ABSTRACT
Many species of bacteria use a cell-cell communication system called quorum sensing (QS) to coordinate group activities. QS systems frequently regulate the production of exoproducts. Some of these products, such as proteases, are “public goods” that are shared among the population and vulnerable to cheating by nonproducing members of the population. Because the QS system of the opportunistic pathogen Pseudomonas aeruginosa regulates several public goods, it can serve as a model for studying cooperation. Bacteria also commonly regulate antimicrobial production through QS. In this study, we focused on the hypothesis that QS-regulated antimicrobials may be important for P. aeruginosa to protect against cheating by another bacterial species, Burkholderia multivorans. We assessed laboratory cocultures of P. aeruginosa and B. multivorans and investigated the importance of three P. aeruginosa QS-regulated antimicrobials, hydrogen cyanide, rhamnolipids, and phenazines, for competition. We found that P. aeruginosa dominates cocultures with B. multivorans and that the three antimicrobials together promote P. aeruginosa competitiveness, with hydrogen cyanide contributing the greatest effect. We show that these QS-regulated antimicrobials are also critical for P. aeruginosa to prevent B. multivorans from cheating under nutrient conditions where both species require a P. aeruginosa quorum-regulated protease for growth. Together our results highlight the importance of antimicrobials in protecting cooperating populations from exploitation by other species that can act as cheaters.
IMPORTANCE Cooperative behaviors are threatened by social cheating, wherein individuals do not produce but nonetheless benefit from shared public goods. Bacteria have been shown to use several genetic mechanisms to restrain the emergence of cheaters from within the population, but public goods might also be used by other bacterial species in the vicinity. We demonstrate that a public good produced by Pseudomonas aeruginosa can be used by another species, Burkholderia multivorans, to obtain carbon and energy. We also show that P. aeruginosa antimicrobials that are coregulated with the public good prevent invasion by the cheating species. Our results demonstrate that cross-species cheating can occur and that coregulation of public goods with antimicrobials may stabilize cooperative behavior in mixed microbial communities.
INTRODUCTION
Many species of Proteobacteria use acyl-homoserine lactone (AHL) quorum sensing (QS) to regulate genes in a cell density-dependent manner (1, 2). In the opportunistic human pathogen Pseudomonas aeruginosa there are two complete AHL QS circuits, LasI-LasR and RhlI-RhlR. The LasI-LasR system is activated by N-3-oxo-dodecanoyl homoserine lactone (3OC12-HSL) (3), and the RhlI-RhlR system is activated by butanoyl homoserine lactone (C4-HSL) (4). LasR is required for RhlR activity. LasR and RhlR together regulate the production of dozens of genes in P. aeruginosa (5, 6), and many of the genes regulated by LasR and RhlR are involved in the production of exoproducts (6). Because they are extracellular, the exoproducts may constitute “public goods” that benefit all of the members of the population, regardless of which individuals are producing them (7–9). Shared public goods are susceptible to social cheating, or defection, by individuals that utilize the public goods while avoiding the cost of their production. A QS-controlled public good in P. aeruginosa is an extracellular protease, elastase (8). Under circumstances where elastase production is required to obtain carbon and energy, LasR mutant social cheaters can arise in the population (7, 8).
Many bacteria also use QS to regulate the production of antimicrobials. Examples include production of bactobolins by Burkholderia thailandensis and of violacein by Chromobacterium violaceum, among others (10, 11). In the case of P. aeruginosa, many antimicrobials are regulated by RhlR; these include peroxides, phenazines, rhamnolipids, and cyanide (6, 12, 13). The benefits of using QS to control antimicrobials are unknown, but it is thought that this regulation may be important for competition with other species. Several studies with laboratory cocultures have shown that QS controls antimicrobials that are important for competition (14–19).
We are interested in understanding why QS commonly controls antimicrobials and how QS-regulated antimicrobials might be important for stabilizing public goods cooperation during interspecies competition. Specifically, our hypothesis is that QS-controlled antimicrobials may offer protection against cheating by other species. For this study, we focused on P. aeruginosa because of the increasing body of work describing the role of QS, and QS-controlled elastase, in cooperation (7, 8). We chose as a competitor the opportunistic pathogen, Burkholderia multivorans. Although B. multivorans and P. aeruginosa are saprophytic opportunistic pathogens that can coexist in soil and in lung infections of patients with the genetic disease cystic fibrosis, we focused on B. multivorans because the growth rate and yield of B. multivorans are similar to those of P. aeruginosa under our conditions. We also chose this strain of B. multivorans because it does not produce a protease similar to elastase, which would confound our interpretation of data. We show that in laboratory cocultures, P. aeruginosa QS-controlled antimicrobials can protect against public-goods cheating by B. multivorans. This may be one explanation for why cooperative public goods and antimicrobial production are coregulated by quorum sensing in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and media.
