Abstract
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of diseases involving hepatic fat accumulation, inflammation with the potential progression to fibrosis and cirrhosis over time. NAFLD is often associated with obesity, insulin resistance, and diabetes. The interactions between the liver and the gut, the so-called ”gut-liver axis”, play a critical role in NAFLD onset and progression. Compelling evidence links the gut microbiome, intestinal barrier integrity, and NAFLD. The dietary factors may alter the gut microbiota and intestinal barrier function, favoring the occurrence of metabolic endotoxemia and low grade inflammation, thereby contributing to the development of obesity and obesity-associated fatty liver disease. Therapeutic manipulations with prebiotics and probiotics to modulate the gut microbiota and maintain intestinal barrier integrity are potential agents for NAFLD management. This review summarizes the current knowledge regarding the complex interplay between the gut microbiota, intestinal barrier, and dietary factors in NAFLD pathogenesis. The concepts addressed in this review have important clinical implications, although more work needs to be done to understand how dietary factors affect the gut barrier and microbiota, and to comprehend how microbe-derived components may interfere with the host’s metabolism contributing to NAFLD development.
Keywords: Dietary factors, intestinal barrier, metabolic endotoxemia, gut microbiome, nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide in both adults and in children, and is considered to be the hepatic manifestation of the metabolic syndrome. NAFLD is generally associated with obesity, insulin resistance, and diabetes. NAFLD includes a spectrum of pathologies from simple steatosis to nonalcoholic steatohepatitis (NASH) characterized by inflammation with the potential progression to fibrosis and cirrhosis over time. The prevalence of steatosis is estimated at 20–30% of the general population of the developed countries, and up to 75 –100% in obese individuals [1, 2]. The prevalence of NAFLD increases in parallel with obesity, and NAFLD is considered to be an emerging epidemic parallel to the dramatic increase in obesity rates in the US and worldwide [3].
Classically, obesity is considered to be due to a surplus of energy intake over energy expenditure, resulting in storage of excess energy as a fat (Figure 1A). Genetic, physiological, and environmental factors (e.g., high fat diet [HFD] and sedentary lifestyle) also play a significant role in the etiology of obesity and obesity-associated metabolic disorders. An innovative concept that has been recently proposed involves the gut-liver axis as a critical component of obesity and NAFLD pathogenesis. The latest advances in the field suggest that the increased consumption of obesogenic foods (particularly those enriched in fat and fructose) may alter the gut microbiota and intestinal barrier function favoring the occurrence of metabolic endotoxemia and low-grade inflammation [4, 5], thereby contributing to the development of obesity and obesity-associated fatty liver disease (Figure 1B). The involvement of the gut microbiota in NAFLD pathogenesis is complex and multifactorial. The data from animal studies support the concept that the gut bacteria may contribute to NAFLD via multiple mechanisms (Table 1), including regulation of energy homeostasis [6, 7] with the increased fermentation of carbohydrates to short chain fatty acids (SCFAs) and subsequent stimulation of de novo synthesis of triglycerides in the liver [8, 9]; modulation of endocannabinoid system [10, 11]; modulation of choline metabolism (which is required for very-low-density lipoprotein synthesis and hepatic lipid export) [12, 13]; modulation of bile acid homeostasis [14, 15]; the ability to generate endogenous ethanol [16, 17]; and bacteria-derived toxins (e.g., lipopolysaccharides (LPS)), which may activate pro-inflammatory cytokine production in the liver macrophages resulting in hepatocellular inflammation [4]. Small intestine bacterial overgrowth (SIBO) has also been linked to NASH pathogenesis [18, 19] [20]. The gut microbiota may also contribute to hepatic fibrosis via stimulation of Toll-like receptor (TLR)-9-dependent profibrotic pathways in hepatic Kupffer cells [21]. It is also important to mention that the link between the gut microbiota and adipose tissue has been recently identified. It has been shown that LPS acts as a master switch to control adipose tissue metabolism both in vivo and ex vivo by blocking cannabinoiddriven adipogenesis [10]. Additionally, LPS inhibited the secretion of adipokines, specifically adiponectin, from the visceral adipose tissue [22], that may affect the onset of metabolic syndrome. Further, it has been reported that decreased production of N-acylethanolamines, an important mediators of metabolic homeostasis and inflammation, in the adipose tissue induced intestinal barrier dysfunction and dysbiosis of the gut microbiota, which in turn participates in the metabolic alterations observed in the adipose tissue [23].
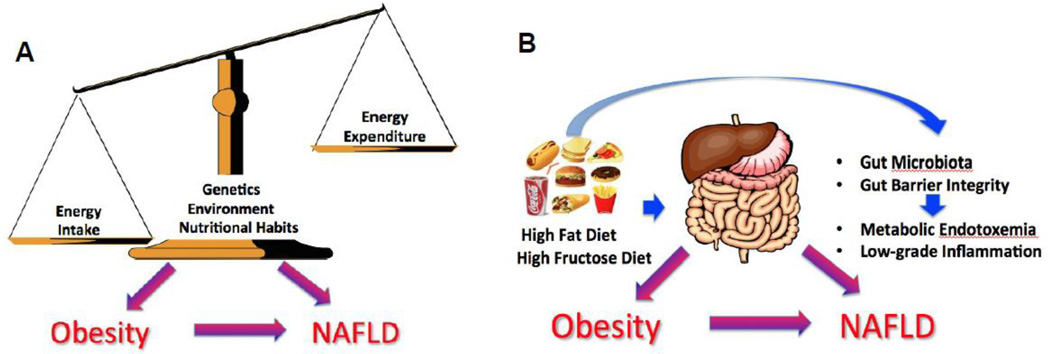
Figure 1.
