the phenotypic profile of tissue-directed autoreactive CD8 T cell populations may act as a useful biomarker of disease activity in type 1 diabetes.
In further experiments, we characterized the TCR repertoire of tetramer-binding CD8 T cells specific for the PPI15-24 epitope. This specificity was selected on the grounds that robust phenotypic differences were detected between type 1 diabetes patients and healthy controls across all analytical strategies. Moreover, quantitative differences in the cognate CD8 T cell population have previously been reported to associate with disease activity (17). The enhanced detection sensitivity afforded by our approach facilitated this analysis, which represents the first ex vivo characterization of autoreactive CD8 T cell clonotypes. In all cases, we observed highly skewed oligoclonal repertoires reminiscent of virus-driven expansions (24). These hierarchical structures could reflect focused autoantigen-specific priming or cross-reactivity with an environmental trigger in the form of a highly immunogenic pathogen-derived epitope (44). However, it is important to note that this clonotypic pattern was not unique to the type 1 diabetes setting. Similar findings in two healthy controls, associated with a small central memory expansion in an otherwise largely naïve landscape in one (Control 2) and a highly differentiated predominant memory phenotype in the other (Control 28), suggest the natural occurrence of heterologous responses. Thus, oligoclonal β-cell-specific CD8 T cell populations can exist in the memory pool of healthy individuals without concomitant evidence of disease activity. It is intriguing to speculate that the highly private nature of these repertoires could provide a molecular explanation for differential TCR-mediated outcomes in the immunopathogenesis of type 1 diabetes, akin to recent descriptions in viral systems (45-47).
Overall, the present study demonstrates that β-cell-specific CD8 T cells are more differentiated in patients with newly diagnosed type 1 diabetes compared to healthy controls. This key result identifies an immune correlate of disease activity that could be further refined at the clonotypic level to monitor organ-specific tissue damage in the periphery.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre Award to Guy’s & St Thomas’ National Health Service Foundation Trust in partnership with King’s College London and by the Juvenile Diabetes Research Foundation (JDRF) Autoimmunity Centers Consortium (1-2007-1803 to MP). AS, GD, AKS and MP are supported by a JDRF R&D Grant (type 1 diabetes 217194; 17-2012-352). MP receives additional funding via the European Commission Seventh Framework Programme (NAIMIT, PEVNET and EE-ASI). JJM is a National Health and Medical Research Council (NHMRC) Fellow. AKS and DAP are Wellcome Trust Senior Investigators. We thank Amanda Bishop and Nicola McClintock (University of Bristol) for assistance with patient recruitment, and Dirk Homann (National Jewish Health and University of Colorado Denver) for helpful discussions.
Footnotes
Parts of this study were presented in a talk at the 13th International Congress of the Immunology of Diabetes Society, Melbourne, Australia (December 2013).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2:a007781. doi: 10.1101/cshperspect.a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roep BO, Peakman M. Diabetogenic T lymphocytes in human Type 1 diabetes. Curr Opin Immunol. 2011;23:746–753. doi: 10.1016/j.coi.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Allen JS, Tree TI, Zhao M, Dayan CM, Sewell AK, Unger WW, Drijfhout JW, Ossendorp F, Roep BO, Peakman M. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronenberg D, Knight RR, Estorninho M, Ellis RJ, Kester MG, de Ru A, Eichmann M, Huang GC, Powrie J, Dayan CM, Skowera A, van Veelen PA, Peakman M. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill beta-cells. Diabetes. 2012;61:1752–1759. doi: 10.2337/db11-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scotto M, Afonso G, Larger E, Raverdy C, Lemonnier FA, Carel JC, Dubois-Laforgue D, Baz B, Levy D, Gautier JF, Launay O, Bruno G, Boitard C, Sechi LA, Hutton JC, Davidson HW, Mallone R. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia. 2012;55:2026–2031. doi: 10.1007/s00125-012-2543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Li H, Chen B, Lu D, Deng W, Jiang Y, Zhou Z, Yang Z. Identification of novel HLA-A 0201-restricted cytotoxic T lymphocyte epitopes from Zinc Transporter 8. Vaccine. 2013;31:1610–1615. doi: 10.1016/j.vaccine.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 11.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattopadhyay PK, Melenhorst JJ, Ladell K, Gostick E, Scheinberg P, Barrett AJ, Wooldridge L, Roederer M, Sewell AK, Price DA. Techniques to improve the direct ex vivo detection of low frequency antigen-specific CD8(+) T cells with peptide-major histocompatibility complex class I tetramers. Cytometry A. 2008 doi: 10.1002/cyto.a.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallone R, Scotto M, Janicki CN, James EA, Fitzgerald-Miller L, Wagner R, Gottlieb P, Thorpe J, Jospe N, Durinovic-Bello I, Boitard C, Lou O, Dayan CM, Wong FS. Immunology of Diabetes Society T-Cell Workshop: HLA class I tetramer-directed epitope validation initiative. Diabetes Metab Res Rev. 2007;27:720–726. doi: 10.1002/dmrr.1243. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, Perfetto SP, Goepfert P, Koup RA, De Rosa SC, Bruchez MP, Roederer M. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12:972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay PK, Gaylord B, Palmer A, Jiang N, Raven MA, Lewis G, Reuter MA, Nur-Ur Rahman AK, Price DA, Betts MR, Roederer M. Brilliant violet fluorophores: A new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytometry A. 2012;81:456–466. doi: 10.1002/cyto.a.22043. