Abstract
In order to assess the status of the volume and composition of the body fluid compartment, the kidney monitors a wide variety of chemical and physical parameters. It has recently become clear that the kidney’s sensory capacity extends well beyond its ability to sense ion concentrations in the forming urine. The kidney also keeps track of organic metabolites derived from a surprising variety of sources and uses a complex interplay of physical and chemical sensing mechanisms to measure the rate of fluid flow in the nephron. Recent research has provided new insights into the nature of these sensory mechanisms and their relevance to renal function.
Keywords: renal physiology, cell signaling, cell and transport physiology
Introduction
It has long been appreciated that we use sensory receptors to detect the chemical and physical properties of the world around us. However, it has recently become clear that we also utilize the same molecular tools to monitor the composition of the world within us. Many sensory receptors, including olfactory receptors (ORs), taste receptors, and other sensory G protein–coupled receptors (GPCRs), as well as receptors that function as mechanosensitive or chemosensitive ion channels, have recently been shown to play key roles in organs and tissues traditionally thought of as “nonsensory.” In the past few years, a large and diverse literature has developed documenting systems in which “sensory” receptors are exploited to serve in a wide variety of physiologic processes. The tongue’s sour taste receptors, for example, are also called upon to sense pH in the spinal column (1), its bitter taste receptors also regulate bronchodilation and ciliary beat frequency in the lung in response to certain inhalants (2,3), and its sweet taste receptors regulate glucose transport in the gut (4,5). In addition to taste receptors, there are also numerous examples of ORs playing a variety of roles in tissues outside of the nose. The OR gene family, in fact, is the largest gene family in the genome (6–8), and thus forms an expansive repertoire of GPCR-based chemical detectors. In addition to odorant detection in the nose, ORs are now understood to also mediate chemical detection in other environments. For example, ORs play roles in muscle cell migration and sperm chemotaxis (9). Ligands for these receptors are often produced as a result of metabolic processes, implying that seemingly inert intermediate metabolites or by-products may have unappreciated signaling roles (1,10–13).
The kidney stands out as a particularly appropriate tissue in which to deploy sensory receptors for chemodetection. The kidney evaluates and maintains a large number of physiologic parameters, including systemic acid-base balance, electrolyte concentrations, volume status, and toxin levels. To achieve this, the kidney must monitor the concentration of numerous substances in the plasma and the early urine. Sensory chemoreceptors are by their nature well suited to play central roles in these renal “chemosensory” tasks.
In addition to sensing chemical cues, the kidney must also monitor physical or mechanical stimuli, such as shear stress, pressure, and flow rate. Recent studies have shown that fluid flow is an important sensory cue in the acute regulation of urine composition (14) and in the proper development and maintenance of the structure of the nephron. Studies in the fruit fly have shown that mechanical stimulation is important in regulating the pathways that control planar cell polarity (15), which is the process through which neighboring epithelial cells acquire distinct structural and functional properties. Planar cell polarity signaling pathways are critically important in the differentiation of the distinct epithelial cell types that constitute the nephron. Impairments in fluid flow detection may play roles in the development of clinically significant diseases of the kidney (16,17). Mechanical forces are important regulators of the activity of renal ion channels (18–25), as well as of the signaling activities that regulate renal ion and fluid transport (14,26–28). Therefore, the capacity to monitor mechanical sensations is exploited by the kidney in its efforts to maintain homeostasis. In this review, we discuss recent studies that reveal surprising and important roles for mechanosensitive and chemosensory molecular machinery in renal function.
Physiology and Pathophysiology of Renal Cilia
With the possible exception of the intercalated cells of the collecting duct (29), every epithelial cell that lines the nephron is endowed with a single primary cilium (30). These cilia arise from the apical surfaces of the epithelial cells and protrude into the tubule lumen. The typical renal epithelial cell cilium is 2- to 3-μm long and <1 μm in cross-sectional width. The cilium’s surface membrane is continuous with that of the apical plasma membrane, although its lipid and protein compositions differ substantially from those of the surrounding apical cell surface (31). Just beneath the ciliary membrane is the ciliary axoneme, which is a scaffolding composed of nine doublet microtubules that are parallel to one another and that extend from the base of the cilium along its entire axial length. In contrast with the motile cilia that are found on airway and oviduct epithelial cells, primary cilia, such as those found on renal epithelial cells, do not beat and do not move fluid. Instead, their function appears to be entirely sensory and their structure reflects their sedentary nature. The axonemes of motile cilia possess a central pair of microtubules that is connected by radial spokes to the nine doublet microtubules at the ciliary periphery. These additional components include the motor proteins that allow motile cilia to generate force. Although primary cilia, such as those found in the kidney, lack these components found in their motile cousins, they are by no means devoid of motor proteins. Large protein assemblies known as intraflagellar transport (IFT) complexes exploit motor proteins to crawl up and down the axonemal microtubules. These IFT complexes serve as delivery machinery that transport newly synthesized proteins into the cilium and carry away components that are destined for removal (32,33). The IFT complexes are absolutely required in order for primary cilia to form and to function. It should also be noted that every cilium arises from a basal body, which is a structure composed of paired cylindrical assemblies of microtubules. The basal body serves as the centriole that organizes the mitotic spindle in dividing cells. A complex array of proteins connects the base of the axoneme to the basal body and forms the ciliary transition zone, which serves as a barrier that helps to maintain the compositional distinctions between the apical and ciliary membrane domains (34,35).
