Abstract
Adolescence is often portrayed as a period of enhanced sensitivity to reward, with long-lasting neurobiological changes upon reward exposure. However, we previously found that time-dependent increases in cue-induced sucrose seeking were more pronounced in rats trained to self-administer sucrose as adults than as adolescents. In addition, adult, but not adolescent sucrose self-administration led to a decreased AMPA/NMDA ratio in the nucleus accumbens core, suggesting that long-lasting changes in glutamatergic transmission may affect adult processing of natural rewards. Here we tested whether altering glutamatergic transmission in the nucleus accumbens core via local injection of an mGluR2/3 agonist and antagonist affects cue-induced sucrose seeking following abstinence and whether this is different in the two age groups. Rats began oral sucrose self-administration training (10 days) on postnatal day (P) 35 (adolescents) or P70 (adults). Following 21 days of abstinence, rats received microinjections of the mGluR2/3 agonist LY379268 (0.3 or 1.0 μg/side) or vehicle into the nucleus accumbens core, and 15 minutes later cue-induced sucrose seeking was assessed. An additional group of rats trained as adults received nucleus accumbens core microinjections of the mGluR2/3 antagonist MPPG (0.12 or 0.5 μg/side).
Confirming our previous results, adult rats earned more sucrose reinforcers, while sucrose intake per body weight was similar across ages. On abstinence day 22, local injection of the mGluR2/3 agonist LY379268 increased cue-induced sucrose seeking only in adult rats, and had no effect in adolescents. Local injections of the mGluR2/3 antagonist MPPG had no effect on sucrose seeking in adult rats. These data suggest an important developmental difference in the neural substrates of natural reward, specifically a difference in glutamatergic transmission in the accumbens in cue-induced responding for sucrose between adolescent and adult rats.
Introduction
Adolescence is a period of continued brain development (Casey et al., 2010, Counotte et al., 2011b, Blakemore and Robbins, 2012) which renders individuals with different reward sensitivity and altered long-lasting consequences of exposure to rewards compared to reward exposure during adulthood (Counotte et al., 2011a, Counotte et al., 2014). Previously, we showed that time-dependent increases in cue-induced sucrose seeking (incubation of sucrose craving) were more pronounced in adult rats that started self-administration at postnatal day (P)70 compared to adolescent rats that started at P35 (Counotte et al., 2014). In addition, we found that AMPA/NMDA ratio in nucleus accumbens core was decreased after 21 days of abstinence in rats that self-administered sucrose as adults, but not adolescents (Counotte et al., 2014). This is the opposite of what was found following cocaine self-administration, where the AMPA/NMDA ratio is increased in accumbens shell medium spiny neurons (MSNs) (Conrad et al., 2008) and blockade of glutamate receptors decreases cue-induced cocaine seeking. Since adult rats showed the most pronounced decrease in AMPA/NMDA ratio after abstinence from sucrose, suggesting an overall decrease in glutamatergic transmission, we predicted that increasing synaptic glutamate levels in the accumbens would decrease cue-induced sucrose seeking in adults, but not adolescents. Conversely, we predict that decreasing glutamatergic transmission would increase cue-induced sucrose seeking in adult rats.