Concepts of obesity and NAFLD pathogenesis are: classical (A) and (B) innovative gut-liver-axis-mediated. Classically, obesity is considered to be due to a surplus of energy intake over energy expenditure, resulting in storage of excess energy as a fat. Genetic, physiological, and environmental factors (e.g., high fat diet [HFD] and sedentary lifestyle) also play a significant role in the etiology of obesity and obesity-associated metabolic disorders. The gut-liver-axis-mediated concept suggests that the increased consumption of obesogenic foods (particularly those enriched in fat and fructose) may alter the gut microbiota and intestinal barrier function favoring the occurrence of metabolic endotoxemia and low-grade inflammation thereby contributing to the development of obesity and obesity-associated fatty liver disease.
Table 1.
Multiple mechanisms by which the gut bacteria may contribute to NAFLD
The present review summarizes the recent insights into the complex interplay between the gut microbiota, intestinal barrier and nutrition; the contribution of these factors to NAFLD pathogenesis is discussed. The central concept addressed in the review is that a diet is a major factor driving the composition and metabolic activity of the gut microbiota. The dietary factors may alter the gut microbiota and intestinal barrier function, favoring the occurrence of metabolic endotoxemia and low-grade inflammation, thereby contributing to the development of obesity and obesity-associated fatty liver disease (Figure 2). The altered gut microbiota may influence the whole-body metabolism by affecting energy balance and by producing microbial metabolites that may become key players in NAFLD development.
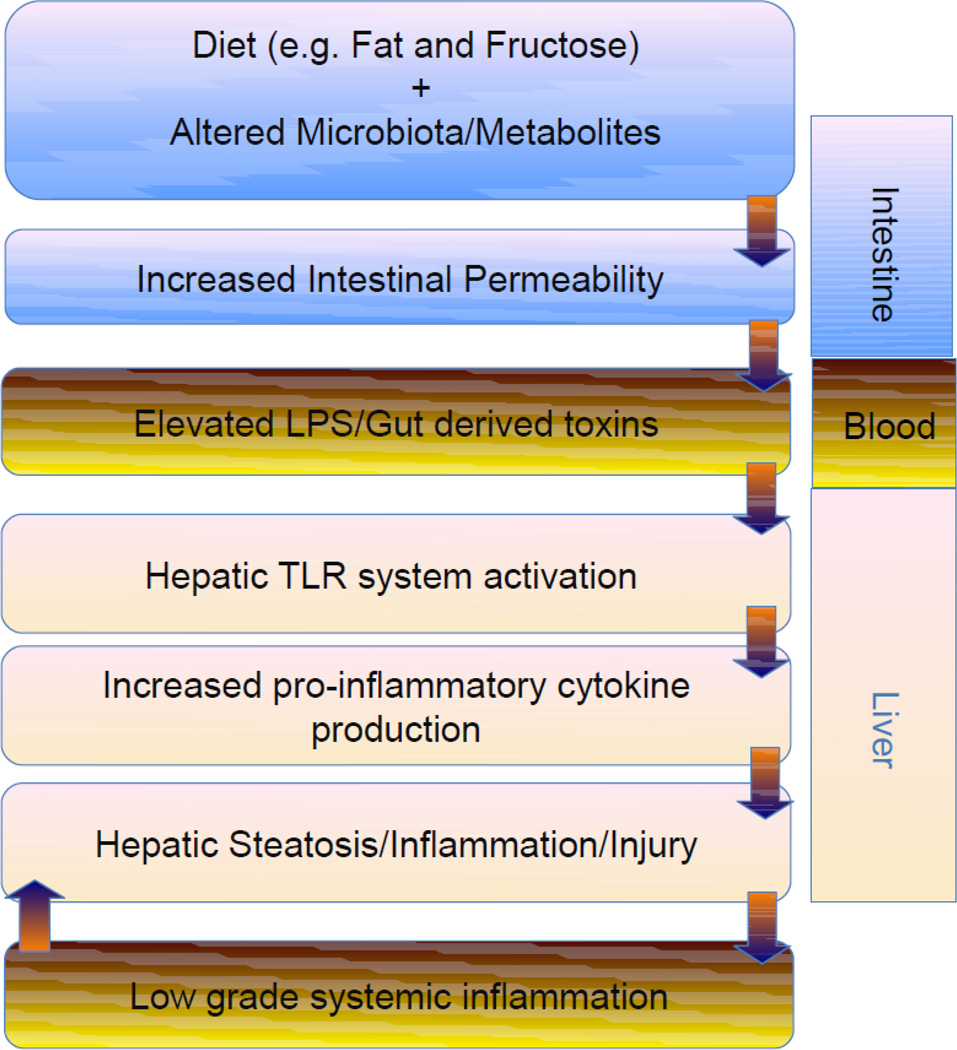
Figure 2.
Diet is a major factor driving the composition and metabolic activity of the gut microbiota. Dietary factors and altered gut microbiota may affect intestinal barrier function resulting in metabolic endotoxemia; gut-derived products/toxins activate hepatic toll-like receptors with a subsequent production of pro-inflammatory mediators and low-grade systemic inflammation contributing to the development of obesity and obesity-associated fatty liver disease. Abbreviations: LPS, lipopolysaccharides; TLR - Toll-like receptors
NAFLD: gut barrier, endotoxemia, and dietary factors
Obesity and NAFLD are closely associated with the disruption of gut barrier integrity, metabolic endotoxemia and TLR-mediated low-grade inflammation [5, 24]. The gut barrier is a direct physical barrier against translocation of luminal bacteria and bacteria-derived products/toxins into the blood. The gut barrier is a complex structure, consisting of the intestinal epithelial cells connected through the intestinal tight junctions (TJs), the mucus layer which coats the intestinal epithelial surface, and the antimicrobial defense system consisting of numerous anti-bacterial peptides produced by Paneth cells (Figure 3A). Numerous factors, including certain dietary components (e.g. dietary fat and fructose) can alter one or more components of this complex structure leading to increased gut permeability to bacteria and bacteria-derived products, including endotoxin lipopolysaccharide (LPS). Diet-induced increase in blood LPS levels is known as metabolic endotoxemia (Figure 3B). Current evidence suggests that diet-induced changes in the gut microbiota [4, 25] and gut barrier function [26, 27] underlie the elevated blood LPS levels. Alteration of intestinal TJ proteins, mainly zonula occludens-1 (ZO-1) and occludin, is a major molecular mechanism contributing to the increased intestinal permeability. The high-fat diets can promote intestinal inflammation [28], which, in turn, might result in TJ alterations and increased intestinal permeability [29]. Glucagon-like peptide 2 (GLP2), a gut peptide, was identified as a regulator of TJ protein expression and localization in obese mice [30]. Activation of the endocannabinoid system was also linked to the increased gut permeability and plasma LPS levels in high-fat-induced obesity animal models [10, 11].
