Abstract
Background
Colorectal cancer (CRC) screening is underutilized by minority populations. Patient navigation increases adherence with screening colonoscopy. We estimated the cost-effectiveness of navigation for screening colonoscopy from the perspective of a payer seeking to improve population health.
Methods
We informed our validated model of CRC screening with inputs from navigation studies in New York City (population 43% African American, 49% Hispanic, 4% White, 4% Other; base case screening 40% without and 65% with navigation, navigation costs $29/colonoscopy completer, $21/non-completer, $3/non-navigated). We compared: 1) navigation vs. no navigation for one-time screening colonoscopy in unscreened persons age ≥50; 2) programs of colonoscopy with vs. without navigation, vs. fecal occult blood testing (FOBT) or immunochemical testing (FIT) for ages 50-80.
Results
In the base case: 1) one-time navigation gained quality-adjusted life-years (QALYs) and decreased costs; 2) longitudinal navigation cost $9,800/QALY gained vs. no navigation, and assuming comparable uptake rates, it cost $118,700/QALY gained vs. FOBT, but was less effective and more costly than FIT. Results were most dependent on screening participation rates and navigation costs: 1) assuming a 5% increase in screening uptake with navigation and navigation cost of $150/completer, one-time navigation cost $26,400/QALY gained; 2) longitudinal navigation with 75% colonoscopy uptake cost <$25,000/QALY gained vs. FIT when FIT uptake was <50%. Probabilistic sensitivity analyses did not alter the conclusions.
Conclusions
Navigation for screening colonoscopy appears to be cost-effective, and one-time navigation may be cost-saving. In emerging healthcare models that reward outcomes, payers should consider covering the costs of navigation for screening colonoscopy.
Keywords: colorectal cancer, colorectal neoplasia, screening, adherence, disparities
Introduction
Colorectal cancer (CRC) screening is highly effective and cost-effective.1-12 Among CRC screening options,13 colonoscopy is the most common test in the U.S., followed by guaiac-based fecal occult blood testing (FOBT), and fecal immunochemical testing (FIT).14
Despite its effectiveness, CRC screening is underutilized,14 especially among minority populations.15, 16 Patient navigation, originally developed to help patients with an abnormal screening test such as mammography receive timely management,17 has been used to overcome CRC screening barriers.18-22 Navigators are trained personnel who facilitate adherence by helping with scheduling, preparation, answering questions and allaying fears. Among predominantly low socioeconomic status African-American and Hispanic urban populations, navigation has increased colonoscopy rates and improved bowel preparations,18, 19, 21 and has yielded high rates of colonic neoplasia.22, 23
There is currently no reimbursement for navigation. In our recent cost analysis, patient navigation proved profitable for our institution by increasing colonoscopy volume.24 Another analysis demonstrated a net financial benefit for two institutions and a net cost for one, depending on the costs of program personnel other than navigators.20
The cost-effectiveness of navigation from the perspective of a health services payer seeking to improve population health has not been assessed. Our first aim was to estimate the long-term cost-effectiveness of navigation to increase the uptake of one-time CRC screening in settings where colonoscopic screening has been championed (e.g. New York City). Given the diversity in CRC screening preferences among minority populations,25 our second aim was to examine navigation as part of a longitudinal colonoscopic screening program compared with fecal testing programs.
Methods
General study design
We adapted our published and validated decision analytic general U.S. population CRC screening Markov model12, 26 to reflect CRC epidemiology and non-CRC age-related mortality in African American, Hispanic and White persons, and aggregated these subpopulations into a cohort reflecting the predominantly minority population in our navigation studies at Mount Sinai Hospital, New York.18, 19, 21
First, we explored navigation for one-time screening colonoscopy in a cohort of previously unscreened persons age 50 or older, with the race/ethnicity and age distributions observed in our recent navigation study.27 This simulation was constructed to reflect real-world, one-time navigation in a population of mixed race/ethnicity and age.
Second, we considered ongoing navigation in a colonoscopy screening program over decades. This simulation was constructed to reflect hypothetical predominantly minority cohorts offered screening from age 50 to 80.13
Navigation costs were added to screening costs.
Decision analytic model
Our original model, data sources, and validation against the Minnesota Colon Cancer Control Study,1, 2 United Kingdom Flexible Sigmoidoscopy Trial,6 SCORE Trial,7 and PLCO Cancer Screening Trial8 have been detailed previously12, 26, 28 (Supplementary Material). The model reproduces the natural history of adenomas and CRC in the general U.S. population without screening. Persons transition between health states of normal, small polyp, large polyp, localized, regional or disseminated CRC, and dead, in 1-year cycles. Screening is superimposed on natural history, resulting in CRC prevention or early detection.12, 26, 28 Model inputs are derived from autopsy data on polyps; Surveillance, Epidemiology, and End Results (SEER) data on CRC incidence and stage from dates preceding widespread CRC screening; studies on test performance characteristics and complication rates, outcomes after CRC treatment, and CRC-related quality of life; U.S. Life Tables; and Medicare payments (Supplementary Table 1).
