Abstract
Background
Simultaneous resection of both the liver and the pancreas carries significant complexity. The objective of this study was to investigate peri-operative outcomes after a synchronous hepatectomy and pancreatectomy (SHP).
Methods
The American College of Surgeons National Surgical Quality Improvement Project database was queried to identify patients who underwent SHP. Resections were categorized as ‘< hemihepatectomy’, ‘≥ hemihepatectomy’ (hemihepatectomy and trisectionectomy), ‘PD’ (pancreaticoduodenectomy and total pancreatectomy) and ‘distal’ (distal pancreatectomy and enucleation).
Results
From 2005 to 2013, 480 patients underwent SHP. Patients were stratified based on the extent of resection: ‘< hemihepatectomy + distal (n = 224)’, ‘≥ hemihepatectomy + distal’ (n = 49), ‘< hemihepatectomy + PD’ (n = 83) and ‘≥ hemihepatectomy + PD’ (n = 24). Although the first three groups had a reasonable and comparable safety profile (morbidity 33–51% and mortality 0–6.6%), the ‘≥ hemihepatectomy + PD’ group was associated with an 87.5% morbidity (organ space infection 58.3%, re-intubation 12.5%, reoperation 25% and septic shock 25%), 8.3% 30-day mortality and 18.2% in-hospital mortality.
Conclusions
A synchronous hemihepatectomy (or trisectionectomy) with PD remains a highly morbid combination and should be reserved for patients who have undergone extremely cautious selection.
Introduction
Hepatic and pancreatic resections are complex operations requiring considerable expertise. Early attempts at liver resection were fraught with mortality rates up to 20% owing to parenchymal haemorrhage and liver failure.1 However, advancements in peri-operative anaesthetic management,2 surgical technique (including portal vein embolization),3 and post-operative care have resulted in substantial improvements in morbidity and mortality, with less than 5% mortality associated with a major hepatectomy in several recent studies.4–9 Similarly, for pancreaticoduodenectomy (PD) while morbidity remains considerably high (41–52%),10–12 mortality has improved from 25% to 1.7% in the last few decades.11–16
What remains controversial is the safety of synchronous hepatectomy with pancreatectomy (SHP). Most studies evaluating outcomes of SHP are limited to single institution series. The first Japanese study on ‘hepato-pancreaticoduodenectomy’ reported a 79% morbidity and 24% mortality in 24 patients with advanced hepatobiliary cancers.17 However, more recent Japanese studies have shown a modest improvement in post-operative morbidity and mortality after SHP.18,19 Ebata et al.18 demonstrated a temporal reduction in mortality from 31% in the 1980s to 14% in the 2000s. While the short- and long-term outcomes of SHP have been extensively studied in Asia, there is a paucity of literature on outcomes of SHP in the West. In 2004, Memorial Sloan Kettering reported an overall post-operative morbidity of 47% and post-operative mortality of 18% in 17 patients who underwent SHP for advanced hepatobiliary cancers.20 More recently, Hemming et al. revealed an overall morbidity of 35% and no peri-operative deaths in 40 patients undergoing SHP.21 However, owing to the small size and heterogeneous mix of surgical procedures in these single-institution studies, the experience of SHP in the West remains limited. Thus, the objective of this study was to determine the short-term outcomes of SHP in North America using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP).
Patients and methods
The ACS-NSQIP Participant Use File was queried to identify all patients who underwent any hepatic or pancreatic resections from 1 January 2005 to 31 December 2013. The ACS-NSQIP is a nationally validated, outcomes-based and risk-adjusted programme used to measure and assess the quality of surgical care in participating institutions. Initially introduced in 1991 to assess post-operative morbidity and mortality in the Veteran Affairs (VA) Health System, the success of the VA-NSQIP subsequently led to the expansion of NSQIP into the private sector. Currently, there are 569 sites enrolled in ACS-NSQIP, which represents 10% of 5686 hospitals in the United States. Trained and certified surgical clinical nurse reviewers prospectively collect and report pre-, intra- and post-operative outcomes on each patient into an electronic clinical registry.