Although the structure of the renal primary cilium is fairly well understood, the same cannot be said of its function. It is clear that the cilia of renal epithelial cells serve extremely important purposes, as evidenced by the large number of renal phenotypes arising from mutations in genes whose products participate in ciliogenesis (36). Bardet–Biedl syndrome, nephronophthisis, Meckel–Gruber syndrome, Joubert’s syndrome, Senior–Løken syndrome, and orofaciodigital syndrome are all caused by loss-of-function mutations in genes that encode distinct subclasses of the components of the cellular machinery that is required to build and maintain the primary cilium (37,38). These pleomorphic conditions are characterized by partially overlapping lists of neurologic, skeletal, metabolic, and sensory phenotypes, including renal cystic disease. In addition, the proteins encoded by the genes responsible for both the autosomal dominant and autosomal recessive forms of polycystic kidney disease localize, at least in part, to the primary cilium (39–41). On the basis of this brief summary, it might be logical to suggest that the cilium participates in sending signals that are required to prevent the development of renal cysts. Recent data, however, suggest that the cilium’s role in renal cystic disease, although critical, is not this straightforward.
The groundbreaking work of Praetorius and Spring revealed that the primary cilium can detect and respond to mechanical stimuli (42). These investigators showed that either direct or flow-induced bending of the primary cilia of cultured renal epithelial cells led to the activation of ion channels that mediated calcium influx, which secondarily activated calcium release from intracellular stores. Studies into the function of the proteins encoded by the autosomal dominant polycystic kidney disease genes (41) have provided insight into the nature of the mechanosensitive ion channels responsible for this cilia-dependent activity. Polycystin-1, the product of the Pkd1 gene, encodes a massive protein. It is composed of 4302 amino acids and spans the membrane 11 times. Polycystin-2, encoded by the Pkd2 gene, is roughly one fourth of the size of polycystin-1 and spans the membrane 6 times. Polycystin-2 belongs to the transient receptor potential (TRP) family of calcium-permeable ion channels. Polycystin-1 and polycystin-2 interact with one another to form a complex that localizes in part to the cilium and that may contribute to the calcium channel activity that is induced by ciliary bending. This activity may depend upon TrpV4, another membrane of the Trp family of cation channels whose channel activity may be regulated in some manner by the polycystin proteins (43). These observations have inspired a model in which the ciliary population of the polycystin-1 and polycystin-2 complex serves as a sensor that transduces tubular fluid flow to produce an elevation of renal epithelial cell cytoplasmic calcium concentrations (44). These observations prompt the further suggestion that loss of this mechanically activated polycystin channel activity, or of the mechanosensitive cilium in which this activity resides, could lead to the perturbations in cell proliferation, differentiation, and fluid secretion that together characterize the formation of autosomal dominant polycystic kidney disease renal cysts. Recent data indicate that close relatives of polycystins, rather than the polycystin-1 and polycystin-2, may mediate ciliary ion currents in at least some cell types (45,46).
Although the role of the ciliary polycystins as flow sensors is intriguing, it seems quite likely that these proteins also participate in other sensory processes. In addition to their connection to cytoplasmic calcium levels, the polycystin proteins have been connected to a very large and diverse collection of signaling pathways that have the potential to influence cellular growth and metabolism (41). Furthermore, polycystin-1 interacts with trimeric G proteins, suggesting the intriguing possibility that it functions as an atypical GPCR (47). Finally, it is worth noting that both polycystin proteins have homologs that have been shown to serve as chemosensors. Polycystin-1–like-3 and polycystin-2–like-1 form a complex that detects low pH, serving both as sour taste receptors in the tongue and as sensors of pH in the central nervous system (1,48,49). Taken together, these facts suggest that the polycystins may serve chemosensory roles in renal epithelial cells. The nature of the ligands to which the polycystins might respond and the subcellular localization in which the polycystins perform this putative activity remain to be determined.
As noted above, the connection between ciliary function and the prevention of renal cystic disease has recently become more complex. Clearly, the pathology associated with the ciliopathies confirms that loss of cilia is sufficient to lead to the development of renal cysts. Surprisingly, however, studies utilizing mouse models reveal that loss of cilia can suppress cyst formation that is caused by the loss of polycystin-1 or polycystin-2 expression (50). This unexpected observation suggests that, in the absence of the polycystin proteins, cilia send a message or messages that activate cystogenic processes. The polycystins appear to counteract or suppress these messages. The nature of these putative procystogenic and countercystogenic messages remains to be determined. Elucidating the molecular details of this apparently antagonistic relationship between the cilium and the polycystins will no doubt provide interesting insights into the mechanisms through which renal epithelial cells exploit cilia and the polycystins to interrogate the chemical composition and flow rate of the tubular fluid.
