Abstract
Purpose
Vocal fold epithelium is composed of layers of individual epithelial cells joined by junctional complexes constituting a unique interface with the external environment. This barrier provides structural stability to the vocal folds and protects underlying connective tissue from injury while being nearly continuously exposed to potentially hazardous insults including environmental or systemic-based irritants such as pollutants and reflux, surgical procedures, and vibratory trauma. Small disruptions in the epithelial barrier may have a large impact on susceptibility to injury and overall vocal health. The purpose of this article is to provide a broad-based review of our current knowledge of the vocal fold epithelial barrier.
Methods
A comprehensive review of the literature was conducted. Details of the structure of the vocal fold epithelial barrier are presented and evaluated in the context of function in injury and pathology. The importance of the epithelial-associated vocal fold mucus barrier is also introduced.
Results/Conclusions
Information presented in this review is valuable for clinicians and researchers as it highlights the importance of this understudied portion of the vocal folds to overall vocal health and disease. Prevention and treatment of injury to the epithelial barrier is a significant area awaiting further investigation.
Keywords: Vocal fold, Epithelium, Cell junctions, Barrier function
Introduction
Vocal folds are a well-defined layered structure consisting of numerous tissue types including epithelium, lamina propria, and muscle that are exposed to nearly constant insults from a multitude of sources including environmental or systemic-based irritants such as pollutants and reflux, surgical procedures, and vibratory trauma (Gray, 2000). In order to preserve vocal function, it is imperative that the vocal folds be able to defend themselves against injury from such insults. The epithelium is an essential, yet underappreciated mechanism for vocal fold defense. As the outermost layer of the vocal folds, the epithelium forms a physical barrier against injury which is maintained through the formation of protein complexes called cell junctions. Cell junctions provide structural support to the epithelium by linking adjacent epithelial cells and sealing the paracellular space. Sustained insults that disrupt vocal fold epithelial cells or junctions diminish the protective capacity offered by this important barrier. Recently, voice researchers have begun to identify an association between disrupted structure of the epithelial barrier and vocal fold injury and pathology. As voice disorders are estimated to affect 3–9% of Americans annually (Roy et al., 2004), it is critical that we recognize the contribution of the vocal fold epithelial barrier to vocal health. In this review, we evaluate our current knowledge of the structure of the vocal fold epithelial barrier and discuss these structures in the context of the function of this barrier in vocal fold injury and pathology. We also highlight the vocal fold epithelial-associated mucus barrier and introduce emerging evidence that this barrier may also be altered in states of vocal fold injury and disease. Our goal is to provide both voice clinicians and researchers a foundation for interpreting current and future work in the vocal fold epithelial biology. We further hope that readers will understand the contribution of the vocal fold epithelial barrier to vocal health and appreciate how prevention and treatment of injury to this important barrier should be an actively pursued area of future voice research.
Structure of the Vocal Fold Epithelial Barrier
Understanding the importance of the vocal fold epithelial barrier to vocal health requires a review of epithelial structure. Laryngeal epithelium is a diverse cellular structure composed of numerous cell types and associated cell junctions.
Cellular Structure
The luminal surface of the membranous vocal folds is covered by stratified squamous epithelium (SSE) (Fisher, Telser, Phillips, & Yeates, 2001; Gill, Buda, Moorghen, Dettmar, & Pignatelli, 2005; Gray, 2000). SSE is composed of multiple layers of closely packed stratified squamous cells (Figure 1). Human vocal fold epithelium normally consists of 5–10 epithelial cell layers (Arens, Glanz, Wonckhaus, Hersemeyer, & Kraft, 2007). Multilayered epithelium is a characteristic of tissues where frequent exposure to a wide range of irritants and mechanical forces require that the epithelium be durable for protection (Stepp, Spurr-Michaud, & Gipson, 1993). SSE of the vocal folds is classified as non-keratinized, in contrast to keratinized SSE, such as that found in skin. Non-keratinized SSE cells are nucleated and living (Morita, Miyachi, & Furuse, 2011). Other tissues with portions of non-keratinized SSE include the oral cavity (Squier & Kremer, 2001), esophagus (Squier & Kremer, 2001), vagina (Houghton & McCluggage, 2009), and cornea (Kinoshita et al., 2001). Within the larynx, SSE is unique to the membranous vocal folds and the superficial surface of the epiglottis (Stell, Gudrun, & Watt, 1981). SSE of the vocal folds transitions to a ciliated pseudostratified columnar epithelium at the anterior and posterior commissures, supraglottis, and subglottis (Bulmer, Ali, Brownlee, Dettmar, & Pearson, 2010; Fisher, et al., 2001; Gray, 2000; Stiblar-Martincic, 1997). Epithelium lining the upper airway is also designated as ciliated pseudostratified columnar which is composed of numerous cell types including ciliated columnar cells and mucus-secreting goblet cells (Knight & Holgate, 2003). Goblet cells are also integrated into the epithelium of the larynx especially in areas of the false vocal folds and subglottis (Kutta, Steven, Varoga, & Paulsen, 2004; Kutta et al., 2008).
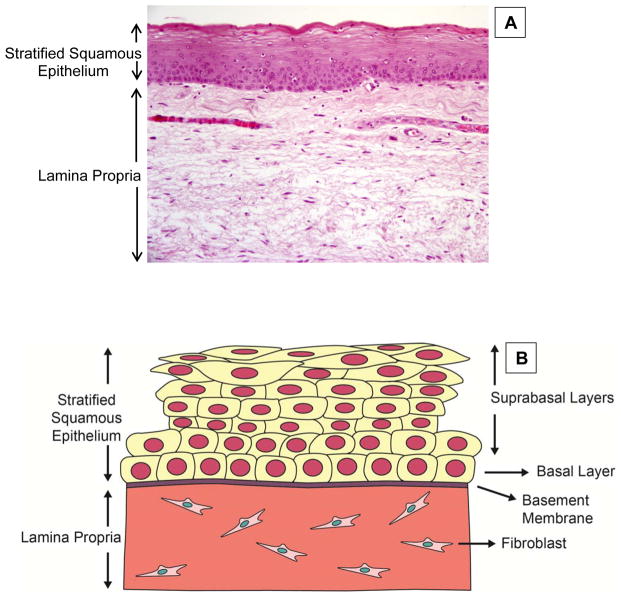
Figure 1.
Structure of the vocal fold epithelial barrier. Vocal fold epithelium is classified as stratified squamous consisting of multiple layers of closely packed squamous cells. (A) Coronal histologic section through membranous portion of a human vocal fold at 20x magnification. Ep – Epithelium, LP – Lamina Propria. (B) Schematic of the vocal fold epithelial barrier. Cell layers are divided into two sections: suprabasal layers and basal layer. The basal layer of epithelial cells is joined to a basement membrane.
Layers of squamous cells of the membranous vocal fold epithelium can be divided into two sections: basal layer and suprabasal, or luminal, layers (Figure 1B). The basal and suprabasal layers are divided histologically through staining for specific stratified squamous epithelial markers called keratins. Specifically, keratin 14 is primarily localized to the basal layer while keratin 13 is primarily localized to the suprabasal layers (Leydon, Selekman, Palecek, & Thibeault, 2013). A state of equilibrium, or epithelial homeostasis, depends on continuous self-renewal of the basal and suprabasal cell layers and represents normal structure and function. Epithelial cell layers experience nearly constant turnover (Gray, 2000; Leydon, Bartlett, Roenneburg, & Thibeault, 2011; Savelli et al., 1991). It has been estimated that complete epithelial turnover occurs in 96 hours (Savelli, et al., 1991). During this process, cells divide in the basal layer and move superiorly and medially into the suprabasal layers. The most luminal epithelial cells are eventually replaced with new cells while old cells are sloughed off into the laryngeal lumen. It is likely that adult stem cells provide the reserve of cells necessary for self-renewal (Leydon, et al., 2011). Adult stem cells are considered a primary component of the tissue regeneration process and have been identified across the length of the vocal folds in humans (Yamashita, et al., 2007) and mice (Leydon, et al., 2011). The basal layer of epithelial cells is joined to a basement membrane (Figure 1B), which is composed primarily of collagen, but includes other proteins such as fibronectin (Gray, Pignatari, & Harding, 1994; Hirschi, Gray, & Thibeault, 2002). Collagenous anchoring structures incorporated into the basement membrane secure the epithelium to the lamina propria (Gray, et al., 1994). The surface of the most superficial layer of epithelial cells supports a series of dense microvilli that increase the epithelial surface area (Gray, 2000; Rousseau, Suehiro, Echemendia, & Sivasankar, 2011). The exact function of microvilli in the epithelium of the vocal folds and other tissues including the cornea and airway remain elusive. It has been hypothesized that these structures promote fluid spreading and adherence (Kahwa, Atwal, & Purton, 1997) and facilitate the absorption of water and other nutrients (Beuerman & Pedroza, 1996). In addition, these structures may perform a unique function in the vocal folds by providing traction during vibration (Gray, 2000).