Bacteria were grown in minimal medium with 1% (wt/vol) sodium caseinate as a carbon source (casein broth) (8) or in Luria-Bertani (LB) broth buffered to pH 7 with 50 mM morpholinepropanesulfonic acid (MOPS) (20). Exogenous elastase was added when required as previously described (7). When appropriate, the following antibiotics were used (per ml): gentamicin at 30 μg or 100 μg (for selection of P. aeruginosa transconjugants made from pUC18 miniTn7 or pEXG2 derivatives, respectively) or 15 μg (for Escherichia coli), and for selection from coculture growth, trimethoprim at 100 μg (to select for P. aeruginosa) and 10 μg gentamicin with 17.3 μg polymyxin B (to select for B. multivorans).
The bacterial strains and plasmids used are listed in Table S1 in the supplemental material. We used wild-type and mutant derivatives of P. aeruginosa strain PAO1-UW (referred to as PAO1) (21) and a wild-type B. multivorans strain (strain AMT 0468-1) (J. Burns, unpublished data). PAO1-derived strains with transposon insertions in phzA1, hcnC, and rhlB were described previously (22), and double and triple mutants of these were constructed by sequentially transferring the genomic DNA containing the mutant allele into the strain of interest using previously described methods (23). In each case, Cre recombination was used to remove the antibiotic resistance marker (24) prior to introducing mutation-containing genomic DNA fragments. PAO1-derived strains with unmarked, in-frame deletions of lasR, hcnC, and rhlR were described previously (25), and we constructed unmarked, in-frame deletions of rhlB using similar methods. Briefly, PCR-amplified DNA fragments flanking rhlB were cloned into pEXG2, which was used to transform Escherichia coli S17-1, and then the pEXG2 derivatives were crossed into PAO1 by mating. Transconjugants were selected on Pseudomonas isolation agar containing gentamicin, and deletion mutants were selected with no-salt LB agar containing 10% (wt/vol) sucrose. The hcnC rhlB double mutant was constructed by introducing the pEXG2 plasmid containing the hcnC deletion fragments into PAO1 ΔrhlB. To complement the hcnC mutation, we introduced an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible hcnC into the neutral att site using the pUC18miniTn7-LAC plasmid as described previously (26). Mutant construction was confirmed in all cases by PCR.
Coculture experiments.
Cocultures with Pseudomonas aeruginosa and Burkholderia multivorans were performed at 37°C. To inoculate cocultures in LB broth, pure cultures were grown to mid-logarithmic phase, subcultured to fresh LB broth to an optical density at 600 nm (OD600) of 0.1, and grown to an OD600 of 1 before combining. The inoculum of each species in the coculture was 1 × 107 to 5 × 107 CFU/ml. Cocultures in LB broth were grown for 24 h before plating to enumerate each species. To inoculate cocultures in casein broth, late-logarithmic-phase cultures of B. multivorans (OD600 of 1.0) were washed twice with phosphate-buffered saline (PBS) and suspended in casein broth prior to combining at the indicated ratio with approximately 5 × 107 CFU/ml P. aeruginosa. The P. aeruginosa inoculum was from a logarithmic-phase culture grown in casein broth (wild type and the triple-antimicrobial mutant) or in LB broth and washed with PBS (LasR mutant). Cocultures were grown for 24 h and then diluted 1:100 into fresh casein broth for three consecutive days. All cocultures were grown in 3 ml of medium in 18-mm glass tubes and incubated with shaking at 250 rpm. The ratio or CFU of each species was determined on LB agar plates with antibiotic selection as described above.
