Abstract
Objective
Developmental dyslexia is presumed to arise from specific phonological impairments. However, an emerging theoretical framework suggests that phonological impairments may be symptoms stemming from an underlying dysfunction of procedural learning.
Method
We tested procedural learning in adults with dyslexia (n=15) and matched-controls (n=15) using two versions of the Weather Prediction Task: Feedback (FB) and Paired-associate (PA). In the FB-based task, participants learned associations between cues and outcomes initially by guessing and subsequently through feedback indicating the correctness of response. In the PA-based learning task, participants viewed the cue and its associated outcome simultaneously without overt response or feedback. In both versions, participants trained across 150 trials. Learning was assessed in a subsequent test without presentation of the outcome, or corrective feedback.
Results
The Dyslexia group exhibited impaired learning compared with the Control group on both the FB and PA versions of the weather prediction task.
Conclusions
The results indicate that the ability to learn by feedback is not selectively impaired in dyslexia. Rather it seems that the probabilistic nature of the task, shared by the FB and PA versions of the weather prediction task, hampers learning in those with dyslexia. Results are discussed in light of procedural learning impairments among participants with dyslexia.
Keywords: Developmental Dyslexia, Probabilistic Category Learning, Feedback, Paired-associate, Weather Prediction Task
Introduction
Developmental dyslexia is a specific developmental disorder in learning to read, which is not a direct result of impairments in general intelligence, gross neurological deficits, uncorrected visual or auditory problems, emotional disturbances or inadequate schooling (American Psychiatric Association, 2000). The usual symptoms of dyslexia are difficulties in reading, writing and spelling, and reading-related sub-skills such as deficits in word identification and phonological decoding (Vellutino, Fletcher, Snowling, & Scanlon, 2004). Despite decades of intensive research, the underlying biological and cognitive causes of dyslexia remain under debate (for a review see, Démonet, Taylor, & Chaix, 2004).
The phonological account has been one of the prominent theories guiding dyslexia research across four decades. By this account, dyslexia is presumed to arise from a deficit of direct access to, and manipulation of, phonemic language units retrieved from long-term declarative memory (Snowling, 2000). Indeed dyslexia is manifested in poor phonological awareness, impaired verbal short term memory, and slow lexical retrieval (Vellutino et al., 2004). However, an accumulating body of research is revealing substantial nonlinguistic deficits in those with dyslexia. Dyslexia has been found to be related to deficits in non-linguistic motor (Nicolson & Fawcett, 1994), procedural learning (Gabay, Schiff, & Vakil, 2012c; Howard, Howard, Japikse, & Eden, 2006; Stoodley, Harrison, & Stein, 2006) and attention skills (Facoetti, Paganoni, & Lorusso, 2000). These impairments are difficult to reconcile with a strictly phonological deficit and have led some to question the ability of the phonological account to serve as the sole explanatory framework of dyslexia (Nicolson & Fawcett, 2011; Stein & Walsh, 1997).
Procedural Learning Deficit in Dyslexia
An emerging perspective in dyslexia research is that a more general deficit, not specific to phonological processing, may underlie dyslexia. The hypothesis is that a selective impairment in procedural learning may result in the difficulties in phonology, reading, writing and spelling that characterize dyslexia (Specific Procedural Learning Deficit; SPLD; Nicolson & Fawcett, 2010; Nicolson & Fawcett, 2007; Nicolson & Fawcett, 2011).
Behavioral studies reveal evidence consistent with procedural learning system impairments among individuals with dyslexia. Much of this work has been carried out in the domain of motor behavior. For example, individuals with dyslexia are impaired in basic motor skills while performing an additional secondary task (Nicolson & Fawcett, 1990; Yap & van der Leij, 1994). Other studies reveal that individuals with dyslexia are impaired on motor adaptation (Brookes, Nicolson, & Fawcett, 2007) and implicit motor sequential learning tasks (Bennett, Romano, Howard, & Howard, 2008; Du & Kelly, 2012; Howard et al., 2006; Stoodley et al., 2006; Stoodley, Ray, Jack, & Stein, 2008; Vicari et al., 2005). Furthermore, procedural motor learning skills of individuals with dyslexia are less stable, more prone to interference (Gabay, Schiff, & Vakil, 2012b) and consolidate less effectively (Gabay, Schiff, & Vakil, 2012a). In contrast, recent studies suggest that declarative learning might be enhanced among individuals with dyslexia (Hedenius, Ullman, Alm, Jennische, & Persson, 2013).
These impairments are hypothesized to arise from disrupted processing in brain areas related to the procedural learning system (Nicolson & Fawcett, 2011). Evidence from neuropsychological and functional neuroimaging studies supports the distinction between task knowledge that is “declarative” (knowing what) and “procedural” (knowing how) (Cohen, Poldrack, & Eichenbaum, 1997) and suggests that the declarative system is sub-served in large part by the medial temporal lobe (especially the hippocampus), whereas neural substrates of the procedural system include the basal ganglia, the cerebellum, and motor-related areas (Cohen et al., 1997; Squire, 2004). In the traditional view, the procedural system has been mainly associated with the learning and formation of motor procedures. However, an accumulating body of evidence implicates this system in learning cognitive, perceptual, and linguistic skills. For example, procedural learning has been implicated in sequence formation (Goschke, Friederici, Kotz, & Van Kampen, 2001) probabilistic category learning (Shohamy, Myers, Onlaor, & Gluck, 2004), and perceptual categorization (Maddox & Ashby, 2004; Seger, 2008). It has also been closely tied to formation of grammar rules (Ullman, 2001). Furthermore, subcortical regions such as the basal ganglia have been shown to be involved in phonological processing (De Diego-Balaguer et al., 2008; Pickett, Kuniholm, Protopapas, Friedman, & Lieberman, 1998). In support of the possibility of impairment in the procedural learning system in dyslexia, several neuroimaging studies report cerebellar impairment in individuals with dyslexia (Nicolson et al., 1999; Rae et al., 1998). Recent research has found that the right cerebellum is the brain region that discriminates best between normal readers and individuals with dyslexia (Pernet, Poline, Demonet, & Rousselet, 2009). Other studies have observed atypical basal ganglia activity in those with dyslexia (Brunswick, McCrory, Price, Frith, & Frith, 1999; Kita et al., 2013; Paulesu et al., 1996).
Despite this evidence, the detailed nature of procedural learning impairment in dyslexia is not yet well established. In the present study, we capitalize on probabilistic category learning, which has been widely used in neuropsychological research of procedural learning impairments (Knowlton, Squire, & Gluck, 1994; Knowlton, Squire, Paulsen, Swerdlow, & Swenson, 1996; Shohamy et al., 2004) to examine procedural learning among adults with dyslexia and matched controls. Using a within-participants design, we manipulate the task demands of probabilistic category learning to examine specifically whether individuals with dyslexia are impaired in learning probabilistic cue-outcome relationships and whether the availability of corrective feedback impacts procedural learning among individuals with dyslexia.