Mounting evidence suggests that activation of group II mGluRs reduces reinstatement to reward seeking (Moussawi and Kalivas, 2010). mGluR2 is expressed outside the active zone on presynaptic terminals and is coupled to Gi signaling, thereby acting as an autoreceptor that reduces glutamate release (Petralia et al., 1996, Xi et al., 2002). mGluR3 is expressed both presynaptic and postsynaptic and its effect on glutamate signaling in the accumbens is as yet unclear (Tamaru et al., 2001, Harrison et al., 2008). The ability of an mGluR2/3 agonist to reduce reward seeking has been shown both in a reinstatement session following extinction training and in the incubation of craving paradigm following forced abstinence from operant self-administration of drugs or food. Specifically, systemic injections of LY379268, an mGluR2/3 agonist, reduce reinstatement to cocaine (Baptista et al., 2004, Peters and Kalivas, 2006, Jin et al., 2010, Cannella et al., 2013), heroin (Bossert et al., 2005), nicotine (Liechti et al., 2007), alcohol (Backstrom and Hyytia, 2005, Zhao et al., 2006, Kufahl et al., 2011), methamphetamine (Kufahl et al., 2013) and food (Bossert et al., 2006b, Peters and Kalivas, 2006, Liechti et al., 2007, Jin et al., 2010) following extinction training. In addition, systemic administration of an mGluR2 positive allosteric modulator has the same effect: it decreases reinstatement to cocaine – but not food – following extinction training (Jin et al., 2010). To localize the effect of group II mGluRs on reinstatement, researchers have used local injections of LY379268, showing that injecting LY379268 in ventral tegmental area (VTA) (Bossert et al., 2004, Lu et al., 2012), accumbens shell (Bossert et al., 2006a) and core (Peters and Kalivas, 2006) also reduces reinstatement to heroin, cocaine and food following extinction training. Of most interest to our results using the incubation of craving paradigm (Grimm et al., 2001, Li et al., 2014) are the findings that systemic and central amygdala injections of LY379268 reduce cue-induced cocaine (Lu et al., 2007) and sucrose seeking (Uejima et al., 2007). Here, we tested whether modulating glutamate release using the mGluR2/3 agonist LY379268 affects cue-induced sucrose seeking following abstinence in rats that were trained to self-administer sucrose. Since we previously observed an increase in cue-induced sucrose seeking following adult compared to adolescent sucrose self-administration, accompanied by differences in synaptic plasticity in the accumbens core between age groups, we set out to determine if LY379268 would have a different effect in the two age groups. In addition, we tested whether the mGluR2/3 antagonist MPPG would conversely change responding following adult sucrose self-administration and abstinence.
Materials and Methods
Animals
Subjects were 84 male Long-Evans rats (Charles Rivers Laboratories, USA) that arrived two weeks before sucrose self-administration experiments started. Adolescent rats started sucrose self-administration at postnatal day (P)35, while adult rats were P70 when self-administration training started. Note that the group that was called “Young adolescents” in our previous paper (Counotte et al., 2014) is now called “Adolescents” as we decided to only look in the two extreme groups in the present study. Rats were housed two per cage under reversed lighting conditions (lights on at 7PM) for the duration of the sucrose self-administration experiment. Following cannulae implantation surgery, rats were single housed. Rats had unlimited access to food and water, except on days before a sucrose self-administration session, when they received 20 g of chow at the end of the day. Since the MPPG and LY379268 experiments in adult animals were run in parallel, the same saline control group was used for both the agonist and antagonist experiments. All experiments were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee and were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
Cannula implantation surgery
Starting 3 days after the last day of sucrose self-administration, rats were anesthetized with isofluorane (5% in oxygen for induction, 2–3% for maintenance) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Permanent guide cannulae (26-gauge, Plastics One, Roanoke, VA, USA) were implanted bilaterally 1 mm above the nucleus accumbens core. The coordinates (Paxinos and Watson, 1998) were AP +1.2 mm, ML ±2.8 mm, DV −6.4 mm (10° angle). Carpofen (5 mg/kg) was administered as an analgesic following surgery, and the rats were allowed at least 14 days to recover.
Intracranial injections
LY379268 ((1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid, Tocris, Bristol, UK) and MPPG ((RS)-α-Methyl-4-phosphonophenylglycine, Sigma-Aldrich, St. Louis, USA) were freshly dissolved in sterile saline on the day of infusion. On the test day, obturators were removed and LY379268 (0.3 or 1.0 μg/side), MPPG (0.12 or 0.5 μg/side) or vehicle at a volume of 0.5 μl was delivered at a flow rate of 0.5 μl/min using 10 μl Hamilton syringes driven by a syringe pump (Harvard apparatus, South Natick, MA, USA). Injectors were left in place for an additional minute to allow diffusion. Next, the obturators were reinserted and the rats were placed in the self-administration chamber, and after 15 minutes the session began. The intracranial doses of LY379268 and MPPG were based on our previous studies (Counotte et al., 2011a). At the end of all experiments (see subsequent sections) the rats were overdosed with pentobarbital and perfused transcardially with 100 ml saline, followed by 250 ml 4% paraformaldehyde in 0.1% sodium phophate buffer. Brains were removed and post fixed for 1 h at room temperature in the same fixative and stored in 30% sucrose plus 0.01% sodium azide. Next, the brains were sectioned, and stained with cresyl violet to verify anatomical placement of the cannulae.
