Abstract
Concentrations of HIV-1 RNA and DNA in mucosal compartments influence the risk of sexual transmission and mother-to-child transmission of HIV-1. Breast milk production is physiologically regulated such that supply is a function of infant demand but whether demand also influences HIV-1 dynamics in breast milk is unknown. We tested whether minor and major changes in feeding frequency influence breast milk viral concentrations in 958 HIV-1-infected women, who were followed with their infants for 24 months as part of a trial in Lusaka, Zambia. Women were randomized to wean abruptly at 4 months or to continue breastfeeding for a duration of their own choosing. Two weeks after breastfeeding cessation i.e. weaning (4.5 months) HIV-1 concentrations in breast milk were substantially higher (median RNA 2,708 copies/ml and DNA 14 copies/ml) than if breastfeeding continued (median RNA <50 copies/ml and DNA <1 copy/ml, p<0.0001). Among those continuing breastfeeding, HIV-1 concentrations in milk were higher if breastfeeding was non-exclusive (median RNA 293 copies/ml and DNA 2 copies/ml, p=0.0006). Elevated milk viral concentrations after stopping breastfeeding explained higher than expected rates of late postnatal HIV transmission in those who weaned early. Changes in the frequency of breastfeeding peri-weaning and with non-exclusive breastfeeding influenced milk viral concentrations. This may explain the reduced risk of HIV-1 transmission associated with exclusive breastfeeding and may explain why early weaning does not achieve the magnitude of HIV prevention predicted by models. Our results support continuation of maternal antiretroviral drug interventions over the full duration of time when any breast milk exposures are likely to occur after planned weaning.
Introduction
Concentrations of HIV-1 RNA and DNA in mucosal fluids strongly influence the risk of sexual and mother-to-child transmission of HIV-1 [1–7]. These concentrations are known to be related to systemic viral burden, usually measured by plasma HIV-1 RNA concentrations, and to immunosuppression measured by systemic CD4 T-cell counts [8]. Mucosal HIV-1 concentrations are dramatically reduced by antiretroviral drugs; although intermittent or lower viral shedding often persists in mucosal compartments despite treatment [9, 10], potentially explaining why antiretroviral interventions are not 100% effective for prevention. Both sexual and mother-to-child HIV-1 transmission are inefficient, stochastic processes with no lower or upper exposure thresholds at which transmission never or always occurs, respectively [1–7].
Human milk production is a highly orchestrated process such that milk volume and composition are dynamically regulated and vary over the course of a feeding [11, 12]. Infant suckling is a major regulator of milk production by stimulating production of prolactin which supports milk production; removal of milk further stimulates more milk production [11, 12]. These feedback loops ensure that within a few days after delivery with regular feeding, human breasts produce almost exactly the amount of milk required by a specific infant. Whether this hormonally-regulated, demand-supply process influences HIV-1 dynamics in breast milk is unknown.
We conducted a randomized clinical trial in Lusaka, Zambia to examine the safety and efficacy of early weaning to reduce HIV-1 transmission and infant mortality [13]. 958 HIV-1-infected women were counseled to breastfeed for at least 4 months at which time half were encouraged to wean abruptly and the other half encouraged to breastfeed for a duration of their own choosing. Infants were followed to 24 months with regular HIV-1 DNA PCR tests to determine the timing of transmission. To ascertain compliance with early weaning, breast milk was pumped for a standard duration at around 4.5 months post-partum from all women regardless of their reported feeding practice [14]. For women who had weaned, pumping was scheduled to occur 2 weeks after the cessation of all breastfeeding. HIV-1 RNA and DNA were quantified in milk collected at 4 and 4.5 months. The unique study design and timing of sample collection, specifically a sample after weaning, allowed us to test the hypothesis that minor and major behavioral changes in feeding frequency influence HIV-1 dynamics in human breast milk.
Results
Randomized groups differed in breast milk HIV-1 concentrations at the post-weaning timepoint
There were dramatic differences in concentrations of HIV-1 RNA and DNA in breast milk at 4.5 months between the two randomized groups. Among 481 women randomized to early weaning, breast milk concentrations of HIV-1 RNA [median 388 copies/ml; interquartile range (IQR): <50–8,624) and HIV-1 DNA (median 6 copies/ml; IQR: <1–69) were significantly higher than among 475 women randomized to continue breastfeeding (RNA median <50 copies/ml [IQR: <50–452] and DNA median <1 copy/ml [IQR: 0–5] p<0.0001 and p<0.0001, respectively). This is a conservative analysis as only 60.5 % of women randomized to stop breastfeeding did so by 4.5 months thus diluting differences between the randomized groups. This conservative, intent-to-treat analysis, clearly demonstrates that differences in viral concentrations between the groups were not due to self-selection associated with women who chose one feeding method over another. In addition, HIV-1 RNA and DNA concentrations in breast milk collected at 4 months, prior to any study-directed changes in feeding practices, did not differ between the groups (Figure 1).
Fig. 1.
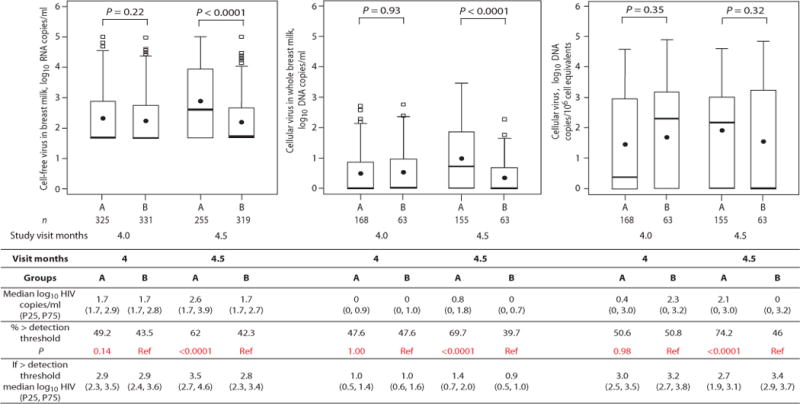
Concentrations of cell-free HIV-1 RNA, cellular HIV-1 DNA per ml of breast milk, and cellular HIV-1 DNA per 106 cell equivalents at 4 and 4.5 months from HIV-1 infected women randomized to wean at 4 months (group A) or to continue breastfeeding (group B). The numbers of samples analyzed at each visit are shown below the box plot. P-values from Wilcoxon tests are shown for corresponding box pairs. The thick horizontal bar represents the median value and the dot the mean; the bottom and top of each box represents the 25th and 75th percentiles; the lower and upper bars of each box represent the minimum values within 1.5 times the inter-quartile range. Observations beyond 1.5 times the IQR are shown as outliers. Actual values for the median and 25th and 75th percentiles (overall and only among those above detection) of cell-free HIV-1 RNA, cellular HIV-1 DNA per ml of breast milk, and cellular HIV-1 DNA per 106 cell equivalents at 4 and 4.5 months by random assignment are shown in the Table. The table also shows the proportions with breast milk HIV-1 concentrations above the detection threshold defined as >50 copies/ml for HIV-1 RNA and >0 for HIV-1 DNA. Chi-squared tests were used to calculate the p-values comparing the proportions above detection across groups.
