Abstract
Myeloid-derived suppressive cells (MDSC) have been reported to promote metastasis, but the loss of cancer-induced B cells/B regulatory cells (tBregs) can block metastasis despite MDSC expansion in cancer. Here, using multiple murine tumor models and human MDSC, we show that MDSC populations which expand in cancer have only partially primed regulatory function and limited pro-metastatic activity unless they are fully educated by tBregs. Cancer-induced tBregs directly activate the regulatory function of both the monocyte and granulocyte subpopulations of MDSC, relying in part on TgfβR1/TgfβR2 signaling. MDSC fully educated in this manner exhibit an increased production of ROS and NO and more efficiently suppress CD4+ and CD8+ T cells, thereby promoting tumor growth and metastasis. Thus, loss of tBregs or TgfβR deficiency in MDSC is sufficient to disable their suppressive function and to block metastasis. Overall, our data indicate that cancer-induced B cells/B regulatory cells are important regulators of the immune suppressive and pro-metastatic functions of MDSC.
Keywords: Stat3-TGFβ axis, tBregs, MDSC, breast cancer
Introduction
The success of metastasis often depends on the ability of disseminating cancer cells to escape immune attack by utilizing the help of regulatory immune cells, a heterogeneous group of specialized cells of granulocytic, myeloid and lymphoid origins with seemingly redundant functions (1). Among these, myeloid-derived suppressive cells (MDSC) are thought to be key inhibitors of antitumor effector cells and, as such, an independent prognostic factor of patient survival (2). As a group of immature cells poised to differentiate into granulocytes, dendritic cells and macrophages, MDSC are subdivided into PMN-MDSC and Mo-MDSC cells (1, 3) based on expression of Ly6G+Ly6CInt/Low CD11b+ and Ly6CHighLy6G− CD11b+ in mice (4, 5) and CD14−CD11b+ CD15+CD33+ and CD14+CD11b+HLA-DRLow/− in humans (2, 6, 7), respectively. By producing GM-CSF, VEGF, TGFβ, IL-6, IL-10, IL-13 and PGE4, cancer not only drastically expands MDSC, but also evokes their regulatory function (for reviews, see ref. (1, 8, 9)) by inducing their production of reactive nitrogen and oxygen species (NO, ROS, H2O2, and peroxinitrite) through the IL4-Stat6-dependent expression of arginase 1 (Arg-1) (10) and Stat1- and Stat3-induced expression of nitric oxide synthase (iNOS) and NADPH oxidase (NOX2) (11, 12). The expansion of MDSC is often used as a criterion of increased tumor burden and metastasis (1, 13). However, using tumor models where MDSC were reported to be essential, we failed to detect the primary importance of MDSC in cancer metastasis. The loss of regulatory T cells (Tregs) or B cells was sufficient to almost completely block the metastasis of the highly aggressive 4T1 cancer in BALB/c mice, a human model of triple negative breast cancer (14), and retard the growth of B16 melanoma in C57BL/6 mice (15–18). In the 4T1 model, cancer produces 5-lipoxygenase metabolites to convert B cells into a new subset of regulatory B cells, termed tumor-evoked regulatory B cells (tBregs) (17, 19), that induce FoxP3+ Tregs to inactivate the anti-metastatic NK and CD8+ T cells (15, 17, 19).
Here, using two different murine models and experimenting with human ex vivo –generated MDSC, we report that cancer only expands MDSC with partially activated regulatory function. As a result, the MDSC cannot support metastasis or promote tumor growth. We show that cancer uses B cells to evoke their full regulatory and thereby pro-metastatic function. Our modeling studies using specific TgfβR1 inhibitor and mice with TgfβR2 deficiency in myeloid cells suggest that cancer-induced B cells/tBregs evoke the full regulatory activity in MDSC via using at least in part the TgfβR1/TgfβR2 signaling axis. These results further underscore B cells/tBregs as key tumor messengers and initiators of the chain of suppressive events needed for metastasis.
Methods
Reagents, cells and mice
TGFβRI (ALK5) inhibitor (SB431542) was purchased from Tocris Bioscience (Ellisville, MO), catalase (1000u/ml) from Sigma Aldrich (St. Louis, MO). L-NMMA and nor-NOHA (0.5mM) were from Cayman Chemical (Ann Arbor, MI). Nitrate and NO were detected with the Griess reagent kit and DAF-FM diacetate, respectively, and ROS was detected with 1μM DHE [dihydroethidium] or DCFDA [2′,7′-dichlorodihydrofluorescein diacetate] were from Molecular Probes (Eugene, OR) and used as described elsewhere (5). α-TGFβ neutralizing antibody (clone 1D11.16.8), α-mouse Gr1 (clone RB6-8C5), mouse IgG and rat IgG2b were purchased from BioXcell.
The following flow cytometry antibodies and their isotype controls (from Biolegend and eBioscience, San Diego, CA, except otherwise specified) were used: CD11b APC or Fitc (M1/70), Gr1 PE or Fitc (RB6-8C5), Ly6G Alexa Fluor700 or PerCP Cy 5.5 (1A8), Ly6C Pacific blue or Fitc (HK1.4), IL4Rα PE (I015F8), F4/80 PerCP Cy5.5 or APC (BM8), CD40 PE Cy7 (3/23), CD115 PE (AFS98), CD80 Brilliant Violet 421 (16-10A1), CD83 Brilliant Violet 650 (Michel-19), GrB Fitc (GB11), IFNγ PE-Cy7 (XMG1.2). Tgfβ receptors antibodies were from R&D (TgfβR1, clone FAB5871A and TgfβR2, clone FAB532F).