Epithelial Ion and Water Transport
The vocal fold epithelial surface is covered by a thin layer of fluid (Fisher et al., 2001). This fluid is believed to substantially contribute to the maintenance of optimal vocal fold hydration which in turn influences the biomechanics of vocal fold vibration and promotes normal voice quality (Leydon, Sivasankar, Lodewyck, Atkins, & Fisher, 2009). Fisher and colleagues (2001) were the first research group to establish that vocal fold surface fluid is maintained, in part, by ion and water transport across the vocal fold epithelia. Ion and water transport occurs through epithelial cells and is mediated by specific pumps and channel proteins located on the apical and basolateral epithelial cell membranes (Leydon, et al., 2009). Ion transport is primarily regulated by sodium (Na+) absorption and chloride (Cl−) secretion. The Na+ K+-ATPase pump protein has been localized to the basolateral membrane of canine vocal fold epithelial cells and creates an electrochemical gradient that is the primary driving force behind active ion transport (Fisher, et al., 2001). The Na+ K+-ATPase transports three Na+ ions out of the cell in exchange for two K+ ions into the cell. Other membrane proteins important for Na+ and Cl− transport across the vocal fold epithelium include the epithelial sodium channel (ENaC) and the cystic fibrosis transmembrane regulator (CFTR), respectively (Fisher, Lodewyck, Menco, Telser, & Yeates, 2002; Leydon, Fisher, & Lodewyck-Falciglia, 2009). Using ovine vocal folds, it has been demonstrated that both ENaC and CFTR are located on the luminal membrane of vocal fold epithelial cells. ENaC provides the primary pathway for Na+ absorption while CFTR provides the primary pathway for Cl− secretion. Transport of Na+ and Cl− ions through the pathways described above creates an osmotic gradient that drives water fluxes across the epithelium (Fisher, et al., 2001). Specifically, basally-directed water fluxes are linked with Na+ absorption, while apically-directed water fluxes are linked with Cl− secretion. These ion-driven water fluxes likely occur, in part, through water channels referred to as aquaporins (Lodewyck, Menco, & Fisher, 2007). Vocal fold epithelial ion transport is influenced by numerous factors including ionic and osmotic perturbations (Sivasankar & Fisher, 2008), simulated reflux (Erickson Levendoski & Sivasankar, 2011), and pollutants (Erickson Levendoski & Sivasankar, 2012). This topic has recently been reviewed in detail elsewhere (Leydon, et al., 2009).
Cell Junctions
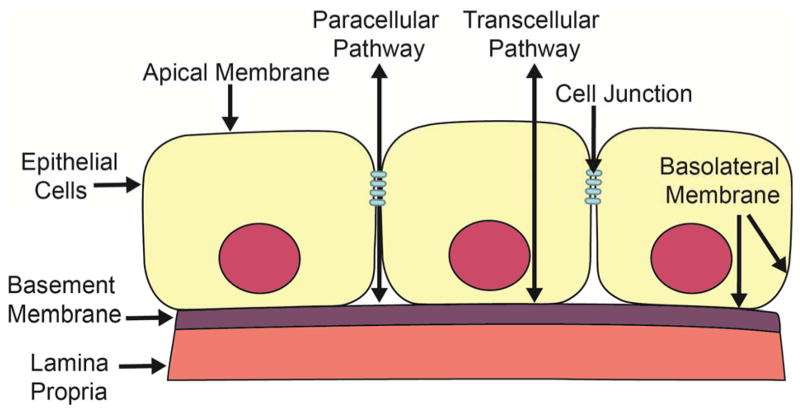
In the SSE of vocal folds, individual cells are joined by protein complexes termed cell junctions (Gill, et al., 2005). Cell junctions are specialized protein complexes that facilitate adherence and communication between two cells or between a cell and the basement membrane and contribute to the maintenance of tissue integrity (Knight & Holgate, 2003). Stratified squamous cells with intervening cell junctions form the basic structure of the vocal fold epithelial barrier. This structural configuration creates two selectively permeable cellular pathways: the transcellular pathway and paracellular pathway (Figure 2). Selective permeability refers to the process of permitting appropriate absorption and secretion of electrolytes and water while limiting permeation of potentially noxious environmental irritants into the vocal folds. While the transcellular pathway is primarily involved with the selective absorption and secretion of ions and water as described in the previous section, the paracellular pathway is associated with transport in the space between adjacent epithelial cells and is regulated by cell junctions. Cell junctions seal the paracellular pathway creating an epithelial barrier. Furthermore, by joining adjacent epithelial cells, cell junctions are also necessary for the mechanical stability of this important barrier, which is essential during vocal fold vibration.
Figure 2.
Two pathways across the vocal fold epithelial barrier. Vocal fold epithelium is a selectively permeable barrier. This schematic demonstrates two distinct pathways for transport across a single sheet of epithelial cells. The transcellular pathway involves selective transport through an epithelial cell. The paracellular pathway involves selective transport through the paracellular space between epithelial cells and is regulated by cell junctions.
Cell junctions are typically grouped by function. Three major groups of cell junctions include occluding, anchoring, and communicating junctions (Table 1). Members of each junction group have been identified in the vocal fold epithelium of humans and numerous animal models using a combination of cellular and molecular biology techniques (Alper, Fu, Erickson-Levendoski, Zheng, & Sivasankar, 2011; Fisher, et al., 2001; Gill, et al., 2005; Hirano et al., 2003; Ling, Raasch, & Welham, 2011; Rousseau, et al., 2011; Schneider, Teschner, Sudermann, Pikula, & Lautermann, 2002; Sivasankar, Erickson, Rosenblat, & Branski, 2010; Van Deusen & Lyon, 2008; Zhang & Fisher, 2012). Alteration in cell junction formation and distribution, destabilization, or all may lead to epithelial barrier dysfunction. For the purpose of this review, we will focus on junction proteins that, to date, have been identified in the vocal folds (Figure 3).
Table 1.
Summary of Vocal Fold Epithelial Junctions
| Junction | Technique | Model | References | |
|---|---|---|---|---|
| Tight | ||||
| Occludin | IHC, RT-PCR, WB | Human, Guinea Pig, Mouse, Pig, Rabbit, Rat, Sheep | Zhang et al., 2012, Sivasankar et al., 2010, Rousseau et al., 2011, Gill et al., 2005 | |
| Zona occludin | IHC, RT-PCR, WB | Human, Guinea Pig, Mouse, Pig, Rabbit, Rat, Sheep | Zhnag et al., 2012, Sivasankar et al., 2010, Rousseau et al., 2011, Gill et al., 2005 | |
| Anchoring | ||||
| E-cadherin | IF, IHC, RT-PCR, WB | Human, Guinea Pig, Mouse, Pig, Rabbit, Rat | Sivasankar et al., 2010, Ling et al.,, 2010, Rousseau et al., 2011, Gill et al., 2005 | |
| B-catenin | IHC, RT-PCR, WB | Human, Guinea Pig, Mouse, Pig, Rat Rabbit | Sivasankar et al., 2010, Rousseau et al., 2011, Gill et al., 2005 | |
| Desmosome | TEM | Human, Pig, Sheep, Rat | Fisher et al., 2001, Hu et al.,, 2004, Martins et al., 2009, Pastuszek et al., Gill et al., 2005 | |
| Communicating | ||||
| Gap | IF, RT-PCR | Human, Rat | Schneider et al., 2002, VanDeusen et al., 2008 | |
IF: Immunofluorescence, IHC: Immunohistochemistry, RT-PCR: Real time - Polymerase chain reaction, WB: Western blotting, TEM: Transmission electron microscopy
Figure 3.
Cell junctions of the vocal fold epithelial barrier. Cell junctions occur at points of cell-cell and cell-matrix contact. Tight junctions are the apical-most cell junctions that seal the paracellular space and are classified as occluding junctions. Adherens junctions, desmosomes, and hemidesmosomes are classified as anchoring junctions. Anchoring junctions join cytoskeletal filaments from cell to cell (adherens junctions, desmosomes) and from cells to extracellular matrix (hemidesmosomes). Gap junctions are classified as communicating junctions that mediate the passage of electrolytes and other small molecules between cells.