Women who weaned early had higher breast milk HIV-1 concentrations post-weaning
To strengthen inferences about the role of changes in breastfeeding behaviors, we analyzed HIV-1 concentrations in breast milk by actual feeding behaviors. These behaviors were more heterogeneous in our study population than usually observed due to the study design. In the intervention group, 69.0% had weaned abruptly by 5 months compared to 7.4% among the controls. The median duration of breastfeeding was 4 months (IQR: 4–14 months) in the intervention group and 16 months (IQR 11–19 months) in the control group [13]. Breast milk HIV-1 RNA and DNA concentrations were significantly higher among those who had fully weaned by the time of milk sampling (median RNA 2,708 copies/ml [IQR: 163–36,790]; DNA 14 copies/ml [IQR: <1–81]) than among those still exclusively breastfeeding at this same age (median RNA <50 copies/ml [IQR: <50–383] and DNA <1 copy/ml [IQR: <1–5] p=0.0006, p<0.0001, respectively). More than three-quarters (77.3%) of those who had stopped breastfeeding had breast milk RNA concentrations above the threshold of detection of 50 copies/ml (median above detection was 8,166 copies/ml [IQR: 1,261–56,568]) compared to 39.5% of those exclusively breastfeeding at this time (median above detection 607 copies/ml [IQR: 225–2,226] p<0.0001). These groups were not different 2 weeks earlier at 4 months (Figure 2). These large differences in breast milk viral concentrations at 4.5 months were still observed after adjustment for potential confounders, including maternal plasma HIV-1 RNA concentrations and CD4+ T cell counts.
Fig. 2.
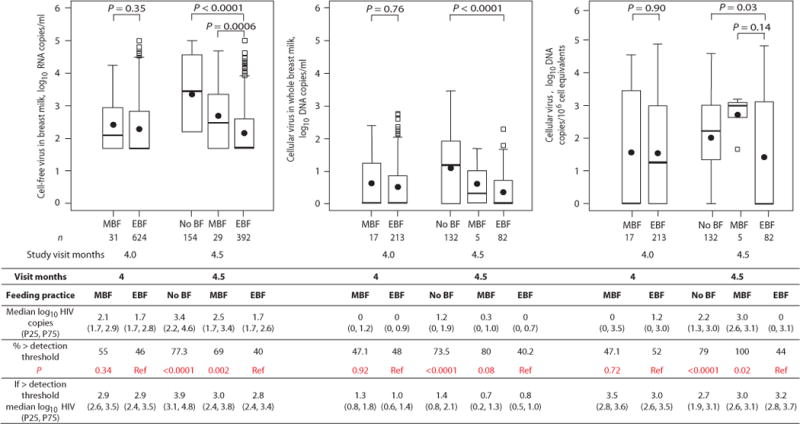
Concentrations of cell-free HIV-1 RNA in copies per ml, cellular HIV-1 DNA in copies per ml of breast milk, and cellular HIV-1 DNA in copies per 106 cell equivalents at 4 and 4.5 months from HIV-1 infected women who had stopped breastfeeding (No BF), were mixed breastfeeding (MBF) or exclusively breastfeeding (EBF). The numbers of samples analyzed in each group are shown below the box plot. P-values from Wilcoxon tests are shown for corresponding box pairs. The thick horizontal bar represents the median value and the dot the mean; the bottom and top of each box represents the 25th and 75th percentiles; the lower and upper bars of each box represent the minimum values within 1.5 times the inter-quartile range. Observations beyond 1.5 times the IQR are shown as outliers. Actual values for the median and 25th and 75th percentiles (overall and among those above detection) of cell-free HIV-1 RNA, cellular HIV-1 DNA per ml of breast milk, and cellular HIV-1 DNA per 106 cell equivalents in each group are shown in the table. The table also shows the proportions with breast milk HIV-1 concentrations above the detection threshold (defined as >50 copies/ml for HIV-1 RNA and >0 for HIV-1 DNA) in each group. Chi-squared tests were used to calculate the p-values comparing the proportions with breast milk HIV-1 concentrations above the detection threshold for each group relative to the EBF group.
A within-individual analysis yielded further compelling results supporting the hypothesis that changes in feeding behaviors lead to changes in mucosal viral concentrations. The median within-woman change between 4 and 4.5 months was an increase of 1.00 log copies/ml (IQR 0, 1.78) of HIV-1 RNA in those who stopped breastfeeding; whereas in those who continued breastfeeding there was no consistent change (median 0 copies/ml; IQR −0.21, 0, p<0.0001). HIV-1 DNA concentrations followed a similar pattern and were significantly elevated after weaning (Figure 3). Among those who had undetectable HIV-1 RNA at 4 months, 58.7% had RNA concentrations above detection 2 weeks after they had stopped breastfeeding (median in those above detection was 2,657 copies/ml [IQR: 1,217–8,624]). In those who had detectable HIV-1 RNA at 4 months, the median increase was 15,973 copies/ml.
Fig. 3.
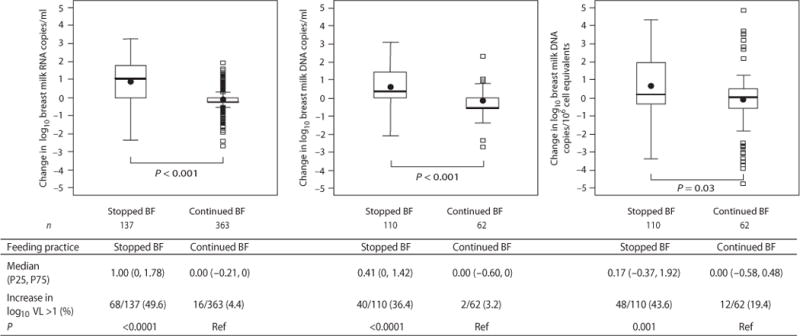
Change in concentrations of breast milk HIV-1 RNA in copies per ml, cellular HIV-1 DNA in copies per ml of breast milk, and cellular HIV-1 DNA in copies per 106 cell equivalents between 4 and 4.5 months stratified by whether breastfeeding (BF) had stopped or continued by 4.5 months. The numbers of paired samples analyzed in each group are shown below the box plot. P-values from Wilcoxon tests are shown for corresponding box pairs. The thick horizontal bar represents the median value and the dot the mean; the bottom and top of each box represents the 25th and 75th percentiles; the lower and upper bars of each box represent the minimum values within 1.5 times the inter-quartile range. Observations beyond 1.5 times the IQR are shown as outliers. Actual values for the median and 25th and 75th percentiles of the change in concentration between 4 and 4.5 months in each group are shown in the table. The table also shows the proportions that increased their breast milk HIV-1 concentration by more than 1 log between 4 and 4.5 months. Chi-squared tests were used to calculate the p-values comparing the proportions with a >1 log increase between those who stopped or continued breastfeeding.
The ratio of viral RNA:DNA concentrations (in log10 units) increased from a median of 1.7 (IQR 1.7, 2.3) at 4 months prior to weaning to 2.2 (IQR 1.7, 2.7) at 4.5 months among those who weaned (p=0.001). Among those still exclusively breastfeeding at 4.5 months, the median RNA:DNA ratio was 1.7 (IQR 1.6, 2.4) at 4 months and remained at a lower median of 1.7 (IQR 1.7, 2.4 p=0.02) at 4.5 months.
Women practicing non-exclusive breastfeeding had higher breast milk HIV-1 concentrations than women practicing exclusive breastfeeding
Women who had given other liquids and solids to the infant in the prior 2 weeks but who were also still breastfeeding (non-exclusive or “mixed” breastfeeding) at 4.5 months had higher concentrations of breast milk HIV-1 RNA (median RNA 293 copies/ml; IQR: <50–2,298) at 4.5 months than women exclusively breastfeeding at this time (Figure 2). Most women (84%) in this study had breastfed exclusively to at least 4 months [15]. Relative to exclusive breastfeeding, non-exclusive breastfeeding was associated with an increase of 0.52 log copies/ml (95% CI: 0.18–0.86, p=0.003) at 4.5 months. Women who were non-exclusively breastfeeding at 4.5 months were doing so against study counseling advice and were more likely to be first-time mothers. There were no other significant differences between the groups. After adjustment for parity, maternal plasma HIV-1 RNA concentrations and CD4+ T cell counts (which were not collinear), there was still an association between non-exclusive breastfeeding and higher HIV-1 RNA concentrations in breast milk (0.41 log increase; 95% CI 0.10–0.72). Breast milk HIV-1 RNA concentrations did not differ between women who were defined as ever vs. never (prior to 4.5 months) mixed breastfeeders.