For intracellular staining of phosphorylated Stat proteins, cells were fixed with 2% paraformaldehyde in PBS for 10 min at 37°C and resuspended in pre-chilled 90% methanol (in water). The cells were stained with anti-mouse CD11b Fitc, Ly6G PE-Cy7, Ly6C Pac blue (Biolegend, San Diego, CA) and rabbit anti-mouse pStat1 or pStat3-Alexa Fluor 647 (Tyr705, Cell Signaling, Danvers, MA) at 1/200 dilution. For lineage negative MDSC sorting, PE conjugated CD19 (clone 6D5), CD3 (clone 145-2C11), B220 (clone RA3-6B2), CD49b (DX5) antibodies were used. Human sorting were performed using CD11b PE (clones M1/70) or CD14 Fitc (clone M5E2), CD19 PE (HIB19), CD3 PE, CD56 PE antibodies (Biolegend and eBioscience, San Diego, CA).
4T1 adenocarcinoma cells and B16F10 melanoma cells were from American Type Culture Collection. 4T1.2 cells, a subset of 4T1 cells, were a gift from Dr. Robin L. Anderson (Peter McCallum Cancer Center, Australia). Non-metastatic 4T1.2-PE cells were described elsewhere (15). Female 8–15 weeks old BALB/c, C57BL/6 mice and JHT mice in C57BL/6 background (JHT; B6.129P2-Igh-Jtm1Cgn/J) were from the Jackson Laboratory (Bar Harbor, ME), μMT mice in BALB/c background were a gift from professor Dr. Thomas Blankenstein (MDC, Berlin, Germany), C57BL/6 mice with spontaneous ovarian cancer due to murine oviduct-specific glycoprotein promoter-driven expression of simian virus 40 large T-antigen (20) were housed at NIA, Baltimore and TGFβRIImyeKO mice at NCI, Bethesda (21).
In vitro manipulations
tBreg generation and T cell suppression assays were performed as previously described (15). Unless specified, control B cells were treated with 100 ng/ml of recombinant mouse BAFF/BlyS (R&D, Minneapolis, MN) in cRPMI. tBregs were characterized as CD81hi CD25+ CD20low 4-1BBLlow cells gated within CD19+ cells using mAbs (CD19-APC-eFluor780, CD81-APC, CD25-Pacific blue, CD20-PE and 4-1BBL-PerCP Cy5.5 (Biolegend and eBioscience). Flow cytometry data were collected on a FACS Canto II (BD) and analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). Cell sorting was performed on FACS Aria III (BD, San Jose, CA) or MoFlo XDP (Beckman Coulter, Inc., Danvers, MA). Mo-MDSC were isolated as Lin−,CD11b+, Ly6G−, Ly6Chi and PMN-MDSC were isolated as Lin−,CD11b+, Ly6G+, Ly6C low-int. Magnetic sorts purity was > 96% and flow cytometry sorts purity was > 98%. All magnetic sorts performed in these experiments were double sorts to increase purity. For the majority of the experiments, PMN- and Mo-MDSC were cell-sorted (purity > 98%) or isolated using the Miltenyi Biotech Myeloid derived suppressor cell isolation kit. MDSC isolated from peripheral blood or spleen yielded similar results. For MDSC education, Gr1+ cells were isolated from tumor bearing mice by magnetic positive selection (Miltenyi Biotech, Auburn, CA) and mixed at experiment ratios (1–10:1) with B cells (B-cont or tBregs) for 5 hours. Then, Gr1 cells were re-sorted by magnetic positive selection prior to assays, such as testing for in vitro T cell suppression or adoptive transfer experiments. When MDSC education was done with B-tumor cells, negatively isolated MDSC (using Miltenyi Biotech MDSC isolation kit) were co-cultured O/N with positively isolated CD19+ B cells, isolated from spleen or LN (yielded similar results) of naïve or 4T1 tumor bearing BALB/c mice. After co-culture, B cells were depleted and MDSC used in T cell suppression assays or adoptive transfer experiments. For suppression assay, MDSC were co-cultured at 1:2–1:16 ratios with splenic CD3+ T cells, which were isolated with the mouse T-cell enrichment columns (R&D Systems), labeled with the cell proliferation dye eFluor450 (eBioscience) and stimulated with anti-CD3/28 beads (Life technologies) for 4–5 days. Dilution of eFluor450 in CD4+ and CD8+ T cells (stained with anti-mouse CD4-PerCPCy5.5, clone GK1.5, and CD8-FITC, clone 53–6.7, Biolegend) is considered proliferation. TGFβ, GrB and IFNγ was detected by intracellular staining of splenic B cells from tumor-bearing mice after 4 h stimulation with phorbol 12-myristate 13-acetate (50 ng/ml PMA and 500 ng/ml ionomycin, Tocris Bioscience) and 10 μM monensin (eBioscience). To test TgfβR signaling, we used 20μM SB431542 in the co-culture of B cells with MDSC. Alternatively, WT B cells were cultured with MDSC from tumor-bearing TgfβR2 KO mice.
Human peripheral blood cell isolation and suppressive myeloid cell assays
Human peripheral blood was collected by the Health Apheresis Unit and the Clinical Core Laboratory, the National Institute on Aging, under Human Subject Protocol # 2003054 and Tissue Procurement Protocol # 2003-071. Peripheral blood mononuclear cells were isolated using Ficoll-Paque (GE Healthcare) density gradient separation and B, T and NK cells were depleted with PE coupled Abs to CD19, CD3, CD56 and anti-PE microbeads following the manufacturer’s instructions (Miltenyi Biotech). To educate myeloid cells, PBMCs (1×106 cells/ml, with or without B cells) were cultured with conditioned media from human breast cancer cell line (MDA-MB-231, 50%, volume) and 20 ng/ml GM-CSF with or without 20 μM SB431542 for 4 days. Then, CD11b+ or CD14+ cells were isolated by positive selection (Miltenyi Biotech) and used in T cell suppression assay. Healthy donor peripheral blood (PB) PBMCs (depleted of T and NK cells) were co-cultured with CD19+ cells from PB of B-CLL patients for 48 h. Then, CD19+ cells were depleted and CD14+ cells were positively isolated by magnetic selection and used in T cell suppression assays.