Probabilistic Category Learning
Probabilistic category learning is believed to involve procedural learning (Knowlton, Mangels, & Squire, 1996). In probabilistic category learning tasks, participants learn to classify multi-featured stimuli into one of two categories based on trial-by-trial corrective feedback. In a common version of this task, known as the “weather prediction” task, the cover story involves predicting an outcome, the weather, based on cues conveyed by a set of geometric features presented on four individual cards presented in all possible combinations. An important aspect of the weather prediction task is its probabilistic nature. Namely, the probabilistic relationships between cues and outcomes make it counterproductive for participants to attempt to recall specific previous trials because repetition of any particular configuration of the cues may lead to different outcomes (Eichenbaum, 2010). Declarative memorization is a less useful strategy in the weather prediction task because of this probabilistic relationship between cues and outcomes. Instead, the probabilities associated with particular cues and combinations of cues, acquired gradually across trials much as habits or skills are acquired, are most predictive of outcome. Knowlton et al. demonstrated that people with amnesia due to damage to the medial temporal lobe exhibit intact learning on the weather prediction task, although their declarative knowledge about the learning situation is impaired. On the other hand, patients with basal ganglia disorders such as Parkinson's and Huntington's disease are impaired in learning in the weather prediction task (Knowlton, Squire, et al., 1996; Shohamy et al., 2004). This dissociation suggests the significance of the so-called procedural learning system (including basal ganglia) for probabilistic category learning.
Since Knowlton et al.'s (1996) seminal findings, many studies have investigated the nature of probabilistic category learning among typical individuals and among those with neurological impairments. Across this literature, there is an acknowledgement that both the probabilistic nature of cue-outcome relationships (Knowlton, Mangels, et al., 1996) and the presence of experimenter-provided feedback, which is typically provided after each classification response, could affect procedural learning in the weather prediction task. In an effort to dissociate these factors, Shohamy et al. (2004) devised two variants of the weather prediction task. A Feedback-based (FB) task mirrored the typical weather prediction task. In this variant, participants initially guess about the relationship between the probabilistic cues and the outcome and subsequently learn from experimenter-provided feedback about the correct outcome that is signaled by the probabilistic cues. This corrective feedback is eliminated in a Paired Associate (PA) variant of the weather prediction task. In this task, participants view a cue and its outcome simultaneously and learning proceeds through observation. In the PA version of the weather prediction task no response is required, except to press a key to advance to the next trial. These two variants of the weather prediction task share the demand to learn outcomes signaled by a set of probabilistic cues. They differ in whether learning takes place by feedback (FB task) or by observation (PA task). Patients with Parkinson's and Huntington disease are impaired on the FB variant of the weather prediction task, but not on the PA variant (Holl, Wilkinson, Tabrizi, Painold, & Jahanshahi, 2012; Shohamy et al., 2004). This suggests that the ability to learn by corrective feedback is selectively impaired in these diseases (but see Wilkinson, Lagnado, Quallo, & Jahanshahi, 2008, for contradicting results).
More generally and relevant to the present aims, the distinct task demands of the two weather prediction task variants present the possibility of a closer examination of procedural learning dysfunction in dyslexia (Nicolson & Fawcett, 2010). Thus, in the current study we examine probabilistic category learning in the FB and PA versions of the weather prediction task among dyslexic adults and age- and cognitive-ability matched control participants with normal reading. Our aim is to inform the nature of procedural learning deficits in dyslexia. If the ability to learn from feedback in this procedural learning task is selectively impaired in dyslexia, then we expect selective disruption of probabilistic category learning in the FB variant of the weather prediction task and intact learning in the PA variant. However, each task involves learning across probabilistic input. Therefore, if the procedural learning impairment in dyslexia is characterized by difficulty in learning across probabilistic input, then we expect poorer learning, relative to controls, among individuals with dyslexia on both task variants. If probabilistic category learning is unimpaired among dyslexic participants, then performance for dyslexic and control participants should not differ.
Methods
Participants
Fifteen participants with developmental dyslexia and a matched control group participated in the study for a total of 30 participants. All were university students in the area of Pittsburgh, PA. All participants were native English speakers with no reported signs of sensory or neurological deficits and came from families with middle to high socioeconomic status. Diagnosis of a comorbid learning disability such as ADHD was an exclusion criterion; two participants with dyslexia who had severe symptoms and a diagnosis of ADHD were excluded from the sample. One participant with dyslexia had a diagnosis of ADD; her data were included in the sample because her main symptoms were in the reading domain (see General Discussion for further details). A well-documented history of dyslexia was the inclusion criterion for the dyslexia group: 1) each individual received a formal diagnosis of dyslexia by a qualified psychologist; 2) each individual's diagnosis was verified by the diagnostic and therapeutic center at their university. As a group, dyslexic individuals differed significantly from matched controls on all literacy measures. The Control group was age matched with the Dyslexia group, with no reading problems and the same level of cognitive ability (as measured by the Raven's Standard Progressive Matrices (SPM) test; Raven, 1992). Written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of Carnegie Mellon University and it was conducted in accordance with the Declaration of Helsinki.
All participants underwent a series of cognitive tests to evaluate general intelligence (as measured by the Raven's SPM tests, verbal working memory (as measured by the forward and backward Digit Span from the Wechsler Adult Intelligence Scale-III; Wechsler, 1997), rapid naming (Wolf & Denckla, 2005) and phonological awareness (Spoonerism; Brunswick et al., 1999). In addition, the Dyslexia and Control groups performed both un-timed and timed (fluency) tests of word reading and decoding skills. Participants performed the Word Identification (WI) and Word Attack (WA) subtests form the Woodcock Reading Mastery Test-Revised (WRMT-R; Woodcock, 1987). In addition, participants performed the Sight Word Efficiency, Forms A+B (i.e., rate of word identification) and Phonemic Decoding Efficiency, Forms A+B (i.e., rate of decoding pseudo words) subtests form the Test of Word Reading Efficiency (TOWRE-II; Torgesen, Wagner, & Rashotte, 1999). Details about these standardized tasks are presented in Table I. Results are shown in Table II.
Table I.
Psychometric Tests
| The following tests were administered according to the test manual instructions: |
| 1. Raven's Standard Progressive Matrices test (Raven, Court & Raven, 1992) – Non-verbal intelligence was assessed by the Raven's-SPM test. This task requires participants to choose the item from the bottom of the figure that would complete the pattern at the top. The maximum raw score is 60. Test reliability coefficient is .9 |
| 2. Digit Span from the Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1997) - In this task participants are required to recall the names of the digits presented auditorily in the order they appeared with a maximum of total raw score 28. Task administration is discontinued after a failure to recall two trials with a similar length of digits. Test reliability coefficient is .9 |
| 3. Rapid Automatized Naming (Denkla, & Rudel, 1976) - The tasks require oral naming of rows of visually-presented exemplars drawn from a constant category (RAN colors, RAN categories, RAN numerals, and RAN letters). It requires not only the retrieval of a familiar phonological code for each stimulus, but also coordination of phonological and visual (color) or orthographic (alphanumeric) information quickly in time. The reliability coefficient of these tests ranging between .98 to .99. |
| 4. Woodcock Reading Mastery Test Word Identification and Word Attack subtests (Woodcock & Johnson, 1990). The Word Identification subtest measures participants’ ability to accurately pronounce printed English words, ranging from high to low frequency of word occurrence with a maximum of total raw score 106. Test reliability coefficient is .97. The Word Attack subtest assesses participants’ ability to read pronounceable nonwords varying in complexity with a maximum total raw score of 45. Test reliability coefficient is .87. Task administration is discontinued when 6 consecutive words are read incorrectly. |
| 5. Sight Word Efficiency (i.e., rate of word identification) and Phonemic Decoding Efficiency, (i.e., rate of decoding pseudowords) subtests from the Test of Word Reading Efficiency (TOWRE-II; Torgesen et al., 1999) were used to measure reading rate. The test contains two timed measures of real word reading and pseudo word decoding. Participants are required to read the words aloud as quickly and accurately as possible. The score reflects the total number of words/nonwords read correctly in a fixed 45-s interval. Task administration is discontinued after 45 seconds. Sight word efficiency maximum raw score is 108. Phonemic decoding efficiency maximum raw core is 65. Test-retest reliability coefficients for these subtests are .91 and .90 respectively. |
| 6. Spoonerism Test (adapted from Brunswick et al., 1999) - This test assesses the participants’ ability to segment single syllable words and then to synthesize the segments to provide new words. For example, the word pair “Basket Lemon” become “Lasket Bemon”. The maximum raw score is 12. |
Table II.