Changes in milk volume, milk cellularity and breast pathology during weaning
Among the women who had weaned by 4.5 months, 26% produced no milk during the protocol-scheduled pumping and were thus excluded from the above analyses. Among the remaining 74% of women who had weaned, the volume of milk produced on timed pumping was significantly lower compared to volumes from women who were still breastfeeding. Weaning also led to significantly higher beta-globin concentrations in milk, used as a marker of milk cellularity (Table 1). To adjust for the cellularity of the post-weaning milk, we standardized HIV-1 DNA copies per million cell equivalents in breast milk. There continued to be a significant increase in HIV-1 DNA copies per million cell equivalents but the elevations were less marked than observed for concentrations of HIV-1 RNA and DNA per ml of breast milk (Figures 1 & 2).
Table 1.
Characteristics of HIV1-infected women at 4.5 months who had stopped or were still breastfeeding at this time
| Stopped Breastfeeding | Still breastfeeding | p-value | |
|---|---|---|---|
| N=227 | N=507 | ||
| N (%) on time for 4.5 month visit | 197 (86.8) | 432 (85.2) | |
| N (%) came late for pumping visit | 30 (13.2) | 75 (14.8) | |
| Median (IQR) infant age at 4.5 month visit (days) | 141 (140–143) | 141 (140–144) | 0.61 |
| Median (IQR) time since stopping BF (days) | 14 (12, 16) | N/A | |
| Abruptness of weaning | |||
| Immediately | 137/218 (62.8%) | N/A | |
| 1 – 2 days | 40/218 (18.4%) | ||
| > 3 days | 41/218 (18.8%) | ||
| No milk produced | 56/215 (26.1%) | 4/440 (0.9%) | <0.0001 |
| Median (IQR) quantity of milk produced (ml) | 14 (1–28) | 20 (11–28) | <0.0001 |
| Any expressed breast milk before 4.5 month study visit | 183/227 (80.6%) | 162/507(32.0%) | <0.0001 |
| Only Exclusive Breastfeedingbefore stopping or 4.5 month visit | 172/227(75.8%) | 394/507 (77.7%) | 0.56 |
| Any breast problem | 26/205 (12.7%) | 17/503 (3.4%) | <0.0001 |
| Maternal fever | 40/208 (19.2%) | 28/501 (5.6%) | <0.0001 |
| Mastitis | 18/209 (8.6%) | 4/503 (0.8%) | <0.0001 |
| n=132 | n=87 | ||
| Beta-globin concentration (copies/2μl) |
7107 (842, 18,023) |
237 (11–774) |
<0.0001 |
Weaning also resulted in significant increases in all breast problems (p<0.0001), maternal fever (p<0.0001) and clinical mastitis (p<0.0001) compared to continued breastfeeding (Table 1). The increases in HIV-1 RNA and DNA in milk after weaning were independent of the increases in breast pathology and maternal fever and were most marked in women with no discernible clinical pathology (Figure 4).
Fig. 4.
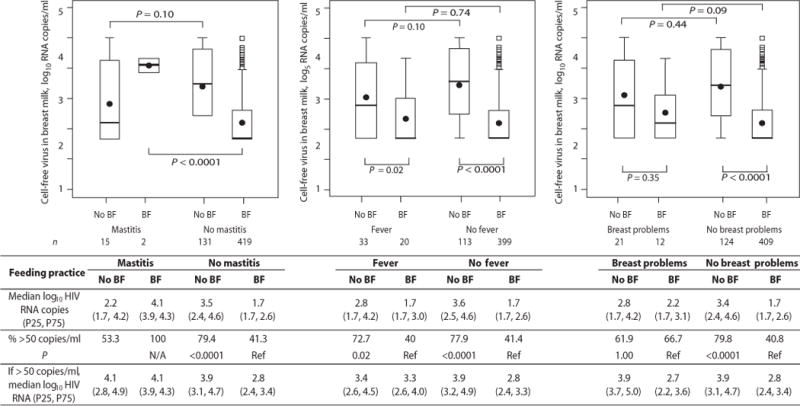
Concentrations of cell-free HIV-1 RNA in breast milk at 4.5 months from HIV-1 infected women who had stopped breastfeeding (No BF) and continued breastfeeding (BF) among those who did and did not have mastitis, fever or any breast problems at 4.5 months. The numbers of samples analyzed in each group are shown below the box plot. P-values from Wilcoxon tests are shown for corresponding box pairs. The thick horizontal bar represents the median value and the dot the mean; the bottom and top of each box represents the 25th and 75th percentiles; the lower and upper bars of each box represent the minimum values within 1.5 times the inter-quartile range. Observations beyond 1.5 times the IQR are shown as outliers. Actual values for the median and 25th and 75th percentiles (overall and only among those above detection) of cell-free HIV-1 RNA in each group are shown in the table. The table also shows the proportions with breast milk HIV-1 RNA concentrations >50 copies/ml in each group. Chi-squared tests were used to calculate the p-values comparing the proportions with breast milk HIV-1 concentrations above the detection threshold by breastfeeding status within each stratum (no mastitis, fever, no fever, breast problems, no breast problems) separately. P-value was not calculated in the mastitis stratum due to small sample size.
Predictors of HIV-1 concentrations in breast milk
Concentrations of HIV-1 RNA and DNA in breast milk at 4.5 months were significantly associated with maternal plasma HIV-1 concentrations, maternal CD4+ T cell counts, volume of milk produced on timed pumping and milk beta-globin concentrations. The slope of the curve describing the relationship between maternal plasma HIV-1 RNA concentrations and breast milk HIV-1 RNA or DNA was almost identical whether or not breastfeeding had stopped but the concentration of milk HIV-1 RNA was an average of 1.16 logs higher among those who had weaned. The relationship between maternal CD4+ T cell counts and breast milk viral concentrations was similarly affected (Figure 5). Breast milk HIV-1 RNA and DNA copies/ml were more strongly related to milk cellularity in those who had weaned than among those who were still breastfeeding (p-interaction<0.001). Pumped milk volume was only associated with breast milk HIV-1 concentrations in those who had weaned (p-interaction<0.001).
Fig. 5.
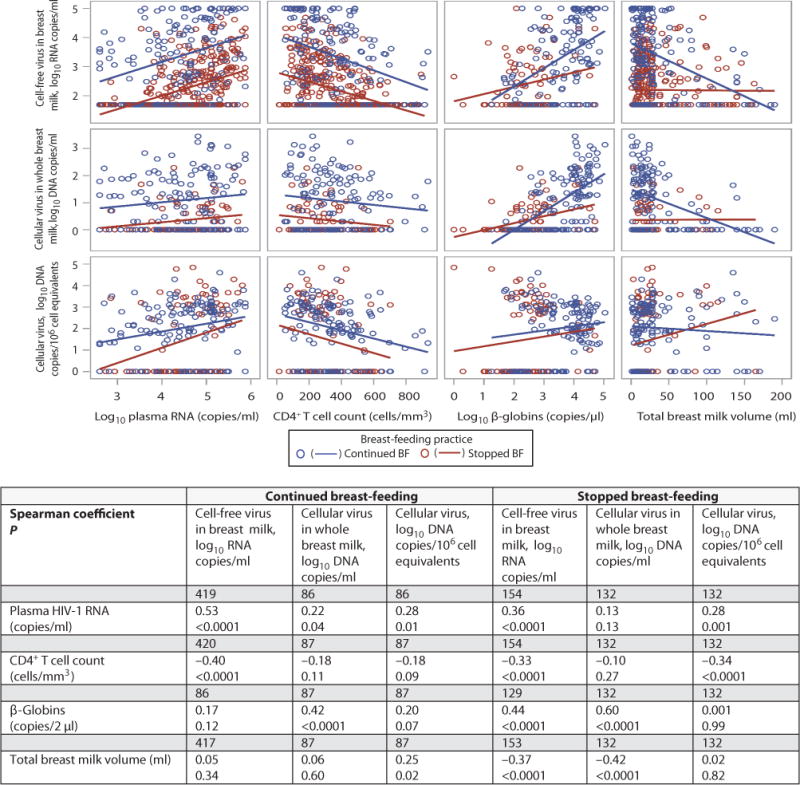
Scatter plots showing associations between breast milk HIV-1 RNA concentrations in copies/ml, HIV-1 DNA concentration in copies/ml and HIV-1 DNA concentration in copies per 106 cell equivalents and plasma HIV-1 RNA, CD4 T cell count, milk beta-globin concentration, and total breast milk volume produced after timed pumping, stratified by feeding practice at 4.5 months. In the table are shown the spearman correlation coefficients and associated p-values for each bivariate association in the continued breastfeeding and stopped breastfeeding groups separately as well as the number of samples analyzed for each association. Associations between breast milk HIV-1 RNA, beta-globin and milk volume were significantly modified by feeding practice (p-value for interactions=0.02 and <0.0001, respectively); as were associations with breast milk HIV-1 DNA copies/ml (p-value for interactions=0.0003 and 0.0008, respectively); association between breast milk HIV-1 DNA copies/106 cell equivalents and milk volume was significantly modified by feeding practice (p-value for interaction=0.03). P-values for the interactions were calculated using linear regression with multiplicative interaction terms of the relevant covariates.
