Abstract
Introduction
IgA nephropathy, the most prevalent glomerular disease in the world, requires a renal biopsy for diagnosis. Reliable biomarkers are needed for the non-invasive diagnosis of this disease and to more fully delineate its natural history and risk for progression.
Areas covered
In this review, the authors examine serum levels of galactose-deficient IgA1 (Gd-IgA1) and glycan-specific IgG and IgA autoantibodies that are integral to pathogenesis of IgA nephropathy. They also explore biomarkers related to alternative and lectin pathways of complement activation and serum and urinary peptide biomarkers detected by mass spectrometric methods. The literature search included review of all publications having IgA nephropathy in the title that were cited in PubMed and Scopus over the past 10 years and a non-systematic review of abstracts published for the annual meetings of the American Society of Nephrology and the International Symposia on IgA Nephropathy.
Expert opinion
Serum Gd-IgA1 level and glycan-specific autoantibody levels are prime candidates to become diagnostic biomarkers for IgA nephropathy because of their central role in the earliest stages of disease pathogenesis. Assays for serum levels of complement proteins C3 and factor H are readily available in clinical practice and deserve continued study, either alone or in tandem with total serum IgA or serum Gd-IgA1 levels, as prognostic biomarkers for patients with IgA nephropathy. Urinary peptidomic data are also reviewed because this approach can successfully differentiate patients with IgA nephropathy from healthy controls and from patients with other forms of renal disease.
Keywords: anti-glycan antibodies, complement, end-stage renal disease, galactose-deficient IgA1, IgA nephropathy, urinary peptidomics
1. Introduction
IgA nephropathy is the most common chronic glomerulonephritis in the world [1]. About 10% of patients with IgA nephropathy progress to end-stage kidney disease within 10 years of diagnosis [2,3]. Renal biopsy showing dominant or co-dominant deposition of IgA in the glomerular mesangium is required for diagnosis [4]. In our opinion, IgA nephropathy is undiagnosed for many people in the USA, particularly those with mild clinical signs and symptoms or those presenting with advanced chronic kidney disease. After diagnosis by renal biopsy, current prognostic markers are clinical: magnitude of proteinuria [5–7], renal function [6,7], hypertension [6,7] and histologic: mesangial hypercellularity, endocapillary hypercellularity, segmental glomerulosclerosis and tubular atrophy/interstitial fibrosis [8]. An absolute renal risk (ARR) score based on presence or absence of hypertension, proteinuria and severe histology demonstrates a strong association between these factors and poor clinical outcome in patients with IgA nephropathy [9]. Reliable biomarkers are needed to allow for the non-invasive diagnosis of this disease and to more fully delineate the natural history and risk for progression.
Recent studies have led to a four-hit hypothesis for the pathogenesis and/or clinical expression of IgA nephropathy [10]. Galactose deficiency of some O-linked glycans in the hinge region of IgA1 is the beginning of a sequence of events that may lead to renal injury. These galactose-deficient O-glycans of IgA1 consist of terminal N-acetylgalactosamine (GalNAc) or sialylated GalNAc [11,12]. Anti-glycan antibodies recognize the hinge-region glycans of IgA1 with terminal Gal-NAc [11,13] to form nephritogenic circulating immune complexes that deposit in the glomerular mesangium, leading to renal injury [11]. Their postulated role in this mesangioproliferative glomerulonephritis is supported by the observation that these complexes stimulate cultured human mesangial cells to proliferate and secrete extracellular-matrix proteins, whereas uncomplexed galactose-deficient IgA1 (Gd-IgA1) or galactose-replete IgA1 does not [14].
A better understanding of the molecular basis of the pathogenesis of IgA nephropathy will likely result in disease-specific serologic and urinary biomarkers. Such biomarkers will potentially lead to earlier diagnosis, improved monitoring of the clinical course or response to treatment, and, eventually, disease-targeted therapy [3].
Because of space limitations, only biomarkers found in serum (or plasma) and urine samples are discussed in this review; microRNA biomarkers are not included. Tissue biomarkers, such as composition of glomerular deposits, and genetic markers are not covered.
2. Biomarkers of IgA nephropathy
2.1 Serum Gd-IgA1 and anti-glycan antibody in the pathogenesis of IgA nephropathy
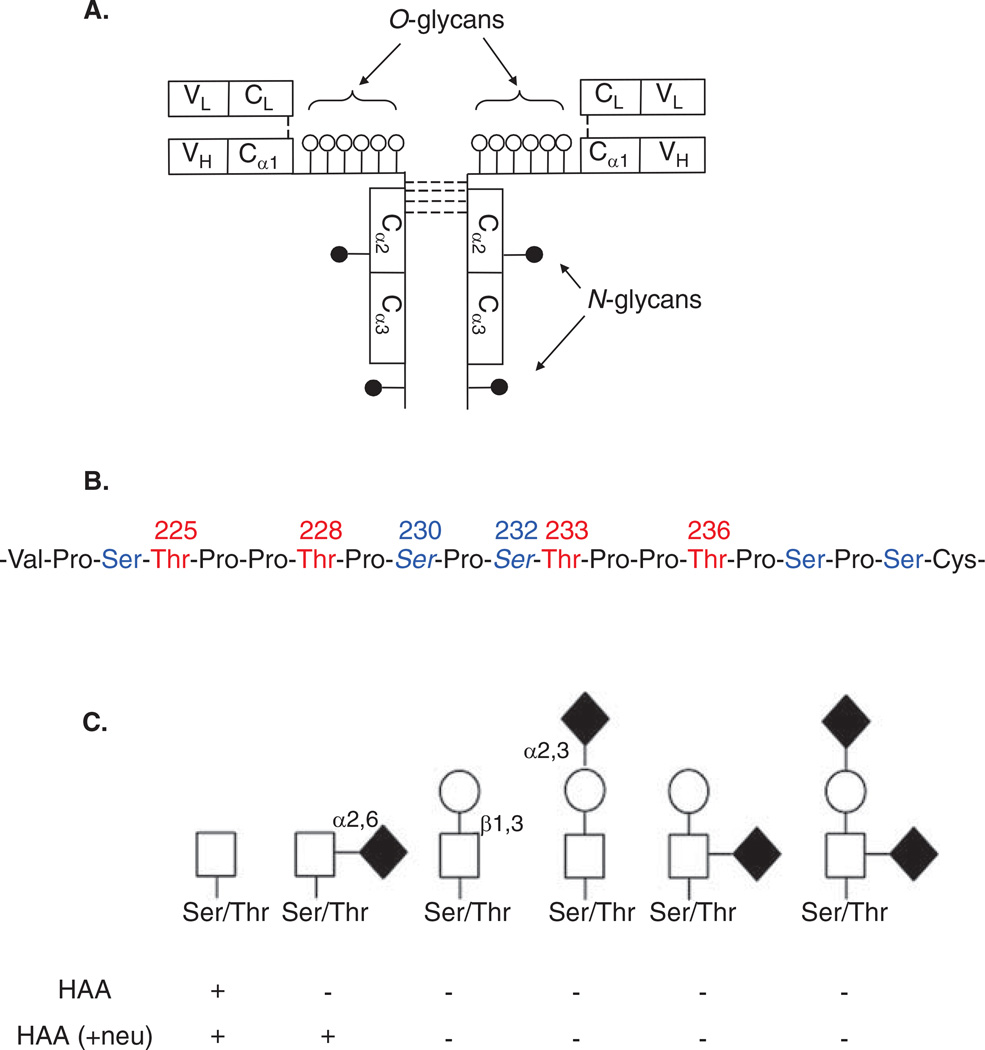
Human circulatory IgA is predominantly of the IgA1 subclass and in its monomeric molecular form, whereas in external secretions, the ratio of IgA1 and IgA2 subclasses varies and both isotypes are in the secretory polymeric form (i.e., dimers or higher oligomers connected by a J-chain with a secretory component) [15]. Heavy chains of IgA1 have a unique hinge region segment between the first and second constant-region domains (CH1 and CH2; Figure 1A). The hinge region of IgA1 has two octapeptide repeats [15–17] and resembles the structure of mucins due to the high content of serine (Ser) and threonine (Thr) residues (Figure 1B). These amino acids are the sites of attachment of the clustered O-glycans.