In vivo manipulations
Mouse experiments were performed in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). 4T1.2 cells and B16-F10 cells (1 × 105) were subcutaneously (s.c.) injected into BALB/c mice or congenic μMT and TgfβR2myeKO or JHT and C57BL/6 mice, respectively. At days 32 and 20 post 4T1 and B16-F10 cell injections, respectively, lung metastasis and tumor weight were evaluated as described elsewhere (15). MDSC were isolated from mice after 25–30 and 18–20 post 4T1 and B16 tumor challenge, respectively. Mice with spontaneous ovarian cancer were 12–15 weeks old. For adoptive transfer experiments, mice were i.v. injected with congenic 1.5–10.0 ×106 MDSC or tBregs at 1–3 days post tumor challenge. For Fig 2B, the mice were tumor inoculated at day 0 and B cell adoptive transfer was performed on day 18 (the late B cell transfer was done to allow sufficient MDSC accumulation). MDSC were sorted 3 days post-B cell transfer to avoid the conversion of naïve B cells due to the tumor pressure.
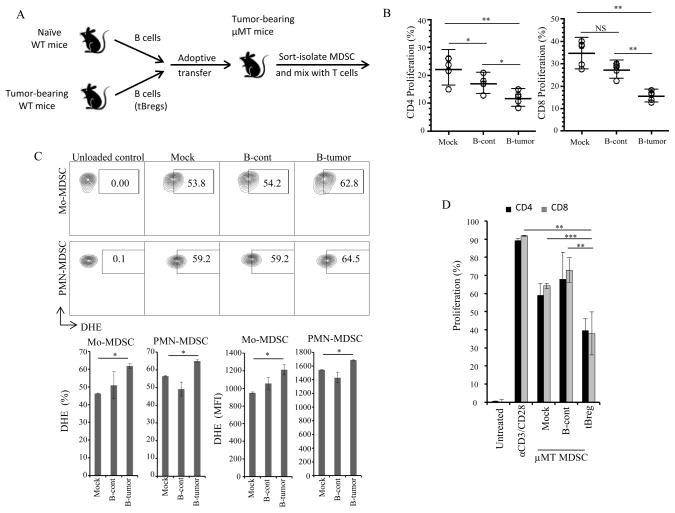
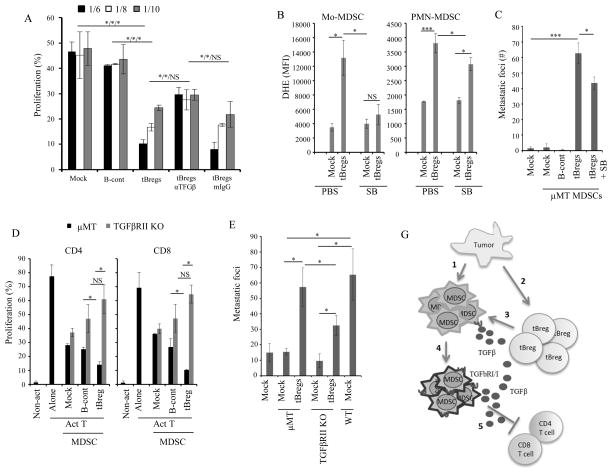
FIGURE 2. The pro-metastatic activity of MDSC requires tBregs.
Adoptive transfer of B cells in μMT mice modulate the T cell suppressive potential of MDSC (B,D) and ROS production (C). Schema of the experiment (A) depicted in (B–D). MDSC were isolated from μMT mice and cultured with T cells in Fig. 1G at 1:8 E:T ratio (B,D), or stained with DHE (C). A representative figure (top) and summary results (as % and MFI, bottom, C) are from 4 mice per group experiment reproduced twice. Y-axis is % of proliferated CD4+ (B) and CD8+ T cells (B,D) ± SEM of four-five mice per group experiments reproduced at least three times. *P<0.05, **P<0.01, ***P<0.001.
α-mouse Gr1 depletion antibody was used at 100μg/mouse intra-peritoneal injections every fourth day. For the B cell depletion experiments, mice were intra-peritonealy injected with α-CD20 antibody (250ug/mouse) on day 7 post-tumor inoculation. PB MDSC were assessed on day 28 post-tumor inoculation.
Statistical Analysis
The results are presented as the mean ± SEM. To assess significance between two groups we used the Mann-Whitney’s test, while the Holm-Sidak method with alpha=5.0% was utilized to correct for multiple comparison tests of tumor growth and titration curve analysis (Prism 6, Graph Pad Software, Inc.).
Results
B-cell deficiency impairs the regulatory activity of MDSC
To understand the pro-metastatic role of MDSC, we subcutaneously implanted 4T1 cancer cells in the mammary gland of WT mice and B-cell deficient μMT mice that do not generate tBregs (16, 17). Tumor growth in the mammary gland was retarded (p<0.005) and lung metastasis was blocked in μMT mice as compared to WT mice (p=0.028, Fig. 1A). However, both mice comparably and strongly expanded MDSC (CD11b+Gr1+, SFig. 1A), such as Mo-MDSC (Ly6G−Ly6C+) and PMN-MDSC (Ly6G+Ly6CInt/Low) in the peripheral blood (PB) as well as in the secondary lymphoid organs, lungs and tumors of both mice (Fig. 1B). Since these results question the importance of MDSC in metastasis, we tested their role by either treating tumor-bearing WT mice with RB6-8C5 Ab that depletes Gr1+ myeloid cells (including MDSC) or, conversely, by the adoptive transfer of MDSC from tumor-bearing WT mice (WT-MDSC) into μMT mice. While the depletion of Gr1+ cells reduced metastasis in WT mice (p=0.004, SFig. 1B), metastasis was restored in μMT mice transferred with WT-MDSC at a similar extent with the mice injected with ex vivo-generated metastasis-supporting tBregs (tBregs, Fig. 1C). The transfer of MDSC from tumor-bearing μMT mice (μMT-MDSC, Fig. 1C) failed to affect metastasis in μMT mice, confirming the importance of B cells/tBregs in the pro-metastatic function of MDSC.