Breast milk HIV-1 RNA and DNA concentrations at 4.5 months were significantly correlated with each other in both women who were breastfeeding and those who had weaned. The relationship between DNA copies/ml and DNA copies per cell equivalent was somewhat weaker, although still significant, in those who had weaned compared to those who were still breastfeeding (Fig S1).
Increases in breast milk HIV-1 RNA concentrations post-weaning were greater if weaning was completely abrupt (1.07; 95% CI −1.21, 3.35) relative to that occurring over >3 days (0.58; 95% CI −1.71, 2.88, p=0.04). Changes in breast milk concentrations of DNA copies/ml were also greater if weaning was abrupt (0.85; 95% CI −1.14, 2.83) relative to that occurring over >1 day (0.31; 95% CI −1.68, 2.30, p=0.006). Adherence to the study protocol which encouraged manual expression of breast milk during the weaning transition was associated with a smaller proportion of women (35.3%) having greater than 1 log increase in breast milk HIV-1 RNA concentrations post-weaning than those who did not follow this advice (51.7%, p=0.02). Whether breastfeeding had or had not been fully exclusive up to the time of weaning did not influence the magnitude of post-weaning viral elevation.
Effects of weaning-associated changes in breast milk HIV-1 concentrations on postnatal transmission
The risk of intrauterine infection, defined as a positive PCR result within the first 3 days of life, was 5.9% (n=56) in the overall cohort and rose to 12.6% (n=117) when combined with intrapartum and very early postnatal transmission (a positive PCR result in the first 42 days of life). Almost all women breastfed to 4 months (95.5%) and by 4 months the transmission rate had further risen to 16.8% (n=154). Late postnatal transmission (defined as infection detected >4 months) occurred among a further 54 infants by 24 months yielding a total transmission rate of 24.4%.
The risk of late postnatal infection (>4 months) was 7.2% by 24 months in those who reported stopping all breastfeeding at 4 months vs 9.7% by those who were still breastfeeding at 4 months (Relative Hazard [RH]=0.65 95% CI: 0.34–1.23). If adjusted for the breast milk HIV-1 RNA concentration at 4.5 months, the reduction in transmission due to early weaning was stronger and became significant (RH=0.28 95% CI: 0.11–0.74). The risk of late postnatal infection in those who reported stopping was 3.5-fold higher (95% CI: 1.65–7.42) than expected if breastfeeding duration is taken into account by censoring follow-up time 30 days after weaning. This excess transmission risk associated with early weaning which we observed after adjusting for breastfeeding duration was attenuated towards the null (RH=1.25 95% CI: 0.42–3.75) once breast milk HIV-1 RNA concentrations at 4.5 months were taken into account, indicating that these breast milk viral elevations post-weaning explained the excess transmission risk occurring among those who weaned early.
Viewed from an intent-to-treat perspective, the risk of late postnatal transmission was 7.6% in the group randomized to stop breastfeeding at 4 months and 10.2% in the group randomized to continue breastfeeding (RH=0.67, 95% CI: 0.39–1.15). However, if adjusted for the HIV-1 RNA concentrations in breast milk collected at 4.5 months, then the benefit of being in the intervention group strengthened (RH=0.34, 95% CI: 0.16–0.76). In other words, if the post-weaning increases in breast milk HIV concentrations were removed, early weaning would have resulted in a more than 3-fold decrease in the risk of late postnatal infection.
Ten of 54 (19%) late postnatal infections (i.e. infections detectable only after 4 months of age in children with negative results at 4 months) occurred among infants of mothers who reported stopping all breastfeeding at 4 months. There was no change in breast milk HIV-1 RNA and DNA concentrations between 4 and 4.5 months (median change in RNA 31 [IQR −721, 201] and DNA −3 [IQR −23, −2]) in these 10 transmissions suggesting these children had not stopped breastfeeding at the time the sample was taken, contrary to the maternal report. This is unlike the substantial changes observed in the group overall (described above).
Both HIV-1 RNA and DNA concentrations in breast milk were strongly associated with postnatal transmission in univariable analysis but only HIV-1 RNA concentrations remained associated with both early and late postnatal HIV transmission after adjusting for maternal CD4+ T cell counts and plasma HIV-1 RNA concentrations (which were not collinear in the model). Non-exclusive breastfeeding, breast problems, low maternal CD4+ T cell counts and a positive syphilis screening test were also independently associated with increased risk of early postnatal HIV transmission. Low maternal CD4+ T cell counts and high plasma HIV-1 RNA were the only factors along with breast milk HIV-1 RNA concentrations independently associated with increased risk of late postnatal HIV transmission (Table 2).
Table 2.