Figure 1.
A. The structure of monomeric IgA1. Three to six O-linked glycans are attached in the hinge region and two N-linked glycans are attached in CH2 and CH3 domains of the heavy chain. B. IgA1 hinge-region amino acid sequence. Amino acid residues are numbered to mark the six common sites of O-linked glycan attachment. Ser residues are in blue and Thr residues are in red. C. O-linked glycans of circulatory IgA1. The first two structures on the left are galactose-deficient O-linked glycans, whereas other structures represent galactosylated variants with or without sialic acid. GalNAc-specific lectin (HAA) reactivity with IgA1 O-glycan variants is shown. GalNAc that has sialic acid attached will not react with HAA unless the sample is treated with neuraminidase (+ neu), which removes the sialic acid residue. Symbols: white square, GalNAc; black circle, Gal; black diamond, sialic acid.
Neu: Neuraminidase; HAA: Helix aspersa agglutinin.
Normal human IgA1 in the circulation has core 1 O-glycans consisting of GalNAc with β1,3-linked galactose (Figure 1C). Each saccharide can be sialylated: GalNAc by an α2,6-linked and galactose by an α2,3-linked sialic acid. The carbohydrate composition of the O-linked glycans on normal serum IgA1 is variable, and the prevailing forms include the GalNAc-galactose disaccharide and its mono- and di-sialylated forms [18–23]. Normal serum IgA1 was thought to contain little or no galactose-deficient O-glycans [20], but it was shown recently that some O-glycans of circulatory IgA1 in healthy individuals are galactose-deficient [24].
Patients with IgA nephropathy have elevated levels of circulatory IgA1 molecules with some O-glycans without galactose, consisting of terminal GalNAc or sialylated GalNAc (Figure 1C, first two structures) [25–29]. This galactosylation feature is specific for IgA1, as other O-glycosylated glycoproteins in sera of patients with IgA nephropathy do not exhibit this abnormality [25,30]. These data are in agreement with the observations that a GalNAc-specific lectin from Helix aspersa (HAA; Figure 1C) binds small amounts of IgA1 from healthy controls [11,25,28,29]. Most Gd-IgA1 is within circulating immune complexes bound by anti-glycan IgG or IgA1 antibodies [11,29,31].
2.2 Serum Gd-IgA1 levels
2.2.1 Serum Gd-IgA1 levels as a diagnostic biomarker
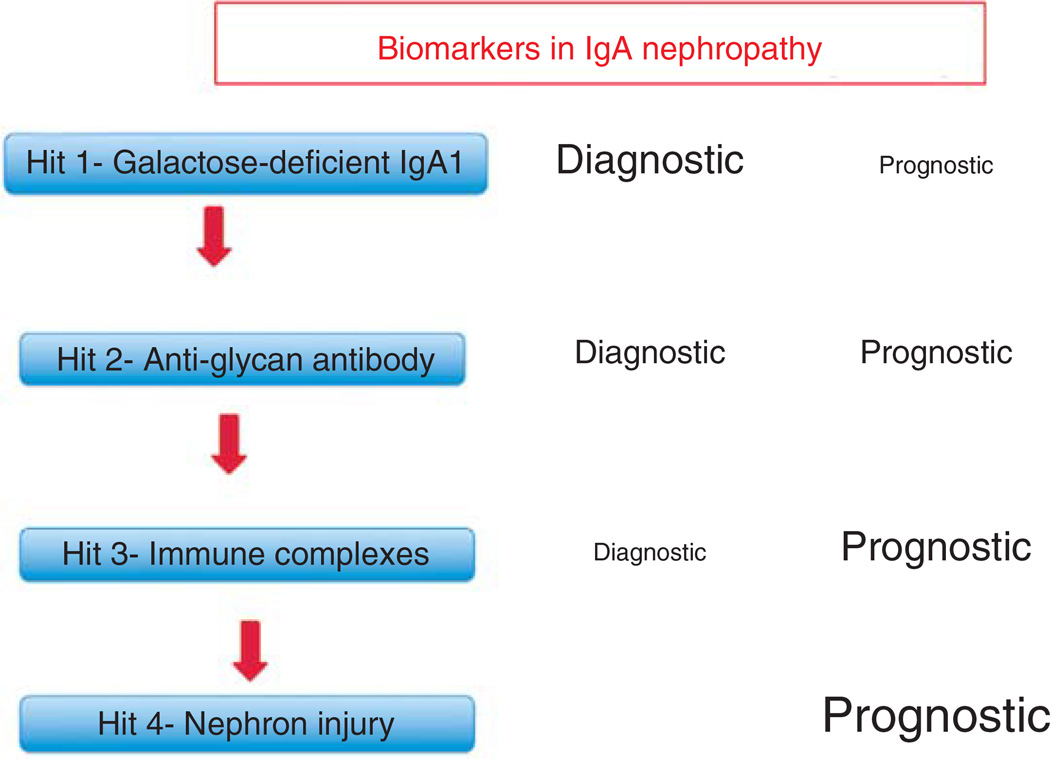
An elevated serum level of Gd-IgA1 is the initial hit in the postulated pathogenesis of IgA nephropathy (Figure 2) and appears necessary but not sufficient for the full clinical expression of the disease [10]. Elevated levels have been reported in patients with IgA nephropathy of Caucasian [28,32,33], Asian [34–36], and African [37] ancestry. The serum level of Gd-IgA1 was above the 90th percentile for healthy controls in 77% of 150 Caucasian adults with IgA nephropathy [28]. The area under the receiver operating characteristic (ROC) curve was 0.90, strongly suggesting that this marker may be of diagnostic significance for the disease [28].
Figure 2. This figure depicts the relationship between the four hits in the pathogenesis of IgA nephropathy [10] and the relative usefulness of a biomarker for diagnosis or prognosis of IgA nephropathy.
We propose that the best diagnostic markers should be related to the earlier hits and that biomarkers related to later hits are more likely to serve as prognostic markers.
Subsequent studies confirmed the finding, but did not find the sensitivity to be as high (Table 1). Lin et al. [35] showed that median serum Gd-IgA1evel in 63 Chinese patients was higher than that in 115 healthy controls and 44 spouses of patients with IgA nephropathy. Shimozato et al. [34] found that 49% of 41 adult Japanese patients with IgA nephropathy had significantly elevated serum Gd-IgA1 levels compared with healthy controls and with patients with other types of kidney disease. In a pediatric cohort including Caucasians and African Americans, serum Gd-IgA1 levels were significantly elevated in 77% of 22 children with IgA nephropathy [38].
Table 1.