Current procedural terminology (CPT) codes were used to determine cases in which patients underwent both hepatic and pancreatic resections. CPT code 47120 (partial lobectomy) was classified as ‘< hemihepatectomy’. CPT codes 47122 (trisegmentectomy), 47125 (total left lobectomy) and 47130 (total right lobectomy) were classified as ‘≥ hemihepatectomy’. CPT codes 48120 (enucleation), 48140 (distal pancreatectomy), 48145 (distal pancreatectomy with pancreatojejunostomy) and 48146 (near total distal pancreatectomy) were classified as ‘distal pancreatectomy’. CPT codes 48150, 48152, 48153, 48154 (different versions of pancreaticoduodenectomy) and 48155 (total pancreatectomy) were categorized as ‘PD’. Patients who underwent wedge biopsy of the liver (CPT 47100) were not included in the study.
Categorical variables were presented as observed counts and percentages and compared using Pearson's chi-square and Fisher's exact test, where applicable. Continuous variables were presented as the median and range compared using the Student's t-test for parametric variables and Wilcoxon's rank-sum test for non-parametric variables. Statistical analyses were performed using SPSS version 22.0 (IBM, Chicago, IL, USA). A two-sided P-value of < 0.05 was considered statistically significant.
Results
Of the 48 568 hepatic and pancreatic resections performed in participating ACS-NSQIP hospitals from 2005 to 2013, 480 (1%) resections were SHP. Patients were stratified into four groups based on the extent of resection: < hemihepatectomy with distal, ≥ hemihepatectomy with distal, < hemihepatectomy with PD and ≥ hemihepatectomy with PD. Patients who were selected to undergo ≥ hemihepatectomy with PD were younger than patients in the other three groups. Otherwise, there were no significant differences in pre-operative clinical characteristics among the four groups (Table 1).
Table 1.
Clinical characteristics
| <Hemi-hepatectomy + Distal (n = 224) | ≥Hemi-hepatectomy + Distal (n = 49) | <Hemi-hepatectomy + PD (n = 183) | ≥Hemi-hepatectomy + PD (n = 24) | P-value | |
|---|---|---|---|---|---|
| Age | 58 (21–84) | 58 (20–86) | 64 (25–88) | 53 (26–78) | 0.001 |
| Race | |||||
| White | 170 (75.9) | 35 (71.4) | 144 (78.7) | 17 (70.8) | 0.460 |
| Black | 26 (11.6) | 5 (10.2) | 12 (6.6) | 2 (8.3) | |
| Asian | 9 (4.0) | 3 (6.1) | 14 (7.7) | 2 (8.3) | |
| Other | 19 (8.5) | 6 (12.2) | 13 (7.1) | 3 (12.5) | |
| Female | 129 (57.6) | 24 (49) | 191 (55.2) | 9 (37.5) | 0.232 |
| BMI | 28 (16–68) | 26 (17–40) | 27 (17–50) | 26 (17–40) | 0.071 |
| Any Comorbidity (n = 380) | 45 (26.5) | 6 (16.2) | 37 (24.5) | 3 (15.8) | 0.528 |