Risk factors* for early (<4 months) and late (>4 months) postnatal HIV transmission among infants born to HIV-infected women in Lusaka, Zambia
| Early transmission N=37/839 | Late transmission† N=54/695 | |||
|---|---|---|---|---|
| Relative Hazard (95% CI) | Relative Hazard (95% CI) | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HIV-1 concentrations in breast milk at 4 months | ||||
| HIV-1 RNA | ||||
| >500 copies/ml | 19.25 (4.43–83.75) | 10.32 (4.88–21.86) | ||
| 50–500 copies/ml | 7.82 (1.52–40.33) | 3.83 (1.60–9.13) | ||
| < 50 copies/ml | 1.0 | 1.0 | ||
| HIV-1 RNA log copies/ml (continuous) | 2.74 (1.90–3.95) | 2.37 (1.54–3.66) | 2.93 (2.18, 3.95) | 2.09 (1.49, 2.95) |
| HIV-1 DNA | ||||
| > 1 copies/ml | 3.66 (1.33–10.06) | 2.79 (1.29, 6.04) | ||
| Undetectable | 1.0 | 1.0 | ||
| HIV-1 DNA log copies/ml (continuous) | 2.12 (1.31–3.45) | 1.52 (1.04, 2.23) | ||
| HIV-1 DNA / cell equivalents | ||||
| > 1 copies/1 million cell equivalents | 3.20 (1.16 – 8.81) | 2.99 (1.34, 6.65) | ||
| Undetectable | 1.0 | 1.0 | ||
| HIV-1 DNA log copies/1M cell equivalents (continuous) | 1.45 (1.09–1.93) | 1.44 (1.15, 1.82) | ||
| HIV-1 concentrations in breast milk at 4.5 months | ||||
| HIV-1 RNA | n/a | |||
| >500 copies/ml | 6.56 (3.07, 13.99) | |||
| 50–500 copies/ml | 1.99 (0.77, 5.15) | |||
| < 50 copies/ml | 1.0 | |||
| HIV-1 RNA log copies/ml (continuous) | 2.37 (1.74, 3.23) | |||
| HIV-1 DNA | n/a | |||
| > 1 copies/ml | 2.34 (1.16, 4.74) | |||
| Undetectable | 1.0 | |||
| HIV-1 DNA log copies/ml (continuous) | 1.90 (1.21, 2.97) | |||
| HIV-1 DNA / cell equivalents | n/a | |||
| > 1 copies/1 million cell equivalents | 2.56 (1.23, 5.32) | |||
| Undetectable | 1.0 | |||
| HIV-1 DNA log copies/1 million cell equivalents (continuous) | 1.32 (1.08, 1.62) | |||
| Feeding Characteristics | ||||
| Non-exclusive breastfeeding‡ | 2.90 (1.43–5.88) | 3.06 (1.19–7.85) | N/A | N/A |
| Exclusive Breastfeeding | 1.0 | |||
| Any breast problems‡ | 3.27 (1.16–9.25) | 1.19 (0.16, 8.81) | ||
| Maternal factors | ||||
| CD4+ T cell counts | ||||
| <350 cells/mm3 | 8.21 (2.91–23.17) | 5.37 (2.76, 10.43) | ||
| >350 cells/mm3 | 1.0 | 1.0 | ||
| CD4 cell count (on continuous scale per 100 cells/mm3) | 0.52 (0.40–0.67) | 0.57 (0.40–0.83) | 0.59 (0.49, 0.71) | 0.69 (0.55, 0.87) |
| Plasma HIV-1 RNA | ||||
| >50,000 copies/ml | 5.73 (2.62–12.53) | 4.60 (2.53, 8.38) | ||
| <50,000 copies/ml | 1.0 | 1.0 | ||
| Plasma HIV-1 RNA log copies/ml (continuous scale) | 3.72 (2.15–6.46) | 3.19 (2.05, 4.96) | 1.96 (1.11, 3.46) | |
| Maternal hemoglobin | ||||
| < 10 g/dL | 2.16 (1.13–4.15) | 1.94 (1.11, 3.39) | ||
| > 10 g/dL | 1.0 | 1.0 | ||
| Rapid Plasma Reagin status during pregnancy§ | ||||
| Positive | 2.53 (1.24–5.14) | 5.00 (2.10–11.93) | 0.84 (0.40, 1.79) | |
| Negative | 1.0 | 1.0 | ||
| Infant factors | ||||
| Birth weight (grams) | ||||
| <2500 | 2.31 (1.01–5.28) | 1.92 (0.90, 4.08) | ||
| >2500 | 1.0 | 1.0 | ||
Calculated using Cox Proportional Hazards models. Adjusted relative hazards are from multivariable models adjusting simultaneously for the variables shown.
Follow-up time was censored 30 days after cessation of breastfeeding for late transmission only
Coded as a time-dependent covariates
A dummy variable for missing Rapid Plasma Reagin results (screening test for syphilis) (n=47) was included in the model
Discussion
We demonstrate that major changes in the frequency of infant feeding that occur around the time of weaning play a critical role in determining concentrations of HIV-1 in breast milk. Sudden reductions in the frequency of infant suckling associated with weaning led to more than one log increase in breast milk HIV-1 RNA and DNA concentrations. Both HIV-1 RNA and DNA were affected, RNA to the greatest extent. Systemic factors, including CD4 T cell counts and plasma HIV-1 RNA concentrations, continued to predict mucosal concentrations peri-weaning but the average milk concentrations were higher than baseline. These increases in mucosal virus shedding were not explained by self-selection of women who stopped breastfeeding; differences remained strong and significant in intent-to-treat analyses, in within-person comparisons and after adjusting for possible confounders. The amount of milk that could be produced on timed pumping, an imperfect but the most direct measurement of the frequency of milk removal,[16] strongly and inversely correlated with concentrations of HIV-1 RNA and DNA among women who had stopped breastfeeding.
Although weaning resulted in maternal morbidity and increased risk of breast problems including mastitis (Table 1), these outcomes did not account for the elevations in milk HIV concentrations. In fact, elevations were most marked in women without clinically-evident pathologies (Figure 4). Consistent with the elevations in breast pathologies, weaning was also associated with increases in milk cellularity which, in turn, was strongly correlated with HIV-1 RNA and DNA concentrations in milk. However, even after standardizing HIV-1 DNA concentrations in milk for cellular content, there continued to be significantly elevated virus concentrations.
We propose that opening of the paracellular tight junctions of the mammary gland, as is known to occur during the establishment of lactation and during weaning [16, 17], is the most parsimonious explanation for the findings. Animal models of lactation, as well as clinical studies in humans, have described this physiological process that occurs during weaning [16–18]. Opening of the tight junctions would facilitate diffusion of cell-free as well as cell-associated virus into breast milk. This may also be associated with inflammatory processes that may serve to up-regulate viral replication in this compartment. We also cannot rule out other changes associated with weaning, such as upregulation of milk protein synthesis, upregulation of lactose and lipid synthesis, and increased oxidative metabolism [18-21] that may explain these elevations.
While weaning is by definition a major change in the frequency of infant suckling, we also observed changes in HIV-1 concentrations in breast milk in association with more minor behavioral changes, namely with non-exclusive breastfeeding at 4.5 months. Virus concentrations among those who were still breastfeeding but not exclusively were higher than those exclusively breastfeeding, but were lower than those who had completely stopped all breastfeeding at this time. It was not possible to determine the extent to which the other fluids and liquids given to the infant during mixed breastfeeding displaced or shortened breast milk feeds or whether they simply disrupted the regularity of breast milk feeds. Since the association was strongest at 4.5 months we hypothesize that this was the period characterized by the most marked changes in feeding frequency and regularity. Clinical studies have found that exclusive breastfeeding during the first few months of life is the most practical method to attain a physiological balance between infant demand and milk supply to prevent “insufficient milk syndrome” and mastitis [22]. This is one of the major reasons to support exclusive breastfeeding in addition to its benefits for infant health [23]. Our data indicate that even minor perturbations from the supply-demand dynamics can lead to an increase in breast milk HIV-1 concentration. This may partially explain the now well-established connection between exclusive breastfeeding and lower risk of postnatal HIV-1 transmission relative to non-exclusive breastfeeding [15, 24–26].
Other studies have reported no association between non-exclusive breastfeeding and breast milk HIV-1 concentrations [5, 27]. However, in each of these studies, the exclusivity of breastfeeding behaviors were not linked specifically to the short time interval immediately prior to the breast milk HIV-1 measurement. Thus, rather than indicating conflicting results, our data suggest that the effects of non-exclusive breastfeeding on breast milk HIV-1 concentrations are closely-linked in time to the disruption of full breastfeeding. That these elevations can still translate to a more than doubling of the postnatal HIV transmission rate [15, 24–26] may seem at first surprising. However, our results are consistent with data showing increased risk of HIV transmission in association with incident HIV infection in the transmitting partner in the context of mother-to-child or sexual transmission [28, 29]. Taken together, these data suggest that sudden spikes in viral concentrations may be more important for determining transmission risk than are cumulative exposures to virus or steady-state concentrations.
Our results have profound implications for prevention of mother-to-child HIV transmission programs in settings where breastfeeding is necessary to protect infant and maternal health. Abrupt weaning is no longer officially recommended as part of infant feeding guidance [30]. However, our data demonstrate that even gradual weaning and more subtle changes, such as those associated with non-exclusive breastfeeding, are associated with breast milk viral elevations. In practice, it will be difficult for mothers to make small and regular enough changes in feeding frequency over a long enough period to entirely smooth out these peaks as they approach full weaning. As part of our study, we advised women to express and discard breast milk after all breastfeeding cessation as a strategy to relieve engorgement. Women who followed this advice had reduced peri-weaning breast milk viral concentrations but the practice did not ensure that breast milk HIV-1 quantity remained at baseline levels. While gradual reductions in breastfeeding frequency over the weeks leading up to the last planned breast milk feed should be encouraged, as should counseling about the benefits of breast milk expression, these interventions alone are unlikely to fully mitigate the risks of HIV transmission around this time in the absence of antiretrovirals.