Here, we constructed a hypothetical cohort based on our recent navigation study27: 43% African American, 49% Hispanic, 4% White, 4% Other. For the African American, Hispanic, and White subpopulations, we adjusted the age-dependent prevalence of lesions at simulation entry, and the transition probabilities from normal to small polyp or localized CRC by a common factor as previously reported29 and calibrated these to reflect the age-dependent CRC incidence in each subpopulation reported in SEER 1992 data, preceding widespread screening.30 The adjustment factors were: African American 1.19, Hispanic 0.65, and White 1.05. Subpopulation-specific age-dependent non-CRC mortality rates were derived from U.S. Life Tables 2008.31
For one-time colonoscopy, persons entered the model at the midpoint of age bands reflecting our recent study: 57% age 50-59, 33% age 60-69, 9% age 70-79 and 1% age ≥80.27 Persons were followed until age 100 or death with no further screening or surveillance.
For screening over decades, all persons entered the model at age 50 and screening and surveillance were offered from age 50 to 80. Persons were followed until age 100 or death.
Navigation for one-time screening colonoscopy
We compared navigation vs. no navigation vs. no screening. At colonoscopy, polyps were removed and CRCs were biopsied if detected. In the base case, colonoscopy uptake without navigation was 40%, based on uptake at Mount Sinai Hospital before navigation was available, and uptake with navigation was conservatively assumed to be 65%, as observed in our early studies.18 A net uptake gain of approximately 20% was reported in three other institutions with higher pre-navigation uptake.20 Sensitivity analyses explored the trade-off between the improvements in colonoscopy uptake with navigation and navigation costs.
Navigation in longitudinal screening
We compared colonoscopy every 10 years with navigation vs. without navigation, vs. annual FOBT or FIT programs.13 For colonoscopy with navigation, navigation was offered every time a person was due for colonoscopy.
Screening and surveillance were offered from ages 50 to 80. If stool-based screening was positive, colonoscopy was offered. If colonoscopy was normal, the stool test was considered false-positive and stool-based screening resumed in 10 years. Polypectomy and biopsy were modeled as above. If screening colonoscopy was normal, it was offered again in 10 years. Surveillance colonoscopy was performed 3 or 5 years after large or small polyp removal respectively,32, 33 and 3 years and then every 5 years after CRC diagnosis.32
In the base case, uptake of the screening colonoscopy program was 40% without navigation and 65% with navigation.18 As comparators, we modeled 40% and 65% uptake for FOBT and FIT programs. For simplicity, in the base case we assumed that persons taking up screening adhered to every testing cycle. In sensitivity analyses, we considered varying levels of screening uptake and per-cycle adherence.34-36
Cost inputs
Base case cost inputs in year 2012 dollars were derived from Medicare reimbursement rates37-39 and estimated CRC care costs40 (Supplementary Material). The base case navigation costs were $29 per colonoscopy completer, $21 per non-completer, and $3 per non-navigated person, as derived in our recent cost analysis.24 In sensitivity analyses we covered the estimates for other New York City institutions ($51, $74 and $287 per patient referred to navigation).20
Clinical and economic outcomes
Primary outputs were quality-adjusted life-years (QALYs) and costs per person,41, 42 discounted by 3%/year.43 Health state utilities for CRC by stage (Supplementary Table 1) were applied for five years after CRC diagnosis. We estimated CRC cases by stage and deaths in 10,000-person cohorts.27
Cost-effectiveness analyses
Analyses from the perspective of a third-party payer were performed in TreeAge Pro (TreeAge Software, Inc., Williamstown, MA) and Excel 2011 (Microsoft Corporation, Redmond, WA). Incremental cost-effectiveness ratios were calculated.41, 42
One-way sensitivity analyses examined all inputs. Threshold and two-way sensitivity analyses addressed influential variables, including uptake and adherence.34-36 To estimate the uncertainty of our projections, we performed Monte Carlo simulations with 1,000 trials, using beta distributions for probabilities derived from parameters in the literature.44 Screening costs were varied by a common factor within a range of 20% of the base case, and CRC care by a different common factor within the same range. Navigation costs were varied as a set, maintaining the base case ratio between them.