Demographic and Psychometric Data of Dyslexia and Control Groups
| Group | ||||||
|---|---|---|---|---|---|---|
| Measure | Dyslexia Mean (SD) | Range | Control Mean (SD) | Range | P | Cohen's d |
| Age (in years) | 21.26 (3.64) | 18-30 | 21.6 (2.94) | 18-30 | n.s. | .1 |
| Raven's SPM | 79.4 (17.51) | 45-95 | 85.06 (13.08) | 50-95 | n.s. | .5 |
| Digit spana (combined) | 10.73 (2.54) | 1-12 | 14 (2.56) | 4-12 | < .01 | .9 |
| RAN objectsa | 104.4 (19.22) | 74-129 | 117.53 (10.73) | 93-133 | < .05 | .8 |
| RAN colorsa | 99.53 (13.3) | 80-124 | 112.4 (6.36) | 101-124 | < .01 | 1.2 |
| RAN numbersa | 103.6 (5.5) | 95-113 | 114.13 (4.24) | 107-120 | < .01 | 1.6 |
| RAN lettersa | 102.92 (6.21) | 85-111 | 112.53 (4.37) | 105-117 | < .01 | 1.9 |
| WRMT-R WIa | 96.26 (3.53) | 91-103 | 101.85 (2.58) | 95-105 | < .01 | 1.6 |
| WRMT-R WAa | 99.4 (5.36) | 92-113 | 112 (7.79) | 100-126 | < .01 | 1.9 |
| TOWRE SA (A+B)a | 97.66 (8.28) | 87-115 | 117 (12.68) | 100-137 | < .01 | 1.88 |
| TOWRE PD (A+B)a | 90.933 (9.19) | 72-112 | 113.2 (8.86) | 100-127 | < .01 | 2.7 |
| Spoonerism time | 136.44 (41.84) | 82-224 | 90.46 (26.06) | 63-150 | < .01 | .6 |
| Spoonerism accuracy | 8.466 (3.37) | 1-12 | 11.06 (2.21) | 4-12 | < .05 | .9 |
Standard scores (whereby smaller numbers are expected for dyslexic group), other scores are raw scores. Raven scores are presented in percentiles.
Groups did not differ according to age or intelligence. However, the Dyslexia group differed significantly from the Control group on word reading and decoding skills across both rate and accuracy measures. In addition, the Dyslexia group was impaired compared with the control group in three major phonological domains: phonological awareness (Spoonerisms), verbal short-term memory (digit span) and rapid naming (rapid automatized naming).
It is noted that all participants in the Dyslexia group were high functioning university students with dyslexia. Prior studies dyslexia reveal that such participants exhibit average performance on standardized reading tests (including that of low frequency words such as word identification from the Woodcock Reading Mastery Test- Revised) but nevertheless differ significantly from matched control groups and continue to present phonological problems that can be assessed by phonological tests such as the Spoonerism test (Wilson & Lesaux, 2001). Our dyslexic participants fit this profile. Each individual had received a former diagnosis of dyslexia by a qualified psychologist. The Dyslexia group differed significantly from the Control group in all literacy measures and exhibited phonological processing impairments (as indicted by the Spoonerism test), despite average performance on standardized tests. This profile is clearly indicative of a sample of dyslexic adults.
Apparatus and materials
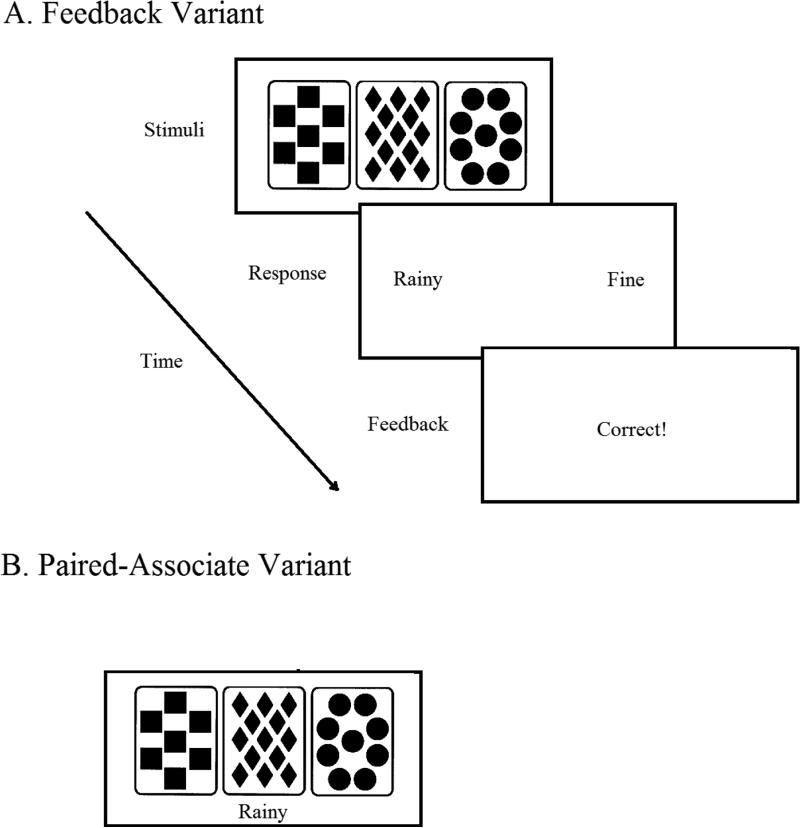
Testing took place in a sound-attenuated chamber with participants seated directly in front of a computer monitor during the entire experiment. Stimulus presentation and the recording of response time and accuracy were controlled by a computer program (E-PRIME; Schneider, Eschman, Zuccolotto, & Guide, 2002). The stimulus material and card arrangements were similar to that used in the study of Holl et al. (2012) and were created from a set of four tarot cards, each with a different geometric pattern (e.g., triangles, circles, diamonds and squares), arranged horizontally across the middle of the computer screen in black against a white background. See Figure 1.
Figure 1.
Schematic illustration of the stimuli and tasks.