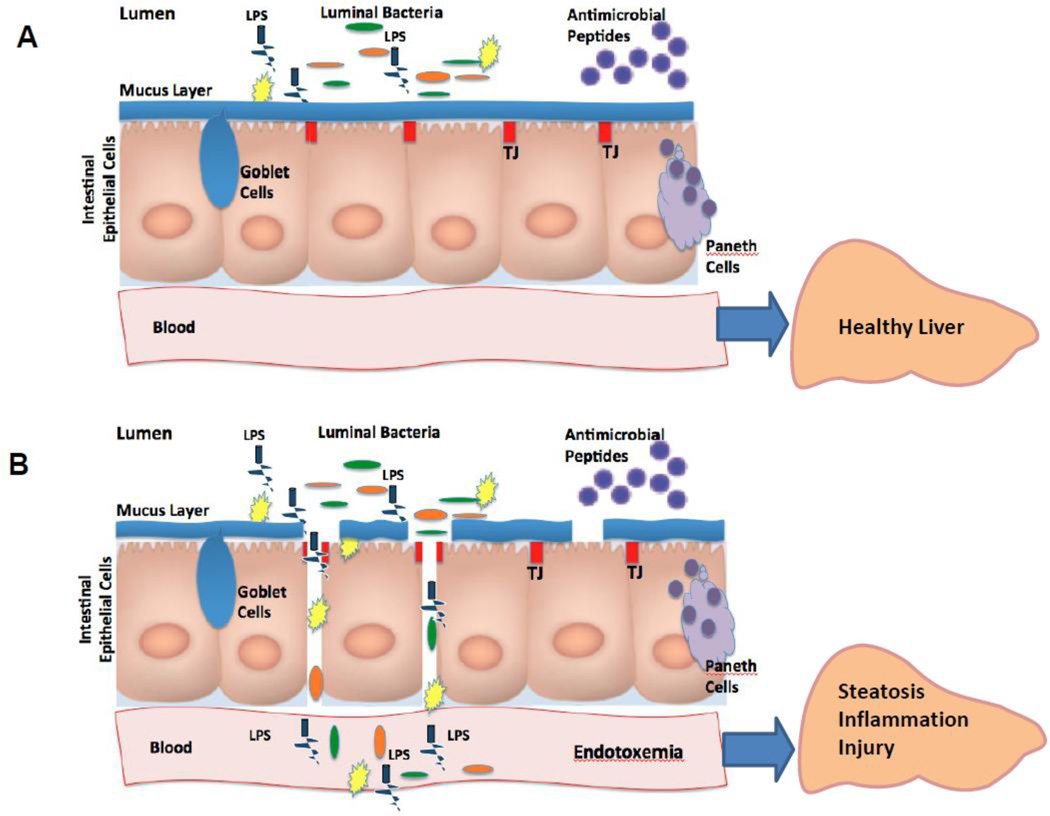
Figure 3.
The intact (A) and disrupted (B) gut barrier. The gut barrier is a complex structure, consisting of epithelial cells sealed with the so-called intestinal tight junctions’, the mucus layer which coats the intestinal epithelial surface (mucins, the major components of mucus layer, are mainly produced by Goblet cells), and the antimicrobial defense system consisting of numerous anti-bacterial peptides produced by Paneth cells. The gut barrier prevents translocation of luminal bacteria and bacteria-derived products into the blood. Numerous factors, including dietary factors, can alter one or more components of the gut barrier structure resulting in the condition known as endotoxemia. Abbreviations: LPS, lipopolysaccharides; TJs, tight junctions.
Evidence supporting the importance of dietary factors in metabolic endotoxemia and in the pathogenesis of obesity and NAFLD has been accumulating over the past decade in experimental and clinical studies. Studies from Cani’s group clearly demonstrated that endotoxemia was permanently increased in a mouse model of high fat-induced obesity and NAFLD [5, 26]. The combination of high sucrose and high fat diets also resulted in elevated circulating LPS levels in parallel with the significantly increased hepatic fat accumulation, and a significant reduction in the expression of the intestinal TJ protein, occludin, in rats [31]. These animals develop the typical metabolic syndrome and NAFLD. Diet-induced endotoxemia is not restricted to increased dietary lipids; fructose-induced NAFLD is also associated with elevated endotoxemia in rodents and primates [32, 33]. Bergheim et al., reported fructose induced experimental NAFLD and endotoxemia. They then demonstrated markedly reduced endotoxemia in parallel with significantly decreased hepatic lipid accumulation in response to administration of antibiotics in fructose fed mice, thus supporting the idea that high fructose consumption contributes to NAFLD not only via overfeeding but also via direct alterations of intestinal permeability and gut microbiota [32]. Another study reported that fructose-induced NAFLD is associated with intestinal bacterial overgrowth and increased intestinal permeability leading to an endotoxin-dependent activation of hepatic Kupffer cells and liver injury in mice [34]. Inactivation of TLR4, a receptor for LPS [34, 35], as well as administration of prebiotics and probiotics to modulate gut microbiota [36, 37] ameliorate fructose-induced hepatic steatosis in rodents.