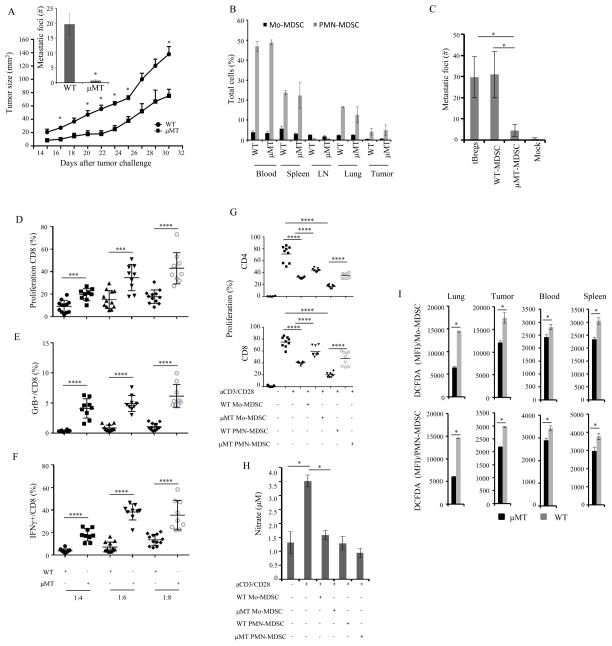
FIGURE 1. B-cell deficiency impairs the regulatory and pro-metastatic function of MDSC.
Tumor size, lung metastasis (inlet, A) and frequency of MDSCs (B) were evaluated in μMT and WT mice with s.c. injected 4T1.2 cancer cells (1×10^5 cells). To assess metastasis inducing ability of tBregs and MDSC (week 4 post-tumor inoculation), they (10×106 cells) were adoptively transferred in μMT mice (n= 4–5 mice per group) after 24h post tumor implantation (C). MDSC (D–F) or MDSC subsets (G–H) were sorted from μMT and WT mice 4 weeks after tumor inoculation and tested for their ability to suppress T cells stimulated with anti-CD3/CD28 Abs at indicated (X-axis, D–F) or 1:8 E:T ratio (G). To test expression of GrB and IFNγ, MDSC were intracellularly stained (E,F); and nitrate production (H) was assessed in conditioned medium of cells used in (G). The DCFDA staining of MDSC subsets in indicated tissues from μMT and WT mice with 4 week tumor is shown in (I). Y-axis shows tumor size (A), or number of metastatic foci (inlet, A), % of total cells per indicated tissue (B), μM of nitrate (H) ± SEM of 3–5 mice per group, experiments reproduced at least three times. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, NS - not significant.
Next, we tested whether the loss of B cells/tBregs can impair the regulatory function of MDSC. To do this, we co-cultured purified WT- and μMT-MDSC isolated from individual mice challenged with 4T1 cancer at the same time (n=9, from here on, unless specified, the MDSC were only isolated from tumor-bearing mice) with naïve mouse T cells stimulated with anti-CD3/CD28 Abs for five days. At the cell-to-cell comparisons, while WT-MDSC strongly inhibited proliferation (Fig. 1D) and production of granzyme B (GrB, Fig. 1E) and IFNγ (Fig. 1F) in CD8+ T cells, μMT-MDSC were significantly less efficient (p<0.001, Fig. 1D–F). To further confirm this result, we also tested suppressive activity of individual subsets of MDSC by co-culturing sort-purified PMN-MDSC and Mo-MDSC with T cells stimulated with anti-CD3/CD28 Abs. Despite overall stronger activity of PMN-MDSC over Mo-MDSC, both μMT-MDSC subsets inhibited less efficiently CD4+ and CD8+ T cells as compared with their respective WT subsets (p<0.001, Fig. 1G and SFig. 1C). Since the suppression involves NO and ROS, their production could be impaired in μMT-MDSC. In support, specific inhibitors NOHA, L-NMMA and, particularly, catalase (scavenges H2O2, dismutation product of ROS), almost completely abolished the ability of MDSC to suppress T cell proliferation (SFig. 1D,E). The relative expression levels of NOX2 and Arg genes were significantly reduced in μMT-MDSC as compared to WT-MDSC (SFig. 2A,B). In concordance, nitrate production (the NO readout) was only detected in WT Mo-MDSC, but not PMN-MDSC or μMT-MDSC, cultured with T cells (p=0.028 as compared μMT-MDSC, Fig. 1H). Both subsets of μMT-MDSC also produced significantly less ROS (detected by staining for O2− with dihydroethidium, DHE, SFig. 2C) and H2O2 (Fig. 1I and SFig. 2D, detected by dichlorofluorescin diacetate conversion assay, DCFDA (22)) as compared to WT-MDSC. For example, the median fluorescence index (MFI) of DCFDA+Mo-MDSC was decreased from 14400±150 to 6459±247 (p<0.001) in the lungs and from 17565 ±1153 to 12109±345 (p<0.001, Fig. 1I) in the tumor of μMT mice compared to WT mice, respectively. For DCFDA+ PMN-MDSC, it was reduced from 14569 ±64 to 6082±41 (p<0.001) and 29616±251 to 21847±74 (p<0.001, Fig. 1I), respectively. Moreover, ROS production in WT-MDSC of tumor-bearing WT mice was further increased (p<0.03, SFig. 2C) if the mice were treated with α-CD20 Ab that enriches for tBregs via depleting B cells (17). Thus, given that μMT mice do not generate tBregs (17), these results suggest that the regulatory function of MDSC requires tBregs.
tBregs activate the regulatory function of cancer-primed MDSC
To test this possibility, we transferred B cells from naïve (B-cont) or tumor-bearing WT mice (B-tumor, which contain tBregs (17)) into μMT mice (n=5/group, Fig. 2A). After 3 days, PB MDSC were isolated and tested for suppression of T cell proliferation. μMT-MDSC from B-cont-transferred mice suppressed proliferation of CD8+ T cells slightly stronger than the ones from mock-treated mice (Fig. 2B), presumably due to some in vivo conversion of B-cont cells into tBregs (16, 17). However, μMT-MDSC from mice transferred with B-tumor inhibited T cell proliferation significantly stronger (p<0.001, Fig. 2B) and produced higher levels of ROS (p=0.029 as compared with B-cont-transferred mouse Mo-MDSC and PMN-MDSC, respectively, Fig. 2C). Thus, these experiments reproduced at least three independent times indicate that the full regulatory function of MDSC in mice requires tBregs. In concordance, the transfer of ex vivo-generated tBregs also significantly increased the suppressive activity of MDSC in μMT mice (p<0.008, Fig. 2D), besides restoring lung metastasis (Fig. 1C and also ref. (16–18)).