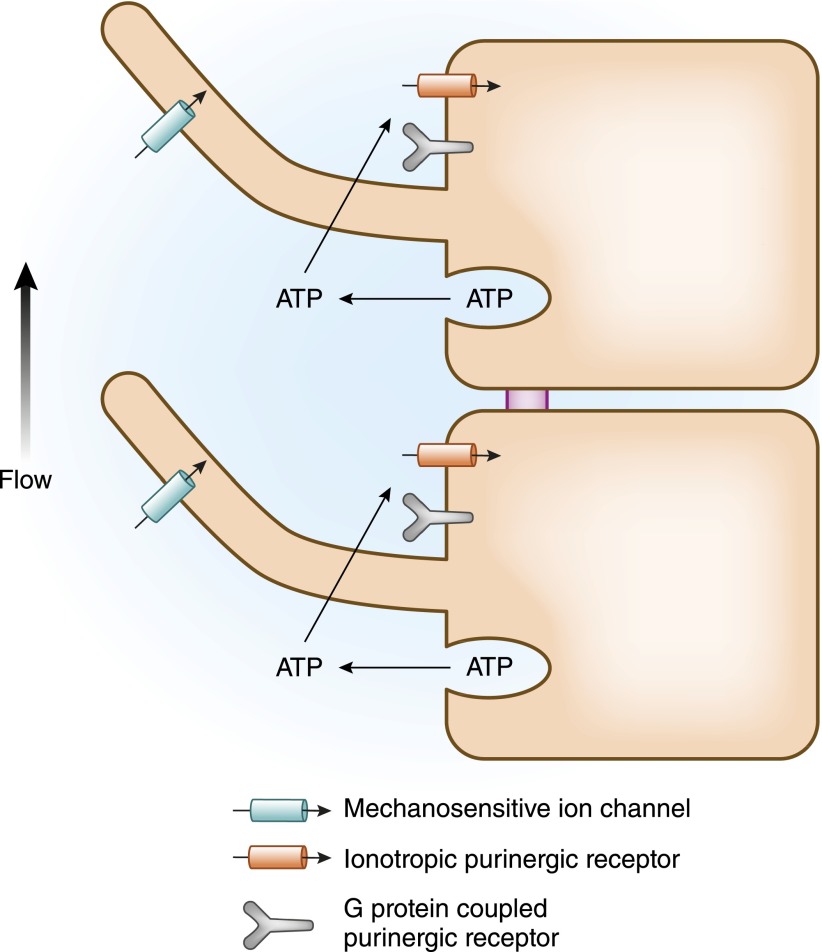
Although flow-induced ciliary bending may activate ion channels directly, it is clear that this mechanical stimulus also initiates a fascinating autocrine chemical signaling process (Figure 1). Bending of the primary cilium causes renal epithelial cells to release ATP into the tubule lumen (51). This ATP appears to be stored inside the cell in vesicular compartments that, upon stimulation, fuse with the apical plasma membrane and release their contents (52). This extracellular ATP can bind to and activate purinergic receptors that are present on the apical plasma membranes of renal epithelial cells. There are two distinct families of purinergic receptors, each of which has many branches. Members of the P2Y family are metabotropic receptors that signal through trimeric G proteins, whereas the P2X receptors are ligand-gated ion channels (53). Both types of purinergic receptors populate the apical and basolateral surfaces of renal epithelial cells. Thus, flow-induced ATP release can activate both ionic currents and intracellular second messenger pathways. Each nephron segment expresses a distinct collection of purinergic receptors, distributed among their apical and basolateral plasma membrane domains, which modulate the tubule segment’s physiologic properties in response to the various physiologic stimuli that are transmitted through elevations in extracellular nucleotide concentrations (54). Activation of the apical P2Y G protein–coupled purinergic receptor in the renal collecting duct, for example, results in activation of protein kinase C, which, in turn, inhibits the epithelial sodium channel (ENaC) and, consequently, reduces transepithelial sodium reabsorption (55). Thus, in this segment, high flow rates can be sensed by the bending of the primary cilium and communicated to the epithelial ion transport machinery through the release of ATP via fusion of intracellular vesicles with the apical plasma membrane. This autocrine and paracrine messenger acts through purinergic receptors to reduce sodium uptake. Hence, high flow rates, which can arise as a consequence of expansion of the extracellular fluid volume leading to an increase in the GFR, reduce the retention of sodium and volume. Through a collaboration between mechanical and chemical sensory tools, this multicomponent and multimodal mechanism thus underlies a logical and elegant physiologic adaptation.
Figure 1.
Flow-induced bending of the primary cilium can be transduced through several mechanisms, including the activation of mechanosensory ion channels and the release of vesicular ATP, which, in turn, activates purinergic receptors.
The intriguing mechanism described in the preceding paragraph by no means constitutes the only example of renal tubule fluid flow being sensed or interpreted to influence the composition of the forming urine. The process of tubuloglomerular feedback is a classic example of flow rates being “sensed” (as an index of changes in tubular fluid electrolyte concentrations) to mediate changes in renal function. In addition, the large-conductance Ca2+-sensitive potassium channel of the renal collecting duct is responsible for the capacity of the late nephron to increase potassium secretion in response to increased flow rates (25,56). Therefore, a variety of renal mechanisms depend upon a complex combination of sensors and signaling pathways that participate in adjusting the kidney’s capacity to modify the forming urine to the rate at which it is flowing through the tubule lumen.
Finally, it is worth noting that cilia are not the only mechanosensitve cellular organelles that extend into the lumen of the renal tubule. The microvilli that constitute the luxuriant brush borders of proximal tubule epithelial cells may also manifest the capacity to monitor and communicate tubular fluid flow rates (14). Flow-induced microvillar bending, communicated through elements of the cytoskeleton to the intracellular signaling machinery that regulates transport processes, may play an important role in governing transepithelial uptake of sodium, bicarbonate, and water in the initial segment of the nephron (57).
Renal Chemosensors
The kidney monitors and responds to the levels of a number of key metabolites. In this review, we focus on the chemosensors that detect succinate, short chain fatty acids (SCFAs), and bicarbonate.