Sucrose self-administration
Rats were tested in self-administration chambers (Med Associates, St. Albans, VT) housed within sound attenuating boxes. Each chamber was equipped with two levers located 9 cm above the floor, a houselight, a tone cue, and a light cue. Rats self-administered a 10% sucrose solution during 10 daily 3-h sessions on Monday through Friday. Presses on the active (and retractable) lever resulted in the activation of the infusion pump, and thus the delivery of 0.6 ml of 10% sucrose solution in the receptacle, accompanied by audiovisual cues (a tone and a light), and the houselight switching off during the 20 sec time-out, during which active lever presses were - recorded but did not lead to the delivery of sucrose (Grimm et al., 2005, Uejima et al., 2007, Karlsson et al., 2013, Counotte et al., 2014). Presses on the inactive (and stationary) lever had no programmed consequences but were recorded. The rats could earn a maximum of 100 reinforcers.
Cue-induced sucrose seeking
The test for cue-induced sucrose seeking is based on previous work (Uejima et al., 2007, Counotte et al., 2014). After 10 days of sucrose self-administration, guide cannulae were implanted in the nucleus accumbens and rats were allowed to recover for at least 2 weeks. 21 days following the last day of sucrose self-administration, rats were subjected to the test for cue-induced sucrose seeking. During testing, active lever-presses resulted in the activation of the tone and light cues (including extinguishment of the house light during the 20 s time-out period), but not sucrose delivery. The number of active and inactive lever presses was recorded.
Statistical analyses
Data were analyzed by ANOVA using the appropriate within-subjects or between-subjects designs (see “Results”). In case of statistically significant main effects or interaction effects (p<0.05), post hoc comparisons were conducted using Fisher PLSD or Bonferroni tests. Data are presented as mean ± SEM.
Results
Sucrose self-administration
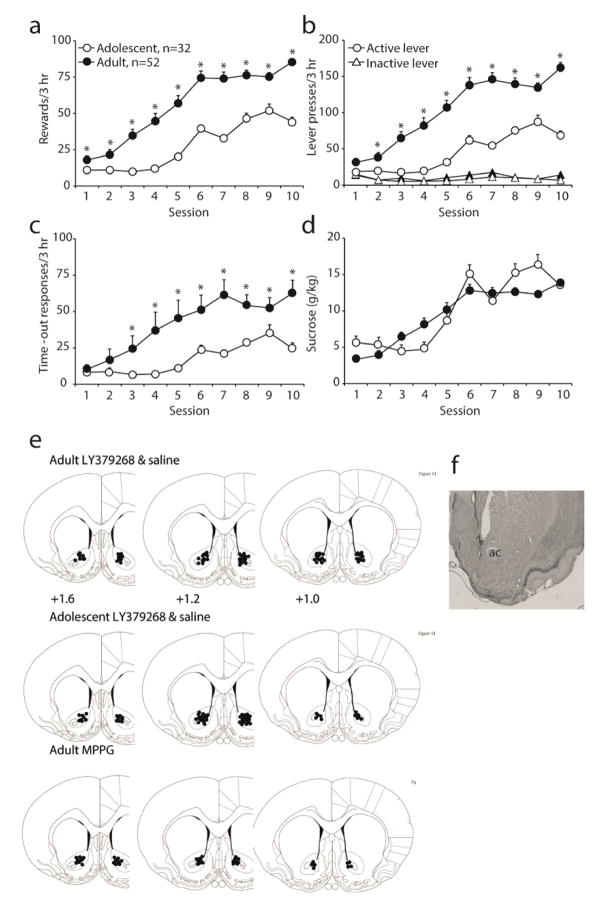
Data were analyzed separately for number of sucrose reinforcers earned, total active lever presses (reinforcers earned + time-out active lever presses), active lever presses during time-out, inactive lever presses and sucrose consumption per body weight. The analyses included the between-subjects factor of age group (adolescent; P35 at start and adult; P70 at start). As previously reported (Counotte et al., 2014), both age groups acquired stable sucrose self-administration (Fig 1a, session: F(9,792)=152.7, p<0.001), with adult animals earning more reinforcers (age group: F(1,88)=70.4, p<0.001). Also, adult animals made more active lever presses (Fig 1b, lever x age group: F(1,88)=94.4, p<0.001) and responded more during the timeout (Fig 1c, session x age group: F(9,792)=11.1, p<0.001). When normalized to bodyweight however, there was no difference in sucrose intake between adolescent and adult animals (Fig 1d).