RESULTS
P. aeruginosa relies on quorum-regulated hydrogen cyanide, rhamnolipids, and pyocyanin to compete with B. multivorans.
P. aeruginosa produces several QS-controlled antimicrobials that are important for coculture competition with other bacterial species, for example, Agrobacterium tumefaciens and Staphylococcus aureus (14, 16, 27). We sought to examine the role of P. aeruginosa QS during competition with B. multivorans. We assessed the competitiveness of the P. aeruginosa wild type or QS receptor mutants (LasR− or RhlR−) in coculture competition with wild-type B. multivorans. We also assessed a P. aeruginosa AHL synthase mutant (LasI− RhlI−) in competition. We found that wild-type P. aeruginosa outcompeted B. multivorans, and this advantage was lost in either the LasR− or RhlR− single mutant (Fig. 1). The AHL synthase-deficient LasI− RhlI− double mutant had a competitive defect similar to that of either of the single receptor mutants, and this defect could be rescued by supplying the P. aeruginosa AHLs 3OC12-HSL and C4-HSL to the growth medium (Fig. 1A). Because RhlR is activated by LasR, these results suggest the possibility that LasR acts through RhlR to regulate factors that are important for P. aeruginosa to compete with B. multivorans.
FIG 1.
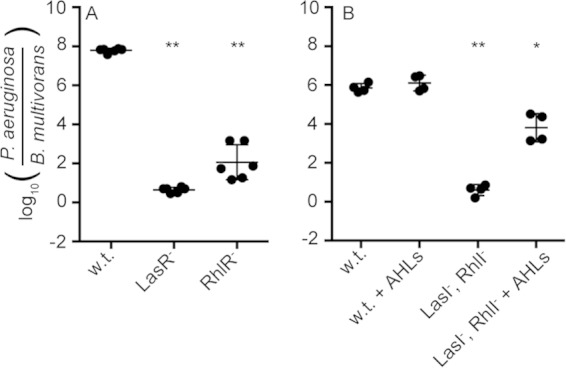
B. multivorans competition with wild-type (w.t.) P. aeruginosa and quorum-sensing mutants. Coculture experiments were carried out in Luria-Bertani (LB) broth. The relative fitness of each strain is shown as the ratio of P. aeruginosa to B. multivorans at 24 h, determined by selective plating and colony counts. (A) B. multivorans in competition with wild-type, LasR−, and RhlR− P. aeruginosa. (B) Complementation of the quorum-null phenotype. The P. aeruginosa AHLs C4-HSL and 3OC12-HSL (5 μM each) were added to culture tubes prior to inoculation. The solid lines represent means for each group. Each symbol represents the outcome of an individual experiment; there were at least four independent experiments for each condition. Statistical analysis by t test compared to wild type: *, P ≤ 0.002; **, P < 1 × 10−7.
Three known RhlR-controlled antimicrobials in P. aeruginosa have been shown to promote competitiveness with other species: the phenazine pyocyanin, hydrogen cyanide, and rhamnolipids (14). We investigated the individual and combined roles of each of these in competition with B. multivorans. Individually, pyocyanin, hydrogen cyanide, and rhamnolipid production had a minimal contribution to competitiveness. However, in combination there was a significant effect. A mutant defective for all three of the antimicrobials was five orders of magnitude less competitive than the wild type, similar to the case for both the LasR− and RhlR− mutants (Fig. 2A). The results of competitions with P. aeruginosa double-antimicrobial mutants indicated that hydrogen cyanide had the greatest contribution to the competitive ability of P. aeruginosa. We were able to restore competitiveness of hydrogen cyanide mutants by expressing the hydrogen cyanide synthase gene hcnC from a neutral site in the chromosome (Fig. 2B). These results indicate that the RhlR QS regulator is important for P. aeruginosa to compete with B. multivorans due to production of RhlR-controlled hydrogen cyanide and, to a lesser extent, pyocyanin and rhamnolipids.
FIG 2.
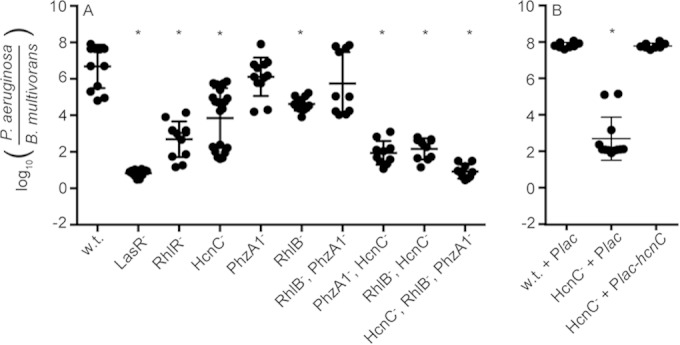
B. multivorans competition with wild-type P. aeruginosa and quorum-sensing and antimicrobial mutants. Coculture experiments were carried out in Luria-Bertani (LB) broth. (A) P. aeruginosa strains are wild type (PAO1) or mutants deficient for production of LasR (lasR), RhlR (rhlR), hydrogen cyanide (hcnC), pyocyanin (phzA1), and/or rhamnolipid (rhlB). The starting ratio of P. aeruginosa to B. multivorans was 1:1. The final (24-h) ratio of P. aeruginosa to B. multivorans was determined by selective plating and colony counts. (B) Hydrogen cyanide complementation restores the wild-type phenotype. IPTG (1 mM final concentration) was added prior to inoculation to induce hydrogen cyanide production from the Plac promoter. The solid lines represent means for each group. Each symbol represents the outcome of an individual experiment; there were at least six independent experiments for each condition. Statistical analysis by t test compared to wild type: *, P ≤ 1 × 10−5.