Consistent with the animal models, several recent studies have reported elevated levels of blood endotoxin in adult patients with simple steatosis and NASH [38, 39], as well as in children with NAFLD [40, 41]. Intestinal permeability was correlated with the liver disease severity; specifically, intestinal permeability was increased in children with steatohepatitis compared to those with steatosis only [42]. A study by Miele at al., provided evidence that the increase in gut permeability in NAFLD patients is caused by disruption of intestinal TJs as documented by decreased expression of one of major TJ proteins – ZO-1— in the intestinal mucosa [43]. A recently published clinical study demonstrating increased endotoxin levels in NAFLD adolescents after consumption of fructose beverages further supports the important role of dietary factors in gut barrier integrity [41]. In this study, postprandial endotoxin levels were acutely increased in adolescents with NAFLD compared to healthy subjects in response to fructose but not glucose beverages (consumed with meals) in a 24-hour feeding challenge. Similarly, endotoxin was significantly increased after adolescents with NAFLD consumed fructose beverages for 2 weeks, and remained high at 4 weeks.
It is important to recognize that dietary factors may facilitate metabolic endotoxemia in healthy individuals. It has been reported that consumption of the Western diet (high fat, high sugar) for one month resulted in increased endotoxemia in healthy individuals compared to the so-called Prudent diet (low fat, high levels of fruits, vegetables, whole-grain, poultry and fish) [44]. The high fat meal [45] or high fat drinks [46] result in low-grade endotoxemia in healthy men over time, possibly by a by a mechanism involving increased intestinal LPS absorption through its incorporation into chylomicrons [47]. Acute postprandial endotoxemia after a high fat, high carbohydrate meal compared to a high-fiber and fruit meal was associated with the increased expression of TLR2, TLR4, suppressor of cytokine signaling-3 (SOCS-3), reactive oxygen species generation, and NF-kB activity in circulating mononuclear immune cells in healthy lean subjects [48], suggesting that the cumulative effects of such a meal may manifest in chronic oxidative and inflammatory stress, and, potentially, in insulin resistance.
Gut Microbiota and NAFLD
The gut microbiota represent a complex and diverse ecosystem. Numerous recent studies using the state-of-the-art metagenomic technologies have revealed that the most abundant bacteria are members of the phyla, Bacteroidetes and Firmicutes [49]. The gut microbiota comprise at least 1013 –1014 microbial cells, and the gut microbiome represents overall more than 100 times the number of genes as in the human genome, and is called the “metagenome” [50]. Comprehensive molecular phylogenetic characterization has revealed the spatial distribution of the intestinal microbiota along the healthy gut, and this is being actively studied in humans with different lifestyles, ages, and diseases [49, 51, 52]. Normally, commensal microbes and their host benefit from a mutually symbiotic relationship. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota [53].
Over the past decade, the intestinal microbiota has been increasingly recognized as a critical factor in the pathogenesis of both obesity and obesity associated NAFLD in mice and humans (see [54, 55]). Using germ free animal models, several research groups have demonstrated that mice lacking gut microbiota are resistant to diet-induced obesity, liver steatosis and insulin resistance [56, 57]. An elegant series of experiments from Gordon’s group demonstrated that colonization of germ free mice with a “normal” gut microbiota harvested from the cecum of “normal” mice produced a 60% increase in body fat content, insulin resistance, and a two-fold increase in hepatic triglyceride content [6]. Administration of the cecal microbiota from ob/ob mice to germ free wild-type recipients resulted in modest fat gain by these mice and extraction of more calories from their food compared to the lean mice having received the gut microbiota from lean donors [58]. The transplanted microbiota from obese mice may decrease phosphorylated AMP-activated protein kinase (AMPK) levels in the liver and its downstream targets involved in fatty acid oxidation [56], as well as selectively suppress fasting-induced adipocyte factor (Fiaf), a circulating lipoprotein lipase inhibitor, facilitating de novo hepatic lipogenesis and deposition of triglycerides in adipocytes and the liver [6]. To further support the role of microbiota in NAFLD development, colonization of germ-free mice with a typical environmental microbial population was characterized by a stimulation of hepatic glycogenesis (as an early response to colonization) which transitioned to triglyceride synthesis as a later adaptive mechanism [59]. Hepatic triglyceride levels were strongly associated with the phylum Actinobacteria (Coriobacteriaceae) in this animal model. An exciting advance in the field has been recently reported: the gut microbiota transplantation from donor mice with NAFLD replicated the phenotype in wild-type recipients, demonstrating that not only obesity [58], but NAFLD is also transmissible [60]. Two bacterial species (Lachnospiraceae bacterium 609 and a relative of Barnesiella intestinihominis) were found to be dominant in mice which developed the NAFLD phenotype [60].
The gut bacteria may facilitate NAFLD progression from simple steatosis to NASH. Thus, mice fed the methionine-choline deficient diet developed a NASH phenotype associated with changes in the gut microbiota composition, specifically, increased Porphyromonadaceae family (primary in the genus Parabacteroides) due to inflammasome deficiency [61]. In this experimental study, dysbiosis (associated with the loss of NLRP3 and NLRP6 inflammasomes) resulted in increased influx of LPS and bacterial DNA to the liver through the hepatic portal circulation. These bacterial products stimulate TLR4 and TLR9, respectively, leading to enhanced hepatic tumor-necrosis factor (TNF)-α expression that drives NASH progression. Importantly, wild-type mice co-housed with inflammasome-deficient mice had exacerbated hepatic steatosis and obesity, providing direct evidence that an altered gut microbiota may be a key mechanism in the pathogenesis of these diseases [61].