tBregs educate MDSC promoting cancer escape and metastasis
To understand how tBregs activate MDSC, we performed in vitro experiments by co-culturing freshly purified μMT-MDSC with naïve mouse B cells (B-cont), or with tBregs, or B cells from tumor-bearing mice (B-tumor). After 5 h incubation, the MDSC were depleted of B cells and mixed with T cells stimulated with anti-CD3/CD28 Ab. Unlike B-cont, the pre-treatment of μMT-MDSC with B-tumor (Fig. 3A,B) or tBregs (Fig. 3C and SFig. 3A) strongly (p=0.029) and in a dose-dependent manner inhibited T cell proliferation. The treatment also blocked production of key factors required for successful antitumor activity of CD8+ T cells (17, 23), such as GrBg and IFNγ (SFig. 3A–C). The B-tumor and tBreg-stimulated μMT-MDSC also expressed higher levels of NOX2, iNOS, and Arg1 genes (SFig. 4A–G) and produced more ROS (P<0.03, Fig. 3D) than B-cont-treated MDSC. They also up regulated expression of IL-10 and TGFβ (SFig. 4E,F), and other factors associated with regulatory MDSC, such as IL4Rα, CD80, CD83, CD40, and phosphorylated Stat1 and Stat3 (Fig. 3E and SFig. 4H). The μMT-MDSC also up regulated surface expression of TgfβR1 (p<0.03) and TgfβR2 (p<0.002) upon stimulation with tBregs (SFig. 4I). When we transferred these μMT-MDSC (pretreated with B-tumor/tBregs) into μMT mice, the mice succumbed to significant lung metastasis (p<0.03, Fig. 3F). In contrast, B-cont-pretreated μMT-MDSC failed to restore metastasis in μMT mice. Thus, these results, which were independently confirmed in multiple experiments, indicate that tBregs directly evoke the regulatory and thereby pro-metastatic function of MDSC.
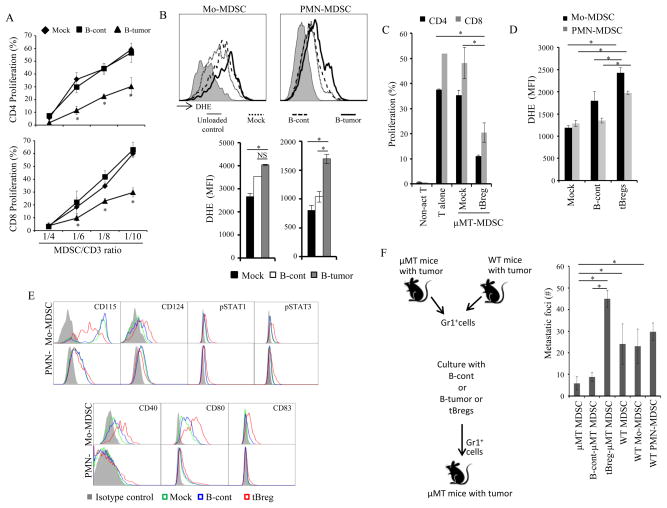
FIGURE 3. tBregs directly activate cancer-expanded MDSC rendering them regulatory.
MDSC from PB of μMT mice with 4T1 cancer were cultured with B naïve/B-cont or B-tumor/tBregs. After 5–16h incubation (longer for B-tumor cells), the cells were stained with DHE (B, D) or for several surface and intra-cellular molecules (E). After B-cell depletion, the MDSC were used in T cell suppression assays as in Fig. 1G (A,C) or adoptively transferred in tumor-bearing μMT mice to assess lung metastasis (F). For the education experiment we used ex vivo-generated tBregs or B-tumor cells sort-purified from at least 3 WT mice with tumor. B cells were mixed with MDSCs isolated from 3 μMT tumor-bearing mice. Each experiment was reproduced at least three times. *P<0.05, **P<0.01, ***P<0.001.
Murine and human cancer B cells can also educate MDSC
Similar “education” of MDSC appears to also occur in other mouse backgrounds and tumor models, as the growth of a highly aggressive B16 melanoma is also retarded in B-cell deficient JHT mice compared to congenic C57BL/6 mice (17–19, 24). To test this possibility, we adoptively transferred JHT mice with B16 melanoma MDSC purified from WT C57BL/6 mice with orthotopic B16 melanoma or with spontaneous ovarian cancer (20) (WT B16-MDSC and OC-MDSC, respectively). Unlike MDSC from JHT mice with B16 melanoma (JHT B16-MDSC), the transfer of WT B16-MDSC and OC-MDSC reversed the retarded tumor growth in JHT mice yielding significantly larger tumors (p<0.05, Fig. 4A). Moreover, WT B16-MDSC and OC-MDSC also produced higher levels of ROS (p<0.03, Fig. 4B) and nitric oxide (p<0.03, Fig. 4C) and strongly inhibited CD4+ and CD8+T cell proliferation (p=0.03, Fig. 4D).
FIGURE 4. Murine and human cancer B cells also educate MDSC.
MDSC (5×106) purified from PB and spleen of B16 melanoma-bearing JHT mice or WT mice (B16-MDSC) or mice with spontaneous ovarian cancer (OC-MDSC) were adoptively transferred into JHT mice (n=4–5 mice per group) 24h after B16 tumor challenge (A). PB MDSCs from WT, JHT, TgfβR2 KO mice with B16 melanoma (WT B16, JHT B16, and TβR2KO B16, respectively) or mice with ovarian cancer (WT OC) were tested for ROS and nitric oxide production by DHE and DAF-FM staining (B,C), or used in T cell suppression assay as in Fig. 1G (D). Experiments in (A) and (C–D) were reproduced 3 and 2 times, respectively. PBMC (n=7, healthy human donors), depleted of CD19+ cells were incubated with conditioned media of MDA-MB-231 cancer cells with or without SB 431542 (20μM), in the presence of 20 ng/ml GM-CSF for 48h. CD11b+ or CD14+ cells were sorted and used in T cell suppression assay (E). In a similar experiment, human PBMC (n=3, healthy human donors), depleted of T cells and NK cells, were incubated with B cells from B-CLL patients. After 48h, CD14+ cells were sorted and used in T cell suppression assays (F). *P<0.05, **P<0.01, ***P<0.001.