Proteins complexes called tight junctions are the primary occluding junction of vocal fold epithelium. Tight junctions encircle the apical ends of epithelial cells and seal together adjacent epithelial cells (Suzuki, 2013). Tight junctions are the main determinant of permeability of the paracellular pathway and are critical to vocal fold defenses as damage to these junctions may result in uncontrolled access of noxious insults into the vocal folds. Transepithelial resistance (TER) and paracellular flux are well-used indicators of tight junction permeability (Balda, Whitney, Flores, González, Cerijido, & Matter, 1996; Hasegawa, et al., 1999). TER measures the “tightness” of the epithelium to the passage of electrolytes (Li, Sheppard, & Hug, 2004). Vocal folds typically exhibit a high TER that is suggestive of a “tight” epithelial barrier (Sivasankar, et al., 2010). Paracellular flux measures the permeability of nonionic molecules, such as mannitol or dextran, through the paracellular pathway (Hasegawa et al., 1999). Changes in TER and paracellular flux typically, but do not always occur in unison. This is likely because TER represents an instantaneous permeability measurment while paracellular flux indicates permeability over a longer period of time (Balda, et al., 1996; Hasegawa, et al., 1999). Consequently, it is important when studying vocal fold tight junction permeability that researchers assess both TER and paracellular flux.
Adherens junctions, desmosomes, and hemidesmosomes are major classes of anchoring junctions. Anchoring junctions play a critical role in the maintenance of epithelial barrier integrity by providing strong adhesive bonds between the cytoskeletal components of adjacent epithelial cells (adherens junctions, desmosomes) or between cellular cytoskeletal components and the basement membrane (hemidesmosomes) (Niessen, 2007). Anchoring junctions are particularly abundant in tissues, such as the vocal folds, that are subjected to significant mechanical forces (Fisher, et al., 2001). Not surprisingly, these junctions are critical for stabilizing epithelial sheets during vibration. Adherens junctions may also play a role in determining paracellular permeability, though to a lesser extent than tight junctions (Niessen, 2007). Further, the adherens junction E-cadherin regulates the assembly of tight junctions (Troxell, et al., 2000; Tunggal, et al, 2005). Consequently, disruption to E-cadherin expression or localization will have negative consequences for the formation and functionality of tight junctions.
Communicating junctions are the final functional class of vocal fold cell junctions. Although communicating junctions do not directly relate to the barrier function of the epithelium, these junctions provide pathways critical for intercellular communication. As the major class of communicating junctions, gap junctions form intercellular channels that facilitate signaling between adjacent cells and permit the passage of small molecules such as ions (Schneider, et al., 2002; Van Deusen & Lyon, 2008). Gap junctions are composed of connexin proteins. At this point, we have very little information regarding the distribution of specific connexin proteins in human or animal vocal fold epithelia. However, initial research suggests that the distribution of connexin proteins differs based upon laryngeal location suggesting unique functional roles for specific proteins (Van Deusen & Lyon, 2008).
Vocal Fold Epithelial Barrier Injury
An intact vocal fold epithelium formed by the squamous cells of the epithelium and adjoining cell junctions acts as a barrier that protects the vocal folds from injury. However, the protective capacity offered by the vocal fold epithelium is contingent upon the maintenance of this robust structure (Gill, et al., 2005). Injury to the vocal fold epithelial barrier from various extracellular stimuli results in changes to epithelial structure and function that are likely closely associated with overall vocal fold health and susceptibility to pathology (Figure 4). Assessment and treatment procedures for vocal fold epithelial injury remain limited as we lack a comprehensive understanding of structural and functional changes that occur to the the vocal fold epithelium as a result of injury. Over the past decade, using in vitro and in vivo animal studies and human biopsy specimens, researchers have begun to identify an association between disrupted structure and function of the epithelial barrier and vocal fold injury and pathology. Such investigations provide a foundation for the development of evidence based assessments and treatments that specifically target vocal fold epithelial barrier injury
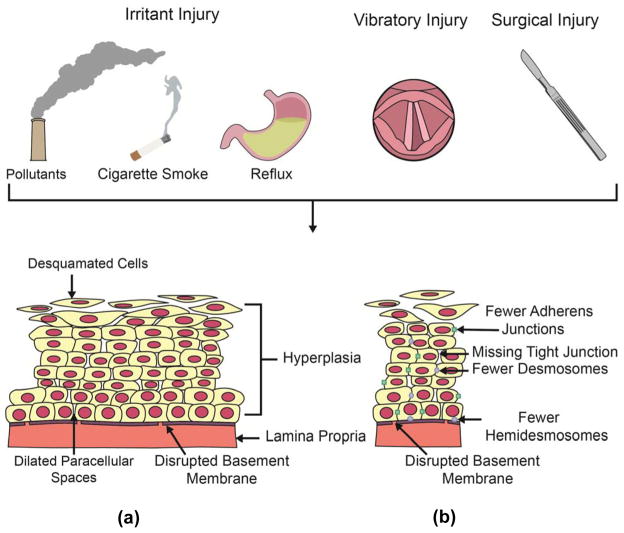
Figure 4.
Summary of factors resulting in vocal fold epithelial barrier dysfunction. Numerous insults including environmental and systemic irritants, vocal fold vibration, and surgical instruments compromise the structure of the vocal fold epithelial barrier. Such structural changes may manifest as either changes in cellular structure or organization (a) or alterations in cell junctions (b). Examples of changes in cellular structure and organization are illustrated in part (a) and may include desquamated cells, dilated paracellular space, disrupted basement membrane, and hyperplasia. Examples of alterations in cells junctions are illustrated in part (b) and may include fewer or missing junctions.
Irritants
The larynx is situated at the separation of the respiratory and digestive tracts (Mouadeb et al., 2009; Thibeault, Rees, Pazmany, & Birchall, 2009). Narrowing at the level of the vocal folds creates an area of highly turbulent airflow that promotes the deposition of a wide-range of inhaled, environmental and sometimes ingested, systemic irritants. As the outermost layer of the vocal folds, the epithelium is the first structure to come in contact with such challenges. Various environmental and systemic irritants that been shown to compromise the vocal fold epithelial barrier.
Environmental irritants
Injury to the vocal fold epithelial barrier may occur as result of drying of the vocal fold surface or may be a product of pollutants such as cigarette smoke. Drying of the vocal fold epithelial surface occurs in everyday home and work environments as a result of factors such as low humidity and mouth breathing (Sivasankar, Erickson, Schneider, & Hawes, 2008; Sivasankar & Fisher, 2002). Vocal fold drying is a common clinical concern as it increases the pulmonary effort required to initiate and sustain vocal fold vibration (Verdolini et al., 2002) as well as alters perturbation measures that are potentially indicative of reduced voice quality (Hemler, Wienke, & Dejonckere, 1997). Vocal fold surface drying can be accompanied by an increase in the tonicity, or concentration, of vocal fold surface fluid (Sivasankar, et al., 2010). To better understand the effects of surface drying on the vocal fold epithelial barrier, Sivasankar and colleagues (2010) investigated the effects of a high tonicity fluid, or hypertonic fluid, on TER in excised porcine vocal fold folds. Paracellular pathway morphology and the expression of cell junction proteins were also investigated. Within two hours, hypertonic surface fluid rapidly decreased TER and increased the length and width of paracellular pathways without altering the expression of tight or adherens junction proteins. This suggests that even short durations of vocal fold drying may compromise the integrity of the vocal epithelial barrier and that with longer challenge durations we may begin to see changes in expression of junction proteins. Such changes to epithelial barrier may make the vocal folds vulnerable to insults from other common inhaled challenges such as pollutants.
Acute and chronic inhalation of pollutants is considered to be hazardous to voice production (Sataloff, 1992). Despite this prevalent clinical belief, very few studies have investigated the effects of pollutant exposures on the vocal fold epithelial barrier. One of the most commonly investigated pollutants challenges in the larynx is cigarette smoke. This is not surprising given that cigarette smoke exposure is a principal risk factor for the development of laryngeal carcinoma (Sadri, McMahon, & Parker, 2006). In a recent study, an acute, 4 hour exposure to cigarette smoke extract did not alter TER in excised porcine vocal folds (Branski, Zhou, Kraus, & Sivasankar, 2011). Authors hypothesized that a more chronic exposure may be required to compromise the functional integrity of the epithelium and increase leakiness. While the effects of chronic cigarette smoke exposures on TER have not been studied, damage to epithelial structure following chronic exposures was observed in rat and rabbit models (Duarte, Faria, Ceolin, Cestari, & Assis, 2006; Gaafar & Al-Mansour, 1981; Isik, Kalender, Yardimci, & Ergun, 2004). Vocal fold epithelium of rats exposed to 30 cigarettes per day for 25, 50, and 75 days demonstrated signs of hyperplasia (Duarte, et al., 2006). Significant disturbances in epithelial structure were also observed with fewer daily cigarettes. Rabbits challenged with cigarette smoke for 20 minutes per day for 90 days exhibited disturbed epithelial stratification, desquamation, and disorganized microridges in addition to epithelial hyperplasia (Gaafar & Al-Mansour, 1981). Similar observations were seen in rats exposed to cigarette smoke for 2 hours per day for 60 days (Isik, et al., 2004). In these animals, reduced numbers of desmosomes and enlargement of the paracellular pathways were also evident.