Results
Navigation for one-time screening colonoscopy, 10,000-person cohort: Base case
Without screening, 551 CRCs were diagnosed, compared with 453 CRCs when colonoscopy uptake was 40% without navigation, and 392 CRCs when navigation increased colonoscopy uptake to 65%; screening shifted CRC stage towards earlier stages (Table 1). The number of CRC deaths was 216 without screening, 174 with colonoscopy without navigation, and 148 with colonoscopy with navigation (Table 1). Screening yielded gains in QALYs and cost savings overall, and in all subpopulations and age-bands (Table 1). Colonoscopy without navigation was more effective and less costly than no screening (i.e. dominant), and colonoscopy with navigation was dominant over colonoscopy without navigation.
Table 1. Clinical and economic outcomes with navigation for one-time screening colonoscopy in a 10,000-person predominantly minority cohort, ages 50 to >80: Base case.
| No Screening | Colonoscopy without Navigation | Colonoscopy with Navigation | |
|---|---|---|---|
| Screening Uptake | 0% | 40% | 65% |
| CRC cases per 10,000 persons from entry until age 100 | 551 | 453 | 392 |
| CRC stage | |||
| Localized | 40% | 41% | 42% |
| Regional | 37% | 37% | 37% |
| Distant | 23% | 22% | 21% |
| CRC deaths per 10,000 persons | 216 | 174 | 148 |
| QALYs/person | 15.4222 | 15.4439 | 15.4575 |
| Cost/person | $2,814 | $2,558 | $2,422 |
| ICER | |||
| No Screening | - | Dominates | Dominates |
| Colonoscopy without Navigation | - | - | Dominates |
| Colonoscopy with Navigation | - | - | - |
| Results by subpopulation | |||
| QALYs/person, African American* | 14.2774 | 14.3012 | 14.3161 |
| Cost/person, African American* | $3,276 | $2,929 | $2,734 |
| QALYs/person, Hispanic* | 16.4264 | 16.4458 | 16.4579 |
| Cost/person, Hispanic* | $2,347 | $2,184 | $2,105 |
| QALYs/person, White* | 15.4349 | 15.4602 | 15.4761 |
| Cost/person, White* | $3,258 | $2,917 | $2,727 |
| QALYs/person, Age 55 at entry** | 17.4717 | 17.4944 | 17.5086 |
| Cost/person, Age 55 at entry** | $2,661 | $2,480 | $2,389 |
| QALYs/person, Age 65 at entry** | 13.7169 | 13.7398 | 13.7540 |
| Cost/person, Age 65 at entry** | $3,058 | $2,697 | $2,495 |
| QALYs/person, Age 75 at entry** | 9.7467 | 9.7600 | 9.7684 |
| Cost/person, Age 75 at entry** | $2,945 | $2,597 | $2,402 |
| QALYs/person, Age 85 at entry** | 5.9554 | 5.9610 | 5.9643 |
| Cost/person, Age 85 at entry** | $2,271 | $2,127 | $2,061 |
57% age 50-59, 33% age 60-69, 9% age 70-79 and 1% age ≥80.
43% African American, 49% Hispanic, 4% White and 4% Other.
CRC, colorectal cancer; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio.
Navigation for one-time screening colonoscopy: Sensitivity analyses
In one-way sensitivity analyses, the cost-effectiveness of navigation depended most on the increase in screening uptake achieved with navigation and the navigation costs (Table 2, Figure 1). Navigation became cost-neutral assuming base case costs at an increase in screening uptake to 43% with navigation vs. 40% without navigation; it cost $6,000/QALY gained assuming base case uptake rates when navigation costs were 10-fold the base case costs ($300 per colonoscopy completer); and it cost $25,000/QALY gained assuming base case uptake rates when navigation costs were approximately 20-fold the base case costs ($622 per colonoscopy completer) (Table 2).
Table 2. Cost-effectiveness of navigation for one-time screening colonoscopy: One-way sensitivity analyses.