To see whether similar education can also occur in human MDSC, healthy donor PBMC depleted of T cells and NK cells (but with or without CD19+B-cell depletion) were treated with conditioned medium (CM) from MDA-MB-231 breast cancer cell line, the procedure that induces the generation of tBreg-like cells. Then, myeloid cells were sort-purified and tested for suppression of human CD8+ T cells stimulated with anti-CD3/CD28 Abs. While myeloid cells strongly inhibited T cell proliferation if pretreated with CM in the presence of B cells (p=0.03, MDA), mock treated cells failed to do so (Mock, Fig. 4E and SFig. 5A,B). Importantly, if we treated PBMCs depleted of B cells, the cells failed to inhibit T cell proliferation (MDA-CD19, Fig. 4E and SFig. 5A,B). To further confirm the link between B cells and the induction of suppressive activity of MDSC, we co-cultured healthy donor PB myeloid cells (depleted of B, T and NK cells) with B cells from patients with B-CLL, which we previously reported either did (PS #154) or did not (PS #174) contain tBreg-like cells (17), for 2 days in the presence of GM-CSF. Then, myeloid cells were re-isolated and tested in T cell suppression assays. Unlike mock or PS #174 co-cultured cells, myeloid cells treated with PS #154 (which contained tBreg-like cells) strongly inhibited proliferation of T cells (p<0.03, Fig. 4F and SFig. 5C). Taken together, these results indicate that both murine and human cancer-exposed B cells/Bregs can evoke the regulatory function of MDSC.
tBregs educate MDSC by triggering TGFβ signaling
Given that murine and human tBregs convert metastasis-promoting FoxP3+Tregs by overexpressing TGFβ (16, 18) and the fact that theTgfβR2 deficiency in myeloid cells abrogates 4T1 cancer metastasis (21), we tested whether TGFβ can be involved in the education of MDSC. As murine tBregs and human tBreg-like cells generated after treatment with CM of MDA-MB-231 cells (16, 18), the B16/OC-associated B cells expressed elevated levels of TGFβ compared to naïve B cells (SFig. 6A). Thus, we tested the role of TGFβ in this process, by co-culturing tBregs with μMT-MDSC in the presence of TGFβ blocking or control Ab. Then MDSC (after removal of B cells) were tested in T cell suppression assay. The TGFβ neutralization during the education of MDSC significantly abrogated the ability of MDSC to inhibit T cell proliferation (p<0.03, Fig. 5A, SFig 3). To confirm this finding, we also co-cultured μMT-MDSC with tBregs in the presence of a specific TgfβR1 inhibitor SB431542. The inhibitor indeed abrogated expression of iNOS, NOX2, and Arg (SFig. 4A–C) and ROS production in MDSC (p<0.03, Fig. 5B and SFig. 6B). Importantly, upon transfer of the μMT-MDSC educated in the presence of SB431542 (after depletion of B cells and several washes with PBS to remove traces of the inhibitor) into μMT mice with 4T1 cancer, they yielded significantly fewer metastatic foci in the lungs than control tBreg-educated μMT-MDSC (p<0.03, Fig. 5C). Note: the fact that the residual metastasis after the transfer of TgfβR1-inactivated μMT-MDSC could be due to the fact that SB431542 only partially blocked ROS production in PMN-MDSC (Fig. 5B and SFig. 6B). To further verify the importance of TGFβ signaling, we also used MDSC from mice with TgfβR2 deficiency in myeloid cells (TgfβR2 KO). Although ROS/NO was reduced in TgfβR2 KO MDSC of mice with B16 melanoma (Fig. 4B,C) and 4T1 cancer (SFig. 6C) as in μMT-MDSC, tBregs failed to up regulate its expression as in μMT-MDSC (SFig. 6C). Importantly, unlike μMT-MDSC, tBregs failed to increase the suppressive activity of TgfβR2 KO MDSC on CD4+ and CD8+ T cells (p<0.03, Fig. 5D). Upon their transfer into μMT mice, they also supported the lung metastasis of 4T1 cancer cells less efficiently (p<0.03, Fig. 5E). In summary, these results confirmed in multiple independent experiments suggest that tBregs educate MDSC at least in part by targeting TgfβR1/2 axis. A similar mechanism appears to be utilized by human myeloid cells, as SB431542 also impaired their education with ex vivo-generated tBregs (Fig. 4E and SFig. 5B) and tBreg-like cells of B-CLL (Fig. 4F and SFig. 5C), significantly (p<0.03) reducing their ability to suppress T cell proliferation.
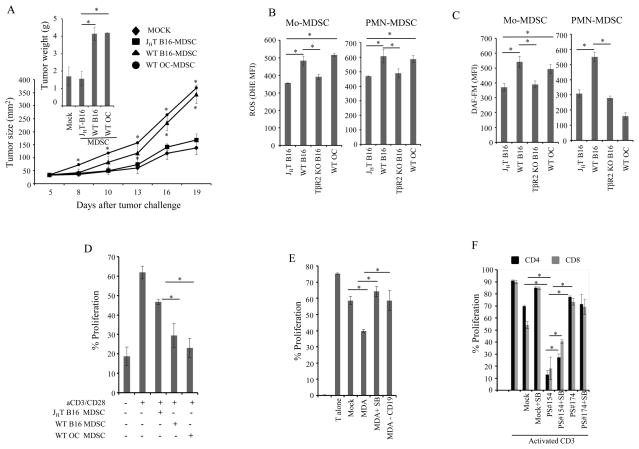
FIGURE 5. tBregs educate MDSC via TGFβ-TgfβR1/TgfβR2 axis.