Analysis (re-analysis in some instances) of data for serum Gd-IgA1 levels from previously published datasets.
| Study | Ethnicity | Age Group | Subjects | Controls | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Moldoveanu et al. 2007 [28] | Caucasian | Adult | 153 | 150 | 76 | 90 | 89 | 79 | 90 |
| Lau et al. 2007 [38] | Caucasian, African | Pediatric | 22 | 16 | 73 | 94 | 94 | 75 | 91 |
| Shimozato et al. 2008 [34] | Asian | Adult | 41 | 38 | 49 | 89 | 83 | 62 | |
| Hastings et al. 2010 [37] | African | Adult | 18 | 65 | 56 | 91 | 63 | 88 | 88 |
| Hastings et al. 2010 [48] | African | Pediatric | 11 | 49 | 82 | 90 | 64 | 96 | 95 |
| Camilla et al. 2011 [32] | Mostly Caucasian | Pediatric Adult | 62* | 69 | 67 | 90 | 76 | 86 | |
| Berthoux et al. 2012 [33] | Caucasian | Adult | 97 | 30 | 37 | 90 | 92 | 31 |
Only patients with complete clinical data; pediatric and adult subjects were combined.
AUC: Area under receiver operating characteristic curve; NPV: Negative predictive value; PPV: Positive predictive value.
Several studies have examined serum Gd-IgA1 levels as a diagnostic marker for IgA nephropathy, but most compared levels in patients with IgA nephropathy to levels in healthy controls (Table 1). The optimal diagnostic test should differentiate patients with IgA nephropathy from those with other glomerular diseases. We have done this by using samples from40 pediatric patients with IgA nephropathy (age < 18 years) with addition of 13 more subjects to the cohort of Lau et al. [38] and 16 non-IgA-nephropathy glomerular-disease controls (excluding patients with systemic lupus erythematosus and Henoch–Schönlein purpura nephritis). The sensitivity and specificity were 63 and 93%, respectively, with a positive predictive value of 96% and negative predictive value of 46%. The area under the ROC curve was 0.84.
Serum levels of Gd-IgA1 are stable over time for patients with IgA nephropathy [39–41]. Biannual serum samples (that date to 1990 and were obtained from all active military personnel) in the United States Department of Defense Serum Repository were accessed for eight personnel who developed IgA nephropathy after entering the service. Gd-IgA1 levels in sera obtained from these eight patients 1000 days prior to renal biopsy and 1000 days after renal biopsy were higher compared to levels in sera obtained at similar time points from 24 matched healthy controls (p = 0.019 and p = 0.046, respectively) [40]. Paired samples collected over a period of 9 – 19 years (median 60 months) showed that serum levels of Gd-IgA1 remained constant for 16 adult patients with IgA nephropathy and 15 healthy controls [39]. Serum Gd-IgA1 levels of healthy children and children with IgA nephropathy have also been noted to remain stable over a 12-month interval [41].
The diagnostic utility of this marker alone is encumbered, as it may be elevated in individuals with no clinical signs of IgA nephropathy and levels vary dependent on age and ethnic group. Increased levels of Gd-IgA1 may be found in up to 5% of normal controls [28] and in 40% of first-degree relatives of patients with IgA nephropathy [35,37,42,43]. Segregation analysis of Gd-IgA1 levels for patients and their first-degree relatives suggested the presence of a major dominant gene on a polygenic background [42].
Median Gd-IgA1 level is significantly higher for Caucasian adults when compared to Caucasian children under age 18 [28,38,44]. Median serum Gd-IgA1 level is also significantly higher in Caucasian American as compared to African American adults [37]. Among pediatric patients, no significant difference in serum Gd-IgA1 level was found based on gender or in African Americans compared to Caucasians [44]. No data were published for Asian and Caucasian patients in the same study, so it is not clear if levels differ between those racial groups. Thus, future studies on the diagnostic utility of serum Gd-IgA1 levels need to carefully consider age, race and possibly gender.
Delineation of the hinge region glycoforms in healthy individuals and patients with IgA nephropathy will provide a better-defined diagnostic biomarker. The current methodology using lectin-based assays cannot provide this degree of detail. High-resolution mass spectrometry has the capacity to define the heterogeneity of the IgA1 O-linked glycans at the molecular level. Renfrow et al. [45] used this approach to localize all O-glycan attachment sites in an IgA1 myeloma protein. Subsequently, several isomeric O-glycoforms in an IgA1 myeloma protein and in IgA1 isolated from normal human serum were identified [46].
Takahashi et al. [47] used high-resolution mass spectrometry to define the hinge region glycosylation patterns and glycosylation sites of IgA1 secreted by immortalized IgA1-producing cells from six patients with IgA nephropathy and seven healthy controls. Four hinge region glycoforms, including three with galactose-deficient O-glycans, predominated in the IgA1 from IgA nephropathy patients. Tandem mass spectrometry revealed that the sites with galactose-deficient O-glycans or non-glycosylated sites included mainly Ser230, Thr233 and Thr236, whereas Thr225, Thr228 and Ser232 were glycosylated predominantly by the GalNAc-galactose disaccharide. This is the first definitive identification of hinge-region O-glycosylation microheterogeneity on IgA1 from IgA nephropathy patients compared to healthy controls. The glycoforms predominating in IgA1 secreted by the cells from patients with IgA nephropathy may be candidates for disease-specific biomarkers.
2.2.2 Serum Gd-IgA1 as a prognostic biomarker
Published data on serum Gd-IgA1 levels as a clinical prognostic marker are not conclusive. Serum Gd-IgA1 level did not associate with degree of proteinuria for adults [28,32] or children [48]. In a study that combined pediatric and adult patients, absolute serum Gd-IgA1 level did not correlate with decline in estimated GFR (eGFR) or degree of proteinuria at biopsy, but an elevated percent of Gd-IgA1/total IgA correlated with these clinical risk factors [32]. A higher Gd-IgA1 quartile associated with progression of renal disease in a large Chinese cohort of patients with IgA nephropathy [36]. A greater degree of under-galactosylation of serum Gd-IgA1 level (as detected by a GalNAc-specific lectin from Vicia villosa) was associated with histologic severity in one study [49]. GalNAc-specific lectins may vary in their precise binding characteristics whereby analyses using different lectins to measure serum levels of Gd-IgA1 may not provide comparable results [50].
2.3 Serum anti-glycan antibody
2.3.1 Serum anti-glycan antibody level as a diagnostic biomarker
The production of unique autoantibodies recognizing Gd-IgA1 (known as anti-glycan antibodies) leads to formation of nephritogenic circulating immune complexes that are central to the pathogenesis of IgA nephropathy. Tomana et al. [11] initially recognized that IgG antibody with specificity to Seror Thr-linked GalNAc residues was present in sera of IgA nephropathy patients, resulting in the classification of the disease as an autoimmune disorder. The IgG anti-glycan autoantibody in patients with IgA nephropathy has an unusual structure that is key for binding to Gd-IgA1; it arises due to a Ser substitution for alanine in the complementarity-determining region 3 of the variable region of the heavy chain [13].
Suzuki et al. [13] developed a dot-blot assay measuring glycan-specific IgG antibody levels and were able to differentiate patients with IgA nephropathy from healthy and renal-disease controls with 88% specificity and 95% sensitivity. Berthoux et al. [33] found that the mean serum levels of IgG autoantibody and IgA autoantibody in an ELISA assay were significantly higher in patients with IgA nephropathy at the time of biopsy than in healthy volunteers and patients with renal disease other than IgA nephropathy (both comparisons, p ≤ 0.01). However, when data from that study are analyzed using 90th percentile for healthy controls as the upper limit of normal, the sensitivity was 29% for serum IgG anti-glycan autoantibody level and 31% for serum IgA anti-glycan autoantibody level (Table 2). Serum IgG anti-glycan antibody level and/or serum IgA anti-glycan antibody level was above the 90th percentile for healthy controls for 40% of the subjects in that study.