| Variable | Base case value | Value in sensitivity analysis |
Colonoscopy with Navigation vs. Colonoscopy without Navigation |
||
|---|---|---|---|---|---|
| Incremental cost/person |
Incremental QALYs/10,000 persons |
ICER | |||
| Navigation cost (Completer cost) | $29 | $199 (approximately 7-fold increase) | $0 | 136 | - |
| $300 (approximately 10-fold increase) | $81 | 136 | $6,000 | ||
| $622 (approximately 21-fold increase) | $340 | 136 | $25,000 | ||
| Uptake if navigated | 65% | 43% | $0 | 17 | - |
| 41% | $13 | 5 | $25,200 | ||
| Colonoscopy sensitivity for small polyp/large polyp/CRC | 0.85/0.90/0.95 | 0.75/0.85/0.92 | (-$113) | 130 | Navigation Dominates* |
| Colonoscopy bleeding/perforation rates | 0.0016/0.00085 | 0.008/0.00425 (five-fold increase) | (-$114) | 130 | Navigation Dominates* |
| Colonoscopy cost/with biopsy or polypectomy | $661/$939 | $1,190/$1,690 (80% increase) | $6 | 136 | $446 |
| $1,322/$1,878 (two-fold increase) | $42 | 136 | $3,100 | ||
| $2,644/$3,756 (four-fold increase) | $398 | 136 | $29,300 | ||
| Colonoscopy bleeding/perforation costs | $6,043/$16,377 | $12,086/$32,754 (two-fold increase) | (-$131) | 136 | Navigation Dominates* |
| Colonoscopy cost/with biopsy or polypectomy/bleeding/perforation | $661/$939/$6,043/$16,377 | $992/$1,409/$9,065/$24,566 (50% increase) | (-$45) | 136 | Navigation Dominates* |
| CRC care costs | (40% decrease) | $1 | 136 | $65 | |
| (50% decrease) | $35 | 136 | $2,600 | ||
| (50% increase) | (-$308) | 136 | Navigation Dominates* | ||
More effective and less costly than comparator.
CRC, colorectal cancer; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio.
Figure 1.
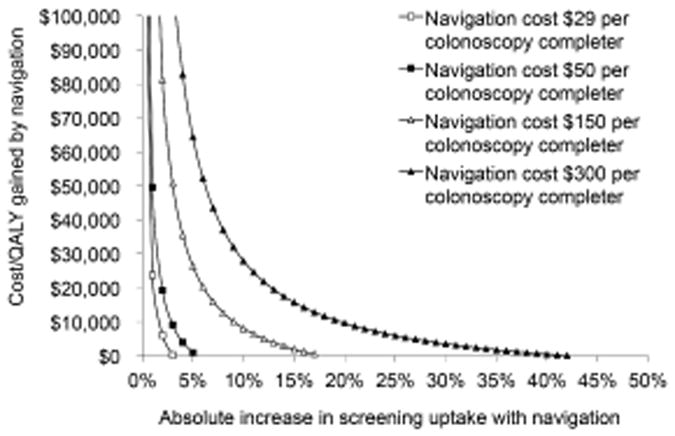
Two-way sensitivity analysis on the cost of navigation and the absolute increase in screening uptake for one-time screening colonoscopy.
In two-way sensitivity analyses, navigation cost $26,400/QALY gained when the navigation cost per colonoscopy completer was $150 and screening uptake with navigation was 45% vs. 40% without navigation; and it cost $28,000/QALY gained when the navigation cost per colonoscopy completer was $300 and screening uptake with navigation was 50% vs. 40% without navigation (Figure 1).
Other variables were less influential. Navigation remained dominant over no navigation with less optimistic colonoscopy sensitivities, specificity and complication rates, a two-fold increase in colonoscopy complication costs, or a 50% increase in colonoscopy and colonoscopy complication costs (Table 2). It cost <$30,000/QALY gained with up to a four-fold increase in colonoscopy costs and <$3,000/QALY gained with a 50% reduction in CRC care costs.
In the Monte Carlo simulation, compared with colonoscopy without navigation, colonoscopy with navigation gained a median of 132 QALYs/10,000 persons (95% range, 119-165), and was cost-saving in all iterations.
Navigation in longitudinal screening, 10,000-person cohort: Base case
Without screening, 580 CRCs were diagnosed, compared with 410 CRCs when colonoscopy uptake was 40% without navigation, and 304 CRCs when navigation increased colonoscopy uptake to 65% (Table 3). Colonoscopy without navigation cost $2,400/QALY gained vs. no screening, and colonoscopy with navigation cost $9,800/QALY gained vs. colonoscopy without navigation (Table 4).
Table 3. Clinical and economic outcomes with navigation as part of a longitudinal screening program in a 10,000-person predominantly minority cohort: Base case.