Each version of the weather prediction task (FB or PA) included 150 trials during the training phase. On each training trial, participants saw a particular arrangement of cards composed of one, two or three of the four possible tarot cards. Four-card and no-card arrangements were not used; as such, the experiment included 14 possible card arrangements. Each arrangement was associated with one of the two weather outcomes (Rainy or Fine). Overall, outcomes were presented with equal frequency. Each individual card was associated with a particular outcome with a fixed, independent probability. The probability assigned to each card was counterbalanced and the probability of an outcome on a particular trial was based on the combined probability of the presented cards (see Table III). Two cards were predictive of fine weather: one strongly (card 4), one weakly (card 3). Two cards were predictive of rainy weather: one strongly (card 1), one weakly (card 2). Overall, participants experienced similar card arrangements, but due to the probabilistic nature of the task the actual outcomes could differ slightly across participants.
Table III.
Probability Structure of the Task
| Cue | P (cue combination) | ||||||
|---|---|---|---|---|---|---|---|
| Pattern | 1 | 2 | 3 | 4 | P (pattern) | Frequency (No. per 200 trials) | P (outcome) |
| A | 0 | 0 | 0 | 1 | 0.0095 | 19 | 0.89 |
| B | 0 | 0 | 1 | 0 | 0.045 | 9 | 0.78 |
| C | 0 | 0 | 1 | 1 | 0.130 | 26 | 0.92 |
| D | 0 | 1 | 0 | 0 | 0.045 | 9 | 0.22 |
| E | 0 | 1 | 1 | 1 | 0.060 | 12 | 0.83 |
| F | 0 | 1 | 1 | 0 | 0.030 | 6 | 0.50 |
| G | 0 | 1 | 1 | 0 | 0.095 | 19 | 0.89 |
| H | 1 | 0 | 0 | 0 | 0.095 | 19 | 0.11 |
| I | 1 | 0 | 0 | 1 | 0.030 | 6 | 0.50 |
| J | 1 | 0 | 1 | 0 | 0.060 | 12 | 0.17 |
| K | 1 | 0 | 1 | 1 | 0.045 | 9 | 0.55 |
| L | 1 | 1 | 0 | 1 | 0.130 | 26 | 0.08 |
| M | 1 | 1 | 0 | 1 | 0.045 | 9 | 0.44 |
| N | 1 | 1 | 1 | 0 | 0.095 | 19 | 0.11 |
| Total | 1.00 | 200 | |||||
On any trial, one of 14 possible combinations of four cues could appear with the probability indicated [P(pattern)]. Each combination of cues predicted one outcome with the probability P(outcome) and predicted the other outcome with a probability of [1–P(outcome)].
Each participant completed the weather prediction task under two different conditions (FB, PA). Thus, two parallel versions of the weather prediction task were employed with different types of cards and different binary outcomes: either Rainy and Fine or Cold and Hot. For half of the participants in each group, Rainy/Fine were the two possible outcomes in the FB condition and Cold/Hot were the outcomes in the PA condition. The remaining participants experienced the reversed pairing. In addition to the set of cards defined by the arrangement of triangles, circles, diamonds and squares, three additional sets of the four tarot cards were also employed during the experiment, with 25% of participants in each group being trained on each set per weather prediction task variant (FB vs. PA) and with the constraint that participants were trained on a different set of cards in each condition.
Procedure
The procedure was similar to that of Holl et al. (2012). Participants performed both the FB and PA tasks one after the other. Task order was counterbalanced across participants.
Weather Prediction Task – FB variant
The training phase consisted of three blocks of 50 trials. On each trial participants saw an arrangement of cards and made a response to predict the weather (Rainy/Fine or Hot/Cold). Feedback appeared immediately after a response, with a written indication presented on the screen to convey whether the weather prediction was correct or incorrect. Participants then requested the next trial with a key press; hence, the task was self-paced. The test phase comprised a further 42 trials with the same structure. On these self-paced trials participants predicted the weather but did not receive feedback.
Weather Prediction Task – PA variant
The training phase consisted of three blocks of 50 trials. On each trial participants saw an arrangement of cards along with its weather outcome (Rainy/Fine or Hot/Cold). No classification response was required. Participants then requested the next trial, eliciting the appearance of the next card arrangement, along with its weather outcome; hence, the task was self-paced. The test phase was identical to the test phase in the FB version.
Awareness tests
Both FB and PA tasks were followed by tests of awareness. Lagnado, Newell, Kahan, and Shanks (2006) differentiate between participants’ insight into the structure of the task (Task-Knowledge) and participants’ insight into their own judgmental processes (Self-Insight). Importantly, the two types of awareness do not necessarily agree. A participant may have an incorrect model of the task, but an accurate model of her own judgments.
Task Knowledge
Participants rated how related each card was to the weather outcome using a continuous scale ranging from 0 to 100 (e.g., 0 =definitely rainy, 50 = could be either rainy or fine, and 100 = definitely fine). After participants made a vocal response the experimenter typed the response on the keyboard.
Self-Insight
Participants then indicated how important each card was for their weather predictions by rating its importance along a continuous scale ranging from 0 to 100, with 0=not important at all, 50=moderately important, 100=very important. The experimenter typed the participant's vocal response on the keyboard.
Results
FB vs. PA, Test Phase
We first compared the accuracy of the two groups during the test phase of the FB and PA tasks. Following prior studies using the weather prediction task, the correct answer was determined according to the most probable outcome (Gluck, Shohamy, & Myers, 2002).
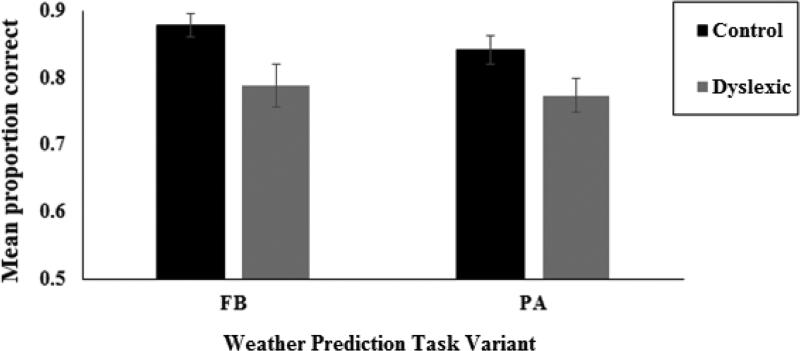
Preliminary analysis revealed that the order in which the two tasks were performed did not interact with the group variable (F<1). Therefore further analyses collapsed data across order. An analysis of variance (ANOVA) was conducted with Task (FB vs. PA) as a within subject factor and Group (Dyslexia vs. Control) as a between-subjects factor and mean proportion correct weather predictions during the test phase as the dependent variable. Results are presented in Figure 2. The main effect of Group was significant, F(1, 28) = 7.51 p = .011, ηp2 = .204., indicating that test-phase accuracy of the Dyslexia group (Mean = .78, SE = .02) was poorer than that of the Control group (Mean = .86, SE = .01). There was no main effect of Task, F(1, 28) = 1.72, p = .203, ηp2 =.054 and no Task by Group interaction, F (1, 28) = .343, p = .563, ηp2 =.16. Overall, this indicates an impairment of the Dyslexia group relative to the Control group on probabilistic category learning. Moreover, the degree of impairment relative to age- and cognitive-ability matched Control participants was statistically equivalent across FB and PA versions of the weather prediction task.