In addition to cigarette smoke a variety of other pollutant challenges have been investigated across acute and chronic timelines. Alper and colleagues (2011) challenged excised porcine vocal folds for two hours with hydrogen peroxide, a common reactive oxygen species. Reactive oxygen species were targeted as these chemically reactive molecules can be produced from a variety of pollutants, tobacco smoke, and radiation. Exposure to hydrogen peroxide did not alter TER or the expression of the tight junction ZO-1. Similarly, a 60 minute exposure to the pollutant acrolein, a common byproduct of mobile exhaust, industrial processes, and tobacco smoke, did not alter TER in excised porcine vocal folds (Erickson Levendoski & Sivasankar, 2012). Neither pollutant challenge caused gross changes in vocal fold epithelial structure. Together, these findings suggest that the vocal fold epithelium may be able to withstand acute pollutant exposures without significant changes in epithelial barrier structure or function. On the other hand, there is evidence that suggests chronic pollutant exposures disturb vocal fold epithelial structure. Calcium carbonate, a main ingredient of chalk dust, induced desquamation, or shedding of the outermost layer of rat vocal fold epithelial cells following 30–90 days of exposure (Marcelino & Oliveira, 2005). In bonnet monkeys, a three month exposure to ozone, an environmentally prevalent pollutant formed by the interaction of automobile exhaust with heat and sunlight, prompted signs of vocal fold epithelial hyperplasia and disorganization (Leonard, Charpied, & Faddis, 1995).
Systemic irritants
Laryngopharyngeal reflux (LPR) is the most commonly investigated systemic-based irritant in the larynx. LPR is the backflow of gastric contents, including acid and pepsin, from the stomach and esophagus into the pharynx and larynx and is estimated to occur is up to 50% of patients with voice problems (Koufman, Amin, & Panetti, 2000). The effects of LPR on vocal function are well recognized (Oguz et al., 2007; Pribuisiene, Uloza, Kupcinskas, & Jonaitis, 2006), however, the mechanisms underlying LPR-induced vocal deficits are less understood. Bulmer and colleagues (2010) used an excised porcine laryngeal damage model to investigate the effects of 60 minute acid and pepsin challenges, designed to mimic LPR, on the integrity of laryngeal epithelial structure using optical density, DNA release measurements, and microscopy. While both acid and pepsin induced epithelial damage, as measured by significant increases in cellular release of intracellular tissue components and DNA and disturbances in gross epithelial structure, acidified pepsin produced the most significant damage to the vocal fold and subglottic epithelium. Laryngeal biopsy specimens from three laryngeal sites (vocal fold, posterior commissure, and ventricle) from patients with LPR were utilized to investigate the effects of this disease on the adherens junction protein, E-cadherin. At all tested sites, there was a significant decrease in E-cadherin expression (Gill et al., 2005; Johnston et al., 2003; Reichel, Mayr, Durst, & Berghaus, 2008). Franchi and colleagues (2007) observed further evidence of structural compromise in laryngeal epithelial biopsy specimens from the posterior commissure in patients with LPR. They noted a widening of the paracellular pathway, obliteration of microvilli, and reduced numbers of desmosomes. Whether such structural changes are seen in the vocal fold epithelium has yet to be determined. Epithelial structural changes are suggestive, but not directly indicative of impaired epithelial barrier function. To test this, Erickson and Sivasankar (2010) challenged excised porcine vocal folds with acidified pepsin and measured TER. Results indicated that within 15 minutes acidified pepsin rapidly decreased TER. A leaky epithelial barrier was associated with acid and but not pepsin exposure and may indicate increased paracellular tissue permeability to further reflux events. Taken together, these findings indicate that exposure to refluxed materials such as acid and pepsin can compromise the structural and functional integrity of the epithelial barrier. With integrity compromised, it is highly possible that the epithelium remains vulnerable to further damage by subsequent reflux events. It may be that repeated exposures to gastric contents lead to the development of LPR. Factors such as reduced expression of E-cadherin and dilated paracellular pathways may be morphologic markers of LPR and prove to be useful diagnostic tools for this disease.
In another example of a systemic challenge to the vocal fold epithelial barrier, Zhang and Fisher (2012) investigated the effect of the inflammatory mediator histamine on vocal fold TER and paracellular pathway permeability in excised ovine vocal folds. Histamine is primarily generated in the granules of mast cells or basophils and, in the airway, is involved in the development of infection and allergic diseases. Within two hours, a histamine challenge significantly decreased vocal fold TER and increased paracellular permeability. These authors hypothesized that changes in TER and permeability were a function of compromised tight junction integrity. The role that histamine may play in the development of vocal fold allergic disease remains to be elucidated, but the results of the current investigation suggest that histamine has the ability to compromise the integrity of the vocal fold epithelial barrier. Similar findings of histamine-related epithelial dysfunction have also been observed in nasal epithelia (Jacobi et al., 1998; Takeuchi, Kishioka, Ishinaga, Sakakura, & Majima, 2001). Future research may prove useful in identifying histamine as a new pathogenic mechanism for vocal fold epithelial barrier dysfunction.
Literature summarized above suggests that in animal models the vocal fold epithelial barrier is sufficiently robust to withstand acute exposures to most tested environmental and systemic irritants. However, consistent epithelial structural breakdown is observed following chronic irritant challenges. Such structural breakdown may hold adverse consequences for various aspects of vocal fold health. For example, reduced expression of cell junctions, disturbed stratification, and desquamation may suggest that the vocal folds cannot withstand the mechanical forces of vibration placing the tissues at risk for development of vocal fold pathology. Hyperplasia, or an increase in cellular proliferation, may be an adaptive response to external stimuli. However, it could also be an early indicator of abnormal cellular proliferation (neoplasia) which may lead to development of laryngeal carcinoma. Finally, it is also possible that breakdown of the epithelial barrier is associated with viral and bacterial infections. In other epithelial tissues such as that found in the stomach and cervix, viral and bacterial infections are linked to impaired epithelial structure and function (Amieva, Vogelmann, Covacci, Tompkins, Nelson, & Falkow, 2003; Stanley, 2012). For example, human papillomavirus (HPV) is a common benign vocal fold disorder in adults and children (Aaltonen, Rihkanen, & Vaheri, 2002). In the cervix, HPV infects squamous epithelium following compromised barrier function (Stanley, 2012). The virus targets basal epithelial cells. While basal cells are usually protected from insult by suprabasal cell layers, disruption to barrier integrity from a microabrasion permits the virus to reach the cells (Doorbar, 2005). In gastric epithelial cells, helicobacter pylori, a Gram-negative bacterium, disrupts epithelial cell junctions; thus, promoting bacterial invasion and growth (Amieva et al., 2003). Whether viral and bacterial infections are associated with breakdown of the vocal fold epithelial barrier is unknown and should be a focus of future research.
Surgical Injury
To date, the majority of studies that investigate vocal fold repair as a result of surgical injury have mostly focused on the recovery of the structure and function of the lamina propria. Researchers are in the early stages of establishing a timeline for epithelial structural reestablishment following surgical injury. In these investigations, animal models of surgical injury are established through vocal fold stripping. This injury-type represents a gross form of barrier loss, where there is direct damage to epithelial cells and exposure of underlying tissues. Following vocal fold stripping in a rabbit model, Branski and colleagues (2005) sacrificed animals at 12 hours, 1, 3, 5, 7, 10, 14, and 21 days post injury. They observed partial epithelial coverage of the injury by day 3 and complete epithelial coverage of the injury at day 5. Despite complete coverage by day 5, the epithelium was not normal in appearance and marked by significant hypertrophy characterized by enlargement of epithelial cells. This structural abnormality is likely indicative of a functionally impaired epithelium. Ling and colleagues (2010) also examined epithelial healing following vocal fold stripping in a rat model of injury. Animals were sacrificed at 1, 3, 5, and 7 days post injury. Partial epithelial cell coverage of the injury was observed at day 1 and complete by day 3. Similar to the findings by Branski and colleagues, the epithelium was characterized by significant hypertrophy during the healing process. However, by day 7, the luminal surface of this thickened epithelium was characterized by decreased cell numbers and partial coverage by squamous-appearing cells. To further investigate epithelial recovery following injury, this group characterized the expression of the adherens junction protein E-cadherin and the protein cross-linking enzyme transglutaminase-1 at the same points as above (Ling, et al., 2011). Like E-cadherin, transglutaminase-1 is important for cell-cell adhesion and epithelial stability. The majority of newly recruited cells at day 1 were negative for E-cadherin; however, by 3–7 days post injury strong E-cadherin signals were present throughout the completely covered epithelium. Similar patterns of recovery were observed for transglutaminase-1. One investigation has been identified that describes the expression of epithelial-related proteins during the chronic phases of wound healing. Hirano and colleagues (2003) investigated the expression of adhering proteins including cadherin, syndecan-1, and syndecan-4 2 and 6 months following vocal fold stripping in canines. Syndecan-4 was increased in the basal layer of epithelial cells at both 2 and 6 months following injury. An important protein during wound healing, syndecan-4 assists in the formation of focal adhesions between cells and the extracellular matrix (Woods & Couchman, 2001), and in this case between the basal epithelial cell layer and the basement membrane. No changes were observed in the expression of cadherin and syndecan-1. Taken together, this finding suggests that the basal layers of epithelial cells continues to experience remodeling during more chronic phases of wound healing, whereas the suprabasal cell layers undergo remodeling during the acute phases of injury.