Succinate
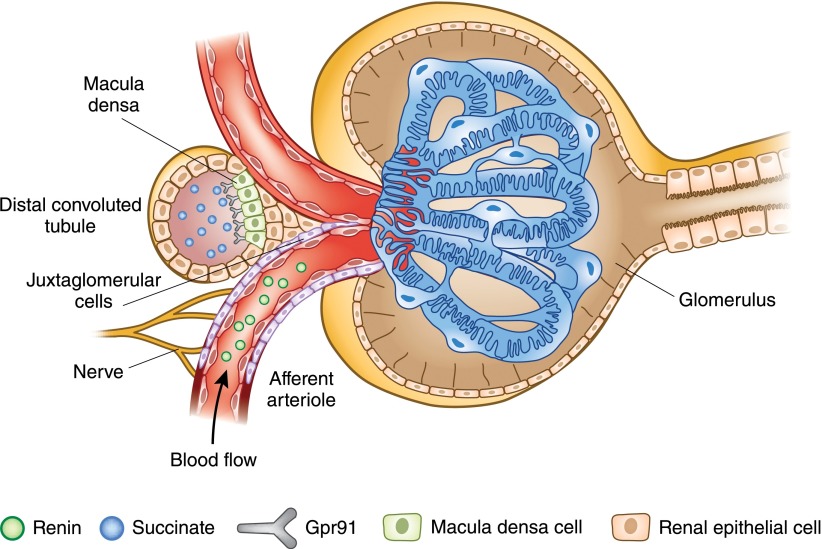
Succinate, which participates in the tricarboxylic acid (TCA; or citric acid) cycle, resides primarily in the mitochondria. However, this metabolite is also found in the plasma in the micromolar range (11), where it plays an important signaling role. It has been suggested that plasma succinate levels are an index of the level of hypoxia and/or of oxidative or mitochondrial stress, based in part on the observation that tissue levels of succinate rise in hypoxic conditions while the levels of other TCA cycle intermediates fall (58,59). Succinate administration increases BP in a dose-dependent manner (11), and the succinate receptor GPCR 91 (Gpr91) was originally identified as a mediator of the hypertensive response to succinate (11,60). The initial characterization of Gpr91 clearly demonstrated that this receptor is highly expressed in the kidney and that the hypertensive response to succinate was dependent on the renin-angiotensin system (RAS) (11). The details of the mechanism underlying this phenomenon were unraveled in several studies that revealed that Gpr91 is localized in the distal nephron, including the macula densa (MD) (61). Succinate increases BP by binding to Gpr91 on the MD cells, which leads to renin release from the adjacent juxtaglomerular apparatus, thus driving the increase in BP (13) (Figure 2). Renin is an enzyme that cleaves angiotensinogen to form angiotensin 1, and this reaction constitutes the rate-limiting step in the RAS pathway. Because elevated succinate levels are associated with hypoxia and oxidative or mitochondrial stress, it may be that the kidney uses succinate as a “cue” to increase systemic BP (and, thus, its own perfusion) in the face of renal energy deprivation.
Figure 2.
Macula densa cell Gpr91 mediates renin release from juxtaglomerular cells in response to succinate binding. Gpr91, G protein–coupled receptor 91.
Intriguingly, this Gpr91–renin-angiotensin signaling pathway has been implicated in the pathophysiology of diabetic nephropathy. It has been appreciated for some time that diabetic nephropathy is a feature of both type 1 and type 2 diabetes, and that pharmacologic blockade of the RAS can slow the progression of diabetic nephropathy in patients with type 1 and type 2 diabetes (62). However, the unifying mechanism underlying this synergy has not been well understood. The connection appears to be high plasma glucose levels, which are associated with elevated succinate levels in both kidney tissue and in urine (63). There is likely also increased localized succinate production (through the TCA cycle) in the kidney under conditions of hyperglycemia. These elevated succinate levels activate Gpr91, increasing renin release. It should be noted that although a minor component of the renin release response may be induced by the osmolar effects of high glucose levels, which is independent of Gpr91 (63), the majority of this response is Gpr91-mediated and succinate-dependent. Therefore, inappropriate activation of the RAS in diabetes is likely tied to elevated plasma glucose, and as a consequence, elevated succinate levels, which activate Gpr91 (and, therefore, renin release). These observations provide an interesting rationale for the well understood benefits of RAS blockade and plasma glucose control in the setting of diabetes.
Adenylate Cyclase 3
Adenylate cyclase 3 (AC3) is best known as a key component of the canonical signaling pathway underlying olfaction (64), but is also expressed in a variety of nonolfactory tissues (9,65), including the kidney (66). In the nose, when an odorant binds to its OR, OR activation leads to downstream activation of the olfactory G protein (Golf), which, in turn, activates AC3, leading to the opening of nucleotide-gated ion channels, and, ultimately, to an action potential (6,64,67). AC3 is indispensable for the signaling pathway underlying olfaction, as evidenced by the fact that AC3 knockout mice are anosmic (64).
Within the kidney, AC3 colocalizes with Golf in the distal convoluted tubule and the MD. The MD is a highly specialized, tightly packed cell type that occurs where each nephron makes physical contact with the vascular pole of its own parent glomerulus. At the junction between the thick ascending limb and the distal convoluted tubule, the renal epithelial cells lining the tubule are referred to as MD cells. These cells have a specialized sensory function that permits them to use chemical cues to deduce the flow rate of the tubular fluid in the distal nephron. These cells measure the chloride concentration of the tubular fluid, which is higher when the flow rate through the thick ascending limb is high. The MD cells can then signal the parent glomerulus to increase or decrease the flow rate, serving as a continuously operating single nephron GFR calibrator, in a process known as tubuloglomerular feedback. In addition, when flow rates are low, MD cells can also signal the juxtaglomerular cells associated with the afferent arteriole that feeds their parent glomerulus to increase renin secretion, which then raises systemic BP. Consistent with the role of the MD cells in tubuloglomerular feedback (and, therefore, GFR regulation) and renin secretion, knockout mice that do not express AC3 have decreased GFR and decreased plasma renin (66) levels, consistent with a defect in MD function. It remains to be determined which receptor and ligand signal through AC3 in the cells of the MD. It is clear, however, that components of the molecular machinery of olfaction play a key role in the kidney to regulate both GFR and renin secretion.