We demonstrated in this study that breast milk HIV-1 RNA and DNA concentrations were strongly correlated with early and late postnatal HIV-1 transmission via breastfeeding, consistent with many other reports [2–7]. In untreated HIV-infected breastfeeding women, HIV-1 RNA above the detection threshold of most assays (>50 copies/ml) is observed in about 50% of breast milk samples with average concentrations about 2 logs lower than observed in blood [31]. Viral shedding may be inconsistent over time and between breasts [32–34]. Viral evolution studies have generated evidence both for and against compartmentalized dynamics [35–37]. Viral populations in breast milk consist of a mixture of locally-produced virus and diffusion of strains circulating in blood. In our analysis, after adjustment for systemic markers of HIV disease progression (CD4 T cell count and plasma HIV-1 RNA concentrations), breast milk RNA concentrations were stronger predictors of transmission than breast milk DNA. Given the strong correlation between these two parameters, as well as between mucosal and systemic viral concentrations, statistical methods have limited capability for determining the underlying biological mechanisms of transmission.
Prior to demonstration by several independent groups of the increased risk of infant morbidity and mortality associated with premature weaning in sub-Saharan Africa [38–44], the practice was widely recommended in many programs. Early weaning was supported as a strategy to reduce the risk of postnatal HIV transmission based on observations that there is continued risk of HIV-1 transmission throughout the duration of breastfeeding [45]. Reducing the duration of exposure to breast milk appears initially to be a reasonable approach to reduce HIV-1 transmission risk. Projections of the likely benefit of this behavior change were generated based on estimates of transmission rates per month observed among women breastfeeding for longer durations and assuming these same transmission rates would apply when breastfeeding was of a shorter duration [46]. Our results demonstrate the limitations of this type of extrapolation and explain why these models seriously over-estimated the benefit of early weaning for HIV prevention by ignoring the more complex biological and behavioral relationships we have described here. For studies that advised early weaning, only a few continued to test infants for HIV who were no longer breastfeeding. In studies that did, some observed continued HIV transmission and attributed this to poor adherence with early weaning [41]. While failure to actually stop breastfeeding undoubtedly occurs, our data suggest that this may not be the prime reason why benefits of early weaning for HIV prevention are less than expected. When women attempt to wean, breast milk HIV exposures continue over the days around this period. Since HIV-1 concentrations in milk at this time are higher than usual, this period is an unusually risky one for HIV-1 transmission. In our data, the elevations in breast milk HIV-1 quantity that occurred peri-weaning entirely explained the higher than expected transmission rates in those who stopped breastfeeding early.
There are several limitations of our analysis. The study was done before antiretroviral therapy even for women with advanced disease became available in the public sector in Zambia. This allowed us to examine viral dynamics without the confounding influence of antiretrovirals. However, the dynamics of HIV in breast milk when antiretroviral drugs are given is a question in urgent need of clarification. Antiretrovirals may dampen weaning-associated breast milk elevations making maternal treatment a more attractive preventive option than infant prophylaxis in some circumstances. Our study also could only examine weaning around 4 months of age which was the age targeted by our study intervention. As WHO guidelines have now shifted to encourage weaning at a later age (~12 months), it is important to consider similarities and differences in weaning-associated mucosal and viral dynamics in an older child.
Weaning can only be conclusively identified in retrospect. In most practical circumstances there is likely to be some period over which a mother reduces the frequency of breastfeeding until such time as the last breast milk exposure occurs. There are no consistent definitions of the start and end of this process and health care workers and community members may differ in terms of their common sense understanding of these fundamental concepts. We propose that for practical purposes the starting point of weaning be defined from the point at which a woman is either instructed to stop all breastfeeding or from the point at which she intends to stop. We recognize the subjective and context-specific limitations of this operational definition. To minimize transmission that may occur over this high risk transition period, HIV-1-infected women should continue the antiretroviral drug interventions that they used through lactation over the full duration of time when any breast milk exposures are likely to occur. Generally, a one to two week period has been considered adequate but this may be too short for many populations. Abrupt weaning, if it could genuinely be obtained, would be effective from an HIV transmission prevention perspective but is associated with maternal morbidity. Although these antiretroviral drug regimens have established efficacy over the full duration of lactation [47], evaluation of intensified regimens targeting the peri-weaning period may be warranted.
Materials and Methods
Study Population
The data and samples were collected from a cohort of 958 HIV-infected women recruited during pregnancy and randomized and followed with their infants prospectively from delivery to 24 months post-partum as part of the Zambia Exclusive Breastfeeding Study (ZEBS) (ClinicalTrials.gov NCT00310726) conducted in Lusaka, Zambia [13]. Recruitment took place between 4 May 2001 and 10 September 2004 at two primary health care clinics in Lusaka and at that time only single-dose nevirapine was available for prevention of mother-to-child HIV-1 transmission. Antiretroviral therapy only became available towards the end of 2004 and thus most of the women in the cohort, even those with advanced disease, did not receive therapy. Breast milk samples collected after the initiation of antiretroviral therapy (n=9) were censored. Follow-up continued through 15 December 2006. All women provided signed informed consent for participation in the study, which was approved by the Institutional Review Boards of all the investigators’ institutions in the U.S. and Zambia.
The primary objective of the overall study was to evaluate the safety and efficacy of exclusive breastfeeding for 4 months followed by abrupt weaning as a strategy to prevent HIV transmission and promote healthy child survival. Only women who intended to breastfeed were enrolled and all women were counseled to breastfeed exclusively to at least 4 months. Half of the cohort was individually randomized to a counseling program that encouraged early abrupt weaning at 4 months. Counseling preparation for weaning started soon after the 5 week postnatal visit and included encouragement of manual expression of milk into a cup during the period of exclusive breastfeeding to begin training the child to adjust to cup feeding. Advice for soothing a non-breastfed child was provided as well as education about nutrition and hygiene. Infant formula and a specially-prepared fortified cereal were provided to all women in the intervention group to support weaning. Manual expression and discarding of breast milk to relieve engorgement after weaning was also encouraged. Women randomized to the control group were encouraged to continue breastfeeding exclusively to 6 months, to gradually introduce complementary foods thereafter and to continue breastfeeding for the duration of their own choosing.
Clinical and social data were collected from women at enrollment during pregnancy. This included body mass index, clinical stage, pregnancy history, parental education, socioeconomic indicators and household composition. Blood was also collected at this time and CD4+ cell counts (FACSCount, BD Immunocytometry Systems, San Jose, CA), hemoglobin (Hemocue® system, Lake Forest, CA) and viral load (Roche Amplicor® 1.5, Roche, Branchburg, NJ). Clinical data were collected at delivery and study visits and counseling conducted weekly in the first month, roughly every 2 weeks to 6 months, and then every 3 months to 24 months. Dried blood spots were collected onto filter paper using heel-sticks from infants at birth, 1 week, and 1, 2, 3, 4, 4.5, 5, 6, 9, 12, 15, 18, 21, 24 months of age regardless of reported feeding practice. Samples were tested in batches after follow-up was complete as infant diagnosis services were not available in Zambia at the time the study was conducted. The last available sample was tested first and if positive, the earliest available samples were tested sequentially until the first positive result was obtained. All positive results were confirmed on at least two separate samples; if a second sample was not available, a different spot from the same time-point was tested to confirm. Beta-globin was amplified from all samples to rule out false negatives on the basis of inadequate sample. Infant diagnosis was done using a validated real-time PCR for HIV-1 DNA [48]. Feeding practices were carefully documented using a standardized questionnaire administered by a study team member not responsible for breastfeeding counseling. Exclusive breastfeeding was defined at each clinic visit as breastfeeding in the absence of all other liquids or solids with the exception of prescribed medications or vitamins since the prior visit. Non-exclusive breastfeeding at the visit would include one or more instances of provision of any liquid or solid to the child if breastfeeding was still continuing. Questions were asked at each visit about breastfeeding cessation (weaning) with questions to determine the last day at which all breastfeeding ended. Clinical outcomes in infants and women, including mortality, morbidity and weight were documented throughout follow-up.