In summary, we are beginning to understand the timeline of epithelial barrier structural reestablishment following surgical injury. From the studies above, it appears that full epithelial coverage following surgical wounding is established fairly quickly, within 3–5 days, and recovery of the adherens junction E-cadherin seems to occur within a similar timeframe. However, we continue to lack a timeline of recovery for other cell junction proteins including tight junctions and other anchoring-type junctions that are critical to epithelial barrier integrity. Furthermore, the nature and extent of epithelial functional recovery following wounding is entirely unknown. Consequently, further studies need to be conducted that include measurements of TER and paracellular permeability. It is likely that during the wound healing process the vocal folds are more susceptible to injury from vibratory stresses and environmental and systemic irritants. Consequently, there is a critical need for establishment of a comprehensive timeline of epithelial structural and functional recovery following surgical injury. Until this time, we do not have a complete understanding of the ability of the epithelial barrier to protect the vocal folds following surgery.
Vibratory Injury
Phonotrauma, or intense vocal fold vibration over prolonged periods, is a major factor that contributes to the development of many mid membranous, benign vocal fold lesions of the lamina propria (Behrman, Rutledge, Hembree, & Sheridan, 2008). It has been suggested that the vocal fold epithelium is important for defending the lamina propria against such mechanical stresses during vocal fold vibration (Rousseau, et al., 2011). To date, three studies, using animal models, have been identified that investigate the effects of simulated phonotrauma, or excessive phonation, on the vocal fold epithelium. Two early studies evaluated the structure of the vocal fold epithelium following excessive phonation. Zhao and colleagues (1991) induced excessive phonation in felines 25 minutes per day, two times per day for 15 weeks, finding that excessive phonation resulted in vocal fold epithelial hyperplasia as well as shedding of surface epithelial cells. Gray and Titze (1988) investigated the effect of two to four hours of excessive phonation in a canine model on vocal fold structure. At two hours, vocal fold surface damage included the destruction and loss of epithelial microvilli and desquamation. By four hours, marked tearing of desmosomes and hemidesmosomes was also noted. More recently, Rousseau and colleagues (2011) investigated the effect of 30 minutes of raised intensity phonation, in rabbits, on the expression of cell junction genes and epithelial structure. Significant reductions in the expression of the tight junction protein occludin and the adherens junction protein β-catenin were observed. Structural changes to the vocal fold epithelium including desquamation, microhole formation, and dilated paracellular spaces were also identified. Together, these results suggest that both short and long durations of excessive phonation may be detrimental to the vocal fold epithelial barrier. Disrupted epithelial barrier structure may not only increase the likelihood of noxious irritants entering the vocal fold mucosa, but also reduce the vocal fold’s ability to tolerate further vibratory stresses and protect the underlying lamina propria from injury. Consequently, it is possible that changes in epithelial structure as a result of vibratory injury are implicated in the development of vocal fold pathology.
Epithelial Barrier Defects Associated with Vocal Fold Pathology
The animal studies discussed in the previous sections suggest that altered epithelial barrier structure and function may be an associative factor predisposing to the development of vocal fold pathologies. However, to date there are no studies in humans that directly demonstrate epithelial barrier changes precede the major structural changes accompanying vocal fold pathologies, only that various research groups have described cellular structure of various vocal fold lesions (Dikkers, Hulstaert, Oosterbaan, & Cervera-paz, 1993; Kotby, Nassar, Seif, Helal, & Saleh, 1988; Martins, Defaveri, Domingues, & de Albuquerque e Silva, 2011). Although a detailed examination of the epithelium is often not the primary goal of these investigations, significant disruptions in epithelial structure have been reported. Dikkers and colleagues (1993) conducted an investigation of the structure of benign laryngeal lesions (nodules, polyps, granulomas, Reinke’s edema, cysts). The basement membrane was characterized by a thickened irregular appearance and the structure of desmosomes and hemidesmosome were altered. Structural changes to the epithelium were most evident in vocal nodules. Additional investigations have focused on characterizing the structure and ultrastructure of single lesions. Kotby and colleagues (1988) and Martins and colleagues (2010) both described the morphological features of vocal fold nodules. Both groups noted dilation of the paracellular spaces and, in places, an absence of the basement membrane. Martins also reported additional findings including a high prevalence of histological alterations including epithelial hyperplasia, basement membrane thickening, and an increase in the number of desquamating cells. Further findings of basement membrane disruption in vocal nodules, including a loss of anchoring structures, has been observed by Gray and colleagues (1995).
To date, inconsistent or few alterations in epithelial structure have been observed in other benign vocal fold lesions including polyps and granulomas, though studies that examine these lesions are limited. In structural analyses of vocal fold polyps, investigators inconsistently observed epithelial changes (Martins, et al., 2011). For example, during gross structural analysis, some polyp epithelia appeared normal while others presented as hyperplastic or atrophic. Analyses of ultrastructure demonstrated similar variability. Desquamation and obliteration of microvilli were only observed in a portion of samples. While epithelial changes are reported more consistently in granulomas, these changes were mild in severity compared with that of nodules. Martins (2009) and Shin (1994) report some mild desquamating surface epithelial cells post endotracheal intubation and in contact granulomas. Martins (2009) further observed some epithelial hyperplasia and altered desmosomal structure in these lesions.
Vocal Fold Mucus Barrier
The epithelium is not the only important vocal fold barrier. Luminal epithelial surfaces of the vocal folds are covered by a thin layer of mucus. Mucus serves as a barrier between the epithelial cell membranes and the environment. Primary functions of mucus include protection, transport, and lubrication (Samuels, et al., 2008). Specifically, mucus binds and traps environmental and systemic irritants for subsequent transport and removal through mucociliary clearance mechanisms. In the larynx, mucus also serves a unique function of lubricating the vocal folds during vibration (Roy, Tanner, Gray, Blomgren, & Fisher, 2003). Unfortunately, our knowledge of the composition and effect of insults on the vocal fold mucus barrier is limited. Mucus is a heterogeneous mixture of salts, carbohydrate-rich glycoproteins (also called “mucins”), and water (Knowles & Boucher, 2002). The functional properties of mucus are mostly influenced by its mucin content. Approximately 20 mucins have been detected in the human airway. These mucins fall into two broad-categories: secreted, gel forming mucins and membrane-associated mucins (Jeffery & Li, 1997). In the larynx, secreted mucins are typically considered to be a product of the false vocal folds and subglottis (Kutta, Steven, Kohla, Tillmann, & Paulsen, 2002; Kutta, et al., 2008). False vocal folds are two mucosal folds located in the supraglottic region immediately superior to the true vocal folds. The false vocal folds and subglottis contain specialized mucus producing cells called goblet cells as well as mucus producing submucosal glands (Figure 5). Secreted mucins are much larger than membrane associated mucins and primarily responsible for the physical properties of airway mucus such as viscosity (Lillehoj & Kim, 2002). Membrane-associated mucins are found on epithelial cell membranes throughout the larynx. Traditionally, the major functions of membrane-associated mucins are thought to include cellular adhesion, pathogen binding, and signal transduction (Rose & Voynow, 2006). However, the extracellular domain of membrane-associated mucins can be proteolytically cleaved or alternatively sliced and released into the mucus layer (Williams, Sharafkhaneh, Kim, Dickey, & Evans, 2006). In this capacity, membrane-associated mucins, like secreted mucins, may contribute to protective physical properties of the mucus layer.
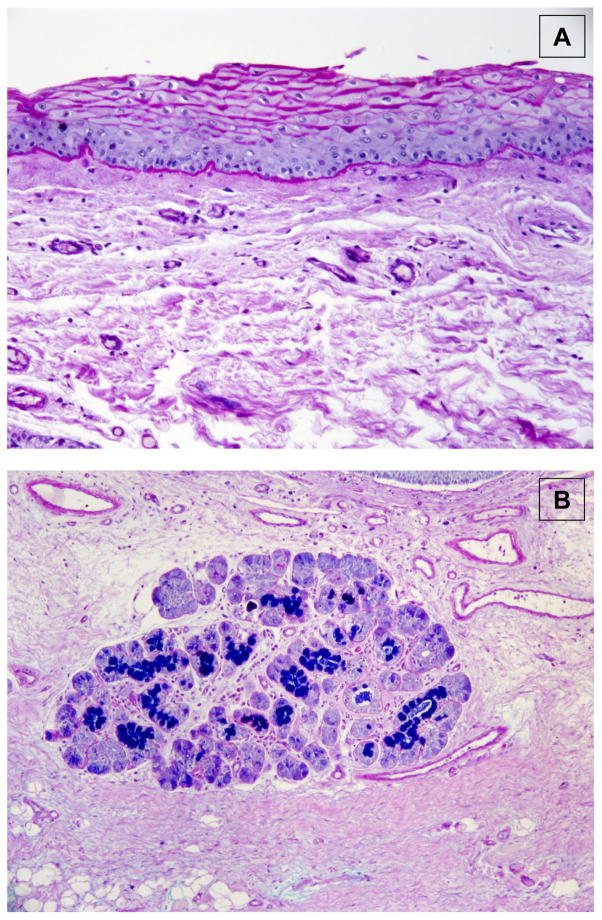
Figure 5.