Table 2.
Analysis (re-analysis in some instances) of data on adult patients with IgA nephropathy for serum anti-glycan antibody levels from previously published datasets.
| Study | Ethnicity | Antibody class | Subjects | Controls | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Suzuki et al. 2009 [13] | Caucasian, Asian | IgG | 56 | 40 | 90 | 90 | 93 | 86 | 96 |
| Berthoux et al. 2012 [33] | Mostly Caucasian | IgG | 97 | 30 | 29 | 90 | 28 | 90 | |
| Berthoux et al. 2012 [33] | Mostly Caucasian | IgA | 97 | 30 | 31 | 90 | 29 | 91 |
AUC: Area under receiver operating characteristic curve; NPV: Negative predictive value; PPV: Positive predictive value.
There is an obvious discrepancy in the results of the two assays. One explanation could be clinical differences between the groups studied, with the cohort of Berthoux et al. [33] having a higher percentage of patients with mild disease. Another possibility is the differences in the assays used to determine serum anti-glycan antibody levels for the two studies. The ELISA in the latter study may not detect antibody binding as well as the former dot-blot assay.
2.3.2 Serum anti-glycan antibody levels as prognostic biomarkers
The anti-glycan autoantibody may play an important role in the clinical expression of IgA nephropathy. In a study evaluating IgG autoantibody at the time of renal biopsy, the intensity of binding to Gd-IgA1 correlated with urine protein/creatinine ratio (p < 0.0001) [13]. Berthoux et al. [33] found that an elevated IgG autoantibody level at the time of biopsy predicted dialysis or death during a maximum of 15 years of follow-up time (p ≤ 0.01). One might surmise that this biomarker may eventually prove useful for monitoring disease progression and/or response to therapy. However, data have not yet been published to support that supposition. Moreover, anti-glycan antibodies might eventually represent a disease-specific marker and potential therapeutic target.
2.4 Serum levels of IgA1-containing circulating immune complexes
The third hit in the postulated pathogenesis of IgA nephropathy is the formation of immune complexes containing Gd-IgA1 bound by IgA1 and/or IgG anti-glycan autoantibody [10]. IgA1-IgG-containing circulating immune complexes were found in 44 to 68% of patients with IgA nephropathy [51,52]. However, these studies did not examine the role of IgA1-containing circulating immune complexes as a diagnostic or prognostic marker for IgA nephropathy, but rather assessed their biological activity using cultured human mesangial cells. C3b is important for solubilization and clearance of immune complexes and is generated during activation of C3 [53].
2.5 Serum levels of complement proteins and plasma levels of complement activation fragments
C3 activation through the alternative and/or lectin pathways occurs in patients with IgA nephropathy [54] and complement-related biomarkers may be important for delineation of disease severity or clinical expression. This possibility is worthy of careful re-evaluation because recent genome-wide association studies point toward the importance of alternative pathway of complement activation in IgA nephropathy [55,56]. Products of factor H gene and the cluster of nearby complement factor H-related genes modulate activation of the alternative complement pathway. The combined deletion of complement factor H-related protein 1 (CFHR1) and CFHR3 confers a substantially reduced risk of IgA nephropathy [55].
In healthy Caucasian subjects, a strong association between serum C3 level and serum factor H level (r = 0.78) [57] led to the hypothesis that serum factor H level determines serum C3 level in the normal state when systemic activation of C3 is not occurring [57]. In a group of 28 patients with IgA nephropathy in Kentucky studied in 1983, Julian et al. [58] found that serum C3, B, H and I concentrations were significantly higher in patients with stable normal renal function as compared to those with chronic kidney disease defined as serum creatinine greater than 2.0 mg/dl at the time of sampling. The single patient with a serum C3 concentration < 90 mg/dl had a partial deficiency of factor H [58].
The finding that the serum C3 concentration was rarely low in patients with IgA nephropathy in the USA or Europe contrasts with results of studies from Asia. In a cohort of 343 Chinese patients with IgA nephropathy, a serum C3 level < 90 mg/dl was associated with poor outcome, defined as end-stage kidney disease or doubling of baseline serum creatinine [59]. Specifically, 19% of these patients had a serum C3 concentration < 90 mg/dl. In a study in Japan, IgA nephropathy patients with a serum C3 concentration that declined over time had a higher rate of loss of renal function, as defined by worse than 20% decline in eGFR, than those with a persistently increased or stable level [60].
Considering the serum C3 concentration may improve precision of the serum IgA level as a diagnostic test for IgA nephropathy. While the serum IgA level tends to be elevated in patients, it is not an adequate diagnostic test for the disease. In our cohort of 153 Caucasian adults with IgA nephropathy, the sensitivity for the serum IgA level was only 33% with a specificity of 90% [28]. Tomino et al. [61] proposed that an elevated serum IgA/C3 ratio would better predict IgA nephropathy prior to biopsy. Using a cutoff ratio of 2.14 that is slightly above the mean value of healthy controls, the sensitivity was 79%, specificity 61% and area under the ROC curve was 0.74. A subsequent study from the same group showed that for 213 patients with IgA nephropathy the mean ratio of 4.55 was significantly higher than that for healthy controls and patients with other glomerular diseases [62].
The serum IgA/C3 ratio may be also of prognostic value. A recent study showed that an elevated ratio was associated with progression of IgA nephropathy, as defined as end-stage kidney disease or 50% decline in eGFR [63]. Earlier studies had suggested that an association existed between elevation of this ratio and disease severity [62,64], but this finding has not been confirmed, even in Asian cohorts.
The IgA/C3 ratio has not been used or advocated for clinical practice in Europe or North America. However, the test could easily be done in the clinical laboratory. Data from European and/or North American cohorts should be examined to determine whether use of the serum IgA/C3 ratio for diagnosis is appropriate in those regions.
Analysis of fragments of complement arising from activation of the complement cascade may be useful in the assessment of patients with IgA nephropathy. When serial plasma samples were analyzed for patients from the USA, levels of iC3b–C3d neoantigen (activated C3) were elevated on at least one occasion for 73% of adult patients and 57% of pediatric patients with IgA nephropathy [65]. Furthermore, a high level of activated C3 was associated with severity of the renal histology [66]. A subsequent German study found that activated C3, measured in an assay using a different monoclonal antibody, was present in only 30% of patients with IgA nephropathy but associated with disease progression [67]. Small studies showed that a high circulating level of C3d, a breakdown fragment of C3, was present in 45 – 50% of patients with IgA nephropathy [68,69]. Mean plasma levels of C3a were significantly higher in patients with IgA nephropathy as compared to healthy controls and controls with non-glomerular renal disease and/or hypertension, although plasma C3a levels were not associated with severity of disease [70].
In a Chinese study of 202 patients with IgA nephropathy, urinary factor H level was significantly higher for patients at the time of biopsy than for healthy controls [71]. In that study, urinary factor H levels were significantly increased in patients with severe histologic findings as compared to those with mild features [71].
Recent studies have implicated the lectin pathway of complement activation in the pathogenesis of severe IgA nephropathy [54]. C4, C3 and the remainder of the complement cascade are activated via this pathway without initiation by immune complexes. The plasma C4d/C4 level was elevated in 20% of adults patients and 5% of pediatric patients with IgA nephropathy [65] and C4 activation occurred in 6% of the patients studied by Zwirner et al. [67]. At the time these studies were performed, C4 activation was attributed to involvement of the classical pathway, but in retrospect the best explanation is that C4 activation fragments were generated through activation of the lectin pathway. Recently, urinary peptidomics (see Section 2.7.1) found a peptide fragment, subsequently identified as C4a desArg, that associated with histologic severity for patients with IgA nephropathy and showed that serum C4a desArg level was significantly higher in patients as compared to healthy controls [72].