| Smoker | 27 (12.1) | 7 (14.3) | 32 (17.5) | 1 (4.2) | 0.213 |
| Diabetes | 43 (19.2) | 3 (6.1) | 35 (19) | 3 (12.5) | 0.114 |
| COPD | 5 (2.2) | 2 (4.1) | 7 (3.8) | 0 (0) | 0.652 |
| HTN on medication | 104 (46.4) | 14 (28.6) | 91 (49.7) | 6 (25) | 0.011 |
| TIA (n = 273) | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0.656 |
| CVA (n-273) | 1 (0.8) | 0 (00 | 2 (1.9) | 0 (0) | 0.766 |
| Sepsis | 4 (1.8) | 3 (6.1) | 6 (3.3) | 0 (0) | 0.303 |
| Pre-operative chemotherapy (n = 272) | 5 (4.0) | 3 (10) | 8 (7.8) | 1 (6.3) | 0.423 |
| Pre-operative Radiotherapy (n = 272) | 4 (3.2) | 0 (0) | 3 (1.9) | 0 (0) | 0.695 |
| Creatinine | 0.8 (0.3–2.4) | 0.9 (0.4–1.6) | 0.8 (0.3–2.43) | 0.84 (0.6–1.3) | 0.472 |
| Albumin | 3.9 (1–5.1) | 3.9 (2–4.8) | 3.8 (1.6–5) | 3.8 (2.1–5.1) | 0.109 |
| Bilirubin | 0.6 (0.1–2.2) | 0.6 (0.1–8.9) | 0.6 (0.6–11.5) | 0.7 (0.1–8.9) | <0.001 |
| Platelet | 228 (40–588) | 225 (106–428) | 250 (23–620) | 278 (166–590) | 0.011 |
| INR | 1 (0.8–1.9) | 1 (0.8–1.7) | 1 (0.74–9.1) | 1 (0.91–1.2) | 0.878 |
| ASA Class > 3 | 158 (70.5) | 37 (75.5) | 126 (68.9) | 20 (83.3) | 0.442 |
ASA, American Society of Anesthesiologist; TIA, transient ischaemic attack; HTN, hypertension; COPD, chronic obstructive pulmonary disease; BMI, body mass index; CVA, cerebral vascular attack; INR, International normalized ratio.
Pre-operative indications for SHP based on the International Statistical Classification of Diseases (ICD-9) codes are shown in Table 2. Of the 480 patients in the entire cohort, 31% of SHP cases were performed for pancreatic malignancy (not otherwise specified), 21% specifically for neuroendocrine tumours, 13% for secondary liver cancers and 8% for cholangiocarcinoma or gallbladder cancer.
Table 2.
Pre-operative diagnoses stratified by extent of hepato-pancreatectomy
| <Hemi-hepatectomy + Distal (n = 224) | ≥Hemi-hepatectomy + Distal (n = 49) | <Hemi-hepatectomy + PD (n = 183) | ≥Hemi-hepatectomy + PD (n = 24) | Total (%) | |
|---|---|---|---|---|---|
| Pancreatic malignancy | 52 (23.2) | 11 (22.4) | 83 (45.4) | 4 (16.7) | 150 (31) |
| Neuroendocrine tumour | 54 (24.2) | 15 (30.6) | 19 (10.4) | 5 (20.8) | 93 (19.3) |
| Secondary liver malignancy | 40 (17.9) | 11 (22.4) | 13 (7.1) | 0 (0) | 64 (13.3) |
| Benign pancreatic disease | 19 (8.5) | 0 (0) | 5 (2.7) | 0 (0) | 24 (5) |
| Bile duct malignancy | 2 (0.9) | 2 (4.1) | 7 (3.8) | 10 (41.7) | 21 (4.4) |
| Gastric malignancy | 17 (7.6) | 1 (2.0) | 1 (0.5) | 0 (0) | 19 (4) |
| Gallbladder malignancy | 0 (0) | 1 (2.0) | 14 (7.7) | 1 (4.2) | 16 (3.3) |