Figure 2.
Learning performance measured by mean proportion correct weather prediction accuracy during FB and PA tests of the Weather Prediction Task for the Dyslexia and Control groups. Error bars represent standard errors.
FB-based learning across training-trial blocks
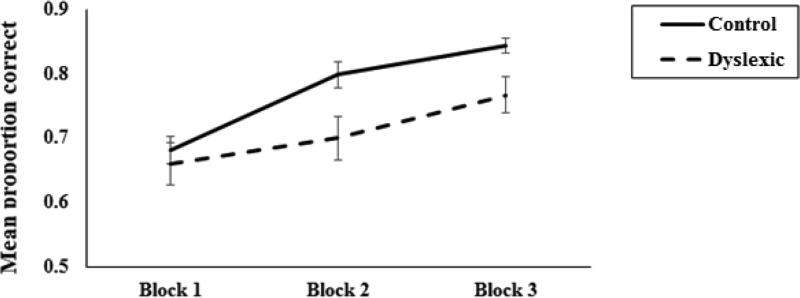
We also compared the learning curve of the Dyslexia and Control groups on the FB task. (Note that the learning curve for the PA could not be evaluated because learning took place via observation and no response was required during training). An analysis of variance (ANOVA) was conducted with Block (trials 1-50, 51-100, 101-150) as a within-subject factor and Group (Dyslexia vs. Control) as a between-subjects factor and mean proportion correct weather predictions during the learning phase as the dependent variable. Results are presented in Figure 3. There was a significant main effect of Group, F(1, 28) = 4.6, p = .0461, ηp2 = .13. The Dyslexia group was significantly less accurate (Mean = .71, SE = .02) compared to the Control group (Mean = .77, SE = .01) in response to the training trials on the FB version of the weather prediction task. There was a significant main effect of Block, F(2, 56) = 33.10, p = .001, ηp2 = .54, indicating that participants improved at predicting the weather across trials. The Group by Block interaction was marginally significant, F(2, 56) = 2.92, p = .062, ηp2 = .08.
Figure 3.
Learning performance measured by mean proportion correct weather predictions during training of the FB task for the Dyslexia and Control groups. Error bars represent standard errors.
We conducted a further analysis to assure that this marginally significant interaction did not suggest that the observed main effect of group arose from a fundamental difference in the baseline performance of Dyslexia versus Control group participants instead of a difference in learning across training. The analysis focused on performance on the first 50 training trials in the FB-version of the weather prediction task across groups. An analysis of variance (ANOVA) was conducted with the first 50 trials binned into 10-trial sets (1-5) as a within subject factor and Group (Dyslexia vs. Control) as a between-subjects factor and mean proportion correct weather predictions across the first five sets of 10 trials (1-10, 11-20, 21-40, 41-50) of the FB weather prediction task as the dependent variable. There was marginally significant main effect for the 10-trial sets, F(4, 112)=2.44, p=.0507, ηp2= .07, consistent with modest improvement across these 50 trials. Of most importance, there were no interactions with Group, F (4, 112) = .444, p =.775, ηp2= .015 and the main effect of group was non-significant, F (1, 28) = .064, p = .801, ηp2= 08. This reassures that the omnibus group main effect across the entire set of training trials was not driven by an a priori group difference instead of a difference in learning within the probabilistic category learning task.
Analysis of Response Strategy
In order to examine if the two experimental groups used different strategies while performing the feedback variant of the weather prediction task (in the PA version there was no manual response during learning phase, so strategies cannot be assessed) we followed the analysis of Gluck et al. (2002). We examined which of three possible strategies accounts best for participants’ responses: (1) an optimal multi-cue strategy, in which participants respond to each pattern on the basis of associations of all four cues with each outcome; (2) a one-cue strategy, in which participants respond on the basis of presence or absence of a single cue, disregarding all other cues; or (3) a singleton strategy, in which participants learn only about the four patterns that have only one cue present and all others absent. A non-parametric χ2 analysis indicated no significant group differences in the number of participants optimally assigned to each strategy (χ2 (1) = 0, p = 1, χ2 (1) = 1.3, p = .24; χ2 (1) = 0, p = 1; for the multi-cue strategy, one-cue strategy and singleton strategies, respectively). Thus, there were no significant differences between the groups in preferred response strategy in the FB variant of the task.
Awareness: Task-knowledge
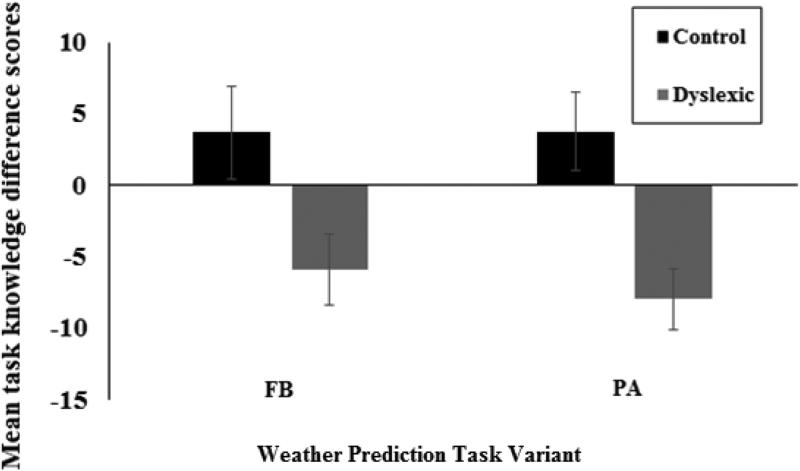
Mean task knowledge difference scores were calculated across the four cards for each participant. A difference score was calculated for each card following the approach of Newell, Lagnado, and Shanks (2007). This was calculated as the actual probability of the negative outcome (.2, .4, .6, .8, for cards 1-4 respectively) subtracted from a participant's own subjective probability estimate. A positive score is indicative of probability overestimates whereas a negative score is indicative of probability underestimation. Preliminary analysis revealed no significant main effects or interactions with the order in which the task-knowledge tasks were performed across FB and PA tasks (minimum p = .168). Therefore, the data were collapsed across task presentation order. An analysis of variance (ANOVA) was conducted on the mean difference scores with Task (FB vs. PA) as a within-subject factor and Group (Dyslexia vs. Control) and as a between-subjects factor. Figure 4 presents task knowledge difference scores for FB and PA tasks for each group. Overall, there was a significant main effect of Group, F(1, 27) = 8.51, p =.003, ηp2 = .233. This effect was not modulated by card strength. Task knowledge of the Dyslexia group on both the FB (t(14) = −2.43, p < .05) and PA (t(14) = −3.92, p < .01) tasks differed significantly from zero whereas task knowledge of the Control group did not differ from zero for either the FB (t(14) = .86, p = .403) or the PA t(14) = 1.37, p = .18) task. This pattern of results indicates that the Control group was accurate at determining probabilities whereas the Dyslexia group significantly underestimated the actual probabilities. All other effects were non-significant (F<1).
Figure 4.