4T1 cancer-bearing mouse μMT-MDSC (A–C) and TgfβR2 KO-MDSC (D,E) were stimulated in vitro with B-cont and tBregs with anti-TGFβ neutralizing antibody or mouse IgG (50 μg/ml) (A) in the presence or absence of SB431542 (20μM, SB, B and C). Then, MDSC were tested for T cell suppression as in Fig. 1G at indicated E:T ratio (A,D), or for expression of ROS (B), or induce metastases upon transfer into μMT mice (C,E). Prior to the transfer or use in suppression assays, MDSC were depleted of B cells. Y-axis shows DHE staining (MFI) in Mo-MDSC and PMN-MDSC (B), % T cell proliferation (gated in CD4+ and CD8+ T cells, A,D), and # of metastatic foci in the lungs of μMT mice (C,E) ± SEM in four-five mice per group experiments reproduced three times. (G) Summary: Tumor initiates an expansion of MDSC (1) and conversion of tBregs from naïve B cells (2). Cancer-activated B cells/tBregs use TGFβ (3) to induce TGFβ receptors on MDSC (4) and to up regulate ROS and NO production in MDSC. As a result, MDSC become fully suppressive for T cells and thereby pro-metastatic (5). *P<0.05, **P<0.01, ***P<0.001.
Discussion
Here, we attempted to mechanistically reconcile the issue raised in our previous studies questioning the importance of MDSC in metastasis. We repeatedly noticed that metastasis was blocked if B cells/tBregs were lost (16–19) despite the fact that cancer drastically expanded MDSC, the key facilitators of cancer escape and metastasis (1, 13). By comparing MDSC in WT BALB/c and C57BL/6 mice with their congenic B-cell deficient mice (μMT and JHT) with highly aggressive tumors, such as 4T1 cancer and B16 melanoma, we demonstrate that tumors only expand and prime the regulatory function of MDSC. As such, MDSC from tumor-bearing μMT and JHT less efficiently produced ROS/NO, inhibited proliferation of CD4+ and CD8+ T cells, and promoted tumor growth and metastasis. However, these impaired functions were completely reversed if the MDSC were briefly stimulated with B cells from WT mice with tumors (which contains tBregs and cancer-induced B cells), but not naïve mouse B cells. Similarly, using modeling studies we demonstrate that human cancer-induced B cells and tBreg-like cells from patients with B-CLL are also required in the activation of human MDSC. Thus, these results clearly indicate that the cancer-primed MDSC require help from tBregs/cancer-associated B cells to empower them fully regulatory and thereby pro-metastatic. Our data also uncouple the regulatory activity of MDSC from their expansion in response to cancer, suggesting that the expansion of MDSC per se is not a criterion of their regulatory and pro-metastatic activity. Although we can readily detect activated MDSC in various sites, such as PB, spleen, the lungs and tumor, the site of their encounter with tBregs remains unknown.
Activated B cells and Bregs are known to up regulate and use TGFβ, for example, in conversion of FoxP3+ T cells and modulation of macrophages (25, 26). Similarly, we recently reported that murine and human tBregs also convert FoxP3+ T cells by overexpressing TGFβ (16, 18). Here, using various complementary in vitro and in vivo modeling studies, we demonstrate that tBregs and cancer-induced B cells also utilize TGFβ-TgfβR1/2 axis in the activation (education) of both Mo and PMN subsets of cancer-expanded MDSC. First, the presence of TGFβ neutralizing, but not control Ab, was sufficient to inhibit the MDSC education in vitro. Second, when we blocked TgfβR1 signaling with a specific inhibitor SB431542 during the education of μMT-MDSC with tBregs, we failed to detect the up regulation of ROS production and suppression of T cells. As a result, these MDSC supported metastasis less efficiently in μMT mice upon their adoptive transfer. Similarly, the TgfβR1 inhibitor also blocked the education of human MDSC by tBregs induced by CM of MDA-MB-231 cells and tBreg-like cells of B-CLL patients. We also confirmed these results using MDSC from tumor-bearing mice with myeloid cells deficient in TgfβR2, which is required for the signaling of TgfβR1 (27). Unlike μMT-MDSC, tBregs failed to educate MDSC with TgfβR2 KO, as shown by the loss of up regulation of ROS production, T cell inhibition and ability to support metastasis upon transfer into μMT mice. Thus, these results unequivocally indicate that cancer-induced B cells and tBregs render the full regulatory function of MDSC by, at least, targeting their TgfβR1/TgfβR2 signaling axis. In support, others recently reported that 4T1 cancer also fails to metastasize in BALB/c mice deficient in TgfβR2 in myeloid cells (21), suggesting that this is due to the inability of their MDSC to get education from tBregs.
Our ex vivo studies with human MDSC and three murine tumor models tested so far, such as mice with 4T1 cancer, B16 melanoma and spontaneous OC, suggest that the MDSC education is a common feature of cancer-activated B cells/Bregs. As in 4T1 cancer model (where tBregs induce MDSC), the loss of mature B cells also impaired the regulatory and tumor-augmenting functions of MDSC in mice with B16 melanoma that does not generate tBregs. Moreover, unlike naïve mouse B cells that cannot educate MDSC nor promote tumor growth (17–19), B cells from mice with B16 melanoma and spontaneous OC not only induced regulatory activity of MDSC and reversed the retarded tumor growth in B-cell deficient mice, but also up regulated TGFβ as in tBregs (18). Similarly, B cells were required for the ex vivo generation of suppressive myeloid cells from the peripheral blood of healthy human donors, as their removal or the blockage of TgfβR1 signaling abrogated the generation of suppressive myeloid cells. Lastly, tBreg-like cells of B-CLL patients, but not B cells from patients without tBregs or from healthy donors, also induced the generation of suppressive myeloid cells utilizing TgfβR1 signaling.