Short Chain Fatty Acids
The succinate pathway nicely exemplifies the kidney’s use of sensory receptors to monitor intermediates and by-products of the body’s own metabolism. Recent studies support the surprising conclusion that the kidney also appears to pay attention to metabolic by-products produced by the gut microbiota. Indeed, the number of cells contained within the human microbiota outnumbers the number of human cells in our bodies by more than a factor of 10 (68–71). The colon is the home to >70% of these microbes (69,71). A major class of metabolites produced by the gut microbiota in the colon are SCFAs (primarily acetate, propionate, and butyrate), which are produced at such a high rate that SCFAs are found in the colon at concentrations of approximately 100 mM (72). After absorption into the bloodstream, SCFAs are found in the plasma at concentrations ranging between 0.1 and 10 mM (73–75). SCFAs produced by gut microbes have recently emerged as signaling molecules in the physiology of the host, where they play roles in modulating physiologic processes, such as metabolism, immune responses, and susceptibility to HIV infection (73,74,76–79).
Gpr41 and Gpr43 are well characterized SCFA receptors (73,74,77) that have been shown to play important roles in regulating the physiology of the host organism in response to gut microbiota metabolite production. For example, in response to changes in plasma SCFA concentrations, Gpr41 regulates host adiposity (77), and Gpr43 modulates host inflammatory responses (73,74). It was recently found that both Gpr41 and Gpr43 are expressed in the kidney and the vasculature, along with a novel SCFA receptor, OR 78 (Olfr78).
The identification and localization of Olfr78 was particularly intriguing, because it was found in the kidney specifically in the afferent arteriole (a key component of the juxtaglomerular apparatus [JGA], which regulates renin secretion), as well as in resistance vasculature beds (80). The identification of Olfr78 as a SCFA receptor (80), together with the distribution of Olfr78 (80), led to the novel hypothesis that SCFA receptors may be responding to gut microbiota–derived SCFAs in order to regulate BP. Indeed, it was found that Olfr78 is responsible for inducing renin release from the JGA upon increases in SCFAs (80) (Figure 3). This effect is detected in isolated JGA cells in culture, and Olfr78 knockout mice exhibit reduced levels of plasma renin and resting BPs. In addition, a separate acute vascular effect of SCFAs was identified: SCFAs delivered intravenously cause a rapid and dose-dependent hypotensive response. This acute vascular effect was found to be mediated primarily by Gpr41, and to be opposed by a milder counter-regulatory hypertensive response to SCFAs driven by Olfr78. In summary, SCFAs have an acute effect to lower BP via Gpr41, which is opposed by Olfr78 in two ways: first, by an acute effect of Olfr78 to support vascular tone; and second, by a more “chronic” effect of SCFAs to increase renin secretion via Olfr78.
Figure 3.
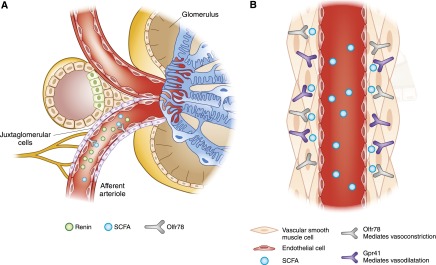
SCFA Receptors and blood pressure. (A) Olfr78 mediates renin release from juxtaglomerular apparatus cells in response to binding of SCFAs. (B) Two different SCFA receptors (Olfr78 and Gpr41) are both expressed in blood vessels. Olfr78 mediates vasoconstriction in response to SCFAs, whereas Gpr41 mediates vasodilation. It should be noted that, whereas Olfr78 has been localized to vascular smooth muscle cells, Gpr41 has not been localized to a specific cell type within blood vessels (it was identified in blood vessels by RT-PCR). Therefore, although it is depicted here in vascular smooth muscle, the precise cell type in which it is localized has not been determined. Gpr41, G protein–coupled receptor 41; Olfr78, olfactory receptor 78; SCFA, short chain fatty acid.
The functional significance of this pathway may be to enhance the capacity of the colon to maximally absorb remaining nutrients after a meal. Although most nutrient absorption occurs in the small intestine, a significant quantity of nutrients are absorbed from the large intestine in animals (72,81–89) and in humans (90–98). After a meal, one would expect SCFA concentrations in the vessels serving the large intestine to peak, driving localized vasodilation that would in turn facilitate nutrient absorption.
It should be noted that Gpr41 and Olfr78 have very different EC50s (half maximal effective concentrations) for SCFAs [propionate: Gpr41, EC50 100–300 μM (73,99) in a guanosine 5′-O-(3-[35S]thio)triphosphate binding assay; Olfr78, EC50 0.9 mM (80) in a cAMP-based reporter assay]. Therefore, under basal conditions in which plasma SCFAs are 0.1–10 mM (73–75), one would expect Gpr41, but not Olfr78, to be tonically active. After a meal, however, as plasma SCFA concentrations rise, Gpr41 would become further active and, thus, would further lower BP until such a point that SCFA concentrations are sufficient to activate Olfr78 as well. Activation of Olfr78 would act as a “brake” on the hypotensive pathway, supporting an increase in BP and, therefore, preventing dangerous hypotension when SCFA concentrations are high.