Figure 1. Self-administration of liquid sucrose in adolescent and adult rats.
A. Number of reinforcers (i.e. lever presses during the active period) earned by adolescent (n=32, white circles) and adult (n=52, black circles) rats. B. Number of active (circles) and inactive (triangles) lever presses during both the active and the time-out period. C. Number of lever presses during the 20s time-out period following sucrose delivery. D. Sucrose consumption normalized to body weight. *p<0.05 compared with adolescent rats. E. Postmortem verification of cannula placements of intracranial LY379268 infusions (white circle depicts 0.3 μg dose, black circle depicts 1.0 μg dose) and saline infusions (triangles). The numbers are in millimeters anterior from Bregma (Paxinos and Watson, 1998). F. Photomicrograph depicting the injection site in an adult saline rat.
Local injection of MPPG has no effect on cue-induced sucrose seeking
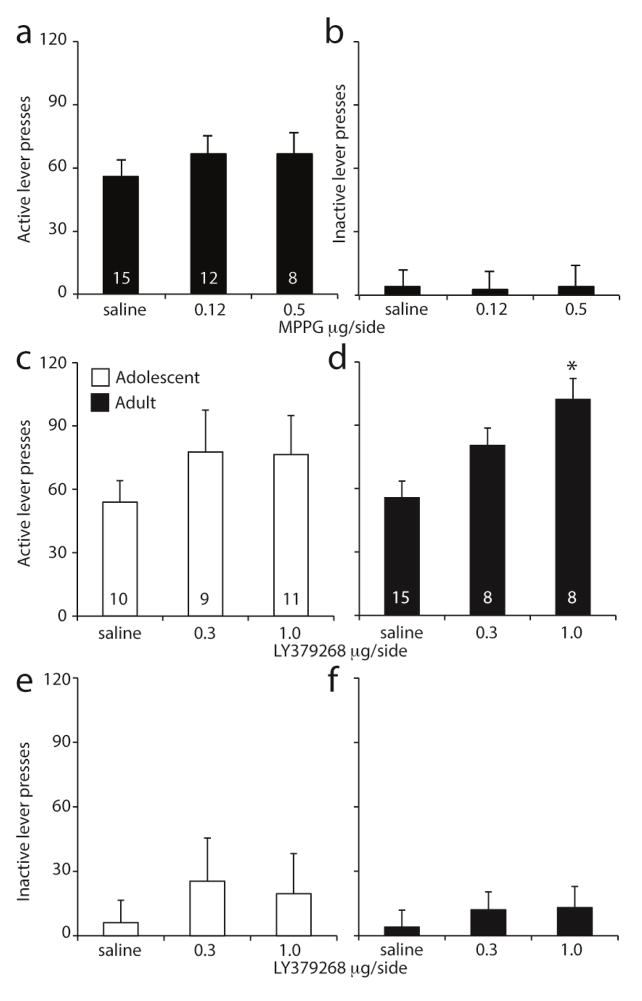
Next, to assess whether the previously found changes in synaptic plasticity in the NAc underlie the age differences in cue-induced sucrose seeking between adolescent and adult rats, we locally injected the mGluR2/3 antagonist MPPG in the NAc prior to cue-induced sucrose seeking (cannula placement shown in Figure 1e). Specifically, rats were trained to self-administer sucrose for 10 days during adulthood – because that is when we found the most pronounced changes in NAc synaptic plasticity - followed by 21 days of abstinence. On day 22, rats received a local injection MPPG or vehicle 15 minutes before cue-induced sucrose seeking was tested. We found that MPPG in the NAc had no effect on cue-induced sucrose seeking 21 days following adult sucrose self-administration (Fig 2a).
