Thromboembolism is central to atrial fibrillation (AF) related morbidity.1 The pathogenesis of intra-cardiac thrombus formation in AF is linked to each component of Virchow’s triad including atrial stasis, endothelial dysfunction, and a systemic hypercoagulable state.2 Although embolism of cardiac thrombi can involve any vascular territory, there has been a historical focus on cerebral embolism, an outcome associated with substantial disability and mortality.3 In contrast to the well-characterized risk and sequelae of cerebral embolism, much less is known regarding the clinical risk factors and outcomes associated with systemic embolic events (SEEs) in AF.1, 4–6
In this issue of Circulation, Bekwelem and colleagues improve our understanding of the epidemiology and prognostic implications of systemic embolism in AF.7 They retrospectively pooled data from four published randomized trials of anti-platelet or anticoagulant therapy in AF patients encompassing a total of 37,973 individuals (Table 1).8–11 The investigators re-adjudicated suspected SEEs using a harmonized classification scheme. An SEE was defined by both clinical and objective evidence of sudden loss of end-organ perfusion. They then examined the risks of both 30-day and long-term morbidity in relation to SEEs.
Table 1.
Baseline characteristics, therapeutic comparison, and embolic endpoints in included studies.
| ACTIVE-A8 (N=7,554) | ACTIVE-W11 (N=6,706) | AVERROES9 (N=5,599) | RE-LY10 (N=18,113) | |
|---|---|---|---|---|
| Therapeutic Comparison | Clopidogrel + ASA vs. ASA | Clopidogrel + ASA vs. VKA | Apixaban vs. ASA† | Dabigatran (110mg vs. 150mg)‡ vs. VKA |
| Baseline Characteristics | ||||
| Age | 71±10 | 70±9 | 70±10 | 71±9 |
| CHADS2 | 2.0±1.1 | 2.0±1.1 | 2.0±1.1 | 2.1±1.1 |
| Male gender | 58 | 66 | 59 | 64 |
| History of Stroke/TIA (%) | 13 | 15 | 14 | 20 |
| Peripheral arterial disease (%) | 3 | 4 | 3 | - |
| Endpoints | ||||
| Follow-up (median) | 3.6 yrs | 1.3 yrs | 1.1 yrs | 2.0 yrs |
| Stroke, all | 2.4 vs. 3.3%/yr | 2.4 vs. 1.4%/yr | 1.6 vs. 3.4%/yr | 1.4 vs. 1.0 vs. 1.6%/yr |
| Stroke, ischemic | 1.9 vs. 2.8%/yr | 2.2 vs. 1.0%/yr | 1.1 vs. 3.0%/yr | 1.3 vs. 0.9 vs. 1.2%/yr |
| SEE | 0.4 vs. 0.4%/yr | 0.4 vs. 0.1%/yr | 0.1 vs. 0.4%/yr | 0.1 vs. 0.1 vs. 0.1%/yr |
TIA, transient ischemic attack; SEE, systemic embolic event; VKA, vitamin K antagonist; AF, atrial fibrillation; ASA, aspirin; yrs, years. ACTIVE, The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events; AVERROES, Apixiban versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy. Continuous measures are reported as mean ± standard deviation.
ASA dose was >81mg in 35% of study patients.
Dabigatran dose was either 110mg or 150mg.
Overall, 221 SEEs occurred in 219 individuals during a mean follow-up of 2.4 years. The incidence of SEEs (0.24/100 person-years) was lower than cerebral embolism (1.92/100 person-years) and comprised 12% of clinically-recognized thromboembolic events. Anatomically, about 60% of SEEs involved the lower extremities, whereas about 30% occurred in the visceral-mesenteric system, and only 11% occurred in the upper extremities. Most patients underwent an invasive procedure as part of their clinical management. SEEs were associated with similar and significant 30-day mortality when compared to stroke alone (24% vs. 25%). SEEs were associated with about a 4-fold increased risk of long-term mortality, as compared to a nearly 7-fold increased risk of mortality associated with ischemic stroke.
This study has a number of strengths that distinguish it from prior literature. Collation of readily available, ethnically diverse patient cohorts yielded the largest reported assessment of AF-related SEEs to date. Careful endpoint ascertainment and independent re-adjudication of events with pre-specified diagnostic criteria enhance the validity of the results. Comprehensive clinical descriptions of SEEs, including anatomic distribution and diagnostic methodology, provide a detailed understanding of the different arterial beds commonly affected by peripheral embolism in AF.