Example of mucus producing cells of the larynx. Coronal histologic section through human false vocal fold at 20x magnification. False vocal fold sections were stained with Alcian Blue/Periodic Acid Schiff (AB/PAS). AB/PAS is a special stain commonly used for the evaluation of mucins. Mucins are stained as either blue or magenta. (A) Positive staining around the cell membrane of apical epithelial cells of the false vocal fold. (B) Positive staining of cells of a submucosal gland.
Given the critical role that mucus plays in vocal fold defense and vibration, a better understanding of the expression of laryngeal mucins in states of health, injury, and pathology is necessitated. In healthy tissue, at the gene level, numerous secreted and membrane-associated mucins including MUCs 1-5AC/B, 7, 9, 13, 15, 16 and 18–20 are expressed in posterior laryngeal biopsy specimens (Samuels, et al., 2008). Similarly, mucin proteins including MUCs 1, 5AC, 5B, 7, 8, and 16 have been detected in the human laryngeal subglottis (Kutta, et al., 2008). There is emerging evidence that the expression of mucin genes is altered in laryngeal disease states. Preliminary findings suggest an improved patient survival advantage when MUC4 is expressed in laryngeal squamous cell cancers (Paleri, et al., 2004). MUCs 2, 3, and 5 are expressed at reduced levels in posterior laryngeal biopsy specimens from patients diagnosed with LPR (Samuels, et al., 2008). In addition, when animals are chronically exposed to pollutants including ozone in bonnet monkeys (Leonard et al., 1995), calcium carbonate in rats (Marcelino & Oliveira, 2005), and tobacco smoke in guinea pigs (Mouadeb, et al., 2009), researchers observed significant alterations in the physical properties of the mucus overlying the laryngeal surfaces. Specifically, pollutant exposures resulted in increased mucus production as well as increased aggregation of viscous mucus on the laryngeal surfaces. Such changes in mucus have multiple implications for laryngeal health. For example, reduced expression of mucin may inhibit the protective properties of this important barrier. Mucin overproduction, on the other hand, may lead to reduced efficiency in clearance of foreign particulates. Furthermore, changes in the viscosity of mucus may significantly impact vocal fold vibration. Researchers have yet to determine whether mucin content is associated with vocal fold pathology. In patients with voice disorders, abnormal aggregation of “rough” and “uneven” mucus is a common observation during laryngeal imaging (Bonilha, White, Kuckhahn, Gerlach, & Deliyski, 2012). As a change in amount mucus could affect vocal fold defenses as well as vibration, it is critical that future research be completed in this area.
Summary and Conclusions
Epithelium constitutes a unique barrier between the external environment and underlying connective tissue of the vocal folds. This paper provided a broad based review of what is currently known about the structure of the vocal fold epithelial barrier and discussed the structure in the context of barrier function in injury and pathology. It is clear that sustained insults from sources such as environmental and systemic insults, surgical instruments, and vibration disrupts vocal fold epithelial barrier structure and likely diminishes the protective capacity offered by this important barrier. Such disturbances may have dramatic consequences for overall vocal health. Despite the increase in our understanding of the importance of the epithelial barrier, we are still in the very early stages of investigation of vocal fold epithelial biology. Future studies are needed that focus on correlating changes in vocal fold epithelial barrier structure and function with changes in voice through perceptual, aerodynamic, and acoustic analyses. Furthermore, we are in need of investigations that seek to re-establish epithelial barrier structure and function following injury.
Acknowledgments
This work was supported by NIH Grants RO1 DC012773, RO3 DC011355, and T32 DC009401 from the National Institute on Deafness and other Communicative Disorders (NIDCD). We thank Anna Pankratz for assistance during illustration preparation.
References
- Aaltonen L, Rihkanen H, Vaheri A. Human papillomavirus in larynx. The Laryngoscope. 2002;112:700–707. doi: 10.1097/00005537-200204000-00020. [DOI] [PubMed] [Google Scholar]
- Alper R, Fu X, Erickson-Levendoski E, Zheng W, Sivasankar M. Acute stress to vocal fold epithelia from reactive oxygen species. The Laryngoscope. 2011;121:2180–2184. doi: 10.1002/lary.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arens C, Glanz H, Wonckhaus J, Hersemeyer K, Kraft M. Histologic assessment of epithelial thickness in early laryngeal cancer or precursor lesions and its impact on endoscopic imaging. European Archives of Oto-Rhino-Laryngology. 2007;264:645–649. doi: 10.1007/s00405-007-0246-8. [DOI] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, González S, Cerijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. The Journal of Cell Biology. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman A, Rutledge J, Hembree A, Sheridan S. Vocal hygiene education, voice production therapy, and the role of patient adherence: A treatment effectiveness study in women with phonotrauma. Journal of Speech Language and Hearing Research. 2008;51:350–366. doi: 10.1044/1092-4388(2008/026). [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Pedroza L. Ultrastructure of the human cornea. Microscopy Research and Technique. 1996;33:320–335. doi: 10.1002/(SICI)1097-0029(19960301)33:4<320::AID-JEMT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bonilha HS, White L, Kuckhahn K, Gerlach TT, Deliyski DD. Vocal fold mucus aggregation in persons with voice disorders. Journal of Communication Disorders. 2012;45:304–311. doi: 10.1016/j.jcomdis.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branski R, Rosen C, Verdolini K, Hebda P. Acute vocal fold wound healing in a rabbit model. Annals of Otology, Rhinology, and Laryngology. 2005;114:19–24. doi: 10.1177/000348940511400105. [DOI] [PubMed] [Google Scholar]
- Branski R, Zhou H, Kraus D, Sivasankar M. The effects of cigarette smoke condensate on vocal fold transepithelial resistance and inflammatory signaling in vocal fold fibroblasts. The Laryngoscope. 2011;121:601–605. doi: 10.1002/lary.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer D, Ali M, Brownlee I, Dettmar P, Pearson J. Laryngeal mucosa: Its susceptability to damage by acid and pepsin. The Laryngoscope. 2010;120:777–782. doi: 10.1002/lary.20665. [DOI] [PubMed] [Google Scholar]
- Dikkers F, Hulstaert C, Oosterbaan J, Cervera-paz F. Ultrastructural changes of the basement membrane zone in benign lesions of the vocal folds. Acta Oto-Laryngologica. 1993;113:98–101. doi: 10.3109/00016489309135774. [DOI] [PubMed] [Google Scholar]
- Doorbar J. The papillomavirus life cycle. Journal of Clinical Virology. 2005;32S:S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Duarte J, Faria F, Ceolin D, Cestari T, Assis G. Effects of passive smoke inhalation on the vocal cords of rats. Revista Brasileira de Otorrinolaringologia. 2006;72:210–216. doi: 10.1016/S1808-8694(15)30057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickon Levendoski E, Sivasankar M. Functional changes in porcine vocal folds induced by a short duration pollutant challenge. Paper presented at the International Conference on Voice Physiology and Biomechanics; Erlangen, Germany. 2012. Jul, [Google Scholar]
- Erickson E, Sivasankar M. Simulated reflux decreases vocal fold epithelial barrier resistance. The Laryngoscope. 2010;120:1569–1575. doi: 10.1002/lary.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson Levendoski E, Sivasankar M. Role for ion transoport in porcine vocal fold epithelial defense to acid challenge. Otolaryngology – Head and Neck Surgery. 2011;146:272–278. doi: 10.1177/0194599811428273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K, Lodewyck D, Menco B, Telser A, Yeates D. Sodium-dependent transepithelial water fluxes of the vocal fold. Paper presented at the 3rd Biennial International Conference on Vocal Fold Physiology and Biomechanics; Denver, CO. 2002. Sep, [Google Scholar]
- Fisher K, Telser A, Phillips J, Yeates D. Regulation of vocal fold transepithelial water fluxes. Journal of Applied Physiology. 2001;91:1401–1411. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- Franchi A, Brogelli B, Massi D, Santucci M, De Campora E, Gallo O. Dilation of intercellular spaces is associated with laryngopharyngeal reflux: An ultrastructural morphometic analysis of laryngeal epithelium. European Archives of Oto-Rhino-Laryngology. 2007;264:907–911. doi: 10.1007/s00405-007-0295-z. [DOI] [PubMed] [Google Scholar]