| No Screening | FOBT, 40% uptake | Colonoscopy without Navigation, 40% uptake | FIT, 40% uptake | FOBT, 65% uptake | Colonoscopy with Navigation, 65% uptake | FIT, 65% uptake | |
|---|---|---|---|---|---|---|---|
| CRC cases per 10,000 persons | 580 | 470 | 410 | 435 | 401 | 304 | 345 |
| CRC stage | |||||||
| Localized | 40% | 46% | 42% | 45% | 51% | 45% | 51% |
| Regional | 37% | 35% | 37% | 35% | 32% | 36% | 32% |
| Distant | 23% | 20% | 21% | 20% | 17% | 19% | 18% |
| CRC deaths per 10,000 persons | 226 | 166 | 153 | 156 | 129 | 108 | 113 |
| QALYs/person | 19.2157 | 19.2423 | 19.2460 | 19.2465 | 19.2589 | 19.2649 | 19.2657 |
| Cost/person | $2,401 | $2,123 | $2,475 | $2,183 | $1,949 | $2,661 | $2,046 |
| ICER | |||||||
| No Screening | - | Dominates | $2,400 | Dominates | Dominates | $5,300 | Dominates |
| FOBT, 40% uptake | - | $95,400 | $14,200 | Dominates | $23,800 | Dominates | |
| Colonoscopy without Navigation | - | Dominates | Dominates | $9,800 | Dominates | ||
| FIT, 40% uptake | - | Dominates | $26,000 | Dominates | |||
| FOBT, 65% uptake | - | $118,700 | $14,200 | ||||
| Colonoscopy with Navigation | - | Dominates | |||||
CRC, colorectal cancer; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; FOBT, fecal occult blood test; FIT, fecal immunochemical test.
Table 4. Cost-effectiveness of navigation as part of a longitudinal screening program: One-way sensitivity analyses.
| Variable | Base case value |
Value in sensitivity analysis |
Colonoscopy with Navigation (65% uptake) vs. Colonoscopy without Navigation (40% uptake) |
Colonoscopy with Navigation (65% uptake) vs. FIT (65% uptake) |
||||
|---|---|---|---|---|---|---|---|---|
| Incremental cost/person |
Incremental QALYs/ 10,000 persons |
ICER | Incremental cost/person |
Incremental QALYs/ 10,000 persons |
ICER | |||
| Navigation cost (Completer cost) | $29 | $90 (approximately 3-fold increase) | $483 | 189 | $25,500 | $912 | (-8) | FIT Dominates* |
| $300 (approximately 10-fold increase) | $1,503 | 189 | $79,400 | $1,932 | (-8) | FIT Dominates* | ||
| Uptake if Navigated | 65% | 50% | $183 | 76 | $24,200 | $612 | (-122) | FIT Dominates* |
| 70% | $187 | 227 | $8,200 | $616 | 30 | $209,000 | ||
| Colonoscopy sensitivity for small polyp/large polyp/CRC | 0.85/0.90/0.95 | 0.75/0.85/0.92 | $193 | 187 | $10,300 | $607 | (-8) | FIT Dominates* |
| FIT sensitivity for small polyp/large polyp/CRC | 0.10/0.24/0.70 | 0.075/0.16/0.50 | $186 | 189 | $9,800 | $555 | 27 | $207,000 |
| Colonoscopy bleeding/perforation rates | 0.0016/0.00085 | 0.008/0.00425 (five-fold increase) | $248 | 164 | $15,100 | $712 | (-50) | FIT Dominates* |
| Colonoscopy cost/with biopsy or polypectomy | $661/$939 | $1,058/$1,502 (60% increase) | $471 | 189 | $24,900 | $1,056 | (-8) | FIT Dominates* |
| $403/$573 (40% decrease) | $1 | 189 | $60 | $329 | (-8) | FIT Dominates* | ||
| Colonoscopy bleeding/perforation costs | $6,043/$16,377 | $12,086/$32,754 (two-fold increase) | $202 | 189 | $10,700 | $639 | (-8) | FIT Dominates* |
| FIT cost** | $23 | $52(base case navigation cost added) | $186 | 189 | $9,800 | $390 | (-8) | FIT Dominates* |
| $81(2-fold base case navigation cost added) | $186 | 189 | $9,800 | $164 | (-8) | FIT Dominates* | ||
| $110(3-fold base case navigation cost added) | $186 | 189 | $9,800 | (-$61) | (-8) | $73,400 for FIT vs.colonoscopy | ||
| Colonoscopy cost/with biopsy or polypectomy/bleeding/perforation | $661/$939/$6,043/$16,377 | $992/$1,40/$9,065/$24,566 (50% increase) | $431 | 189 | $22,800 | $994 | (-8) | FIT Dominates* |
| CRC care costs | (50% decrease) | $408 | 189 | $21,600 | $667 | (-8) | FIT Dominates* | |
| (40% increase) | $9 | 189 | $460 | $574 | (-8) | FIT Dominates* | ||
More effective and less costly than the comparator.
Higher FIT-based screening cost can be interpreted to reflect the cost of an organized program designed to increase uptake.
CRC, colorectal cancer; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; FOBT, fecal occult blood test; FIT, fecal immunochemical test.
When compared with fecal testing, colonoscopy with navigation and 65% uptake cost $23,800/QALY gained vs. FOBT with 40% uptake, and $26,000/QALY vs. FIT with 40% uptake (Table 3). At 65% uptake for fecal testing, colonoscopy with navigation and 65% uptake cost $118,700/QALY gained vs. FOBT, and FIT was dominant over colonoscopy with navigation.