| Colorectal malignancy | 6 (2.7) | 0 (0) | 7 (3.8) | 1 (4.2) | 14 (2.9) |
| Ampullary malignancy | 0 (0) | 0 (0) | 11 (6.0) | 0 (0) | 11 (2.3) |
| Duodenal malignancy | 2 (0.9) | 0 (0) | 6 (3.3) | 2 (8.3) | 10 (2.1) |
| Primary liver malignancy | 4 (1.8) | 2 (4.1) | 3 (1.6) | 1 (4.2) | 10 (2.1) |
| Retroperitoneal tumour | 8 (3.6) | 0 (0) | 4 (2.2) | 0 (0) | 12 (1.5) |
| Benign biliary disease | 4 (1.8) | 1 (2.0) | 1 (0.5) | 0 (0) | 6 (1.3) |
| Adrenal malignancy | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) |
| Ovarian malignancy | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) |
| Other | 14 (6.3) | 5 (10.2) | 9 (4.9) | 0 (0) | 28 (5.8) |
| Total | 224 | 49 | 183 | 24 | 480 |
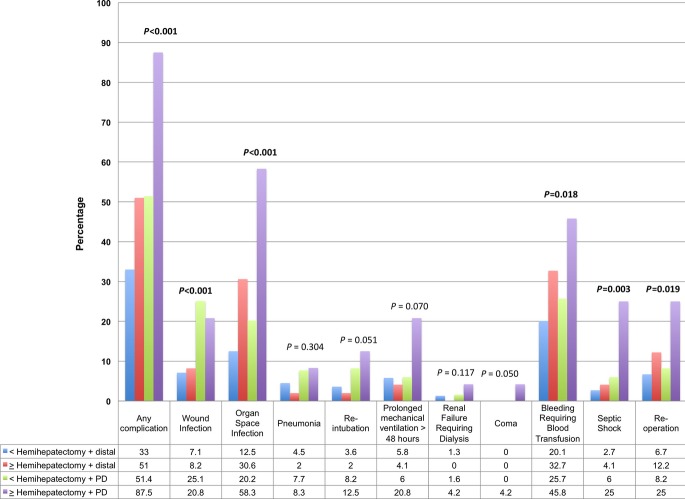
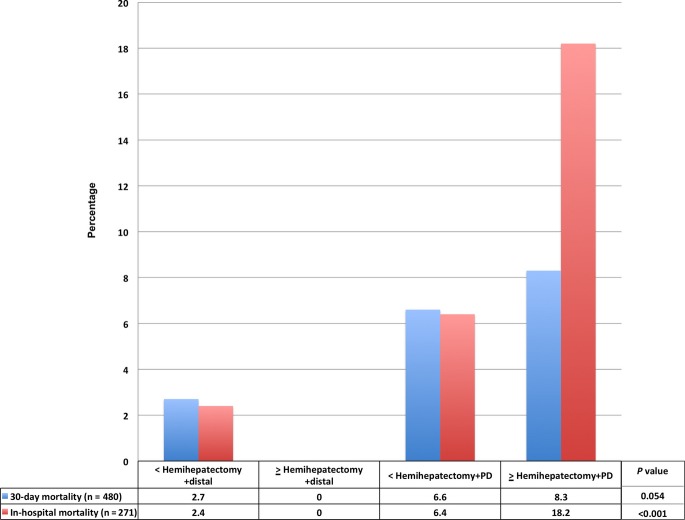
Figure 1 demonstrates the rates of post-operative complications based on the extent of resection. As extent of resection increased, post-operative morbidity was appreciably higher (33%, 51%, 51.4% and 87.5%; P < 0.001), the operative time was longer (270, 341, 385 and 461 min; P < 0.001), as was the hospital length of stay (7.5, 8, 9 and 14 days; P < 0.001). Rates of organ space infection (P < 0.001), post-operative bleeding requiring a blood transfusion (P = 0.018), systemic sepsis (P = 0.003), septic shock (P = 0.001) and re-operation (P = 0.019) were significantly higher as an extent of the resection increased. The in-hospital mortality rates increased significantly with extent of resection (2.4%, 0%, 6.4% and 18.2%; P < 0.001), whereas the corresponding increase in 30-day mortality only approached statistical significance (2.7%, 0%, 6.6% and 8.3%, P = 0.054) (Fig. 2).
Figure 1.
Morbidity of the entire cohort stratified by extent of resection
Figure 2.
Mortality of the entire cohort stratified by extent of resection
Discussion
Synchronous hepatectomy and PD is a procedure that is rarely performed, accounting for only 1% of all hepatic and pancreatic cases in the United States. Worldwide, there are fewer than 400 cases reported from the East17,19,22–34 and 100 cases from the West20,21,35–39 in the last 25 years. In the present study of 480 patients treated in North America, more extensive resections correlated with higher morbidity, longer hospital stay and longer operative time. More specifically, ≥ hemihepatectomy (i.e. lobectomy or trisectionectomy) with PD was associated with profoundly higher morbidity of 87% and in-hospital mortality of 18.2%. In contrast, we found that PD with ≤ hemihepatectomy was associated with a complication rate of 51.4% and in-hospital mortality rate of 6.4%.