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velthuis JH, Unger WW, Abreu JR, Duinkerken G, Franken K, Peakman M, Bakker AH, Reker-Hadrup S, Keymeulen B, Drijfhout JW, Schumacher TN, Roep BO. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes. 2010;59:1721–1730. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridgeman JS, Sewell AK, Miles JJ, Price DA, Cole DK. Structural and biophysical determinants of alphabeta T-cell antigen recognition. Immunology. 2012;135:9–18. doi: 10.1111/j.1365-2567.2011.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulek AM, Cole DK, Skowera A, Dolton G, Gras S, Madura F, Fuller A, Miles JJ, Gostick E, Price DA, Drijfhout JW, Knight RR, Huang GC, Lissin N, Molloy PE, Wooldridge L, Jakobsen BK, Rossjohn J, Peakman M, Rizkallah PJ, Sewell AK. Structural basis for the killing of human beta cells by CD8(+) T cells in type 1 diabetes. Nat Immunol. 2012;13:283–289. doi: 10.1038/ni.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppieters KT, Harrison LC, von Herrath MG. Trials in type 1 diabetes: Antigen-specific therapies. Clin Immunol. 2013;149:345–355. doi: 10.1016/j.clim.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS, Pickersgill L, de Koning E, Ziegler AG, Boehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castano L, Wagner A, Lervang HH, Perrild H, Mandrup-Poulsen T, Pociot F, Dinarello CA. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzzetti R. Diabetes: Immunotherapy for T1DM--still not there yet. Nat Rev Endocrinol. 2013;9:697–698. doi: 10.1038/nrendo.2013.221. [DOI] [PubMed] [Google Scholar]
- 24.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lissina A, Ladell K, Skowera A, Clement M, Edwards E, Seggewiss R, van den Berg HA, Gostick E, Gallagher K, Jones E, Godkin AJ, Peakman M, Price DA, Sewell AK, Wooldridge L. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 2009;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley MF, Almeida JR, Price DA, Douek DC. Unbiased molecular analysis of T cell receptor expression using template-switch anchored RT-PCR. Curr Protoc Immunol. 2011:33. doi: 10.1002/0471142735.im1033s94. Chapter 10:Unit10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, Thouvenin-Contet V, Lefranc G. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 28.Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, Roederer M. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 30.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Roederer M, Hardy RR. Frequency difference gating: a multivariate method for identifying subsets that differ between samples. Cytometry. 2001;45:56–64. doi: 10.1002/1097-0320(20010901)45:1<56::aid-cyto1144>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001;45:47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Miles JJ, Douek DC, Price DA. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol Cell Biol. 2011;89:375–387. doi: 10.1038/icb.2010.139. [DOI] [PubMed] [Google Scholar]
- 35.Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 36.Coppieters KT, von Herrath MG. Viruses and cytotoxic T lymphocytes in type 1 diabetes. Clin Rev Allergy Immunol. 2011;41:169–178. doi: 10.1007/s12016-010-8220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 39.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirmer M, Goldberger C, Wurzner R, Duftner C, Pfeiffer KP, Clausen J, Neumayr G, Falkenbach A. Circulating cytotoxic CD8(+) CD28(−) T cells in ankylosing spondylitis. Arthritis Res. 2002;4:71–76. doi: 10.1186/ar386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Z, Zhong W, Lu X, Shi B, Zhu Y, Chen L, Zhang G, Zhang X. Association of Graves’ disease and prevalence of circulating IFN-gamma-producing CD28(−) T cells. J Clin Immunol. 2008;28:464–472. doi: 10.1007/s10875-008-9213-4. [DOI] [PubMed] [Google Scholar]
- 42.Pedroza-Seres M, Linares M, Voorduin S, Enrique RR, Lascurain R, Garfias Y, Jimenez-Martinez MC. Pars planitis is associated with an increased frequency of effector-memory CD57+ T cells. Br J Ophthalmol. 2007;91:1393–1398. doi: 10.1136/bjo.2007.116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chattopadhyay PK, Chelimo K, Embury PB, Mulama DH, Sumba PO, Gostick E, Ladell K, Brodie TM, Vulule J, Roederer M, Moormann AM, Price DA. Holoendemic malaria exposure is associated with altered Epstein-Barr virus-specific CD8(+) T-cell differentiation. J Virol. 2013;87:1779–1788. doi: 10.1128/JVI.02158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, Dolton G, Clement M, Llewellyn-Lacey S, Price DA, Peakman M, Sewell AK. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2011;287:1168–1177. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price DA, Asher TE, Wilson NA, Nason MC, Brenchley JM, Metzler IS, Venturi V, Gostick E, Chattopadhyay PK, Roederer M, Davenport MP, Watkins DI, Douek DC. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J Exp Med. 2009;206:923–936. doi: 10.1084/jem.20081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM, Walker BD. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol. 2012;13:691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladell K, Hashimoto M, Iglesias MC, Wilmann PG, McLaren JE, Gras S, Chikata T, Kuse N, Fastenackels S, Gostick E, Bridgeman JS, Venturi V, Arkoub ZA, Agut H, van Bockel DJ, Almeida JR, Douek DC, Meyer L, Venet A, Takiguchi M, Rossjohn J, Price DA, Appay V. A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity. 2013;38:425–436. doi: 10.1016/j.immuni.2012.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.