(a) Mean task knowledge difference scores for the Dyslexia and Control groups. Error bars represent standard errors.
Awareness: Self-Insight
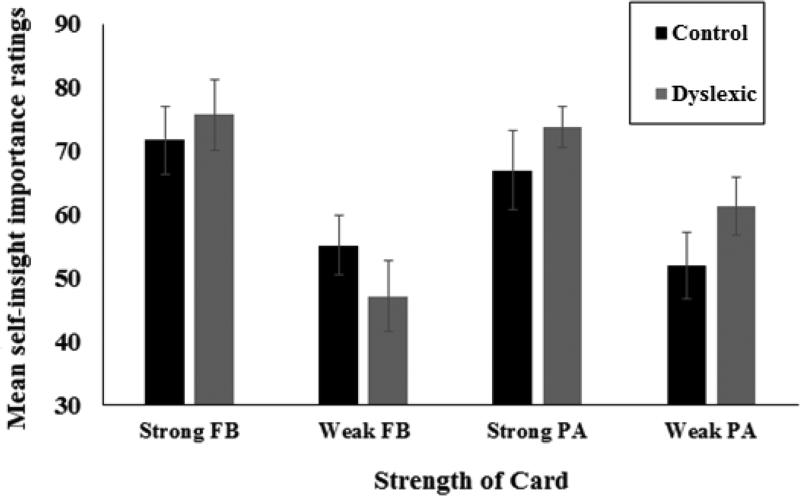
The main test of self-insight awareness is whether participants’ ratings discriminate between strongly and weakly predictive cards. Ratings for two strongly predictive cards (cards 1, 4) were combined and ratings for the two weakly predictive cards (cards 2, 3) were combined. Preliminary analysis revealed no significant main effects or interactions with the order in which the self-insight tasks were performed across FB and PA tasks (minimum p = .127). The results, therefore, were analyzed across order. An analysis of variance (ANOVA) was conducted on ratings, with Task (FB vs. PA) and Strength of association between card and outcome (Strong vs. Weak) as within-subject factors and Group (Dyslexia vs. Control) as a between-subjects factor. Figure 5 presents participant's ratings for strongly and weakly predictive cards for the FB and PA tasks for each group. There was a significant main effect for card strength, F(1, 27) = 47.54 , p = .000, ηp2 = .14, indicating that participants gave higher importance ratings to strong cards compared to weak cards. There was also a marginally significant interaction of Strength of association and Task, F(1, 27) = 3.81, p = .061, ηp2 = .11, such that the tendency to rate strong cards was greater in the FB task compared with the PA task. All other effects were non-significant. There were no significant Group differences.
Figure 5.
Mean self-insight ratings for strong and weak cards for Dyslexia and Control groups. Error bars represent standard errors.
General Discussion
To the best of our knowledge the present study is the first observe impairments in probabilistic category learning among participants with dyslexia. We examined two versions of the weather prediction task that shared the probabilistic association of cues and outcomes, but differed in whether learning proceeded via explicit feedback (FB version) or through observation of cues and their outcomes (PA version) among a group of adults with dyslexia and matched controls. In other domains, the FB and PA versions of the weather prediction task have served to examine the task characteristics that engage procedural learning (Knowlton et al., 1994; Knowlton, Squire, et al., 1996; Shohamy et al., 2004). Both versions of the weather prediction task rely on probabilistic relationships between cues and outcomes. They key difference between the PA and FB versions of the WPT is whether learning takes place via observation (PA) or corrective feedback (FB), but each task requires learning across probabilistic cue-outcome relationships. Comparison of categorization accuracy at test revealed that dyslexic participants learned significantly less than age- and cognitive-ability matched controls in both the FB and PA versions of the weather prediction task. In the FB task, for which responses were gathered during training trials, it was possible to observe impaired performance among participants with dyslexia during the learning phase.
We observed no dissociation of impairment across the FB and PA versions of the weather prediction task among the dyslexic participants in the present study. This suggests that poorer learning among dyslexic participants relative to controls was related to a task characteristic common to FB and PA versions of the weather prediction task: learning across probabilistic cues. Individuals with dyslexia are not specifically impaired in learning from feedback, but rather have difficulty in learning relationships across probabilistic input. In fact, evidence from other procedural learning tasks such as the serial reaction time task supports this possibility; dyslexic learners are significantly impaired in learning probabilistic sequences (Du & Kelly, 2013; Howard et al., 2006), but not deterministic ones (Deroost et al., 2010; Rüsseler, Gerth, & Münte, 2006). Identification of procedural learning impairments in such high functioning participants indicates the involvement and centrality of procedural learning impairments in the etiology of dyslexia. Further evidence will be necessary to determine whether these impairments extend to younger samples of dyslexics and those with more severe impairments. In all, these results indicate impairment in probabilistic category learning among participants with dyslexia that is not a result of a selective deficit of feedback-based learning, since poorer learning relative to control participants was observed across both FB and PA tasks. Future studies should explore the possibility that additional learning trials could bridge the learning gap as the dyslexia group was able to reach to a relatively high level of accuracy on each task at the end of training.
The pattern of awareness rankings for dyslexic and control participants is interesting in this regard. At the end of learning, the Control group exhibited explicit task knowledge, as observed in fairly accurate estimates of how well the cues predicted weather outcomes. This is consistent with previous reports that unimpaired participants have good explicit knowledge of the probabilistic relationships learned in the weather prediction task (Holl et al., 2012). However, the dyslexic participants were significantly less accurate in task knowledge, tending to underestimate the probability that particular cues predicted the associated outcomes. This is particularly interesting in light of the fact that dyslexic participants did not differ significantly from the Control group in explicit rankings of strongly versus weakly predictive cards in the Self-Insight awareness task. Both groups rated strongly predictive cards as the more important predictors. The Dyslexia group thus accurately ranked the cues according to the predictive value, but nonetheless underestimated how relevant the cue was to predicting the outcome.
The current results are consistent with procedural learning impairments in dyslexia and raise the possibility that probabilistic relations present a particular learning challenge for those with dyslexia. A general impairment in probabilistic learning, not specific to language, may have cascading effects on how language is processed among those with dyslexia because language acquisition and subsequent processing rely heavily on probabilistic mappings from the input. In reading, for example, the co-occurrence of letters can help to predict the next letter in a word, albeit not deterministically (Arciuli & Simpson, 2012). Likewise, transitional probabilities across syllables may help listeners to discover word boundaries in continuous spoken language (Saffran, Aslin, & Newport, 1996). Indeed, the ability to integrate across probabilistic acoustic information is essential in learning to map the substantial signal variability present in spoken language to consistent linguistic units (see Holt & Lotto, 2010). Impairment in the general cognitive mechanisms involved in learning from probabilistic input could be expected to have important repercussions in acquiring and processing linguistic materials due to the high demands language places on learning and using probabilistic relationships. Common with this are the findings of impaired probabilistic category learning in populations with linguistic deficits such as individuals with Specific Language Impairments (Kemény & Lukács, 2010).