The findings of Bekwelem et al.7 should be interpreted in the context of the cohorts included and study design. First, while the collated studies have similar baseline characteristics (Table 1) there was significant heterogeneity in therapeutic exposure (anticoagulant vs. anti-platelet) as well as relatively short follow-up time. Thus, the reported SEE rate represents a ‘blended’ rate among patients taking either anticoagulation or anti-platelet therapy and does not reflect the long-term ‘natural history’ of AF. Second, given the relatively low incidence of SEEs, the study was likely underpowered for several of the subgroup analyses presented, including age, sex, and location of peripheral embolism. Third, the reliance on ‘clinically-evident’ end-organ ischemia is practical though likely underestimates the ‘true’ incidence of systemic embolism, particularly to abdominal visceral organs which have a rich network of collateral circulation which may mitigate the ischemic insult of embolic phenomenon.1, 6 Fourth, some peripheral embolic events could reflect lower baseline vascular reserve (eg. related to prior SEEs, atherosclerotic burden, or anatomically smaller vessels) or more significant embolic burden. This may explain the identification of prior SEEs and peripheral arterial disease as risk factors for incident SEEs and may confound analyses of mortality.
Systemic Embolism in AF – Unique Events or Additional Embolic Destinations?
Should an SEE be regarded as a distinct AF outcome or yet another embolic destination? Given that the vast majority of patients with thromboembolism in this study experienced either stroke or an SEE alone (~97% of embolic events), one might speculate that patients are uniquely predisposed to either incident stroke or SEEs. The authors identify several clinical characteristics more prevalent in patients with incident SEEs compared to stroke including female gender and white ethnicity as well as a history of smoking, prior myocardial infarction, previous SEEs, and peripheral arterial disease. Female gender and SEEs have been linked previously4 and others have identified increasing age, severe left ventricular dysfunction and echocardiographic evidence of left atrial appendage thrombi as additional risk factors for SEEs compared to stroke in AF.12
The incidence and anatomic location associated with cardioembolism may also be related to other factors. Anatomic distribution is generally influenced by the character of arterial branching and the course of blood flow.1, 6 Increased thrombus size has been reported to favor peripheral rather than cerebral embolic destination.12 Limited histochemical comparison of central versus peripheral thrombi in AF is suggestive of differing biology with in situ thrombi comprised primarily of amorphous debris and fibrin in contrast to embolic thrombi comprised of fibrin and platelets.13 Regardless of whether patients are predisposed to specific embolic destinations, the contribution by Bekwelem and colleagues underscores the substantial morbidity associated with embolism of any kind.
Clinical and Therapeutic Implications of Identifying SEE Risk in AF
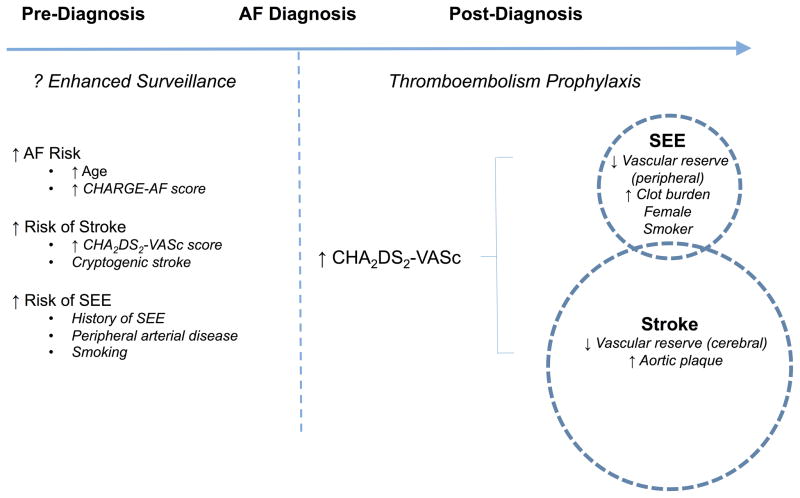
Given that several SEE risk factors (eg. female gender, peripheral vascular disease, prior myocardial infarction) are included in stroke prediction algorithms it is unlikely that the report by Bekwelem et al.7 will significantly modify the decision to initiate anticoagulation. Indeed, the CHA2DS2-VASc score demonstrated similar discrimination and reclassification of clinical outcomes when risk was defined either as stroke-alone or a composite of stroke and SEEs.14 Nevertheless, the study suggests that current efforts being considered for stroke prevention might have extended value for SEE prevention. For example, given the morbidity attributable to SEEs, should cardiac rhythm monitoring be implemented to identify subclinical AF in patients with SEE risk factors such as peripheral arterial disease (Figure 1)?15, 16 Such initiatives may be cost-effective for stroke prevention17 and are currently being evaluated.18–20 Similarly, would enhanced cardiac rhythm monitoring to detect AF alter management in patients with visceral embolism or peripheral ischemia without known AF, akin to efforts to identify a cause for cryptogenic stroke?21 At what expense would AF prevention prove cost-effective altogether for the reduction of embolic morbidity, if it were achievable? Practically, answering such questions may be challenging owing to the relatively low event rates and large sample sizes necessary. Therefore, at present it appears prudent to exercise increased vigilance for AF detection in patients either at high-risk for systemic embolic events or with unexplained peripheral ischemia that may be of embolic origin.