Figure 2. Effects of nucleus accumbens core injections of MPPG and LY379268 on cue-induced sucrose seeking after 21 days of abstinence.
Number of active (A) and inactive (B) lever presses following accumbens core injections of MPPG during the 1-h extinction session that was used to assess cue-induced sucrose seeking following adult sucrose self-administration. Number of active (C,D) and inactive (E,F) lever presses following accumbens core injections of LY379268 following adolescent (white bars, C,E) and adult (black bars, D,F) sucrose self-administration. During testing, lever presses led to contingent presentation of a tone-light cue previously paired with sucrose delivery, but not sucrose. The numbers indicate number of rats per group. *p<0.05 compared with saline injection.
Local injection of LY379268 increases cue-induced sucrose seeking in adult rats
Next, we tested the effect of intra-NAc injection of the mGluR2/3 agonist LY379268 on cue-induced sucrose seeking following adolescent and adult sucrose self-administration. Surprisingly, LY379268 in the NAc had no effect on cue-induced sucrose seeking 21 days following adolescent sucrose self-administration (Fig 2c), whereas it increased cue-induced sucrose seeking following adult self-administration training (Fig 2d, dose: F(2,29)=3.629, p=0.039). The adolescent and adult animals were not run simultaneously, which is why we deemed it appropriate to test them separately. Analyzing the combined data showed a near-significant trend of LY379268 (F(2,55)=2.992, p=0.058), but no interaction of age and LY379258 (F(2,55)=0.394, p=0.676). Analysis of presses on the inactive lever showed no effect of LY379268 following adolescent self-administration (Fig 2e, dose: F(2,27)=2.76, p=0.081) but an overall effect of LY379268 in animals that self-administered as adults (Fig 2f, dose: F(2,29)=3.58, p=0.04). However, post-hoc analyses showed no significant differences between the two doses of LY379268 and saline controls on inactive lever presses.
Discussion
Previously, we found differences in incubation of sucrose craving between rats that self-administered sucrose as adults or adolescents, with more incubation in adults (Counotte et al., 2014). This increased incubation of sucrose craving was accompanied by a decreased AMPA/NMDA ratio in the nucleus accumbens, which was only observed following adult, but not adolescent, sucrose self-administration (Counotte et al., 2014). Here, we extend these findings by demonstrating that local injection of the mGluR2/3 agonist LY379268 in the accumbens core has a different effect on cue-induced sucrose seeking in rats that self-administered sucrose during adulthood than during adolescence. Surprisingly, we found that intra-NAc LY379268 increases cue-induced sucrose seeking in rats that self-administered sucrose as adults, and had no effect on rats that were trained as adolescents. The mGluR2/3 antagonist MPPG had no effect on sucrose seeking in adult rats.
We reproduced our previous results showing that adult animals make more active lever presses for 10% sucrose solution during self-administration training, but in this cohort, when normalizing to body weight we did not observe a difference between rats starting self-administration at P35 (adolescents) or at P70 (adults). Another aspect of our previous study that we failed to replicate is the increased cue-induced sucrose seeking after 21 days of abstinence following adult versus adolescent sucrose self-administration. The reason might be that for this study the adult and adolescent rats were not trained and tested simultaneously. Another reason for the cue-induced sucrose seeking to be lower in the adult animals compared with the previous cohort of rats could be the stress of undergoing surgery, the presence of the guide cannula in the accumbens or the stress associated with the local injection of vehicle. In addition the rats were placed in the operant chamber for 15 minutes following the local injection, whereas in our previous study the session was started immediately upon placement of the rat in the test chamber. Experiencing the test chamber for 15 minutes without being able to make a lever press may have reduced subsequent cue-induced sucrose seeking behavior.
We had hypothesized that local injection of the group II mGluR antagonist MPPG would reduce cue-induced sucrose seeking following adult sucrose self-administration by increasing synaptic glutamate, since we observed a reduction of AMPA/NMDA ratio in the previous study. However, we saw no effect of MPPG on reinstatement, at a dose that had been shown to affect attention when infused in the prefrontal cortex (Counotte et al., 2011a). The fact that this cohort of adult animals showed less reinstatement to sucrose seeking may obscure the effects of MPPG in this instance, and a higher dose of MPPG might have been sufficient to overcome this. Future experiments would have to clarify whether MPPG is able to block the increased cue-induced sucrose seeking provoked by local injection of LY379268 to prove that indeed group II mGluRs are the site of action of this effect.