Navigation in longitudinal screening: Sensitivity analyses
In one-way sensitivity analyses reflecting plausible upper-end and lower-end estimates for model inputs, colonoscopy with navigation was not cost-saving vs. colonoscopy with no navigation, but in most scenarios it cost <$26,000/QALY gained (Table 4). In most scenarios, FIT with 65% uptake remained dominant over screening colonoscopy with navigation and comparable uptake (Table 4).
Colonoscopy with navigation and 65% uptake cost $25,500/QALY gained vs. colonoscopy with no navigation and 40% uptake when navigation cost $90 per colonoscopy completer.
In two-way sensitivity analyses, navigation cost $240,000/QALY gained when the navigation cost per colonoscopy completer was $150 and screening uptake with navigation was 45% vs. 40% without navigation; and it cost $228,500/QALY gained when the navigation cost per colonoscopy completer was $300 and screening uptake with navigation was 50% vs. 40% without navigation (Figure 2).
Figure 2.
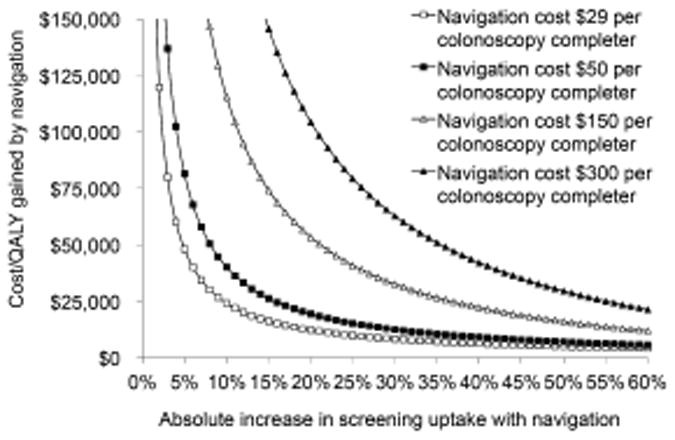
Two-way sensitivity analysis on the cost of navigation and the absolute increase in screening uptake for a program of screening colonoscopy over decades.
Colonoscopy with navigation at uptake rates of 25%, 50%, 75% and 100% cost <$25,000/QALY gained vs. FIT when FIT uptake rates were <17%, <33%, <50% and <66%, respectively. If navigation increased colonoscopy uptake to >86%, then it cost <$50,000/QALY gained vs. FIT with 65% uptake. This uptake threshold remained relatively stable even at the extreme low-end of navigation costs ($5-10 per colonoscopy completer). Assuming a higher cost for FIT-based screening of $52 (reflecting, for instance, the cost of an organized program to increase uptake), colonoscopy with navigation at uptake rates of 25%, 50%, 75% and 100% cost <$25,000/QALY gained vs. FIT when FIT uptake rates were <20%, <39%, <58% and <77%, respectively.
Among persons taking up screening, the cost-effectiveness of navigation for colonoscopy compared with fecal strategies depended on the per-cycle adherence rates (Figure 3). Assuming an uptake rate for FIT 1.35-fold that of colonoscopy,34 and illustrating 100% per cycle adherence among those taking up screening colonoscopy, FIT became dominant over colonoscopy with navigation at a FIT per-cycle adherence >35% among those taking up FIT.
Figure 3.
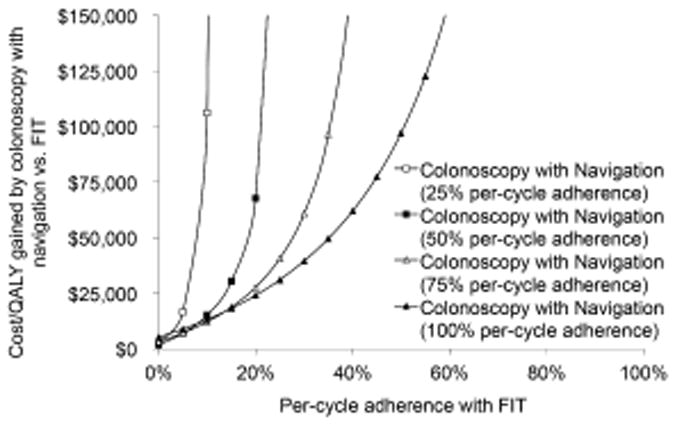
Two-way sensitivity analysis on the relative per-cycle adherence with screening for persons taking up a colonoscopy-based vs. a FIT-based screening program.