Procedural learning impairments have been also implicated in ADHD (Adi-Japha, Fox, & Karni, 2011). Furthermore, basal ganglia/cerebellar abnormalities and specifically disruption to cortico-striatal loops have been strongly implicated in ADHD (Berquin et al., 1998; Teicher et al., 2000) Based on this and the high comorbidity between dyslexia and ADHD, there might be concern that patterns observed in the current research originated from attention impairments within the Dyslexia group. However, the presence of an additional ADHD comorbid learning disability was an exclusion criterion. Moreover, although one participant with dyslexia that had also been diagnosed with ADD was included in the sample excluding her from the analysis only strengthened the observed group difference (F(1, 28)=8.589, p=.007, M = .78 for the Dyslexia group, M = .85 for the Control group). Taken together this lessens the possibility that attention problems are the driving force behind the present results. Nevertheless, the comorbidity of dyslexia and ADHD remains of interest and it has been suggested that both ADHD and dyslexia may belong to a family of neurodevelopmental disorders which are associated with procedural learning impairments (Nicolson & Fawcett, 2007; Ullman, 2004). Future studies with both populations will be informative in delineating the nature of procedural learning impairments observed across dyslexia and ADHD.
The task structure of the weather prediction task raises the possibility that the present results may arise from an impairment in paired associate learning, which has been implicated in dyslexia (Mayringer & Wimmer, 2000; Messbauer & de Jong, 2003). In a typical paired associate learning task participants are presented with several visual pictures and have to learn the relationship of each image to a real word or nonsense name that is presented auditorily (visual-verbal associations) or to visually-presented symbols (visual-visual associations). In visual-verbal paired associate tasks, participants must repeat the auditorily-presented verbal label. Individuals with dyslexia are impaired in learning visual-verbal, but not visual-visual, associations (Mayringer & Wimmer, 2000; Messbauer & de Jong, 2003) and visual-verbal paired associate learning is related to learning to read (Hulme, Goetz, Gooch, Adams, & Snowling, 2007).
It could be argued that learning the association between cue and outcome in the weather prediction task draws on paired associate learning, which is known to be impaired in dyslexia (Mayringer & Wimmer, 2000; Messbauer & de Jong, 2003). However, although there is superficial similarity between these tasks, the weather prediction task (as used in our study) is arguably more similar to the visual-visual paired associate learning in which individuals with dyslexia are unimpaired. In the weather prediction task, participants learn an association between visual character (cue presented visually on a card) and outcome (presented orthographically). There is no verbal response, no information is presented auditorily, and there is no nonsense verbal label to retain. This is important as previous research has consistently demonstrated that paired associate learning that does not involve phonological output is not disrupted in dyslexia (Litt & Nation, 2014) and that only tasks that involve verbal-auditory output are found to be significantly correlated with reading (Litt, de Jong, van Bergen, & Nation, 2013). In fact Litt et al. (2014) suggest that dyslexics’ paired associate learning impairments do not arise from problems in associative learning per se, but rather from deficits in phonological form learning that is engaged by the auditory-phonological aspects of visual-verbal paired associate tasks. Since our tasks did not involve a high verbal demand (such as unfamiliar nonsense words presented auditorily to be paired with associated symbols) as is typical in paired associate learning tasks, it is unlikely that paired associate learning impairments could account for the current results.
Impaired learning of the relationship of probabilistic nonlinguistic visual cues to outcomes among adults with dyslexia is difficult to reconcile with a purely phonological account, but is consistent with a procedural learning deficit in dyslexia (Nicolson & Fawcett, 2011). However, there remain many important open questions as to the nature of the procedural learning impairment in dyslexia to be answered. Indeed, the wide range of learning tasks considered to be “procedural” is unlikely to draw on identical learning mechanisms. In further development of the taxonomies of deficits observed in dyslexia and other disorders it will be important to develop more detailed conceptualization of the nature of the tasks that fall into the class of procedural learning. The present results contribute to this by demonstrating that it is the probabilistic nature of the weather prediction task that causes difficulty for learners with dyslexia, and not a disruption of feedback-based learning.
Acknowledgements
We would like to thank Prof. Marjan Jahanshahi for sharing a sample of cards used in her previous study. The work was supported by a grant from the National Institutes of Health to to LLH, R01DC004674.
References
- Adi-Japha E, Fox O, Karni A. Atypical acquisition and atypical expression of memory consolidation gains in a motor skill in young female adults with ADHD. Research in Developmental Disabilities. 2011;32(3):1011–1020. doi: 10.1016/j.ridd.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Romano JC, Howard JH, Jr, Howard DV. Two forms of implicit learning in young adults with dyslexia. Annals of the New York Academy of Sciences. 2008;1145(1):184–198. doi: 10.1196/annals.1416.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin P, Giedd J, Jacobsen L, Hamburger S, Krain A, Rapoport J, Castellanos F. Cerebellum in attention-deficit hyperactivity disorder A morphometric MRI study. Neurology. 1998;50(4):1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Brookes RL, Nicolson RI, Fawcett AJ. Prisms throw light on developmental disorders. Neuropsychologia. 2007;45(8):1921–1930. doi: 10.1016/j.neuropsychologia.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price C, Frith C, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics A search for Wernicke's Wortschatz? Brain. 1999;122(10):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5(1-2):131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- De Diego-Balaguer R, Couette M, Dolbeau G, Dürr A, Youssov K, Bachoud-Lévi A-C. Striatal degeneration impairs language learning: evidence from Huntington's disease. Brain. 2008;131(11):2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démonet J-F, Taylor MJ, Chaix Y. Developmental dyslexia. The Lancet. 2004;363(9419):1451–1460. doi: 10.1016/S0140-6736(04)16106-0. [DOI] [PubMed] [Google Scholar]
- Deroost N, Zeischka P, Coomans D, Bouazza S, Depessemier P, Soetens E. Intact first-and second-order implicit sequence learning in secondary-school-aged children with developmental dyslexia. Journal of Clinical and Experimental Neuropsychology. 2010;32(6):561–572. doi: 10.1080/13803390903313556. [DOI] [PubMed] [Google Scholar]
- Du W, Kelly SW. Implicit sequence learning in dyslexia: a within-sequence comparison of first-and higher-order information. Annals of Dyslexia. 2012:1–17. doi: 10.1007/s11881-012-0077-1. [DOI] [PubMed] [Google Scholar]
- Du W, Kelly SW. Implicit sequence learning in dyslexia: a within-sequence comparison of first-and higher-order information. Annals of dyslexia. 2013;63(2):154–170. doi: 10.1007/s11881-012-0077-1. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Memory systems. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1(4):478–490. doi: 10.1002/wcs.49. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Paganoni P, Lorusso ML. The spatial distribution of visual attention in developmental dyslexia. Experimental Brain Research. 2000;132(4):531–538. doi: 10.1007/s002219900330. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Attentional requirements during acquisition and consolidation of a skill in normal readers and developmental dyslexics. Neuropsychology. 2012a;26(6):744. doi: 10.1037/a0030235. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Dissociation between online and offline learning in developmental dyslexia. Journal of Clinical and Experimental Neuropsychology. 2012b;34(3):279–288. doi: 10.1080/13803395.2011.633499. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Dissociation between the Procedural Learning of Letter Names and Motor Sequences in Developmental Dyslexia. Neuropsychologia. 2012c;50(10):2435–2441. doi: 10.1016/j.neuropsychologia.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: Individual variability in strategies for probabilistic category learning. Learning & Memory. 2002;9(6):408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T, Friederici AD, Kotz SA, Van Kampen A. Procedural learning in Broca's aphasia: Dissociation between the implicit acquisition of spatio-motor and phoneme sequences. Journal of Cognitive Neuroscience. 2001;13(3):370–388. doi: 10.1162/08989290151137412. [DOI] [PubMed] [Google Scholar]