B. multivorans can cheat on the P. aeruginosa protease elastase.
Because LasR is required for the production of the protease elastase, and elastase is needed to proteolyze casein to liberate nutrients for growth, LasR mutants show poor or no growth on casein as the sole carbon and energy source (Fig. 3A). We found that our strain of B. multivorans also did not proteolyze casein (Fig. 3A) and demonstrated poor growth in casein medium compared to identically grown cultures with the elastase supplied exogenously (Fig. 3B). Thus, B. multivorans does not produce proteases that promote growth on casein but can benefit from exogenous elastase.
FIG 3.
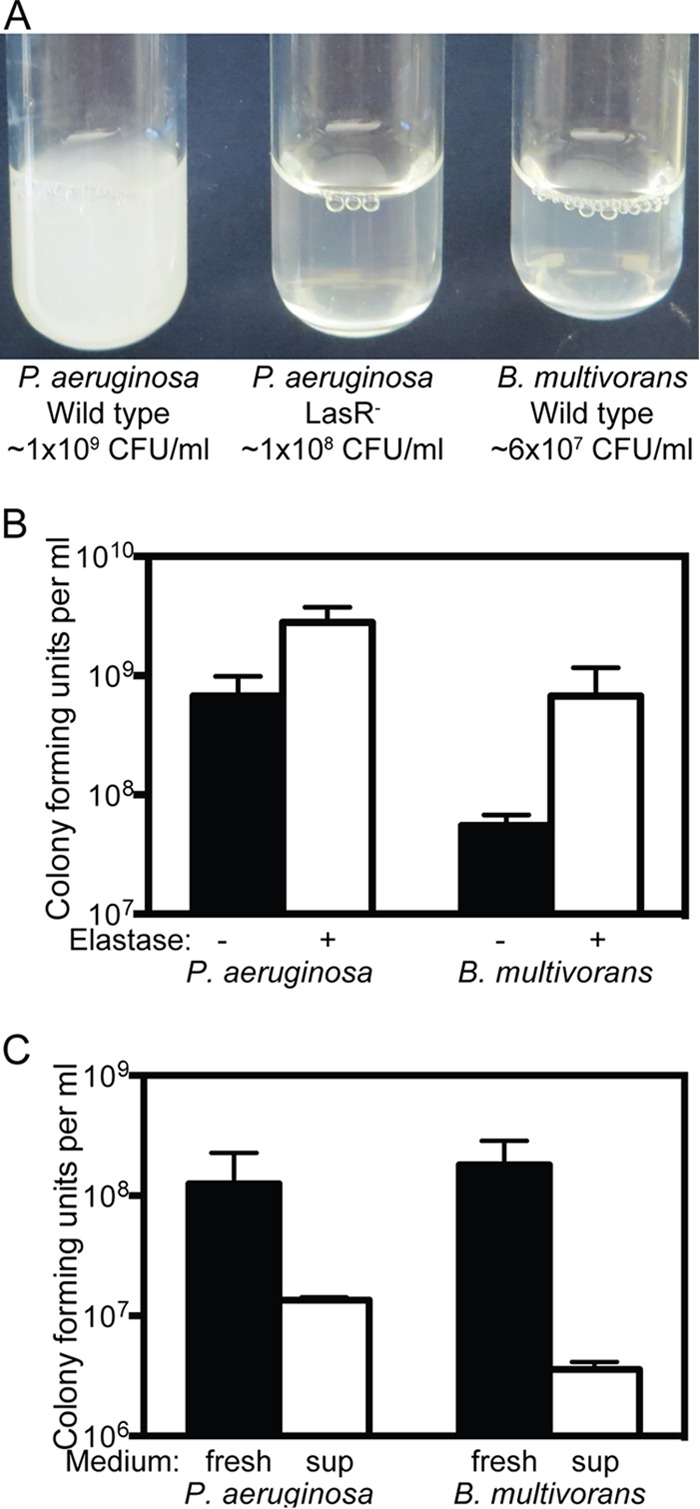
Bacterial growth with casein or Casamino Acids. (A) P. aeruginosa and B. multivorans cultures grown in casein medium. The turbidity and growth yields at 24 h are shown. (B) Final cell densities of P. aeruginosa and B. multivorans after 24 h of growth in casein medium (black bars) or casein medium with exogenously added elastase protease (0.095 U/ml) (white bars). (C) Final cell density of B. multivorans or P. aeruginosa after 24 h of growth in 0.01% Casamino Acids (black bars) or in filtered fluid from casein-grown stationary-phase cultures of the other species (white bars) (see the text). In each case, the final cell density was determined by dilution plating.