The alterations of gut microbiota characterized by the increase in fecal Firmicutes-to-Bacteroidetes ratio [49] (although debatable), and a dramatic fall in the number of gut microbial genes, and thus gut bacterial richness [62], have been described in the murine models of obesity and humans. However, very little is known about the composition of the intestinal microbiota in patients with NAFLD, as well as the metabolic activity of the gut bacteria related to liver steatosis and inflammation. A recent study by Zhu et al., has revealed the different microbial diversities (alpha and beta) among healthy, obese and NASH children and adolescents; Proteobacteria/Enterobacteriaceae/Escherichia was similarly represented between healthy and obese microbiomes, but was significantly elevated in NASH [17]. Elevated representation of Escherichia (alcohol-producing bacteria) was observed in parallel with the increased blood alcohol concentration in NASH patients, suggesting a novel mechanism for the pathogenesis of NASH. Thus, gut microbiota enriched in alcohol-producing bacteria (e.g., E. coli) constantly produce more alcohol, which is known to play an important role in the disruption of intestinal TJs [63], causing hepatic oxidative stress and inducing liver inflammation [17]. Decreased Bacteroidetes, which was independent of body mass index and energy intake from dietary fat has recently been reported in NASH patients compared to those with simple steatosis and healthy controls [64]. In an a Chinese cohort of histology-proven NASH patients, Wong et al., found lower fecal abundance of Faecalibacterium and Anaerosporobacter but higher abundance of Parabacteroides and Allisonella; no bacterial biodiversity was found between NASH patients and controls [65]. These authors have reported that improvement in hepatic triglyceride content was associated with a reduction in the abundance of Firmicutes and an increase in Bacteroidetes. The inverse correlation between Bacteroidetes and steatohepatitis may be partially due to the possibility that a lower percentage of Bacteroidetes may facilitate extension of other bacteria that are more efficient in extracting energy from the diet. It has been shown that a 20% decrease in fecal Bacteroidetes is associated with an increased energy harvest from the diet of approximately 150 kcal [7].
Nutrition and gut microbiota: implication for obesity and NAFLD
Dietary factors and dietary patterns play a critical role in the modulation of the gut microbiota [66–72]. Remarkably, these dietary factors can modify the gut microbiota very rapidly. For example, shifting to a high-fat/high-sugar diet from a low-fat, plant polysaccharide-rich diet in mice [68], and from a high-fat/low-fiber diet to a low-fat/high-fiber diet in humans [70] caused marked changes in the gut microbiota within a day. Increasing bodies of evidence show that a high-fat diet substantially modulates the intestinal microbiota. The population levels of Bifidobacterium spp. and E.rectale/Cl.coccoides group were significantly reduced in animals fed a high fat diet vs mice receiving the standard high carbohydrate diet. These events were accompanied with a significant increase in plasma LPS levels, increased liver fat accumulation and expression of the hepatic inflammatory mediators, including TNF-α, interleukin 1 (IL-1), and plasminogen activator inhibitor-1 (PAI-1) [5]. In a study involving resistin-like molecule β-knockout (RELM-β-KO) mice, which are resistant to diet-induced obesity, switching to a high-fat diet resulted in a decrease in Bacteroidetes and an increase in both Firmicutes and Proteobacteria in both RELM-β-KO and Wild-type mice, suggesting that diet is the critical factor determining the gut microbiota [66].
Due to the difficulty of standardizing the diet during sampling, there are a limited number of studies investigating the microbial response to the specific nutrients or dietary patterns in humans. A study exploring the effects of choline depletion in healthy human subjects consuming a rigorously controlled diet has shown that decreased levels of Gammaproteobacteria and increased levels of Erysipelotrichi were directly associated with elevated accumulation of hepatic fat [73]. A recently published paper in Nature has demonstrated that the animal-based diet (composed of meat, eggs, and cheese) consumed by the healthy individuals for 5 days increased the abundance of bile-tolerant microorganisms (Alistipes, Bilophila, and Bacteroides) and decreased the levels of Firmicutes that metabolize dietary plant polysaccharides such as Roseburia, Eubacterium rectale, and Ruminococcus bromii [72]. The long-term impact of different amounts and quality of dietary fat and carbohydrate on gut microbial composition was studied in subjects with increased risk of metabolic syndrome [74]. In this study, the low-fat, high carbohydrate diet increased fecal Bifidobacterium in parallel with the reduced fasting glucose and cholesterol levels; high carbohydrate/high glycemic index diet also increased fecal Bacteroides, whereas high carbohydrate/low glycemic index diet and high saturated fat diet increased Faecalibacterium prausnitzii; high monounsaturated fat diet did not affect individual bacterial population but reduced total bacteria number [74]. Significant diet-dependent reductions in a group of butyrate-producing Firmicutes (e.g., Roseburia) were detected in fecal samples from obese subjects on reduced carbohydrate weight loss diets [71].
Modulation of the gut microbiota: new therapeutic strategies in the management of obesity and NAFLD
The treatment of obesity and NAFLD is challenging. One of the effective strategies developed for morbid obesity is bariatric surgery, which consistently achieves and sustains substantial weight loss [75]. Several recent studies have reported changes in the gut microbial community after a surgical weight-loss procedure, suggesting involvement of the gut microbiota in a bariatric surgery-mediated weight loss. Thus, Zhang et al., have shown that Firmicutes were dominant in normal-weight and obese individuals but significantly decreased in post-gastric-bypass individuals, who had a proportional increase of Gammaproteobacteria [76]. Another study demonstrated that Bacteroides/Prevotella group was increased 3 month after bariatric surgery, as well as Escherichia coli species that were inversely correlated with fat mass and leptin levels, independent of changes in food intake [77]. Increased richness/diversity of gut microbiota after gastric bypass surgery has also been reported [78]. The causal link between the gastric bypass surgery-mediated changes in the gut microbiota and reduced weight and adiposity is provided in a recently published study by Liou et al. [79]. The authors showed that transferring the gut microbiota from mice that underwent bypass surgery to non-operated, germ-free mice resulted in weight loss and decreased body and liver fat mass in the recipient mice compared to mice receiving the gut microbiota from sham surgery donors, potentially due to altered microbial production of short-chain fatty acids.