- Gaafar HA, Al-Mansour AH. The effect of cigarette smoke on the vocal cord mucosa of the rabbit: An electron microscopic study. The Journal of Laryngology and Otology. 1981;95:721–729. doi: 10.1017/s0022215100091349. [DOI] [PubMed] [Google Scholar]
- Gill G, Buda A, Moorghen M, Dettmar P, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. Journal of Clinical Pathology. 2005;58:1265–1270. doi: 10.1136/jcp.2004.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G, Johnston N, Buda A, Pignatelli M, Pearson J, Dettmar P, Koufman J. Laryngeal epithelial defenses against laryngopharyngeal reflux: Investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Annals of Otology, Rhinology & Laryngology. 2005;114:913–921. doi: 10.1177/000348940511401204. [DOI] [PubMed] [Google Scholar]
- Gray S. Cellular Physiology of the Vocal Folds. In: Rosen C, Murry T, editors. The Otolaryngologic Clinics of North America. Philadelphia, PA: W.B. Saunders Company; 2000. [DOI] [PubMed] [Google Scholar]
- Gray S, Hammond E, Hanson D. Benign pathologic response of the larynx. Annals of Otology, Rhinology, & Laryngology. 1995;104:8–13. doi: 10.1177/000348949510400103. [DOI] [PubMed] [Google Scholar]
- Gray S, Pignatari S, Harding P. Morphologic ultrastructure of anchoring fibers in normal vocal fold basement membrane zone. Journal of Voice. 1994;8:48–52. doi: 10.1016/s0892-1997(05)80318-2. [DOI] [PubMed] [Google Scholar]
- Gray S, Titze I. Histologic investigation of hyperphonated canine vocal cords. The Annals of Otology, Rhinology, and Laryngology. 1988;97:381–388. doi: 10.1177/000348948809700410. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Fugita H, Katoh H, Aoki K, Nakamura K, Negishi M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by rho in madin-darby canine kidney cells. The Journal of Biological Chemistry. 1999;274:20982–20988. doi: 10.1074/jbc.274.30.20982. [DOI] [PubMed] [Google Scholar]
- Hemler R, Wienke G, Dejonckere P. The effect of relative humidity of inhaled air on acoustic parameters of voice in normal subjects. Journal of Voice. 1997;11:295–300. doi: 10.1016/s0892-1997(97)80007-0. [DOI] [PubMed] [Google Scholar]
- Hirano S, Bless D, Rousseau B, Welham N, Scheidt T, Ford C. Fibronectin and adhesion molecules on canine scarred vocal folds. The Laryngoscope. 2003;113:966–972. doi: 10.1097/00005537-200306000-00010. [DOI] [PubMed] [Google Scholar]
- Hirschi S, Gray S, Thibeault S. Fibronectin: An interesting vocal fold protein. Journal of Voice. 2002;16:310–316. doi: 10.1016/s0892-1997(02)00102-9. [DOI] [PubMed] [Google Scholar]
- Houghton O, McCluggage WG. The expression and diagnostic utility of p63 in the female genital tract. Advances in Anatomic Pathology. 2009;16:316–321. doi: 10.1097/PAP.0b013e3181b507c6. [DOI] [PubMed] [Google Scholar]
- Isik AC, Kalender Y, Yardimci S, Ergun A. Environmental tobacco smoke in rats. Journal of Otolaryngology. 2004;33:382–386. [PubMed] [Google Scholar]
- Jacobi HH, Skov PS, Kampen GT, Poulsen LK, Reimert CM, Bindslev-Jensen C, Mygind N. Histamine and tryptase in nasal lavage fluid following challenge with methacholine and allergen. Clinical & Experimental Allergy. 1998;28:83–91. doi: 10.1046/j.1365-2222.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Jeffery P, Li D. Airway mucosa: Secretory cells, mucus and mucin genes. European Respiratory Journal. 1997;10:1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- Johnston N, Bulmer D, Gill G, Panetti M, Ross P, Pearson J, Koufman J. Cell biology of laryngeal epithelial defenses in health and disease: Further studies. Annals of Otology, Rhinology and Laryngology. 2003;112:481–491. doi: 10.1177/000348940311200601. [DOI] [PubMed] [Google Scholar]
- Kahwa CK, Atwal OS, Purton M. Transmission electron microscopy of the epithelium of distal airways and pulmonary parenchyma of the goat lung. Research in Veterinary Science. 1997;63:49–56. doi: 10.1016/s0034-5288(97)90157-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Adachi W, Sotozono C, Nishida K, Yokoi N, Quantock AJ, Okubo K. Characteristics of the human ocular surface epithelium. Progress in Retinal and Eye Research. 2001;20:639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Knight D, Holgate S. The airway epithelium: Structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- Knowles M, Boucher R. Mucus clearance as a primary innate defense mechanism for mammalian airways. Journal of Clinical Investigation. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotby M, Nassar A, Seif E, Helal E, Saleh M. Ultrastructural features of vocal fold nodules and polyps. Acta Oto-Laryngologica. 1988;105:477–482. doi: 10.3109/00016488809119505. [DOI] [PubMed] [Google Scholar]
- Koufman J, Amin M, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngology—Head & Neck Surgery. 2000;123:385–388. doi: 10.1067/mhn.2000.109935. [DOI] [PubMed] [Google Scholar]
- Kutta H, Steven P, Kohla G, Tillmann B, Paulsen F. The human false vocal folds: An analysis of antimicrobial defense mechanisms. Anatomy and Embryology. 2002;205:315–323. doi: 10.1007/s00429-002-0255-8. [DOI] [PubMed] [Google Scholar]
- Kutta H, Steven P, Varoga D, Paulsen F. TFF peptides in the human false vocal folds of the larynx. Peptides. 2004;25:811–818. doi: 10.1016/j.peptides.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kutta H, Willer A, Steven P, Brauer L, Tsokos M, Paulsen F. Distribution of mucins and antimicrobial substances lysozyme and lactoferrin in the laryngeal subglottic region. Journal of Anatomy. 2008;213:473–481. doi: 10.1111/j.1469-7580.2008.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R, Charpied G, Faddis B. Effects of chronic ozone (O3) exposure on vocal-fold mucosa in bonnet monkeys. Journal of Voice. 1995;9:443–448. doi: 10.1016/s0892-1997(05)80208-5. [DOI] [PubMed] [Google Scholar]
- Leydon C, Bartlett R, Roenneburg D, Thibeault S. Localization of label-retaining cells in murine vocal fold epithelium. Journal of Speech, Language, and Hearing Research. 2011;54:1060–1066. doi: 10.1044/1092-4388(2010/10-0267). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leydon C, Fisher K, Lodewyck-Falciglia D. The cystic fibrosis transmembrane conductance regulator (CFTR) and chloride-depedent ion fluxes of ovine vocal fold epithelium. Journal of Speech, Language and Hearing Research. 2009;52:745–754. doi: 10.1044/1092-4388(2008/07-0192). [DOI] [PubMed] [Google Scholar]
- Leydon C, Selekman JA, Palecek S, Thibeault SL. Human embryonic stem cell-derived epithelial cells in a novel in vitro model of vocal mucosa. Tissue Engineering Part A. 2013;19:2233–2241. doi: 10.1089/ten.tea.2012.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leydon C, Sivasankar M, Lodewyck D, Atkins C, Fisher K. Vocal fold surface hydration: A review. Journal of Voice. 2009;23:658–665. doi: 10.1016/j.jvoice.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sheppard D, Hug M. Transepithelial electrical measurements with the Ussing chamber. [Review] Journal of Cystic Fibrosis. 2004;3:123–126. doi: 10.1016/j.jcf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Lillehoj E, Kim K. Airway mucus: Its components and function. Archives of Pharmacal Research. 2002;25:770–780. doi: 10.1007/BF02976990. [DOI] [PubMed] [Google Scholar]
- Ling C, Raasch JL, Welham NV. E-cadherin and transglutaminase-1 epithelial barrier restoration precedes type IV collagen basement membrane reconstruction following vocal fold mucosal injury. Cells Tissues Organs. 2011;193:158–169. doi: 10.1159/000318605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair and Regeneration. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodewyck D, Menco B, Fisher K. Immunolocalization of aquaporins in vocal fold epithelia. Archives of Otolaryngology-Head and Neck Surgery. 2007;133:557–563. doi: 10.1001/archotol.133.6.557. [DOI] [PubMed] [Google Scholar]