In summary, our data indicate that the generation of regulatory MDSC is a two-step process (Fig. 5G). While drastically expanding MDSC, probably using GM-CSF, IL-1β, VEGF, TGFβ and other factors (1, 13, 28), cancer also activates their inducers, such as tBregs in mice with 4T1 cancer and OC (16, 17) or/and yet to be identified Bregs in mice with B16 melanoma. Naïve B cells do not educate MDSC. Although the nature and induction of cancer-induced B cells in mice with B16 melanoma is a focus of a separate study, we recently reported that 4T1 cancer directly induces the generation of tBregs from B2 cells via production of 5-lipoxygenase metabolites (19). The reasons behind the two-step activation process for MDSC remain unclear. This can be a way to limit the use of MDSC only at the sites of inflammation and metastasis, such as the tumors and lungs, via triggering the feedback loop induced by TGFβ and ROS/NO, as shown for other cells (29–31) where the activation of TGFβ and production ROS/H2O2/NO is mutually regulated. In concordance, μMT-MDSC became fully regulatory and pro-metastatic within a brief (<5h) encounter with tBregs. The education not only increased a number of surface molecules linked with regulatory MDSC (for example, IL-4Rα, TGFβ, and IL-10), but also up regulated surface expression of TgfβR-s presumably as a part of the feedback loop. Thus, the education may also bring additional factors that further augment the differentiation and regulatory function of MDSC, the focus of a different study. On the other hand, it is tempting to speculate that the early stage of cancer requires tBregs to support metastasis of 4T1 cancer via inducing metastasis-protecting FoxP3+Tregs (17), as we detect tBregs at least one week before the massive expansion of MDSC. As such, the loss of Tregs mostly blocks 4T1 cancer metastasis without affecting primary tumor growth (15, 17). At the later stage of cancer, tBregs appear to be needed to counteract the induction of antitumor myeloid cells (8) by switching and enhancing their regulatory function. Overall, the data presented here further underscore the importance of B cells in cancer (24, 32–36) by adding for the first time the education of MDSC to a growing list of pro-tumorigenic functions, such as the inhibition of cytotoxic CD8+ T and NK cells (37), Treg conversion (38) and M1-to-M2 macrophage polarization (39, 40). As such, B cells have to be targeted to enhance antitumor immune responses.
Supplementary Material
Acknowledgments
We are grateful Dr. Kathy Perdue (NIA/NIH) for the help with re-derivation of μMT mice, Dr. Charles Hesdorffer (VA, Washington, DC) for B-CLL cells, and Drs. Salman Tajuddin and Ilya Goldberg (NIA/NIH) for help with statistical analysis, Ana Lustig and Dr. Nicole N. Hooten (NIA/NIH) for critical reading our the manuscript. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH, and CRADA with Janssen Research Development program.
Footnotes
Conflict-of-interest disclosure: The authors declare no conflicting financial interests
Authorship
Contribution: M.B., C.L-C., K.M., R.W. and C.H.H. performed the research C.A.S-B, Y.A, M.L., L.Y., and G.T. contributed to vital new reagents; M.B. and A.B. designed the experiments, analyzed and interpreted the results; A.B. and M.B. wrote the paper; and A.B. conceived and supervised the research.
Supplementary Materials include S. Fig. 1–6.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer immunology, immunotherapy : CII. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serafini P, De SC, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer ImmunolImmunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 5.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. JImmunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. Journal of immunology. 2009;182:6562–8. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 7.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. Journal of cancer research and clinical oncology. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallberg E, Stenstrom M, Liberg D, Ivars F, Leanderson T. CD11b+Ly6C++Ly6G− cells show distinct function in mice with chronic inflammation or tumor burden. BMC Immunol. 2012;13:69. doi: 10.1186/1471-2172-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 10.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. Journal of immunology. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 11.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of immunology. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. JImmunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 14.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–70. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 15.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer research. 2013;73:2127–38. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. Journal of immunology. 2013;191:4141–51. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha. Journal of immunology. 2013;190:2575–84. doi: 10.4049/jimmunol.1201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman-Baust CA, Kuhn E, Valle BL, Shih Ie M, Kurman RJ, Wang TL, et al. A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development. The Journal of pathology. 2014;233:228–37. doi: 10.1002/path.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang Y, Gara SK, Achyut BR, Li Z, Yan HH, Day CP, et al. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer discovery. 2013;3:936–51. doi: 10.1158/2159-8290.CD-12-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai H, Dikalov S, Griendling KK, Harrison DG. Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods in molecular medicine. 2007;139:293–311. doi: 10.1007/978-1-59745-571-8_20. [DOI] [PubMed] [Google Scholar]
- 23.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. JExpMed. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Medicine. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 25.Bao Y, Cao X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. Journal of autoimmunity. 2014 doi: 10.1016/j.jaut.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Reyes JL, Wang A, Fernando MR, Graepel R, Leung G, van Rooijen N, et al. Splenic B cells from Hymenolepis diminuta-infected mice ameliorate colitis independent of T cells and via cooperation with macrophages. J Immunol. 2015;194:364–78. doi: 10.4049/jimmunol.1400738. [DOI] [PubMed] [Google Scholar]
- 27.Taylor AW. Review of the activation of TGF-beta in immunity. J Leukoc Biol. 2009;85:29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuPre SA, Redelman D, Hunter KW., Jr The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–60. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. American journal of physiology Lung cellular and molecular physiology. 2011;300:L295–304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem. 1999;274:33881–7. doi: 10.1074/jbc.274.48.33881. [DOI] [PubMed] [Google Scholar]
- 31.Ayache N, Boumediene K, Mathy-Hartert M, Reginster JY, Henrotin Y, Pujol JP. Expression of TGF-betas and their receptors is differentially modulated by reactive oxygen species and nitric oxide in human articular chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10:344–52. doi: 10.1053/joca.2001.0499. [DOI] [PubMed] [Google Scholar]
- 32.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–8. [PubMed] [Google Scholar]
- 33.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. Journal of immunology. 1978;121:359–62. [PubMed] [Google Scholar]
- 34.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer research. 2013;73:2468–79. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 37.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer research. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 38.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237:264–83. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 39.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. European journal of immunology. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 40.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.