All standard-of-care antenatal interventions were provided as part of the study including screening for syphilis and treatment of the women and her partner with penicillin if necessary, malaria prophylaxis, folate and iron supplementation. Co-trimoxazole prophylaxis for women with low CD4 counts (<200 cells/μL) was introduced during the course of the study [49]. All standard-of-care child interventions were provided as part of the study including all vaccines, vitamin A supplementation, growth monitoring with access to a high energy soy protein supplement with any evidence of failure to thrive. Co-trimoxazole was provided for all infants from 6 weeks to 12 months of age.
Breast milk samples and testing
Breast milk samples were collected by manual expression from all women at 4 months post-partum. At the 4.5 month visit, scheduled to correspond to 2 weeks after the cessation of all breastfeeding for women in the intervention group, both breasts were pumped using a pulsatile electric breast pump (Medela Lactina, McHenry, IL) set at a standard rate for 10 minutes. Milk volume produced on timed pumping was calculated by adding the volume produced by each breast. The pumping visit could be delayed for up to 2 weeks to accommodate women who delayed their planned weaning or who missed the visit and it was still called the 4.5 month visit. The attempt to collect milk after all breastfeeding had ended was intended as a biological marker of the extent of changes in feeding practices across the groups.
Milk was kept refrigerated at the site until transported to the local study laboratory later in the day where it was processed within 4 hours of collection. Milk was centrifuged and the cellular portion stored separately. The aqueous portion, including the lipid, was frozen at −80°C until later testing. HIV-1 RNA was quantified in breast milk using the Ultrasensitive Roche Amplicor Monitor® assay with a lower level of detection of 50 copies/ml (Roche Molecular Systems, Branchburg, NJ) [48]. HIV-1 DNA was quantified using a real-time PCR assay as previously described [48]. Beta-globin was quantified from all samples as a marker of the cellular concentration of milk. The concentration of viral DNA was quantified as copies per ml of breast milk as well as copies per 1 million cell equivalents using the beta-globin concentration as the standard consistent with previous studies[3, 50].
All available samples collected at 4 months and 4.5 months were tested for HIV-1 RNA. This included 659 samples at 4 months and 575 samples at 4.5 months. Either the left or the right breast sample was tested based on random selection. This excluded maternal or infant deaths, loss to follow-up, missed visits, failure to collect the sample, and, at the 4.5 months visit, failure to produce > 1ml of milk (Fig S2). A stratified sample of 232 four month samples and 215 four and a half month samples were tested for HIV-1 DNA. These samples were selected to include all those who had stopped breastfeeding by the 4.5 month visit, all the mothers who transmitted HIV to their infants through breastfeeding, and a random sample of all others.
Statistical methods
Breast milk RNA and DNA concentrations were normalized with log10 transformation. RNA concentrations below 50 copies/ml were imputed as 49 copies/ml and DNA concentrations below detection were imputed as 1 copy/ml. Descriptive statistics, medians, inter-quartile ranges (IQR) displaying the 25th and 75th percentiles and standard box plots were generated stratifying by breastfeeding group. At 4 months, we compared exclusive to mixed breastfeeding based on reported practices since the prior visit (previous month). At 4.5 months, we compared 3 groups: those who had stopped all breastfeeding (weaned), and those who were either exclusive or mixed breastfeeders since the prior visit (previous 2 weeks). Student’s t and nonparametric Wilcoxon tests were used to test for differences across the groups for continuous variables and Pearson’s χ2 statistics and Fisher’s exact test were used for categorical variables. Spearman rank order correlation coefficients were used to describe associations between continuous variables. To test for interactions by feeding modality, linear regression with multiplicative interaction terms of relevant parameters were conducted. Risks of postnatal transmission were described using Kaplan-Meier probabilities taking time to first positive result for infections and censoring those without a positive result at their last negative PCR result. To take into account the duration of breastfeeding, follow-up time was censored 30 days after the cessation of breastfeeding in some analyses of postnatal transmission occurring after 4 months. Univariable and multivariable Cox Proportional Hazard models were used to describe associations between risk factors and postnatal transmission. Unadjusted and adjusted hazard ratios (HR) were reported with 95% confidence intervals. All statistical analyses were performed using SAS (version 9.2).
Supplementary Material
Fig. S1. Scatter plots showing associations between breast milk HIV-1 RNA concentrations in copies/ml, HIV-1 DNA concentration in copies/ml and HIV-1 DNA concentration in copies per 1 million cell equivalents (1M) stratified by feeding practice at 4.5 months.
Fig. S2. Flowchart of HIV-infected women who had breast milk HIV measurements.
Breastfeeding and weaning questionnaires.
Acknowledgments
We would like to thank the Zambian families who participated in the research and all the study staff and volunteers. We gratefully acknowledge assistance with aspects of the conduct and write-up of this study from: Drs. Sally Lederman, Lynne Mofenson, Ellen Piwoz, Daniel Raiten, Katherine Semrau.
Funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) (HD39611, HD40777, HD57617). GMA is a recipient of the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) Scientist Award.
Footnotes
Author contributions: The study was designed by LK and DT. Fieldwork and clinical oversight was provided by MS, CK and MM. All laboratory work was conducted by GA, DD, JW. The analysis was done by LK and HYK. All authors contributed to the interpretation of the findings and to the write up of the manuscript.
Competing interests: None of the authors have any conflicts of interest to declare.