Diet-induced weight loss is associated with the changes in the gut microbiota composition in mice and humans [71, 80], reviewed in [81]. Energy-restricted diet significantly increased microbial gene richness in obese individuals [82]. Although dietary modification in conjunction with exercise to control weight gain remains the primary therapeutic approach for obesity and obesity-associated NAFLD, several studies in both animal models and humans have demonstrated that modulation of the gut microbiota by prebiotics (non-digestible food substances that can promote growth of beneficial bacteria) and probiotics (live microorganisms that are favorable to the host) are beneficial in these conditions. The exact mechanisms of beneficial effects of pre- and probiotics on the gut-liver axis are not yet fully elucidated; however, many of the favorable therapeutic effects may result from modulation of the intestinal microflora composition and antibacterial factor production, modification of intestinal epithelial permeability and function, and modulation of the immune system at both local and systemic levels. For example, restoration of Bifidobacteria [4, 83] and Akkermansia muciniphila [84] levels in high-fat fed mice by dietary supplementation with the prebiotic, oligofructose, significantly reduced metabolic endotoxemia and metabolic syndrome features, including reduced hepatic fat accumulation. These effects were facilitated by increased production of endogenous glucagonlike peptide-2 (GLP-2), which reduced intestinal barrier permeability [30]; stimulation of fatty acid oxidation via proliferator-activated receptor α (PPAR-α), lessened cholesterol accumulation by inhibiting sterol regulatory element binding protein 2 (SREBP-2)-dependent cholesterol synthesis [83]; and increased intestinal levels of endocannabinoids that control inflammation and gut barrier integrity [84].
Accumulating data suggest beneficial effects of probiotics (either a combination of several bacterial species or individual bacteria) in experimental animal models of NAFLD. The most commonly used microorganisms are within the genera Bifidobaceria and Lactobacillus. Supplementation with VSL#3, a multi-strain preparation composed of Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infants, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophiles improved high fat diet-induced liver steatosis and insulin resistance in mice by modulation of hepatic natural killer T cells (NKT cells) and suppression of the TNF-α/IKK-β signaling pathway [85]; decreased serum alanine aminotransferase (ALT) levels; increased insulin sensitivity; and improved hepatic inflammation by reducing activity of Jun N-terminal kinase and decreasing DNA binding activity of NF-kB in ob/ob mice [86]. Several strains of Lactobacillus have shown beneficial effects on experimental NAFLD. Eight weeks of oral administration of Lactobacillus rhamnosus PL60 showed anti-obesity effects and improved liver steatosis in a mouse model of diet-induced obesity [87]. Lactobacillus acidophilus and Lactobacillus casei administration for 8 weeks demonstrated anti-oxidant effects in the liver and pancreatic tissues in high fructose diet fed mice [88]. Wang et al., have reported that 5 weeks administration of Lactobacillus plantarum MA2 decreased both liver cholesterol and triglycerides in rats fed a cholesterol-enriched diet [89]. Lactobacillus paracasei F19 significantly attenuated liver injury induced by both ischemia-reperfusion and a methionine/choline-deficient diet in rats by restoring gut microbiota and reducing inflammation and steatosis [90]. A recent study by Xu et al., has demonstrated that Bifidobacterium longum supplementation was superior to Lactobacillus acidophilus in terms of attenuating liver fat accumulation in a diet-induced rat model of NAFLD [91], supporting the importance of careful evaluation of probiotic strains for therapeutic use.
Endo et al., recently reported that the Clostridium butyricum strain, MIYAIRI 588—a butyrate-producing probiotic, prevents progression of choline-deficient/L-amino acid-defined (CDAA)-diet-induced NAFLD and tumorigenesis in rats [92]. In this study MIYAIRI 588 significantly reduced the CDAA-diet induced hepatic lipid deposition, increase in endotoxin levels in the portal vein, and restored intestinal TJ protein levels (ZO-1 and occludin) to levels comparable to the control group. MIYAIRI 588 substantially increased the activation of hepatic adenosine 5’-monophosphate-activated protein kinase (AMPK) and AKT and the expression of lipogenesis- or lipolysis related proteins. Further, the MIYAIRI 588-treated rats also showed a remarkable induction of nuclear factor (erythoid-derived 2)-like 2 (Nrf2) and its targeted antioxidant enzymes, which suppressed hepatic oxidative stress. This study is an excellent example pointing out that not only restoration of gut bacteria composition but also modulation of the microbiota metabolic activity should be taken into consideration while designing pre- or probiotic preventive and/or therapeutic interventions for NAFLD.
Although current evidence is limited, there is sufficient proof of concept that the modification of the intestinal bacteria with pre-, pro-, and symbiotics (a combination of pre- and probiotics) can be used as a therapeutic approach in human NAFLD. A recent meta-analysis of four randomized trials involving 134 NAFLD/NASH patients demonstrated that probiotic therapies can reduce liver aminotransferases, total cholesterol, TNF-α and improve insulin resistance in NAFLD patients [93]. Two randomized double-blind placebo-controlled studies have shown a significant decrease in liver aminotransferases in response to 2 months of Lactobacillus rhamnosus strain GG supplementation in children [94], and to 3 months of Lactobacillus bulgaricus and Streptococcus thermophilus treatment in adults [95]. In a randomized controlled double-blind clinical trial, a 4-month supplement of VSL#3 significantly improves NAFLD in children; the VSL#3-dependent GLP-1 increase could be responsible for these beneficial effects [96]. Administration of the probiotic formula containing Lactobacillus plantarum, Lactobacillus deslbrueckii, Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacterium bifidum significantly reduced hepatic fat accumulation and serum aspartate aminotransferase (AST) levels in patients with histology-proven NASH [97]. Malaguarnera et al., have reported that 24 weeks of Bifidobacterium longum with fructo-oligosaccharides supplementation together with lifestyle modification (i.e., diet and exercise), when compared to lifestyle modification alone, significantly reduces serum AST and TNF-α levels, serum endotoxin, steatosis and the NASH activity index [98]. Eslamparast et al., reported beneficial effects of a symbiotic preparation consisting of 7 strains of bacteria (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum, and Lactobacillus bulgaricus) along with prebiotic fructooligosaccharide on hepatic inflammation and overall liver function in patients with NAFLD. In this study, the symbiotic supplementation for 28 weeks in addition to lifestyle modification was superior to lifestyle modification alone for the treatment of NAFLD, at least partially through the inhibition of NF-kB activation and the reduction of TNF-α production [99]. These promising results strongly indicate the great potential of pre-, pro- and symbiotics in the treatment of NAFLD, but larger studies that include serial liver biopsies are needed.