In a Chinese study of 162 patients with IgA nephropathy, urinary mannan-binding lectin (MBL) level significantly associated with impaired renal function and magnitude of proteinuria [73]. Urinary MBL level at the time of renal biopsy was significantly lower for those who showed clinical improvement as compared to patients with disease progression and was associated with more severe renal histologic lesions [73].
2.6 Soluble CD89-IgA complexes
A role for soluble CD89–IgA complexes in the pathogenesis of IgA nephropathy is not fully understood. Soluble CD89–IgA complexes can induce an influx of mononuclear cells into the glomerular mesangium and renal interstitium in IgA nephropathy [74]. Recently, an amplification loop involving transglutaminase 2 was described in mesangial cells and it was postulated that this pathway facilitates IgA1-sCD89 deposition and mesangial cell activation [75]. These observations are contrasted with results of another publication that circulating CD89–IgA complexes are not specific for IgA nephropathy [76]. Furthermore, in a Swedish cohort, patients with IgA nephropathy without disease progression had stable but high levels of soluble CD89, whereas the patients with disease progression had low levels of soluble CD89 [77]. The same study also reported that levels of soluble CD89 complexes correlated with one of the five CD89 genetic variants in 212 patients with IgA nephropathy and 477 healthy controls; furthermore, the same single-nucleotide polymorphism was associated with lower expression of soluble CD89. However, no association between CD89 genetic polymorphisms and susceptibility to IgA nephropathy was found [77]. There is clearly more work to be done to better understand the interplay between CD89 and IgA1 in IgA nephropathy.
2.7 Urinary biomarkers
2.7.1 Urinary peptidomics
Peptidomics offers the opportunity to develop a non-invasive and unbiased diagnostic tool without a priori assumptions as to the pathogenesis of a disease. Peptidomics is the assessment (profiling) of peptides within a specific compartment, a peptidome. For such an analysis, urine has several advantages compared with plasma or serum because it is less complex and more stable. Analyses of urine samples from patients with IgA nephropathy and controls by SDS-PAGE and Western blotting for urinary protein patterns and molecular masses of the IgA and IgG heavy chains and their fragments has been undertaken. Patients with severe IgA nephropathy excreted large amounts of immunoglobulins and their fragments [78,79] that were presumably generated by proteases activated in the kidney [80]. As these polypeptides and fragments could represent disease-specific markers, capillary electrophoresis coupled with mass spectrometry (CE-MS) analysis of urinary peptidome was performed for a more detailed characterization. Samples from 58 patients with IgA nephropathy (reference set) were compared to samples from patients with various renal diseases (n = 253) and healthy controls (n = 207). A profile with 25 IgA nephropathy-specific biomarkers was defined; this pattern showed 92% sensitivity and 93% specificity [78,79]. These results thus validated an earlier study that also found disease-specific urinary peptides [81]. Thus, a CE-MS pattern of urinary polypeptides may serve as a biomarker for IgA nephropathy. If validated, it may be feasible to use these biomarkers to develop novel tests to detect renal injury at earlier stages, assess clinical manifestations, and monitor responses to therapy. New developments, such as comprehensive characterization of human urinary peptidome and availability of a urinary standard for healthy controls, will enable further development of disease-specific biomarkers [82–86].
Other peptidomic or proteomic approaches have been tested as well. For example, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis identified a fragment of uromodulin, m/z 1913.14, in urine samples that showed an area under the curve (AUC) of 0.998 for distinguishing patients with IgA nephropathy from healthy controls and of 0.815 from patients with other glomerulopathies [87]. MALDI-TOF MS analysis of urine samples from 19 patients with IgA nephropathy and 14 healthy controls found 16 IgA nephropathy-associated peptides, including uromodulin (m/z 1913) [88].
In another study, urine samples from 49 patients with IgA nephropathy were analyzed by surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI-TOF MS) and differentially expressed proteins were identified by MALDI-TOF MS. The IgA nephropathy patients had lower excretion of laminin G-like 3 and free κ light chains compared to patients with other chronic kidney diseases and healthy controls [89]. Urinary laminin G-like 3 and free κ light chain concentrations were inversely correlated with severity of clinical and histologic features [89].
Urine peptidome analysis has great potential for identification and measurement of clinically useful biomarkers for diagnosis and perhaps even prognosis of IgA nephropathy. Most of the data to date were generated in small patient cohorts from single centers. Large multi-center studies with clearly characterized clinical and histologic features are needed to advance this field of investigation.
2.7.2 Other urinary biomarkers
Torres et al. [90] measured urine levels of epidermal growth factor (EGF) and monocyte chemotactic peptide 1 (MCP-1) at the time of renal biopsy in 132 consecutive European patients with IgA nephropathy. The ROC analysis showed that the AUCs were 0.83, 0.57, and 0.91, for EGF, MCP-1, and EGF/MCP-1, respectively, for the outcome of end-stage kidney disease or doubling of serum creatinine concentration. In a cohort of 65 patients from the Netherlands, urinary excretion of KIM-1 and baseline serum creatinine had an AUC of 0.86 when used to predict end-stage kidney disease, although the specificity of 90% was accompanied by a sensitivity of only 60% [91].
Podocalyxin, present on the apical cell membrane of podocytes, is shed in urine following podocyte injury [92]. For 51 Japanese patients with IgA nephropathy, the urinary podocalyxin level on the day of biopsy significantly correlated with the severity of acute extracapillary abnormalities. Furthermore, the number of urinary podocytes was significantly higher in patients with segmental glomerular sclerosis as compared to those without segmental sclerosis [92].
2.8 Other serum biomarkers
Various other serum biomarkers have been studied and correlated with disease prognosis. Levels of advanced oxidative protein products in 292 patients with IgA nephropathy from the USA and Italy correlated with the rate of decline in renal function (r = −0.33, p = 0.008) measured by eGFR [32]. An increased serum level of interleukin-18, a cytokine associated with renal injury in ischemia-reperfusion models, was significantly correlated with the rate of loss of renal function (r = 0.242, p = 0.021), also measured by eGFR, in a cohort of 76 Chinese patients with IgA nephropathy with moderate to severe histologic changes on kidney biopsy (greater than 25% of glomeruli with crescents, segmental sclerosis, or global sclerosis) [93].
The endothelial ligand VCAM-1 is involved in adhesion of circulating leukocytes to endothelial cells and is up-regulated in response to inflammatory stimuli [94]. Plasma VCAM-1 levels were significantly increased in 327 patients with IgA nephropathy compared to levels in 55 healthy controls [94]. Increased VCAM-1 level in these patients associated with lower eGFR (p < 0.001), worse proteinuria (p < 0.001), more severe tubular atrophy/interstitial fibrosis (p < 0.001), and the presence of cellular crescents, fibrocellular crescents, and fibrinoid necrosis (p < 0.001) [95]. IgA1 from the sera of patients increased VCAM-1 expression by cultured endothelial cells, suggesting a link between undergalactosylated IgA1 and endothelial injury [95].
FGF23 is a circulating hormone involved in phosphate homeostasis [96], and high levels have been associated with increased mortality [97], cardiovascular events [98] and renal allograft loss [99]. In a Swedish cohort of 180 patients with IgA nephropathy, FGF23 levels in the highest tertile (> 23 RU/ml) were significantly associated (p < 0.001) with disease progression (defined as 50% rise in serum creatinine or progression to stage 5 chronic kidney disease) by Kaplan–Meier analysis [100].