References and Notes
- 1.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. Journal of Infectious Diseases. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau CM, Nduati R, Richardson B, John-Stewart G, Mbori-Ngacha D, Kreiss J, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. Journal of Infectious Diseases. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manigart O, Crepin M, Leroy V, Meda N, Valea D, Janoff EN, et al. Effect of perinatal zidovudine prophylaxis on the evolution of cell-free HIV-1 RNA in Breast Milk and on postnatal transmission. J Infect Dis. 2004;190:1422–1428. doi: 10.1086/424569. [DOI] [PubMed] [Google Scholar]
- 5.Lunney KM, Iliff P, Mutasa K, Ntozini R, Magder LS, Moulton LH, et al. Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clin Infect Dis. 2010;50:762–769. doi: 10.1086/650535. [DOI] [PubMed] [Google Scholar]
- 6.Ndirangu J, Viljoen J, Bland RM, Danaviah S, Thorne C, Van de Perre P, et al. Cell-Free (RNA) and Cell-Associated (DNA) HIV-1 and Postnatal Transmission through Breastfeeding. PLoS One. 2012;7:e51493. doi: 10.1371/journal.pone.0051493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koulinska IN, Villamor E, Chaplin B, Msamanga G, Fawzi W, Renjifo B, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;41:93–99. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- 8.Fiscus SA, Aldrovandi GM. Virologic determinants of breast milk transmission of HIV-1. Adv Exp Med Biol. 2012;743:69–80. doi: 10.1007/978-1-4614-2251-8_5. [DOI] [PubMed] [Google Scholar]
- 9.Slyker JA, Chung MH, Lehman DA, Kiarie J, Kinuthia J, Holte S, et al. Incidence and correlates of HIV-1 RNA detection in the breast milk of women receiving HAART for the prevention of HIV-1 transmission. PLoS One. 2012;7:e29777. doi: 10.1371/journal.pone.0029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Politch JA, Mayer KH, Welles SL, O’Brien WX, Xu C, Bowman FP, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012 doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManaman JL, Neville MC. Mammary physiology and milk secretion. Advanced Drug Delivery Reviews. 2003;55:629–641. doi: 10.1016/s0169-409x(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 12.Neville MC. Determinants of milk volume and composition. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 87–114. [Google Scholar]
- 13.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt cessation of breastfeeding on HIV-free survival of children in Zambia. New England Journal of Medicine. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thea DM, Aldrovandi G, Kankasa C, Kasonde P, Decker WD, Semrau K, et al. Post-weaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. AIDS. 2006;20:1539–1547. doi: 10.1097/01.aids.0000237370.49241.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn L, Sinkala M, Kankasa C, Semrau K, Kasonde P, Scott N, et al. High Uptake of Exclusive Breastfeeding and Reduced Early Post-natal HIV Transmission. PLOS ONE. 2007;2(12):e1363. doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neville MC, Allen JC, Archer P, Casey CE, Seacat J, Keller RP, et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. American Journal of Clinical Nutrition. 1991;54:81–92. doi: 10.1093/ajcn/54.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:233–246. doi: 10.1023/a:1018707309361. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen DA, Parlow AF, Neville MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. Journal of Endocrinology. 2001;170:347–356. doi: 10.1677/joe.0.1700347. [DOI] [PubMed] [Google Scholar]
- 19.Neville MC. Classic studies of mammary development and milk secretion: 1945–1980. J Mammary Gland Biol Neoplasia. 2009;14:193–197. doi: 10.1007/s10911-009-9151-7. [DOI] [PubMed] [Google Scholar]
- 20.Mellenberger RW, Bauman DE. Metabolic adaptations during lactogenesis. Lactose synthesis in rabbit mammary tissue during pregnancy and lactation. Biochem J. 1974;142:659–665. doi: 10.1042/bj1420659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellenberger RW, Bauman DE. Metabolic adaptations during lactogenesis. Fatty acid synthesis in rabbit mammary tissue during pregnancy and lactation. Biochem J. 1974;138:373–379. doi: 10.1042/bj1380373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastitis: Causes and Management. Geneva: World Health Organization; 2000. [Google Scholar]
- 23.Fetherston C. Mastitis in lactating women: physiology or pathology? Breastfeeding Review. 2001;9:5–12. [PubMed] [Google Scholar]
- 24.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 25.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 26.Iliff P, Piwoz E, Tavengwa N, Zunguza C, Marinda E, Nathoo K, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 27.Rossenkhan R, Novitsky V, Sebunya TK, Leidner J, Hagan JE, Moyo S, et al. Infant feeding practices were not associated with breast milk HIV-1 RNA levels in a randomized clinical trial in Botswana. AIDS Behav. 2012;16:1260–1264. doi: 10.1007/s10461-011-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey JH, Marinda E, Mutasa K, Moulton LH, Iliff PJ, Ntozini R, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. doi: 10.1136/bmj.c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New England Journal of Medicine. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Perre P, Rubbo PA, Viljoen J, Nagot N, Tylleskar T, Lepage P, et al. HIV-1 Reservoirs in Breast Milk and Challenges to Elimination of Breast-Feeding Transmission of HIV-1. Science Translational Medicine. 2012;4:143sr143. doi: 10.1126/scitranslmed.3003327. [DOI] [PubMed] [Google Scholar]
- 32.Semrau K, Ghosh M, Kankasa C, Sinkala M, Kasonde P, Mwiya M, et al. Temporal and lateral dynamics of HIV shedding and elevated sodium in breast milk among HIV-positive mothers during the first 4 months of breast-feeding. J Acquir Immune Defic Syndr. 2008;47:320–328. doi: 10.1097/qai.0b013e31815e7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willumsen JF, Newell ML, Filteau SM, Coutsoudis A, Dwarika S, York D, et al. Variation in breastmilk HIV-1 viral load in left and right breasts during the first 3 months of lactation. AIDS. 2001;15:1896–1898. doi: 10.1097/00002030-200109280-00026. [DOI] [PubMed] [Google Scholar]
- 34.Willumsen JF, Filteau SM, Coutsoudis A, Newell ML, Rollins NC, Coovadia HM, et al. Breastmilk RNA viral load in HIV-infected South African women: effects of subclinical mastitis and infant feeding. AIDS. 2003;17:407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 35.Heath L, Conway S, Jones L, Semrau K, Nakamura K, Walter J, et al. Restriction of HIV-1 genotypes in breast milk does not account for the population transmission genetic bottleneck that occurs following transmission. PLoS One. 2010;5:e10213. doi: 10.1371/journal.pone.0010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray RR, Salemi M, Lowe A, Nakamura KJ, Decker WD, Sinkala M, et al. Multiple independent lineages of HIV-1 persist in breast milk and plasma. AIDS. 2011;25:143–152. doi: 10.1097/QAD.0b013e328340fdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson GJ, Hoffman NG, Ping LH, Fiscus SA, Hoffman IF, Kitrinos KM, et al. HIV-1 populations in blood and breast milk are similar. Virology. 2004;330:295–303. doi: 10.1016/j.virol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, et al. Elevations in mortality due to weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clinical Infectious Diseases. 2010;54:437–444. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, et al. Frequency of Gastroenteritis and Gastroenteritis-Associated Mortality with Early Weaning in HIV-1-Uninfected Children Born to HIV-Infected Women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 40.Taha TE, Hoover DR, Chen S, Kumwenda NI, Mipando L, Nkanaunena K, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–395. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagaayi J, Gray RH, Brahmbhatt H, Kigozi G, Nalugoda F, Wabwire-Mangen F, et al. Survival of infants born to HIV-positive mothers by feeding modality in Rakai, Uganda. PLOS One. 2008;3:e3877. doi: 10.1371/journal.pone.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- 44.Onyango-Makumbi C, Bagenda D, Mwatha A, Omer SB, Musoke P, Mmiro F, et al. Early Weaning of HIV-Exposed Uninfected Infants and Risk of Serious Gastroenteritis: Findings from Two Perinatal HIV Prevention Trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–27. doi: 10.1097/QAI.0b013e3181bdf68e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekpini ER, Wiktor SZ, Satten GA, Adjorlolo-Johnson GT, Sibailly TS, Ou CY, et al. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire. Lancet. 1997;349:1054–1059. doi: 10.1016/s0140-6736(96)06444-6. [DOI] [PubMed] [Google Scholar]
- 46.Nagelkerke NJ, Moses S, Embree JE, Jenniskens F, Plummer FA. The duration of breastfeeding by HIV-1-infected mothers in developing countries: balancing benefits and risks. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:176–181. [PubMed] [Google Scholar]
- 47.Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antiviral Therapy. 2010;15:537–553. doi: 10.3851/IMP1574. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh MK, Kuhn L, West J, Semrau K, Decker D, Thea DM, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. Journal of Clinical Microbiology. 2003;41:2465–2470. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter J, Mwiya M, Scott N, Kasonde P, Sinkala M, Kankasa C, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. Journal of Infectious Diseases. 2006;194:1510–1518. doi: 10.1086/508996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gantt S, Shetty AK, Seidel KD, Matasa K, Musingwini G, Woelk G, et al. Laboratory indicators of mastitis are not associated with elevated HIV-1 DNA loads or predictive of HIV-1 RNA loads in breast milk. J Infect Dis. 2007;196:570–576. doi: 10.1086/519843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Scatter plots showing associations between breast milk HIV-1 RNA concentrations in copies/ml, HIV-1 DNA concentration in copies/ml and HIV-1 DNA concentration in copies per 1 million cell equivalents (1M) stratified by feeding practice at 4.5 months.
Fig. S2. Flowchart of HIV-infected women who had breast milk HIV measurements.
Breastfeeding and weaning questionnaires.