These observations imply that BP is regulated not only by the genetics of an individual, but potentially by the genetics of that individual’s microbiota as well. The potential clinical implications are numerous and prompt a variety of interesting questions. For example, do alterations in the diet of the host alter gut flora metabolite production and therefore BP? Can purposeful alteration of the gut microbiota composition alter BP? Similarly, are gut microbiota altered secondary to other clinical treatments (e.g., antibiotics), and might this influence BP regulation? Future studies will be required to better understand the complex and unexpected ways in which gut microbiota affect host physiology in general, and the renal regulation of BP in particular.
Bicarbonate
The proximal and distal segments of the renal tubule utilize distinct sensory mechanisms to detect the concentration of the bicarbonate ion and to tune ion transport processes accordingly. Bicarbonate transport in the proximal tubule is governed by several physiologic regulatory mediators, including angiotensin II, which acts through its GPCR to increase the activity of the apical sodium proton exchanger NHE3 and, thus, to increase bicarbonate reabsorption (100). Bicarbonate itself also appears to serve as a regulatory signal that modulates the activity of proximal tubule transporters. Although the molecular nature of the receptor that detects and responds to bicarbonate has yet to be determined, the activity of a tyrosine kinase appears to be involved in this process (101,102).
In more distal segments of the renal tubule, an ion transport protein appears to serve as a key component of a bicarbonate sensory mechanism that regulates sodium transport. Pendrin is a Na+-independent Cl−/HCO3− exchanger expressed in the cochlea of the ear, the thyroid gland, and the kidney. In the kidney, pendrin plays a role in sensing and maintaining both volume status and acid-base balance. The gene encoding pendrin (solute carrier family 26, member 4 [SLC26A4]) is mutated in the autosomal recessive disorder known as Pendred syndrome. The pathophysiology of Pendred syndome reflects the tissue localization of the pendrin protein, as patients typically have hearing loss, goiter, and hypothyroidism. Pendrin mediates iodide flux in the thyroid (103), and it plays a key role in generating the endocochlear potential in the inner ear (104). Although patients with Pendred syndrome do not typically present with a clinically significant renal phenotype, recent studies have shown that pendrin does indeed play important roles in renal physiology. In the kidney, pendrin is expressed in the collecting duct, where it localizes apically in interacalated cells (105)—in particular, in type B and non-A non-B intercalated cells (105,106). The transport activity of pendrin in these cells appears to serve two distinct purposes. In its most straightforward role, pendrin is a coupled cotransport protein that mediates both Cl− reabsorption and HCO3− secretion (107) and, thus, contributes to the collecting duct’s compendium of ion transport machinery. Pendrin activity is upregulated by angiotensin II (108) and aldosterone (109). By mediating Cl− absorption in the collecting duct, pendrin plays a role in volume homeostasis and, therefore, in BP control (109–111). As a direct consequence of chloride/bicarbonate exchange activity, however, pendrin also plays a central role in a recently defined signaling pathway. By modulating the bicarbonate concentration in the lumen of the collecting duct, pendrin sends a message to the neighboring principal cells that, in turn, regulates the functional expression of the ENaC sodium channels.
Evidence in support of this conclusion derives from observations made on mice that are deficient for pendrin expression. Although pendrin knockout mice are normal at baseline, they do not gain weight and do not become hypertensive upon treatment with an aldosterone analog (unlike their wild-type counterparts) (109). If, however, the bicarbonate concentration of the collecting duct tubule fluid in the pendrin knockout mice is raised artificially through pharmacologic manipulations, then these animals respond normally to aldosterone by increasing their ENaC-mediated sodium absorption. It appears that the bicarbonate ions that pendrin secretes into the collecting duct lumen act through an as-yet-mysterious signaling pathway to stimulate the upregulation of ENaC expression and activity in principal cells (107).
In addition, pendrin also acts as the “downstream” partner in a paracrine signaling scheme. A recent study demonstrated that pendrin activity is altered by changes in proximal tubule transport (112). It was reported that changes in acid-base status alter proximal tubule transport of α-ketoglutarate (αKG), and lead to changes in the concentration of αKG in the distal tubular fluid. In the connecting tubule and collecting ducts, αKG is sensed in intercalated cells by a receptor (oxoglutarate receptor 1), which then stimulates pendrin. Therefore, although pendrin itself is not found in the proximal tubule, it is responsive to acid-base effects on proximal tubule transport.
Thus, pendrin appears to serve in a variety of physiologic functions. By mediating Cl− absorption, pendrin collaborates with ENaC to participate in transepithelial NaCl uptake, thus playing a direct role in regulating volume status. By mediating HCO3− secretion, pendrin participates directly in the regulation of acid-base balance. It also determines the luminal concentration of bicarbonate, which acts as the critical determinant in a chemosensory pathway that helps to control the capacity of the principal cells to increase their sodium absorptive capacity in response to aldosterone. Finally, pendrin activity is also regulated by the acid-base status of the proximal tubule. Although many of the receptors and signaling pathways involved in this bicarbonate chemosensory process have yet to be elucidated, these schemes beautifully illustrate a fascinating cross-talk between transport and sensory processes in the kidney.
The kidney must respond to a wide variety of physiologic cues in its efforts to maintain homeostasis. It has only recently been appreciated that both mechanosensors and chemosensors play important roles in controlling a variety of renal functions in response to a number of novel stimuli. Future studies will no doubt provide us with a clearer understanding of the physiologic and pathophysiologic implications of these pathways in the governance of renal function.