A literature review of morbidity and mortality after being combined PD with ≥ hemihepatectomy reveals variable short-term results. Two studies from North America have described outcomes after hepato-pancreaticoduodenectomy. A small study of six patients revealed a morbidity of 83% and mortality of 50%.20 In contrast, another study of 40 patients who underwent SHP, in general, demonstrated an overall mortality of 0% and morbidity of 35% for the entire cohort; however, the exact number of patients who actually underwent ≥ hemihepatectomy with PD could not be discerned. The Asian experience for hepato-pancreaticoduodenectomy is more extensive and is summarized in Table 3. The morbidity rates ranged from 57% to 95% while the mortality rates ranged from 0% to 31%. This wide variation in mortality rates in Asian centres merits further attention. A careful review of these studies shows that the use of pre-operative portal vein embolization (PVE) prior to hepato-pancreaticoduodenectomy is related to lower mortality rates in the range of 0 to 5%.18,30,31,33,34 In contrast, in the two studies that did not employ PVE, the mortality rates were significantly higher (21% to 31%).19,32 Although cross-study comparisons are limited to securing definitive conclusions, it appears that pre-operative portal vein embolization can be an effective way to decrease post-operative liver failure and mortality after SHP.
Table 3.
Selected publications reporting outcomes of hepato-pancreaticoduodenectomy
| Series | Number of patients | Morbidity | Mortality | Fraction of patients who underwent preoperative PVE | 5 year OS |
|---|---|---|---|---|---|
| Miwa et al.33 | 26 | 30.8% | 0% | 77% | 25% (GBC) 51.9% (BDC) |
| Wakai et al.19 | 28 | 82% | 21% | 0 | 9% (GBC) 12%(BDC) |
| Kaneoka et al.31 | 14 | 57% | 0% | 43% | 50% |
| Ebata et al.30 | 78 | 77% | 2.4% | 79% | 54% (BDC) |
| Lim et al.32 | 23 | 91% | 31% | 0 | 10% (GBC) 32.3% (BDC) |
| Sakamoto et al.34 | 19 | 95% | 5% | 89% | 0% (GBC) 53% (BDC) |
PVE, portal vein embolization; GBC, gallbladder cancer; BDC, bile duct cancer; OS, overall survival.
Long-term survival outcomes after SHP for advanced hepatobiliary malignancies remain unclear. A review of Table 3 shows SHP for gallbladder cancer is associated with 5-year survival rates between 0% and 25%,19,32–34 whereas SHP for cholangiocarcinoma has been associated with 5-year survival rates ranging from 12% to 54%.19,30,32,34 In contrast, SHP for more indolent malignancies such as neuroendocrine tumors is associated with more favourable 5-year survival rates ranging from 64% to 69%.35,40 The superior long-term outcomes associated with aggressive resection of neuroendocrine tumours may justify performing SHP in this group. Given the lack of favourable long-term survival outcomes, selection of patients with gallbladder cancer and cholangiocarcinoma for hepato-pancreaticoduodenectomy remains extremely challenging.
The present study has several important limitations. First, ACS-NSQIP is a national clinical registry that obtains only 30-day post-operative outcomes. This limits our ability to detect mortality after 30 days, particularly as 90-day mortality is currently considered a more reliable metric of surgical outcomes.41 Second, ACS-NSQIP lacks detailed pathology data (exact diagnosis, margin, grade and lymph node status) as well as long-term oncologic data (recurrence and survival) that would have provided further insight into the selection of patients for this extensive procedure. Third, specific hepatectomy and pancreatectomy complications could not be deciphered using the ACS-NSQIP database. For example, although ‘organ space infection’ is available in the database, it remains unclear if this complication is representative of a pancreatic fistula, bile leak or another source of intra-abdominal abscess. Along the same lines, post-hepatectomy liver failure is not captured in the ACS-NSQIP database. Lastly, although PVE appears critical in decreasing mortality after hepato-pancreaticoduodenectomy, we could not assess the effect of PVE using ACS-NSQIP. Nevertheless, the use of this large and multi-institutional registry provides useful and generalizable insight on the outcomes of SHP in North American patients.