3. Conclusion
Reliable biomarkers are needed for the non-invasive diagnosis of IgA nephropathy disease and to more fully delineate its natural history and risk for progression. Serum Gd-IgA1 level is a particularly good candidate for a diagnostic biomarker for individuals suspected of having the disease. As there are 3–6 O-glycans per hinge region of IgA1, molecular studies are needed to understand the heterogeneity of the aberrant IgA1 O-glycosylation. We envision that approaches using high-resolution mass spectrometry can define pathogenic glycoforms of Gd-IgA1 associated with the risk for IgA nephropathy.
Limited data for serum levels of anti-glycan antibody indicate great promise as both a diagnostic and prognostic marker. Future studies need to define the heterogeneity of anti-Gd-IgA1 antibodies and answer a basic question whether all potential glycoforms of Gd-IgA1 are recognized by the same set of autoantibodies or whether there are specific sets of Gd-IgA1 glycoforms that are recognized by unique autoantibodies with specific features.
Answering these questions should lead to a better characterization of pathogenic Gd-IgA1-containing complexes. However, additional studies are necessary to define the serum components required for formation of pathogenic (nephritogenic) immune complexes and to determine the respective roles of the alternative and lectin complement pathways in the pathogenesis of IgA nephropathy.
The application of urinary peptidomic techniques shows the potential to differentiate patients with IgA nephropathy from patients with other glomerular diseases and may be useful both for diagnosis and therapeutic monitoring of this disease. Future studies will need to define the origin of diagnostic urinary peptides (e.g., glomeruli vs. tubuli) and determine the prognostic significance of these peptides. Numerous other biomarkers have been examined and reported in the literature, but most lack specificity for the pathogenesis of IgA nephropathy.
4. Expert opinion
IgA nephropathy is the most common chronic glomerulonephritis in the world, but without a universally accepted non-invasive diagnostic biomarker its true prevalence and impact cannot be appreciated. Reliable biomarkers will potentially lead to earlier diagnosis, better monitoring of the clinical course or response to treatment, and, eventually, disease-targeted therapy. Serum Gd-IgA1 and glycan specific autoantibody levels are prime candidates to become diagnostic biomarkers for IgA nephropathy. These tests are attractive because of the postulated sequence of events in the autoimmune pathogenesis of the disease. However, currently the assays for these biomarkers have been performed only in research laboratories. The standardization of batches of commercially available GalNAc-specific lectins, such as those from Helix aspersa, will be necessary. The greatest need at this time is the development of an accurate and reproducible assay that can be performed in a clinical reference laboratory.
The organization and implementation of treatment trials for IgA nephropathy are severely limited by the lack of prognostic biomarkers. Prognostic biomarkers are requisite to select appropriate subjects for these trials and to serve as surrogate outcome markers that will shorten the length of the study. An ideal prognostic biomarker would identify high-risk patients for treatment prior to irreparable kidney damage that is manifested by clinical and histologic prognostic indicators used as inclusion criteria in most current trials.
Serum levels of the complement proteins C3 and factor H can easily be measured in the clinical laboratory. Levels of these proteins, either alone or in tandem with total serum IgA or serum Gd-IgA1 levels, deserve continued study as prognostic biomarkers for patients with IgA nephropathy. In contrast to serum C3 and factor H levels, most complement activation fragment assays require EDTA plasma samples to be frozen shortly after being obtained and not thawed until just before the assay is performed.
Article highlights.
Serum Gd-IgA1 level is a potential candidate for a useful diagnostic biomarker for individuals suspected to have IgA nephropathy.
Limited data for serum levels of anti-glycan antibody indicate some promise for it as both a diagnostic and prognostic marker.
The role of activation of the alternative complement pathway may be underappreciated in IgA nephropathy. Serum IgA and C3 levels are readily available in clinical practice and the serum IgA/C3 ratio may have utility as both a diagnostic and prognostic marker.
Urinary peptidomic techniques have successfully differentiated patients with IgA nephropathy (even those with only modest proteinuria) from healthy controls and from patients with other forms of renal disease.
Urinary levels of epidermal growth factor, kidney injury molecule-1, mannose binding lectin, and excreted podocytes and podocalyxin have been proposed as biomarkers for IgA nephropathy.
Serum levels of advanced oxidative protein products, interleukin-18, soluble CD89-IgA complexes, vascular cell adhesion have been studied as possible biomarkers for IgA nephropathy.
This box summarizes key points contained in the article.
Acknowledgments
Supported in part by NIH grants DK078244, DK082753, and GM098539, by a grant from the IgA Nephropathy Foundation of America, and by a generous gift from Anna and Don Waite.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.D’Amico G. The commonest glomerulonephritis in the world: igA nephropathy. Q J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 2.Hastings MC, Delos Santos NM, Wyatt RJ. Renal survival in pediatric patients with IgA nephropathy. Pediatr Nephrol. 2007;22:317–318. doi: 10.1007/s00467-006-0303-3. [DOI] [PubMed] [Google Scholar]
- 3. Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. •• General review of the manifestations and pathogenesis of IgA nephropathy from a clinician’s perspective.
- 4.Wyatt RJ, Julian BA, Bhathena DB, et al. IgA nephropathy: presentation, clinical course, and prognosis in children and adults. Am J Kidney Dis. 1984;4:192–200. doi: 10.1016/s0272-6386(84)80071-2. [DOI] [PubMed] [Google Scholar]
- 5. Reich HN, Troyanov S, Scholey JW, et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. •• Landmark paper that found that time-average proteinuria has much better prognostic value than proteinuria at the time of renal biopsy.
- 6.D’Amico G, Minetti L, Ponticelli C, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–378. [PubMed] [Google Scholar]
- 7.Radford MG, Donadio JV, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 8. Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. •• Multi-national study that identified four light-microscopic histological features that exhibited independent value as prognostic markers of IgA nephropathy.
- 9.Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–761. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. • Overview of the laboratory findings for patients with IgA nephropathy and discussion of the four-hit hypothesis of the autoimmune pathogenesis of the disease.
- 11.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. • This paper describes the imbalance of glycosyltransferases that leads to secretion of Gd-IgA1 by cell lines derived from cells in the peripheral blood of patients with IgA nephropathy.
- 13. Suzuki H, Fan R, Zhang Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. • A detailed description of the anti-glycan autoantibodies that form nephritogenic circulating immune complexes in IgA nephropathy.
- 14.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 15.Mestecky J, Moro I, Kerr MA, Woof JM. Mucosal immunoglobulins. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology. 3rd edition. Amsterdam: Elsevier Academic Press; 2005. pp. 153–181. [Google Scholar]
- 16.Frangione B, Wolfenstein-Todel C. Partial duplication in the "hinge" region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972;69:3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putnam FW. Structure of the human IgA subslasses and allotypes. Protides Biol Fluids. 1989;36:27–37. [Google Scholar]
- 18.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 19.Field MC, Dwek RA, Edge CJ, Rademacher TW. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989;17:1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- 20.Mattu TS, Pleass RJ, Willis AC, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 21.Tomana M, Niedermeier W, Mestecky J, Hammack WJ. The carbohydrate composition of human myeloma IgA. Immunochemistry. 1972;9:933–940. doi: 10.1016/0019-2791(72)90166-8. [DOI] [PubMed] [Google Scholar]
- 22.Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976;13:325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- 23.Tomana M, Niedermeier W, Spivey C. Microdetermination of monosaccharide in glycoproteins. Anal Biochem. 1978;89:110–118. doi: 10.1016/0003-2697(78)90731-5. [DOI] [PubMed] [Google Scholar]
- 24. Renfrow MB, Cooper HJ, Tomana M, et al. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. • The first study using high-resolution mass spectrometry to analyze heterogeneity of IgA1 O-glycosylation.