Our finding that an mGluR2/3 agonist leads to increased cue-induced sucrose seeking is unexpected, as local injection of an mGluR2/3 agonist will likely decrease glutamate release in the accumbens (Moussawi and Kalivas, 2010). Reduced glutamate levels in the accumbens have been shown previously to lead to decreased reinstatement for drugs and food (Bossert et al., 2006a, Peters and Kalivas, 2006). However, LY379268 has also been shown to increase surface expression of GluA1 and GluA2 subunits in cultured prefrontal neurons (Wang et al., 2013). If the same occurs in accumbens MSNs, injection of LY379268 could increase the AMPA/NMDA ratio, which also happens during incubation of sucrose craving (Counotte et al., 2014), thereby exacerbating cue-induced sucrose seeking. Whether this happens in vivo, and whether this is only the case following adult and not adolescent sucrose self-administration remains to be determined. Similarly, examining the effect of intra-NAc LY379268 in a no forced-abstinence control group would answer whether a period of incubation in which the AMPA/NMDA ratio has been shown to increase, is necessary for LY379268 to increase cue-induced sucrose seeking.
For both cocaine and food reinstatement, it has been shown that intra-accumbens LY379268 injections decrease reinstatement, but following extinction training, while the rats in our paradigm undergo abstinence. Careful examination of the data from Peters et al. (2006) show that a low dose of LY379268 increases food seeking (albeit not statistically significant), and only a high dose decreases food seeking. It has been shown that reinstatement to food does not increase accumbens glutamate, whereas reinstatement to cocaine does (McFarland et al., 2003). Because the rats in our experiment underwent abstinence rather than forced extinction of sucrose seeking, it is hard to interpret our data in the context of these studies. It is also possible that cue-induced sucrose seeking invokes different glutamatergic substrates in the NAc than response behaviors for water, food, or other natural reinforcers, and this warrants further study.
Most importantly, our data show that LY379268-induced decrease in cue-induced sucrose seeking only happens in rats that self-administered sucrose as adults, which has been shown to lead to a reduced AMPA/NMDA ratio in the accumbens (Counotte et al., 2014). This may be due to the absence of glutamate release after mGluR2/3 agonist administration, or due to an increased expression of AMPA receptors upon LY379268 injection related to our methodology.
In conclusion, our data demonstrate age-dependent sensitivity to mGluR2/3-induced changes in the level of cue-induced sucrose seeking. When considering activation of group II mGluRs as treatment to reduce reinstatement to drug seeking it is important to determine whether this increased cue-induced sucrose seeking might interfere with therapeutic benefits.
Intra-NAc injection of mGluR2/3 agonist increased cue-induced sucrose seeking in adult rats.
No effect of local mGluR2/3 agonist after adolescent sucrose self-administration.
No effect of mGluR2/3 antagonist in either age group.
Acknowledgments
The authors would like to thank Dr. Yavin Shaham for helpful comments on experimental design and the manuscript. The research and writing of this paper was supported by R01 DA014020 to PO’D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nat Neurosci. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006a;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue- induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006b;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, Deroche-Gamonet V, Hansson AC, Spanagel R. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011a;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, O’Donnell P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology (Berl) 2014;231:1675–1684. doi: 10.1007/s00213-013-3294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Developmental Cognitive Neuroscience. 2011b;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. Journal of psychopharmacology. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Kircher DM, Shaham Y, O’Donnell P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology (Berl) 2013;226:45–51. doi: 10.1007/s00213-012-2882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Olive MF. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addiction biology. 2014 doi: 10.1111/adb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lu L, Xue Y, Steketee JD, Rebec GV, Sun W. Regulation of cocaine-induced reinstatement by group II metabotropic glutamate receptors in the ventral tegmental area. Psychopharmacology (Berl) 2012;220:75–85. doi: 10.1007/s00213-011-2455-5. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Li YC, Snyder MA, Wang H, Li F, Gao WJ. Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons. PloS one. 2013;8:e61787. doi: 10.1371/journal.pone.0061787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]