In conclusion, less extensive combined pancreatic and hepatic resections can be performed with reasonable morbidity and mortality, with the exception of combined PD with hemihepatectomy, which is associated with almost universal morbidity (87%) and considerable in-hospital mortality (18%). Patients should be selected for this extensive procedure with extreme caution when surgical candidacy is robust, and the long-term oncological benefit is anticipated to outweigh the peri-operative risks significantly. This appears to be the case more commonly for patients with a pancreatic neuroendocrine tumour with liver metastases and less so for patients with biliary tract adenocarcinoma. Furthermore, although not supported directly from our data, a review of the literature suggests that pre-operative PVE might decrease the incidence of mortality after hepato-pancreaticoduodenectomy. The role of PVE in reducing post-operative mortality in this setting warrants further investigation.
Funding sources
None.
Conflict of interest
None declared.
References
- 1.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1–342. [PubMed] [Google Scholar]
- 2.Redai I, Emond J, Brentjens T. Anesthetic considerations during liver surgery. Surg Clin North Am. 2004;84:401–411. doi: 10.1016/S0039-6109(03)00229-9. [DOI] [PubMed] [Google Scholar]
- 3.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–380. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. discussion 206. [DOI] [PubMed] [Google Scholar]
- 5.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 06-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725. doi: 10.1001/archsurg.139.7.718. discussion 25-7. [DOI] [PubMed] [Google Scholar]
- 11.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. doi: 10.1097/00000658-199305010-00002. discussion 35-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. discussion 57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102:763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monge JJ, Judd ES, Gage RP. Radical pancreatoduodenectomy: a 22-year experience with the complications, mortality rate, and survival rate. Ann Surg. 1964;160:711–722. doi: 10.1097/00000658-196410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilsdorf RB, Spanos P. Factors influencing morbidity and mortality in pancreaticoduodenectomy. Ann Surg. 1973;177:332–337. doi: 10.1097/00000658-197303000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. doi: 10.1016/j.jamcollsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Nimura Y, Hayakawa N, Kamiya J, Maeda S, Kondo S, Yasui A, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology. 1991;38:170–175. [PubMed] [Google Scholar]
- 18.Ebata T, Nagino M, Nishio H, Arai T, Nimura Y. Right hepatopancreatoduodenectomy: improvements over 23 years to attain acceptability. J Hepatobiliary Pancreat Surg. 2007;14:131–135. doi: 10.1007/s00534-006-1106-4. [DOI] [PubMed] [Google Scholar]
- 19.Wakai T, Shirai Y, Tsuchiya Y, Nomura T, Akazawa K, Hatakeyama K. Combined major hepatectomy and pancreaticoduodenectomy for locally advanced biliary carcinoma: long-term results. World J Surg. 2008;32:1067–1074. doi: 10.1007/s00268-007-9393-8. [DOI] [PubMed] [Google Scholar]
- 20.D'Angelica M, Martin RC, 2nd, Jarnagin WR, Fong Y, DeMatteo RP, Blumgart LH. Major hepatectomy with simultaneous pancreatectomy for advanced hepatobiliary cancer. J Am Coll Surg. 2004;198:570–576. doi: 10.1016/j.jamcollsurg.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Hemming AW, Magliocca JF, Fujita S, Kayler LK, Hochwald S, Zendejas I, et al. Combined resection of the liver and pancreas for malignancy. J Am Coll Surg. 2010;210:808–14. doi: 10.1016/j.jamcollsurg.2009.12.007. 14-6. [DOI] [PubMed] [Google Scholar]
- 22.Nanashima A, Nagasaki T, Sumida Y, Abo T, Tobinaga S, Takeshita H, et al. An experience of hepatopancreatoduodenectomy in patients with hepatobiliary malignancies. Hepatogastroenterology. 2008;55:1691–1694. [PubMed] [Google Scholar]