- Marcelino F, Oliveira D. Histopathological changes of vocal folds induced by chronic pollutant exposure: An experimental study. Journal of Voice. 2005;19:529–533. doi: 10.1016/j.jvoice.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Martins RH, Defaveri J, Custodio Domingues MA, de Albuquerque ESR, Fabro A. Vocal fold nodules: Morphological and immunohistochemical investigations. Journal of Voice. 2010;24:531–539. doi: 10.1016/j.jvoice.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Martins RH, Defaveri J, Domingues MA, de Albuquerque e Silva R. Vocal polyps: clinical, morphological, and immunohistochemical aspects. Journal of Voice. 2011;25:98–106. doi: 10.1016/j.jvoice.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Martins RH, Dias NH, Santos DC, Fabro AT, Braz JR. Clinical, histological and electron microscopic aspects of vocal fold granulomas. Brazilian Journal of Otorhinology. 2009;75:116–122. doi: 10.1016/S1808-8694(15)30842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Miyachi Y, Furuse M. Tight junctions in epidermis: From barrier to keratinization. European Journal of Dermatology. 2011;21:12–17. doi: 10.1684/ejd.2010.1192. [DOI] [PubMed] [Google Scholar]
- Mouadeb D, Belafsky P, Birchall M, Hood C, Konia T, Pinkerton K. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngology-Head and Neck Surgery. 2009;140:493–497. doi: 10.1016/j.otohns.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. Journal of Investigative Dermatology. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- Oguz H, Tarhan E, Korkmaz M, Yilmaz U, Safak MA, Demirci M, Ozluoglu LN. Acoustic analysis findings in objective laryngopharyngeal reflux patients. Journal of Voice. 2007;21:203–210. doi: 10.1016/j.jvoice.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Paleri V, Pearson J, Bulmer D, Jeannon J, Wight R, Wilson J. Expression of mucin gene products in laryngeal squamous cancer. Otolaryngology--Head and Neck Surgery. 2004;131:84–88. doi: 10.1016/j.otohns.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Pribuisiene R, Uloza V, Kupcinskas L, Jonaitis L. Perceptual and acoustic characteristics of voice changes in reflux laryngitis patients. Journal of Voice. 2006;20:128–136. doi: 10.1016/j.jvoice.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Reichel O, Mayr D, Durst F, Berghaus A. E-cadherin but not beta-catenin expression is decreased in laryngeal biopsies from patients with laryngopharyngeal reflux. European Archives of Otolaryngology. 2008;265:937–942. doi: 10.1007/s00405-007-0568-6. [DOI] [PubMed] [Google Scholar]
- Rose M, Voynow J. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiological Reviews. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Suehiro A, Echemendia N, Sivasankar M. Raised intensity phonation compromises epithelial barrier integrity. The Laryngoscope. 2011;121:346–351. doi: 10.1002/lary.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N, Merrill R, Thibeault S, Parsa R, Gray S, Smith E. Prevalence of voice disorders in teachers and the general population. Journal of Speech, Language, and Hearing Research. 2004;47:281–293. doi: 10.1044/1092-4388(2004/023). [DOI] [PubMed] [Google Scholar]
- Roy N, Tanner K, Gray S, Blomgren M, Fisher K. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure. Journal of Voice. 2003;17:331–342. doi: 10.1067/s0892-1997(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Sadri M, McMahon J, Parker A. Laryngeal dysplasia: aetiology and molecular biology. The Journal of Laryngology and Otology. 2006;120:170–177. doi: 10.1017/S0022215105005360. [DOI] [PubMed] [Google Scholar]
- Samuels T, Handler E, Syring N, Blumin J, Kerschner J, Johnston N. Mucin gene expression in human laryngeal epithelia: Effect of laryngopharyngeal reflux. Annals of Otology, Rhinology & Laryngology. 2008;117:688–695. doi: 10.1177/000348940811700911. [DOI] [PubMed] [Google Scholar]
- Sataloff R. The impact of pollution on voice. Otolaryngology Head and Neck Surgery. 1992;106:701–705. doi: 10.1177/019459989210600614. [DOI] [PubMed] [Google Scholar]
- Savelli V, Rizzoli R, Rizzi E, Galanzi A, Buffa A, Rana R, Baratta B. Cell kinetics of vocal fold epithelium in rats. Boll Soc Ital Biol Sper. 1991;67:1081–1088. [PubMed] [Google Scholar]
- Schneider B, Teschner M, Sudermann T, Pikula B, Lautermann J. Expression of gap junction proteins (connexin 26, 30, 32, 43) in normal mucosa, hyperkeratosis and carcinoma of the human larynx. Journal for Oto-Rhino-Laryngology and its Related Specialties. 2002;64:324–329. doi: 10.1159/000066086. [DOI] [PubMed] [Google Scholar]
- Shin T, Watanabe H, Oda M, Umezaki T, Nahm I. Contact granulomas of the larynx. European Archives of Oto-rhino-laryngology. 1994;251:67–71. doi: 10.1007/BF00179894. [DOI] [PubMed] [Google Scholar]
- Sivasankar M, Erickson E, Rosenblat M, Branski R. Hypertonic challenge to the vocal folds: Effects on barrier function. Otolaryngology-Head and Neck Surgery. 2010;142:79–84. doi: 10.1016/j.otohns.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar M, Erickson E, Schneider S, Hawes A. Phonatory effects of airway dehydration: Preliminary evidence for impaired compensation to oral breathing in individuals with vocal fatigue. Journal of Speech, Language, and Hearing Research. 2008;51:1494–1506. doi: 10.1044/1092-4388(2008/07-0181). [DOI] [PubMed] [Google Scholar]
- Sivasankar M, Fisher K. Oral breathing increases Pth and vocal effort by superficial drying of the vocal fold mucosa. Journal of Voice. 2002;16:172–181. doi: 10.1016/s0892-1997(02)00087-5. [DOI] [PubMed] [Google Scholar]
- Sivasankar M, Fisher K. Vocal folds detect ionic perturbation on the luminal surface: An in vitro investigation. Journal of Voice. 2008;22:408–419. doi: 10.1016/j.jvoice.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. Journal of the National Cancer Institutes Monographs. 2001;29:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- Stanley MA. Epithelial cell responses to infection with human papillomarivus. Clinical Microbiology Reviews. 2012;25:215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell PM, Gudrun R, Watt J. Morphology of the human larynx. III. The supraglottis. Clinical Otolaryngology and Allied Sciences. 1981;6:389–393. doi: 10.1111/j.1365-2273.1981.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investigative Ophthalmology & Visual Science. 1993;34:1829–1844. [PubMed] [Google Scholar]
- Stiblar-Martincic D. Histology of laryngeal mucosa. ACTA Oto-laryngologica (Stockholm), Suppl. 1997;527:138–141. [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cellular and Molecular Life Scieces. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Kishioka C, Ishinaga H, Sakakura Y, Majima Y. Histamine alters gene expression in cultured human nasal epithelial cells. Journal of Allergy and Clinical Immunology. 2001;107:310–314. doi: 10.1067/mai.2001.112127. [DOI] [PubMed] [Google Scholar]
- Troxell ML, Gopalakrishnan S, McCormack J, Poteat BA, Pennington J, Garringer SM, Marrs JA. Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. Journal of Cell Science. 2000;113:985–996. doi: 10.1242/jcs.113.6.985. [DOI] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarx H, Günzel D, Fromm M, Niessen CM. E-cadherin is essention for in vivo epidermal barrier function by regulating tight junctions. The European Molecular Biology Organization Journal. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault S, Rees L, Pazmany L, Birchall M. At the crossroads: Mucosal immunology of the larynx. Mucosal Immunology. 2009;2:122–128. doi: 10.1038/mi.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deusen MB, Lyon MJ. Connexins within the rat larynx. Otolaryngology-Head and Neck Surgery. 2008;139:823–828. doi: 10.1016/j.otohns.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Verdolini K, Min Y, Titze I, Lemke J, Brown K, van Mersbergen M, Fisher K. Biological mechanisms underlying voice changes due to dehydration. Journal of Speech, Language, and Hearing Research. 2002;45:268–281. doi: 10.1044/1092-4388(2002/021). [DOI] [PubMed] [Google Scholar]
- Williams O, Sharafkhaneh A, Kim V, Dickey B, Evans C. Airway mucus: From production to secretion. American Journal of Respiratory Cell and Molecular Biology. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Current Opinion in Cell Biology. 2001;13(5):578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Hirano S, Kanemaru S, Tsuji S, Suehiro A, Ito J. Side population cells in the human vocal fold. Annals of Otology, Rhinology and Laryngology. 2007;116:847–852. doi: 10.1177/000348940711601110. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Fisher K. Tight junction-related barrier contributes to the electrophysiological asymmetry across vocal fold epithelium. Plos ONE. 2012;7:e34017. doi: 10.1371/journal.pone.0034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Cai Y, Wang H. Pathological changes of hyperphonated cat vocal folds. Auris Nasus Larynx. 1991;18:55–59. doi: 10.1016/s0385-8146(12)80250-1. [DOI] [PubMed] [Google Scholar]