Conclusions and Future Directions
To summarize, the gut-liver axis and the interplay between the dietary factors, gut microbiota, and intestinal barrier integrity play an important role in the development of obesity and obesity-associated NAFLD. Dietary nutrients may alter the gut microbiota and intestinal barrier function favoring the occurrence of metabolic endotoxemia and low-grade inflammation contributing to the development of obesity and NAFLD. Low-grade systemic inflammation involves a complex network of signals interconnecting several organs, including liver, adipose tissue, and skeletal muscles (Figure 4). Further meta-transcriptomic, metabolomic and meta-proteomic studies are needed to determine the changes in the microbial metabolic activity as a result of dietary challenge. Understanding which dietary factor(s) affect gut microbiota, and how they do so, and identifying which component(s) of microbial metabolic activity influence the host’s metabolism, and how they contribute to obesity and obesity-associated NAFLD may help to develop new nutritional approach for prevention and/or treatment of these conditions.
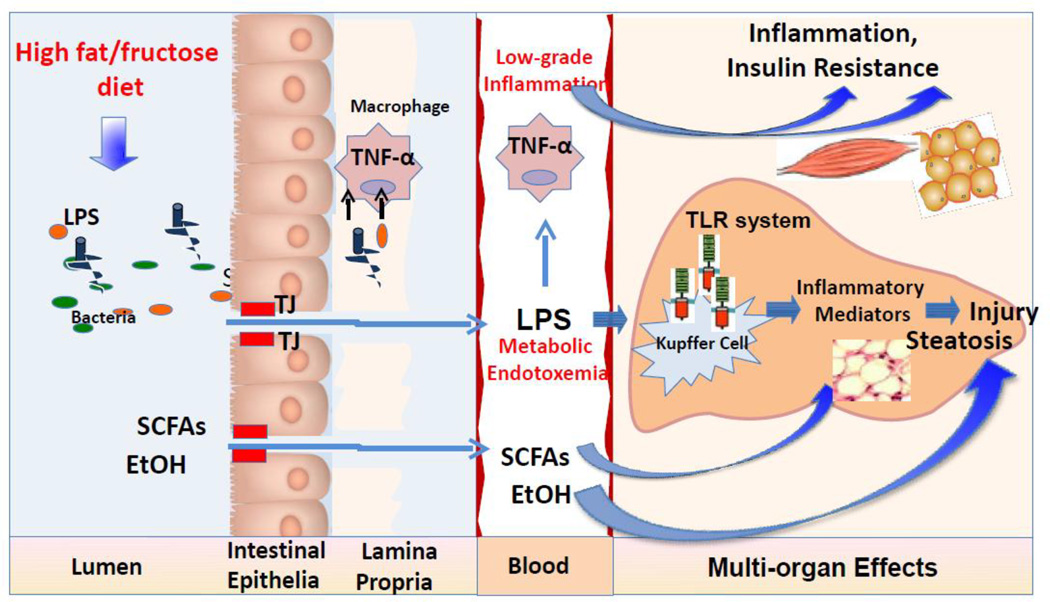
Figure 4.
The interplay between dietary factors, gut microbiota, and gut barrier integrity in the development of NAFLD. Both high fat or high fructose diets may cause dysbiosis and bacterial overgrowth. Intestinal bacteria alterations result in disruption of gut barrier integrity with the subsequent increase in gut permeability to bacteria-derived pathogens, including LPS. The altered gut microbiota may change its metabolic activity, including an increase in fermentation of dietary polysaccharides to SCFAs, and production of endogenous EtOH. The produced metabolites are absorbed and transported to the liver where SCFAs may stimulate de novo synthesis of triglycerides and EtOH may elevate ROS production. The presence of LPS in the systemic circulation results in the activation of the innate immune system and a massive secretion of pro-inflammatory cytokines, particularly TNF-α. Low-grade systemic inflammation involves a complex network of signals interconnecting several organs, including the liver, adipose tissue, and skeletal muscles. In the liver, LPS causes hepatocellular inflammation by stimulating distinct cell types to release pro-inflammatory cytokines via TLR-4-mediated mechanisms leading to liver injury. Abbreviations: EtOH, ethanol; LPS, lipopolysaccharides; ROS, reactive oxygen species; SCFAs, short chain fatty acids; TJs, tight junctions; TLR, Toll like receptor; TNF-α, tumor-necrosis factor alpha
Highlights.
Obesity and NAFLD are closely associated with the disruption of gut barrier integrity, metabolic endotoxemia and low-grade inflammation
Gut microflora plays a significant role in the development of NAFLD
The increased consumption of obesogenic foods (e.g. fat and fructose) may alter the gut microbiota and intestinal barrier function favoring the occurrence of metabolic endotoxemia and low-grade inflammation, thereby contributing to the development of obesity and obesity-associated fatty liver disease
Therapeutic manipulations with prebiotics and probiotics to modulate the gut microbiota and maintain intestinal barrier integrity are potential agents for NAFLD management
Acknowledgements
The authors thank Marion McClain for the manuscript proofreading.