- 25.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andre PM, Le Pogamp P, Chevet D. Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal. 1990;4:115–119. doi: 10.1002/jcla.1860040208. [DOI] [PubMed] [Google Scholar]
- 27. Mestecky J, Tomana M, Crowley-Nowick PA, et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. • The findings in this paper introduced the importance of a reduced content of galactose on IgA1 O-glycans as the basis for slow hepatic clearance of IgA1 from the circulation. The multiple studies that followed from centers around the world have clarified the structure of the IgA1 hinge region glycans that is the crux of the autoimmune nature of the pathogenesis of IgA nephropathy.
- 28. Moldoveanu Z, Wyatt RJ, Lee J, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. • This paper describes application of the first quantitative assay for Gd-IgA1.
- 29. Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. • The first report of the autoantigen nature of the Gd-IgA1 in patients with IgA nephropathy.
- 30.Smith AC, de Wolff JF, Molyneux K, et al. O-Glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol. 2006;17:1192–1199. doi: 10.1681/ASN.2005101115. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Fan R, Zhang Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camilla R, Suzuki H, Daprà V, et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011;6:1903–1911. doi: 10.2215/CJN.11571210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berthoux F, Suzuki H, Thibaudin L, et al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23:1579–1687. doi: 10.1681/ASN.2012010053. • This paper documents the clinical importance of IgG or IgA anti-glycan antibodies on the clinical course of patients with IgA nephropathy.
- 34.Shimozato S, Hiki Y, Odani H, et al. Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant. 2008;23:1931–1939. doi: 10.1093/ndt/gfm913. [DOI] [PubMed] [Google Scholar]
- 35.Lin X, Ding J, Zhu L, et al. Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant. 2009;24:3372–3375. doi: 10.1093/ndt/gfp294. [DOI] [PubMed] [Google Scholar]
- 36. Zhao N, Hou P, Lv J, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790–796. doi: 10.1038/ki.2012.197. • This paper describes a cohort of Chinese patients with IgA nephropathy with a long follow-up in which serum levels of Gd-IgA1 were associated with disease progression.
- 37.Hastings MC, Moldoveanu Z, Julian BA, et al. Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability. Clin J Am Soc Nephrol. 2010;5:2069–2074. doi: 10.2215/CJN.03270410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau KK, Wyatt RJ, Moldoveanu Z, et al. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol. 2007;22:2067–2072. doi: 10.1007/s00467-007-0623-y. [DOI] [PubMed] [Google Scholar]
- 39.Dickinson SJ, Mendichovzsky I, Molyneux K, et al. O-galactosylation patterns of serum IgA1 in patients with IgA nephropathy remain constant over long periods of time. J Am Soc Nephrol. 2008;19:659A. [Google Scholar]
- 40.Olson SW, Novak J, Suzuki H, et al. Evaluation of chronic serum galactose-deficient IgA1 levels prior to diagnosis of IgA nephropathy. J Am Soc Nephrol. 2009;20:149A. [Google Scholar]
- 41.Hastings MC, Sanders JT, Moldoveanu Z, et al. Serial measurement of galactose-deficient IgA1 (Gd-IgA1) in children. J Am Soc Nephrol. 2010;21:635A. [Google Scholar]
- 42.Gharavi AG, Moldoveanu Z, Wyatt RJ, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiryluk K, Moldoveanu Z, Sanders JT, et al. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2011;80:79–87. doi: 10.1038/ki.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders JT, Moldoveanu Z, Wen-Qiang H, et al. Galactose-deficient IgA1 in normal pediatric subjects. American Pediatric Society/Society for Pediatric Research. 2010 Available from: http://www.abstracts2view.com/pasall/search. [Google Scholar]
- 45.Renfrow MB, Mackay CL, Chalmers MJ, et al. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem. 2007;389:1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 46. Takahashi K, Smith AD, Poulsen K, et al. Naturally occurring structural isomers in serum IgA1 o-glycosylation. J Proteome Res. 2012;11:692–702. doi: 10.1021/pr200608q. • This paper reveals the existence of isomeric glycoforms of IgA1 hinge region glycopeptides.
- 47.Takahashi K, Suzuki H, Koshi Y, et al. Molecular characterization of IgA1 secreted by IgA1-producing cell lines from patients with IgA nephropathy. J Am Soc Nephrol. 2012;23:853A. [Google Scholar]
- 48.Hastings MC, Afshan S, Sanders JT, et al. Serum galactose-deficient IgA1 level is not associated with proteinuria in children with IgA nephropathy. Int J Nephrol. 2012;2012:315467. doi: 10.1155/2012/315467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu LX, Zhao MH. Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int. 2005;68:167–172. doi: 10.1111/j.1523-1755.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 50.Moore JS, Kulhavy R, Tomana M, et al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czerkinsky C, Koopman WJ, Jackson S, et al. Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. J Clin Invest. 1986;77:1931–1938. doi: 10.1172/JCI112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schena FP, Pastore A, Ludovico N, et al. Increased serum levels of IgA1-IgG immune complexes and anti-F(ab’) 2 antibodies in patients with primary IgA nephropathy. Clin Exp Immunol. 1989;77:15–20. [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi M, Takahashi S, Hirose S. Solubilization of antigen-antibody complexes: a new function of complement as a regulator of immune reactions. Prog Allergy. 1980;27:134–166. [PubMed] [Google Scholar]
- 54.Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 55.Gharavi AG, Kiryluk K, Choi M, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. doi: 10.1371/journal.pgen.1002765. A summary of the genetic loci associated with IgA nephropathy and their variation in prevalence in different regions of the world.