- Hedenius M, Ullman MT, Alm P, Jennische M, Persson J. Enhanced recognition memory after incidental encoding in children with developmental dyslexia. PloS one. 2013;8(5):e63998. doi: 10.1371/journal.pone.0063998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl AK, Wilkinson L, Tabrizi SJ, Painold A, Jahanshahi M. Probabilistic classification learning with corrective feedback is selectively impaired in early Huntington's disease—Evidence for the role of the striatum in learning with feedback. Neuropsychologia. 2012;50(9):2176–2186. doi: 10.1016/j.neuropsychologia.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Holt LL, Lotto AJ. Speech perception as categorization. Attention, Perception, & Psychophysics. 2010;72(5):1218–1227. doi: 10.3758/APP.72.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44(7):1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hulme C, Goetz K, Gooch D, Adams J, Snowling MJ. Paired-associate learning, phoneme awareness, and learning to read. Journal of Experimental Child Psychology. 2007;96(2):150–166. doi: 10.1016/j.jecp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kemény F, Lukács Á . Impaired procedural learning in language impairment: Results from probabilistic categorization. Journal of Clinical and Experimental Neuropsychology. 2010;32(3):249–258. doi: 10.1080/13803390902971131. [DOI] [PubMed] [Google Scholar]
- Kita Y, Yamamoto H, Oba K, Terasawa Y, Moriguchi Y, Uchiyama H, Inagaki M. Altered brain activity for phonological manipulation in dyslexic Japanese children. Brain. 2013;136(12):3696–3708. doi: 10.1093/brain/awt248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1(2):106–120. [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Paulsen JS, Swerdlow NR, Swenson M. Dissociations within nondeclarative memory in Huntington's disease. Neuropsychology. 1996;10(4):538. [Google Scholar]
- Lagnado DA, Newell BR, Kahan S, Shanks DR. Insight and strategy in multiple-cue learning. Journal of Experimental Psychology: General. 2006;135(2):162. doi: 10.1037/0096-3445.135.2.162. [DOI] [PubMed] [Google Scholar]
- Litt RA, de Jong PF, van Bergen E, Nation K. Dissociating crossmodal and verbal demands in paired associate learning (PAL): What drives the PAL–reading relationship? Journal of Experimental Child Psychology. 2013;115(1):137–149. doi: 10.1016/j.jecp.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Litt RA, Nation K. The nature and specificity of paired associate learning deficits in children with dyslexia. Journal of Memory and Language. 2014;71(1):71–88. [Google Scholar]
- Maddox WT, Ashby FG. Dissociating explicit and procedural-learning based systems of perceptual category learning. Behavioural Processes. 2004;66(3):309–332. doi: 10.1016/j.beproc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Mayringer H, Wimmer H. Pseudoname learning by German-speaking children with dyslexia: Evidence for a phonological learning deficit. Journal of Experimental Child Psychology. 2000;75(2):116–133. doi: 10.1006/jecp.1999.2525. [DOI] [PubMed] [Google Scholar]
- Messbauer V, de Jong PF. Word, nonword, and visual paired associate learning in Dutch dyslexic children. Journal of Experimental Child Psychology. 2003;84(2):77–96. doi: 10.1016/s0022-0965(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Newell BR, Lagnado DA, Shanks DR. Challenging the role of implicit processes in probabilistic category learning. Psychonomic Bulletin & Review. 2007;14(3):505–511. doi: 10.3758/bf03194098. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Fawcett A. Automaticity: A new framework for dyslexia research? Cognition. 1990;35(2):159–182. doi: 10.1016/0010-0277(90)90013-a. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Fawcett A. Dyslexia, learning, and the brain. The MIT Press; 2010. [Google Scholar]
- Nicolson RI, Fawcett AJ. Comparison of deficits in cognitive and motor skills among children with dyslexia. Annals of Dyslexia. 1994;44(1):147–164. doi: 10.1007/BF02648159. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Procedural learning difficulties: reuniting the developmental disorders? Trends in Neurosciences. 2007;30(4):135–141. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex. 2011;47(1):117–127. doi: 10.1016/j.cortex.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet. 1999;353(9165):1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119(1):143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC neuroscience. 2009;10(1):67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett ER, Kuniholm E, Protopapas A, Friedman J, Lieberman P. Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: a case study. Neuropsychologia. 1998;36(2):173–188. doi: 10.1016/s0028-3932(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Stein JF. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. The Lancet. 1998;351(9119):1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Raven J, Court JH, Raven J. Standard progressive matrices. Manual for Raven's Progressive Matrices and Vocabulary Scales. 1992.
- Rüsseler J, Gerth I, Münte TF. Implicit learning is intact in adult developmental dyslexic readers: Evidence from the serial reaction time task and artificial grammar learning. Journal of Clinical and Experimental Neuropsychology. 2006;28(5):808–827. doi: 10.1080/13803390591001007. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A, Guide E.-P. U. s. Psychology Software Tools Inc.; Pittsburgh, USA: 2002. [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience & Biobehavioral Reviews. 2008;32(2):265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers C, Grossman S, Sage J, Gluck M, Poldrack R. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127(4):851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers C, Onlaor S, Gluck M. Role of the basal ganglia in category learning: how do patients with Parkinson's disease learn? Behavioral Neuroscience. 2004;118(4):676. doi: 10.1037/0735-7044.118.4.676. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. Dyslexia. Blackwell Publishing; 2000. [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends in Neuroscience. 1997;20(4):147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Harrison EPD, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2006;44(5):795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Ray NJ, Jack A, Stein JF. Implicit Learning in Control, Dyslexic, and Garden-Variety Poor Readers. Annals of the New York Academy of Sciences. 2008;1145(1):173–183. doi: 10.1196/annals.1416.003. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nature Medicine. 2000;6(4):470–473. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. TOWRE–2 Test of Word Reading Efffciency. 1999.
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92(1):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(10):1392–1397. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wilkinson L, Lagnado DA, Quallo M, Jahanshahi M. The effect of feedback on non-motor probabilistic classification learning in Parkinson's disease. Neuropsychologia. 2008;46(11):2683–2695. doi: 10.1016/j.neuropsychologia.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Lesaux NK. Persistence of phonological processing deficits in college students with dyslexia who have age-appropriate reading skills. Journal of Learning Disabilities. 2001;34(5):394–400. doi: 10.1177/002221940103400501. [DOI] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests. 2005. Pro-ed.
- Woodcock RW. Woodcock reading mastery tests, revised. American Guidance Service Circle Pines; MN: 1987. [Google Scholar]
- Yap RL, van der Leij A. Testing the automatization deficit hypothesis of dyslexia via a dual-task paradigm. Journal of Learning Disabilities. 1994;27(10):660–665. doi: 10.1177/002221949402701006. [DOI] [PubMed] [Google Scholar]