Funding: The work presented in this study was supported by NIH grants R21 AA020849-01A1 (IK), 1UO1AA022489 (CJM), 1U01AA021901-01 (CJM), 1U01AA021893-01 (CJM), R01 AA023681 (CJM), R01 AA018869 (CJM), the Department of Veterans Affairs (CJM), and the Department of Defense (CJM)
Abbreviations
- ALT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- AST
aspartate aminotransferase
- CDAA diet
choline-deficient/L-amino acid-defined diet
- GLP-2
glucagon-like peptide-2
- HFD
high fat diet
- IL-1
interleukin 1
- IL-6
interleukin 6
- Fiaf
fasting-induced adipocyte factor
- LA
linoleic acid
- LPS
lipopolysaccharides
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-kB
Nuclear factor-κB
- Nrf2
nuclear factor (erythoid-derived 2)-like 2
- PAI-1
plasminogen activator inhibitor-1
- RELM-β-KO
resistin-like molecule β-knockout
- SCFAs
short chain fatty acids
- SIBO
small intestine bacterial overgrowth
- SOCS-3
suppressor of cytokine signaling-3
- TJs
tight junctions
- TLR
Toll like receptor
- TNF-α
tumor-necrosis factor alpha
- ZO-1
zonula occludens-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Irina A. Kirpich has no conflict of interest to declare; Craig J. McClain has no conflict of interest to declare.
Contributor Information
Luis S. Marsano, Email: luis.marsano@louisville.edu.
Craig J. McClain, Email: cjmccl01@louisville.edu.
References
- 1.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126(2):137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Kelly T, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 6.Backhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jumpertz R, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr. 1986;116(9):1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 9.Wolin MJ. Fermentation in the rumen and human large intestine. Science. 1981;213(4515):1463–1468. doi: 10.1126/science.7280665. [DOI] [PubMed] [Google Scholar]
- 10.Muccioli GG, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect. 2012;18(Suppl 4):50–53. doi: 10.1111/j.1469-0691.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 12.Dumas ME, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swann JR, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125(1):386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119(5):1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 18.Wigg AJ, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabate JM, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18(4):371–377. doi: 10.1007/s11695-007-9398-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferolla SM, et al. The Role of Intestinal Bacteria Overgrowth in Obesity-Related Nonalcoholic Fatty Liver Disease. Nutrients. 2014;6(12):5583–5599. doi: 10.3390/nu6125583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura K, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–334. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taira R, et al. Bacterial cell wall components regulate adipokine secretion from visceral adipocytes. J Clin Biochem Nutr. 2015;56(2):149–154. doi: 10.3164/jcbn.14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geurts L, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6:6495. doi: 10.1038/ncomms7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9(6):737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho BM, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55(10):2823–2834. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- 26.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 27.Brun P, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 28.Ding S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 30.Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, et al. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PLoS One. 2014;9(12):e115148. doi: 10.1371/journal.pone.0115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergheim I, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48(6):983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Kavanagh K, et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr. 2013;98(2):349–357. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spruss A, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50(4):1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin Exp Pharmacol Physiol. 2014;41(7):482–488. doi: 10.1111/1440-1681.12241. [DOI] [PubMed] [Google Scholar]
- 36.Wagnerberger S, et al. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem. 2013;24(3):531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Ritze Y, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9(1):e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harte AL, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farhadi A, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2008;28(7):1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alisi A, et al. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2010;50(6):645–649. doi: 10.1097/MPG.0b013e3181c7bdf1. [DOI] [PubMed] [Google Scholar]
- 41.Jin R, et al. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgio V, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46(6):556–560. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Miele L, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 44.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142(5):1100–1101. e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erridge C, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 46.Deopurkar R, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–997. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghoshal S, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Ghanim H, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32(12):2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ley RE, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 50.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balmer ML, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6(237):237ra66. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- 54.Moschen AR, Kaser S, Tilg H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol Metab. 2013;24(11):537–545. doi: 10.1016/j.tem.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 56.Backhed F, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabot S, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24(12):4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 58.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 59.Claus SP, et al. Colonization-induced host-gut microbial metabolic interaction. MBio. 2011;2(2):e00271–e00210. doi: 10.1128/mBio.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Roy T, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 61.Henao-Mejia J, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 63.Kirpich IA, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36(5):835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mouzaki M, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58(1):120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 65.Wong VW, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8(4):e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hildebrandt MA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–1724. e1–e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turnbaugh PJ, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Wit N, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 70.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duncan SH, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32(11):1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 72.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer MD, et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fava F, et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome 'at-risk' population. Int J Obes (Lond) 2013;37(2):216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 75.Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furet JP, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kong LC, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 79.Liou AP, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravussin Y, et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012;20(4):738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 82.Cotillard A, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 83.Pachikian BD, et al. Prebiotic approach alleviates hepatic steatosis: implication of fatty acid oxidative and cholesterol synthesis pathways. Mol Nutr Food Res. 2013;57(2):347–359. doi: 10.1002/mnfr.201200364. [DOI] [PubMed] [Google Scholar]
- 84.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma X, H J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. Journal of Hepatology. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 87.Lee HY, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochimica et biophysica acta. 2006;1761(7):736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 88.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, et al. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2009;84(2):341–347. doi: 10.1007/s00253-009-2012-x. [DOI] [PubMed] [Google Scholar]
- 90.Nardone G, et al. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. American journal of physiology. Gastrointestinal and liver physiology. 2010;299(3):G669–G676. doi: 10.1152/ajpgi.00188.2010. [DOI] [PubMed] [Google Scholar]
- 91.Xu RY, et al. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50(1):72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Endo H, et al. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8(5):e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma YY, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19(40):6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vajro P, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52(6):740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 95.Aller R, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15(9):1090–1095. [PubMed] [Google Scholar]
- 96.Alisi A, et al. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong VW, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12(2):256–262. [PubMed] [Google Scholar]
- 98.Malaguarnera M, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 99.Eslamparast T, et al. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99(3):535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]