- 57.Wyatt RJ, Forristal J, Davis CA, et al. Control of serum C3 levels by beta 1 H and C3b inactivator. J Lab Clin Med. 1980;95:905–917. [PubMed] [Google Scholar]
- 58.Julian BA, Wyatt RJ, McMorrow RG, Galla JH. Serum complement proteins in IgA nephropathy. Clin Nephrol. 1983;20:251–258. [PubMed] [Google Scholar]
- 59.Kim SJ, Koo HM, Lim BJ, et al. Decreased circulating C3 levels and mesangial c3 deposition predict renal outcome in patients with IgA nephropathy. PLoS ONE. 2012;7:e40495. doi: 10.1371/journal.pone.0040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki H, Ohsawa I, Kodama F, et al. Fluctuation of serum C3 levels reflects disease activity and metabolic background in patients with IgA nephropathy. J Nephrol. 2013;26:708–715. doi: 10.5301/jn.5000278. [DOI] [PubMed] [Google Scholar]
- 61.Tomino Y, Suzuki S, Imai H, et al. Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J Clin Lab Anal. 2000;14:220–223. doi: 10.1002/1098-2825(2000)14:5<220::AID-JCLA4>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishiguro C, Yaguchi Y, Funabiki K, et al. Serum IgA/C3 ratio may predict diagnosis and prognostic grading in patients with IgA nephropathy. Nephron. 2002;91:755–758. doi: 10.1159/000065043. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Wang C, Tang Y, et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 2013;18:125–131. doi: 10.1111/nep.12010. [DOI] [PubMed] [Google Scholar]
- 64.Maeda A, Gohda T, Funabiki K, et al. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J Clin Lab Anal. 2003;17:73–76. doi: 10.1002/jcla.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyatt RJ, Kanayama Y, Julian BA, et al. Complement activation in IgA nephropathy. Kidney Int. 1987;31:1019–1023. doi: 10.1038/ki.1987.101. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt RJ, Julian BA. Activation of complement in IgA nephropathy. Am J Kidney Dis. 1988;12:437–442. doi: 10.1016/s0272-6386(88)80042-8. [DOI] [PubMed] [Google Scholar]
- 67.Zwirner J, Burg M, Schulze M, et al. Activated complement C3: a potentially novel predictor of progressive IgA nephropathy. Kidney Int. 1997;51:1257–1264. doi: 10.1038/ki.1997.171. [DOI] [PubMed] [Google Scholar]
- 68.Lagrue G, Branellec A, Intrator L, et al. Measurements of serum C3d in primitive chronic glomerular nephropathies (author’s transl)] Nouv Presse Med. 1979;8:1153–1156. [PubMed] [Google Scholar]
- 69.Sølling J. Circulating immune complexes and complement breakdown product C3d in glomerulonephritis and kidney transplantation. Acta Pathol Microbiol Immunol Scand C. 1984;92:213–220. doi: 10.1111/j.1699-0463.1984.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 70.Janssen U, Bahlmann F, Köhl J, et al. Activation of the acute phase response and complement C3 in patients with IgA nephropathy. Am J Kidney Dis. 2000;35:21–28. doi: 10.1016/S0272-6386(00)70296-4. [DOI] [PubMed] [Google Scholar]
- 71.Zhang JJ, Jiang L, Liu G, et al. Levels of urinary complement factor H in patients with IgA nephropathy are closely associated with disease activity. Scand J Immunol. 2009;69:457–464. doi: 10.1111/j.1365-3083.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 72.Sogabe A, Uto H, Kanmura S, et al. Correlation of serum levels of complement C4a desArg with pathologically estimated severity of glomerular lesions and mesangial hypercellularity scores in patients with IgA nephropathy. Int J Mol Med. 2013 doi: 10.3892/ijmm.2013.1390. [DOI] [PubMed] [Google Scholar]
- 73.Liu LL, Jiang Y, Wang LN, Liu N. Urinary mannose-binding lectin is a biomarker for predicting the progression of immunoglobulin (Ig)A nephropathy. Clin Exp Immunol. 2012;169:148–155. doi: 10.1111/j.1365-2249.2012.04604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Launay P, Grossetête B, Arcos-Fajardo M, et al. Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-IgA complexes in patients and CD89 transgenic mice. J Exp Med. 2000;191:1999–2009. doi: 10.1084/jem.191.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793–806. doi: 10.1084/jem.20112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Boog PJ, De Fijter JW, Van Kooten C, et al. Complexes of IgA with FcalphaRI/CD89 are not specific for primary IgA nephropathy. Kidney Int. 2003;63:514–521. doi: 10.1046/j.1523-1755.2003.00756.x. [DOI] [PubMed] [Google Scholar]
- 77.Vuong MT, Hahn-Zoric M, Lundberg S, et al. Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int. 2010;78:1281–1287. doi: 10.1038/ki.2010.314. [DOI] [PubMed] [Google Scholar]
- 78.Julian BA, Wittke S, Novak J, et al. Electrophoretic methods for analysis of urinary polypeptides in IgA-associated renal diseases. Electrophoresis. 2007;28:4469–4483. doi: 10.1002/elps.200700237. [DOI] [PubMed] [Google Scholar]
- 79.Julian BA, Wittke S, Haubitz M, et al. Urinary biomarkers of IgA nephropathy and other IgA-associated renal diseases. World J Urol. 2007;25:467–476. doi: 10.1007/s00345-007-0192-5. [DOI] [PubMed] [Google Scholar]
- 80.Candiano G, Musante L, Bruschi M, et al. Repetitive fragmentation products of albumin and alpha1-antitrypsin in glomerular diseases associated with nephrotic syndrome. J Am Soc Nephrol. 2006;17:3139–3148. doi: 10.1681/ASN.2006050486. [DOI] [PubMed] [Google Scholar]
- 81. Haubitz M, Wittke S, Weissinger EM, et al. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int. 2005;67:2313–2320. doi: 10.1111/j.1523-1755.2005.00335.x. • An early description of the diagnostic utility of mass spectrometry in analysis of the urinary proteome to generate a disease-specific signature pattern for IgA nephropathy.
- 82.Mischak H, Coon JJ, Novak J, et al. Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spectrom Rev. 2009;28:703–724. doi: 10.1002/mas.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Good DM, Zurbig P, Argiles A, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. • A comprehensive characterization of human urinary peptidome.
- 84.Mischak H, Kolch W, Aivaliotis M, et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl. 2010;4:464–748. doi: 10.1002/prca.200900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mischak H, Ioannidis JP, Argiles A, et al. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest. 2012;42:1027–1036. doi: 10.1111/j.1365-2362.2012.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mischak H, Allmaier G, Apweiler R, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2:46ps2. doi: 10.1126/scitranslmed.3001249. [DOI] [PubMed] [Google Scholar]
- 87.Wu J, Wang N, Wang J, et al. Identification of a uromodulin fragment for diagnosis of IgA nephropathy. Rapid Commun Mass Spectrom. 2010;24:1971–1978. doi: 10.1002/rcm.4601. [DOI] [PubMed] [Google Scholar]
- 88.Graterol F, Navarro-Muñoz M, Ibernon M, et al. Poor histological lesions in IgA nephropathy may be reflected in blood and urine peptide profiling. BMC Nephrol. 2013;14:82. doi: 10.1186/1471-2369-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rocchetti MT, Papale M, d’Apollo AM, et al. Association of urinary laminin g-like 3 and free k light chains with disease activity and histological injury in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8:1115–1125. doi: 10.2215/CJN.05950612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torres DD, Rossini M, Manno C, et al. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008;73:327–333. doi: 10.1038/sj.ki.5002621. [DOI] [PubMed] [Google Scholar]
- 91.Peters HP, Waanders F, Meijer E, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:3581–3588. doi: 10.1093/ndt/gfr135. [DOI] [PubMed] [Google Scholar]
- 92.Asao R, Asanuma K, Kodama F, et al. Relationships between levels of urinary podocalyxin, number of urinary podocytes, and histologic injury in adult patients with IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(9):1385–1393. doi: 10.2215/CJN.08110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi B, Ni Z, Cao L, et al. Serum IL-18 is closely associated with renal tubulointerstitial injury and predicts renal prognosis in IgA nephropathy. Mediators Inflamm. 2012;2012:728417. doi: 10.1155/2012/728417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fotis L, Giannakopoulos D, Stamogiannou L, Xatzipsalti M. Intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 in children. Do they play a role in the progression of atherosclerosis? Hormones (Athens) 2012;11:140–146. doi: 10.14310/horm.2002.1340. [DOI] [PubMed] [Google Scholar]
- 95.Zhu L, Shi S, Liu L, et al. Increased plasma sVCAM-1 is associated with severity in IgA nephropathy. BMC Nephrol. 2013;14:21. doi: 10.1186/1471-2369-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 97.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olauson H, Qureshi AR, Miyamoto T, et al. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. 2010;25:3033–3038. doi: 10.1093/ndt/gfq191. [DOI] [PubMed] [Google Scholar]
- 99.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lundberg S, Qureshi AR, Olivecrona S, et al. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7:727–734. doi: 10.2215/CJN.10331011. [DOI] [PMC free article] [PubMed] [Google Scholar]