Disclosures
M.J.C is a consultant for Allertein Pharmaceuticals and Portage Pharmaceuticals, serves on the Telethon Foundation Scientific Advisory Board, and is an editorial consultant for Elsevier Publishing.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS: The cells and logic for mammalian sour taste detection. Nature 442: 934–938, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB: Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ: Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM: Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 104: 15069–15074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP: T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A 104: 15075–15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck L, Axel R: A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65: 175–187, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Malnic B, Godfrey PA, Buck LB: The human olfactory receptor gene family. Proc Natl Acad Sci U S A 101: 2584–2589, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey PA, Malnic B, Buck LB: The mouse olfactory receptor gene family. Proc Natl Acad Sci U S A 101: 2156–2161, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin CA, Kafadar KA, Pavlath GK: MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 17: 649–661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magistroni R, Furci L, Albertazzi A: [Autosomal dominant polycystic kidney disease: From genes to cilium]. G Ital Nefrol 25: 183–191, 2008 [PubMed] [Google Scholar]
- 11.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L: Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G: Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108: 8030–8035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J: Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol 20: 1002–1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM: Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper JC, Jülicher F, Eaton S: Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142: 773–786, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA: Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 132: 1907–1921, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O’Neil RG: Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J Biol Chem 287: 8782–8791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neil RG, Heller S: The mechanosensitive nature of TRPV channels. Pflugers Arch 451: 193–203, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC: Coupled ATP and potassium efflux from intercalated cells. Am J Physiol Renal Physiol 300: F1319–F1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtzclaw JD, Liu L, Grimm PR, Sansom SC: Shear stress-induced volume decrease in C11-MDCK cells by BK-alpha/beta4. Am J Physiol Renal Physiol 299: F507–F516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM: Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Satlin LM, Sheng S, Woda CB, Kleyman TR: Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Woda CB, Bragin A, Kleyman TR, Satlin LM: Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pluznick JL, Wei P, Grimm PR, Sansom SC: BK-{beta}1 subunit: Immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ortiz PA, Hong NJ, Garvin JL: Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. II. Role of PI3-kinase and Hsp90. Am J Physiol Renal Physiol 287: F281–F288, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ortiz PA, Hong NJ, Garvin JL: Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ: Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433–1443, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice WL, Van Hoek AN, Păunescu TG, Huynh C, Goetze B, Singh B, Scipioni L, Stern LA, Brown D: High resolution helium ion scanning microscopy of the rat kidney. PLoS ONE 8: e57051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Dong Z: Primary cilia and kidney injury: Current research status and future perspectives. Am J Physiol Renal Physiol 305: F1085–F1098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satir P, Christensen ST: Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Pedersen LB, Rosenbaum JL: Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85: 23–61, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL: Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol 164: 255–266, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szymanska K, Johnson CA: The transition zone: An essential functional compartment of cilia. Cilia 1: 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Q, Nelson WJ: Ciliary diffusion barrier: The gatekeeper for the primary cilium compartment. Cytoskeleton (Hoboken) 68: 313–324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pazour GJ, Rosenbaum JL: Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol 12: 551–555, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Gascue C, Katsanis N, Badano JL: Cystic diseases of the kidney: Ciliary dysfunction and cystogenic mechanisms. Pediatr Nephrol 26: 1181–1195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters AM, Beales PL: Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039–1056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Chapin HC, Caplan MJ: The cell biology of polycystic kidney disease. J Cell Biol 191: 701–710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Praetorius HA, Spring KR: The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G: TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 45.DeCaen PG, Delling M, Vien TN, Clapham DE: Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504: 315–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE: Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP: The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 48.LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL: Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem 98: 68–77, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Ishii S, Misaka T, Kishi M, Kaga T, Ishimaru Y, Abe K: Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem Biophys Res Commun 385: 346–350, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Praetorius HA, Leipziger J: Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 24: 3–10, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Bjaelde RG, Arnadottir SS, Overgaard MT, Leipziger J, Praetorius HA: Renal epithelial cells can release ATP by vesicular fusion. Front Physiol 4: 238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Booth JW, Tam FW, Unwin RJ: P2 purinoceptors: Renal pathophysiology and therapeutic potential. Clin Nephrol 78: 154–163, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Unwin RJ, Bailey MA, Burnstock G: Purinergic signaling along the renal tubule: The current state of play. News Physiol Sci 18: 237–241, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ: Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM: Mechanoregulation of BK channel activity in the mammalian cortical collecting duct: Role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan Y, Weinstein AM, Weinbaum S, Wang T: Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci U S A 107: 21860–21865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peti-Peterdi J: High glucose and renin release: The role of succinate and GPR91. Kidney Int 78: 1214–1217, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Goldberg ND, Passonneau JV, Lowry OH: Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem 241: 3997–4003, 1966 [PubMed] [Google Scholar]