In the Monte Carlo simulation, compared with screening colonoscopy without navigation, colonoscopy with navigation gained a median of 184 QALYs/10,000 persons (95% range, 167-226), and its median cost/QALY gained was $10,100 (95% range, $8,100-11,500). Compared to screening colonoscopy with navigation and 65% uptake, FIT with 65% uptake yielded more QALYs/person and was cost-saving in 72% of iterations.
Discussion
Our results indicate that patient navigation for one-time screening colonoscopy among a predominantly minority population may increase life-expectancy while decreasing costs. In settings with higher navigation costs in which navigation does not prove to be cost-saving, navigation is still likely to be considered cost-effective. Navigation in the context of a longitudinal screening program is likely to be cost-effective, but probably not cost-saving. These results highlight the high impact of the initial colonoscopy in previously unscreened persons. The overall results were robust across a wide range of sensitivity analyses, provided that navigation increased uptake by a clinically significant increment.
Our findings suggest that there is an overall favorable balance between the increase in screening uptake achieved by navigation and the costs required to provide navigation. Although the precise incremental benefits and costs differed somewhat by racial/ethnic group (Table 1), the overall conclusions about the cost-effectiveness of navigation were robust across these groups. While an insurer may not realize the estimated long-term savings in CRC care associated with screening a specific individual who later switches to another insurer, the savings would be realized by the health system as a whole (and, under a steady state of balanced switching between insurers, also by specific insurers).
In our simulation, as expected, the cost-effectiveness of navigation for colonoscopy compared with stool-based screening was highly dependent on the relative screening uptake and adherence rates. With comparable screening uptake and adherence, similar clinical benefits may be realized by colonoscopy or FIT programs,45 but each program's impact will depend on overall uptake, adherence over time, incremental yield from cycle to cycle, and the rate of colonoscopy follow-up after an abnormal screening fecal test.28, 34-36, 46 Health systems grappling with decisions regarding CRC screening may weigh the merits of a colonoscopy navigation program vs. those of opportunistic or organized programs of fecal-based testing, as well as considering how to accommodate patient preferences.25, 34
The costs of navigation are currently not reimbursed by insurance. Our analysis of navigation at our institution24 yielded modest cost estimates ($29 per colonoscopy completer, $21 per non-completer, and $3 per non-navigated). The estimates for three other institutions in New York City were higher ($51, $74 and $287 per patient referred to navigation), with differences attributable primarily to costs of program personnel other than navigators.20 We explored the potential cost-effectiveness of navigation if the incremental costs of navigation were covered by payers. In the base case, navigation for one-time screening colonoscopy was cost-saving. Navigation for one-time screening colonoscopy cost $6,000/QALY gained at a cost of $300 per colonoscopy completer and a 25% absolute increase in screening uptake, $28,000/QALY gained at $300 per colonoscopy completer and 10% increase in screening uptake, and $26,400/QALY gained at $150 per colonoscopy completer and 5% increase in screening uptake. These are all cost-effective by traditional standards.
Current reforms in the U.S. focus on accountable care organizations, population health management, and cost containment. Our analyses suggest that incremental payments for patient navigation for screening colonoscopy are likely to be considered cost-saving or cost-effective across a broad range of assumptions about effectiveness and cost. Thus, in emerging healthcare models that reward outcomes, payers should strongly consider covering the costs of patient navigation for CRC screening.
Our study has limitations. It is not clear if future payment models will cover navigation. The benefits of all longitudinal screening programs may be overestimated if adherence decreases substantially over time. The benefits of fecal-based screening programs may be overestimated by our assumption of independence between testing cycles. We did not explicitly model fecal-based screening with navigation as separate strategies because of the lack of data to inform a base case, but our sensitivity analyses on fecal testing uptake and cost reflect hypothetical scenarios of navigation for fecal-based screening.
In conclusion, the balance between the clinical benefits of patient navigation for screening colonoscopy and the incremental costs required to provide navigation is likely to be highly favorable. Navigation for one-time screening colonoscopy may be cost-saving, and navigation in a longitudinal screening colonoscopy program is likely to be cost-effective. In emerging healthcare models that reward outcomes, payers should strongly consider covering the incremental costs of patient navigation for screening colonoscopy.
Supplementary Material
Acknowledgments
The authors thank Dr. William H. Redd for access to raw data from previous studies.
Support: Funded in part by grant R01 CA120658 from the National Cancer Institute, and the Icahn School of Medicine at Mount Sinai.
Footnotes
Disclosures: Dr. Ladabaum: consultant, Exact Sciences Corporation, Given Imaging; scientific advisor, Mauna Kea Technologies. Dr. Itzkowitz: scientific advisor, research support, Exact Sciences Corporation.