Figure 1.
Schematic representation of risk assessment in atrial fibrillation (AF). Pre-diagnosis, the role of enhanced surveillance may be considered for patients at increased risk of AF, stroke, and systemic embolic events (SEEs). The CHARGE-AF score (inclusive of age, race, height, weight, blood pressure, smoking status, use of anti-hypertensive medication, diabetes, history of myocardial infarction and heart failure) has demonstrated discrimination in the prediction of AF.15 Post-diagnosis, risk assessment and implementation of thromboembolism prophylaxis is often guided by the CHA2DS2-VASc algorithm.16 The circles display the relatively increased incidence of stroke as compared to SEE. Enclosed in the circles are risk factors that may selectively predispose to either stroke or SEE. AF, atrial fibrillation; CHARGE-AF, Cohorts for Heart and Aging Research in Genomic Epidemiology Atrial Fibrillation; SEE, systemic embolic event; LA, left atrial; LAA, left atrial appendage.
Bekwelem and colleagues have contributed significantly to our understanding of the spectrum of thromboembolic risk in patients with AF. Their report provides us with estimates of the incidence of SEEs, the arterial beds most commonly affected, and the morbidity associated with such events. By opening our eyes, these data help us “SEE” embolic risk more clearly. Ultimately such clarity will guide more effective application of therapies to stem the rising tide of AF22 and thromboembolic morbidity.
Footnotes
Disclosures: None.
References
- 1.Menke J, Luthje L, Kastrup A, Larsen J. Thromboembolism in atrial fibrillation. Am J Cardiol. 2010;105:502–10. doi: 10.1016/j.amjcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–66. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 3.Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A, Wolfe CD, Inzitari D. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32:392–8. doi: 10.1161/01.str.32.2.392. [DOI] [PubMed] [Google Scholar]
- 4.Frost L, Engholm G, Johnsen S, Moller H, Henneberg EW, Husted S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161:272–6. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]
- 5.Vohra R, Zahrani H, Lieberman DP. Factors affecting limb salvage and mortality in patients undergoing femoral embolectomy. J R Coll Surg Edinb. 1991;36:213–5. [PubMed] [Google Scholar]
- 6.Wasilewska M, Gosk-Bierska I. Thromboembolism associated with atrial fibrillation as a cause of limb and organ ischemia. Adv Clin Exp Med. 2013;22:865–73. [PubMed] [Google Scholar]
- 7.Bewelem WCS, Halperin JL, Adabag S, Duval S, Chrolavicius S, Pogue J, Ezekowitz MD, Eikelboom J, Wallentin L, Yusuf W, Hirsch A. Extracranial Systemic Embolic Events in Patients with Nonvalvular Atrial Fibrillation: Incidence, Risk Factors, and Outcomes. Circulation. 2015;132:XX–XXX. doi: 10.1161/CIRCULATIONAHA.114.013243. [DOI] [PubMed] [Google Scholar]
- 8.Investigators A, Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–78. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 10.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 11.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 12.McBane RD, Hodge DO, Wysokinski WE. Clinical and echocardiographic measures governing thromboembolism destination in atrial fibrillation. Thromb Haemost. 2008;99:951–5. doi: 10.1160/TH07-12-0734. [DOI] [PubMed] [Google Scholar]
- 13.Wysokinski WE, Owen WG, Fass DN, Patrzalek DD, Murphy L, McBane RD., 2nd Atrial fibrillation and thrombosis: immunohistochemical differences between in situ and embolized thrombi. Thromb Haemost. 2004;2:1637–44. doi: 10.1111/j.1538-7836.2004.00899.x. [DOI] [PubMed] [Google Scholar]
- 14.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 15.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 17.Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, Frykman-Kull V, Levin LA. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023–9. doi: 10.1093/europace/euv083. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed July 17, 2015];Detection of Subclinical Atrial Fibrillation in High Risk Patients Using Implantable Loop Recorder. at https://clinicaltrials.gov/ct2/show/NCT02041832.
- 19. [Accessed July 17, 2015];Prevalence of Sub-Clinical Atrial Fibrillation in Elderly Patients With Hypertension, Detected Using an External Loop Recorder (ASSERT-III) at https://clinicaltrials.gov/ct2/show/NCT02401854.
- 20.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–84. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 21.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, Investigators CA. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 22.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]