- 60.Hebert SC: Physiology: Orphan detectors of metabolism. Nature 429: 143–145, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, Milligan G: Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int 76: 1258–1267, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J: Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR: Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27: 487–497, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H: Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299: 2054–2058, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ: Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones DT, Reed RR: Golf: An olfactory neuron specific-G protein involved in odorant signal transduction. Science 244: 790–795, 1989 [DOI] [PubMed] [Google Scholar]
- 68.Sekirov I, Russell SL, Antunes LC, Finlay BB: Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Ley RE, Peterson DA, Gordon JI: Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Savage DC: Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31: 107–133, 1977 [DOI] [PubMed] [Google Scholar]
- 71.Whitman WB, Coleman DC, Wiebe WJ: Prokaryotes: The unseen majority. Proc Natl Acad Sci U S A 95: 6578–6583, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bugaut M: Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987 [DOI] [PubMed] [Google Scholar]
- 73.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M: Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samuel BS, Gordon JI: A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 103: 10011–10016, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill MJ, Drasar BS: The normal colonic bacterial flora. Gut 16: 318–323, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI: Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A 105: 16767–16772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI: A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J: Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94: 58–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ: Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Armstrong DG, Beever DE: Post-abomasal digestion of carbohydrate in the adult ruminant. Proc Nutr Soc 28: 121–131, 1969 [DOI] [PubMed] [Google Scholar]
- 82.Demigne C, Remesy C: Influence of unrefined potato starch on cecal fermentations and volatile fatty acid absorption in rats. J Nutr 112: 2227–2234, 1982 [DOI] [PubMed] [Google Scholar]
- 83.Demigné C, Rémésy C, Rayssiguier Y: Effect of fermentable carbohydrates on volatile fatty acids, ammonia and mineral absorption in the rat caecum. Reprod Nutr Dev 20: 1351–1359, 1980 [DOI] [PubMed] [Google Scholar]
- 84.Hintz HF, Hogue DE, Walker EF, Jr, Lowe JE, Schryver HF: Apparent digestion in various segments of the digestive tract of ponies fed diets with varying roughage-grain ratios. J Anim Sci 32: 245–248, 1971 [DOI] [PubMed] [Google Scholar]
- 85.Keys JE, Jr, DeBarthe JV: Site and extent of carbohydrate, dry matter, energy and protein digestion and the rate of passage of grain diets in swine. J Anim Sci 39: 57–62, 1974 [DOI] [PubMed] [Google Scholar]
- 86.Orskov ER, Fraser C, Kay RN: Dietary factors influencing the digestion of starch in the rumen and small and large intestine of early weaned lambs. Br J Nutr 23: 217–226, 1969 [DOI] [PubMed] [Google Scholar]
- 87.Orskov ER, Fraser C, McDonald I: Digestion of concentrates in sheep. 3. Effects of rumen fermentation of barley and maize diets on protein digestion. Br J Nutr 26: 477–486, 1971 [DOI] [PubMed] [Google Scholar]
- 88.Orskov ER, Fraser C, Mason VC, Mann SO: Influence of starch digestion in the large intestine of sheep on caecal fermentation, caecal microflora and faecal nitrogen excretion. Br J Nutr 24: 671–682, 1970 [DOI] [PubMed] [Google Scholar]
- 89.Topping DL, Illman RJ, Taylor MN, McIntosh GH: Effects of wheat bran and porridge oats on hepatic portal venous volatile fatty acids in the pig. Ann Nutr Metab 29: 325–331, 1985 [DOI] [PubMed] [Google Scholar]
- 90.Anderson IH, Levine AS, Levitt MD: Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med 304: 891–892, 1981 [DOI] [PubMed] [Google Scholar]
- 91.Bond JH, Levitt MD: Quantitative measurement of lactose absorption. Gastroenterology 70: 1058–1062, 1976 [PubMed] [Google Scholar]
- 92.Englyst HN, Cummings JH: Digestion of the polysaccharides of some cereal foods in the human small intestine. Am J Clin Nutr 42: 778–787, 1985 [DOI] [PubMed] [Google Scholar]
- 93.McNeil NI, Bingham S, Cole TJ, Grant AM, Cummings JH: Diet and health of people with an ileostomy. 2. Ileostomy function and nutritional state. Br J Nutr 47: 407–415, 1982 [DOI] [PubMed] [Google Scholar]
- 94.Perman JA, Modler S: Role of the intestinal microflora in disposition of nutrients in the gastrointestinal tract. J Pediatr Gastroenterol Nutr 2[Suppl 1]: S193–S196, 1983 [DOI] [PubMed] [Google Scholar]
- 95.Pomare EW, Branch WJ, Cummings JH: Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest 75: 1448–1454, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandberg AS, Andersson H, Kivistö B, Sandström B: Extrusion cooking of a high-fibre cereal product. 1. Effects on digestibility and absorption of protein, fat, starch, dietary fibre and phytate in the small intestine. Br J Nutr 55: 245–254, 1986 [DOI] [PubMed] [Google Scholar]
- 97.Saunders DR, Wiggins HS: Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol 241: G397–G402, 1981 [DOI] [PubMed] [Google Scholar]
- 98.Wiggins HS: Nutritional value of sugars and related compounds undigested in the small gut. Proc Nutr Soc 43: 69–75, 1984 [DOI] [PubMed] [Google Scholar]
- 99.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ: The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Capasso G, Unwin R, Rizzo M, Pica A, Giebisch G: Bicarbonate transport along the loop of Henle: Molecular mechanisms and regulation. J Nephrol 15[Suppl 5]: S88–S96, 2002 [PubMed] [Google Scholar]
- 101.Zhao J, Zhou Y, Boron WF: Effect of isolated removal of either basolateral HCO-3 or basolateral CO2 on HCO-3 reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol 285: F359–F369, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Zhou Y, Bouyer P, Boron WF: Role of a tyrosine kinase in the CO2-induced stimulation of HCO3- reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol 291: F358–F367, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP: The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21: 440–443, 1999 [DOI] [PubMed] [Google Scholar]
- 104.Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED: Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10: 153–161, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW: Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM: Pendrin modulates ENaC function by changing luminal HCO3-. J Am Soc Nephrol 21: 1928–1941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM: Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Wall SM, Pech V: Pendrin and sodium channels: Relevance to hypertension. J Nephrol 23[Suppl 16]: S118–S123, 2010 [PubMed] [Google Scholar]
- 111.Wall SM, Weinstein AM: Cortical distal nephron Cl(-) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol 305: F427–F438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D: α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest 123: 3166–3171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]