References
- 1.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 3.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 6.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 7.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 8.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 11.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharaf RN, Ladabaum U. Comparative Effectiveness and Cost-Effectiveness of Screening Colonoscopy vs. Sigmoidoscopy and Alternative Strategies. Am J Gastroenterol. 2013;108:120–132. doi: 10.1038/ajg.2012.380. [DOI] [PubMed] [Google Scholar]
- 13.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 14.Vital signs: colorectal cancer screening test use--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton JJ, Tancredi DJ, Green P, Franks P, Baldwin LM. Persistent racial and ethnic disparities in up-to-date colorectal cancer testing in medicare enrollees. J Am Geriatr Soc. 2009;57:412–418. doi: 10.1111/j.1532-5415.2008.02143.x. [DOI] [PubMed] [Google Scholar]
- 16.Semrad TJ, Tancredi DJ, Baldwin LM, Green P, Fenton JJ. Geographic variation of racial/ethnic disparities in colorectal cancer testing among medicare enrollees. Cancer. 2011;117:1755–1763. doi: 10.1002/cncr.25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21:1614–1617. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 18.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Braschi CD, Sly JR, Singh S, Villagra C, Jandorf L. Increasing Colonoscopy Screening for Latino Americans Through a Patient Navigation Model: A Randomized Clinical Trial. J Immigr Minor Health. 2013 doi: 10.1007/s10903-013-9848-y. [DOI] [PubMed] [Google Scholar]
- 20.Elkin EB, Shapiro E, Snow JG, Zauber AG, Krauskopf MS. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer. 2012;118:5982–5988. doi: 10.1002/cncr.27595. [DOI] [PubMed] [Google Scholar]
- 21.Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing african americans' adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1577–1587. doi: 10.1158/1055-9965.EPI-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebwohl B, Capiak K, Neugut AI, Kastrinos F. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Aliment Pharmacol Ther. 2012;35:1467–1473. doi: 10.1111/j.1365-2036.2012.05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KK, Jandorf L, Thelemaque L, Itzkowitz SH. Colorectal neoplasia detection among black and Latino individuals undergoing screening colonoscopy: a prospective cohort study. Gastrointest Endosc. 2014;79:466–472. doi: 10.1016/j.gie.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandorf L, Stossel LM, Cooperman JL, et al. Cost analysis of a patient navigation system to increase screening colonoscopy adherence among urban minorities. Cancer. 2013;119:612–620. doi: 10.1002/cncr.27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151–1162. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 27.Pelto DJ, Sly JR, Winkel G, et al. Predicting Colonoscopy Completion among African Americans and Latinos Participating in a Patient Navigation Program. 2014 doi: 10.1007/s40615-014-0053-z. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladabaum U, Allen J, Wandell M, Ramsey SD. Colorectal Cancer Screening with Blood-Based Biomarkers: Cost-Effectiveness of Methylated Septin 9 DNA vs. Current Strategies. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-13-0204. [DOI] [PubMed] [Google Scholar]
- 29.Ladabaum U, Ferrandez A, Lanas A. Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy. Cancer Epidemiol Biomarkers Prev. 2010;19:2765–2776. doi: 10.1158/1055-9965.EPI-10-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2011 Sub, Vintage 2009 Pops (1992-2009), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission. Accessed at http://www.seer.cancer.gov on March 26, 2014.
- 31.Arias E. United States life tables, 2008 National vital statistics reports. 3. Vol. 61. Hyattsville, MD: National Center for Health Statistics; 2012. Accessed at http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_03.pdf on March 28, 2014. [PubMed] [Google Scholar]
- 32.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 35.Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C. High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol. 2012;10:633–638. doi: 10.1016/j.cgh.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 36.van Roon AH, Goede SL, van Ballegooijen M, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut. 2013;62:409–415. doi: 10.1136/gutjnl-2011-301583. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services. Physician Fee Schedule 2012. Accessed at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/on on March 4, 2014.
- 38.Centers for Medicare & Medicaid Services. Inpatient Prospective Payment System 2012. Accessed at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/on on March 4, 2014.
- 39.Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule 2010. Accessed at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/on on March 4, 2014.
- 40.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Technology Assessment: Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. 2007 [PubMed] [Google Scholar]
- 41.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 43.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. pp. 214–235. [Google Scholar]
- 44.Briggs A, Claxton K, Sculpher M. Making decision models probabilistic Decision modelling for health economic evaluation. Cambridge, MA: Oxford University Press; 2006. pp. 77–120. [Google Scholar]
- 45.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of Genetic Testing by Relatives of Lynch Syndrome Probands: A Systematic Review. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 46.Day LW, Bhuket T, Allison J. FIT testing: an overview. Curr Gastroenterol Rep. 2013;15:357. doi: 10.1007/s11894-013-0357-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