- 23.Chijiiwa K, Nishiyama K, Takashima M, Mizumoto K, Noshiro H, Shimizu S, et al. Diffuse bile duct carcinoma treated by major hepatectomy and pancreatoduodenectomy with the aid of pre-operative portal vein embolization. Report of two cases. Hepatogastroenterology. 1999;46:1634–1638. [PubMed] [Google Scholar]
- 24.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukada K, Yoshida K, Aono T, Koyama S, Shirai Y, Uchida K, et al. Major hepatectomy and pancreatoduodenectomy for advanced carcinoma of the biliary tract. Br J Surg. 1994;81:108–110. doi: 10.1002/bjs.1800810139. [DOI] [PubMed] [Google Scholar]
- 26.Todoroki T, Kawamoto T, Takahashi H, Takada Y, Koike N, Otsuka M, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999;86:622–627. doi: 10.1046/j.1365-2168.1999.01085.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Sakaguchi S, Suzuki S, Muro H. Aggressive surgery for carcinoma of the gallbladder. Surgery. 1989;106:467–473. [PubMed] [Google Scholar]
- 28.Ota T, Araida T, Yamamoto M, Takasaki K. Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. J Hepatobiliary Pancreat Surg. 2007;14:155–158. doi: 10.1007/s00534-006-1110-8. [DOI] [PubMed] [Google Scholar]
- 29.Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Combined pancreaticoduodenectomy and hepatectomy for patients with locally advanced gallbladder carcinoma: long term results. Cancer. 1997;80:1904–1909. [PubMed] [Google Scholar]
- 30.Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 2012;256:297–305. doi: 10.1097/SLA.0b013e31826029ca. [DOI] [PubMed] [Google Scholar]
- 31.Kaneoka Y, Yamaguchi A, Isogai M, Kumada T. Survival benefit of hepatopancreatoduodenectomy for cholangiocarcinoma in comparison to hepatectomy or pancreatoduodenectomy. World J Surg. 2010;34:2662–2670. doi: 10.1007/s00268-010-0702-2. [DOI] [PubMed] [Google Scholar]
- 32.Lim CS, Jang JY, Lee SE, Kang MJ, Kim SW. Reappraisal of hepatopancreatoduodenectomy as a treatment modality for bile duct and gallbladder cancer. J Gastrointest Surg. 2012;16:1012–1018. doi: 10.1007/s11605-012-1826-5. [DOI] [PubMed] [Google Scholar]
- 33.Miwa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, Kusama K, et al. Is major hepatectomy with pancreatoduodenectomy justified for advanced biliary malignancy? J Hepatobiliary Pancreat Surg. 2007;14:136–141. doi: 10.1007/s00534-006-1107-3. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto Y, Nara S, Kishi Y, Esaki M, Shimada K, Kokudo N, et al. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 2013;153:794–800. doi: 10.1016/j.surg.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Gaujoux S, Gonen M, Tang L, Klimstra D, Brennan MF, D'Angelica M, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. 2012;19:4270–4277. doi: 10.1245/s10434-012-2462-8. [DOI] [PubMed] [Google Scholar]
- 36.Birnbaum DJ, Vigano L, Ferrero A, Langella S, Russolillo N, Capussotti L. Locally advanced gallbladder cancer: which patients benefit from resection? Eur J Surg Oncol. 2014;40:1008–1015. doi: 10.1016/j.ejso.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Edwards J, Scoggins C, McMasters K, Martin R. Combined pancreas and liver therapies: resection and ablation in hepato-pancreatico-biliary malignancies. J Surg Oncol. 2013;107:709–712. doi: 10.1002/jso.23318. [DOI] [PubMed] [Google Scholar]
- 38.Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 39.Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. 2007;110:2484–2492. doi: 10.1002/cncr.23074. [DOI] [PubMed] [Google Scholar]
- 40.Poultsides GA, Huang LC, Chen Y, Visser BC, Pai RK, Jeffrey RB, et al. Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol. 2012;19:2295–2303. doi: 10.1245/s10434-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 41.Mayo SC, Shore AD, Nathan H, Edil BH, Hirose K, Anders RA, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB. 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]