Our results are consistent with the idea that B. multivorans can utilize nutrients liberated by P. aeruginosa elastase. However, in cooperating systems, true cheaters must compete with the cooperators for the same resources. To test whether B. multivorans and P. aeruginosa are competing for carbon and energy in casein medium, we assessed growth of each species in a defined medium with limiting Casamino Acids (the breakdown product of casein). We grew each species in Casamino Acids, filter sterilized the culture fluid, and used the spent supernatant as a growth medium for the other species. When grown on Casamino Acids, each species increased from a starting density of 5 × 106 to about 108 cells per ml; however, when grown on spent filtrates, neither species increased in cell density (Fig. 3C). These results indicate that pregrowth in Casamino Acids depletes the medium of the nutrients required for growth of the other species. This is consistent with the idea that in casein medium, B. multivorans and P. aeruginosa compete for the nutrients liberated by elastase. Together, our results support the conclusion that B. multivorans is able to cheat on the P. aeruginosa public good elastase.
Quorum-controlled antimicrobials protect P. aeruginosa cooperators from B. multivorans invasion.
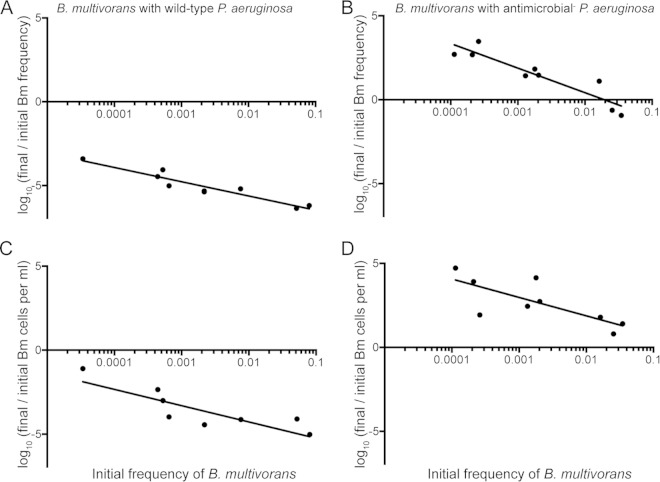
Our results showed that P. aeruginosa QS-controlled antimicrobials are important for competitiveness with B. multivorans under nutrient-rich conditions (Fig. 2A). We also showed that P. aeruginosa QS-controlled elastase is a public good that can be cheatable by B. multivorans (Fig. 3). We hypothesized that during coculture growth, B. multivorans can avail itself of resources liberated by P. aeruginosa elastase and that the P. aeruginosa QS-controlled antimicrobials can protect against B. multivorans cheating. We tested this hypothesis by growing B. multivorans with either wild-type or triple-antimicrobial mutant (hcnC rhlB phzA1) P. aeruginosa in casein medium and varying the B. multivorans starting frequency with respect to P. aeruginosa. We found that regardless of the starting frequency, B. multivorans was essentially eliminated from the culture with wild-type P. aeruginosa after 48 h of passage (Fig. 4A and C and Table 1). However, the P. aeruginosa triple-antimicrobial mutant was unable to prevent B. multivorans from increasing in the population; with the triple-antimicrobial mutant, B. multivorans came to comprise 10% of the final population, regardless of the starting frequency (Fig. 4B and Table 1). Not surprisingly, because the P. aeruginosa LasR− mutant does not produce proteases needed for maximal growth in the casein medium, B. multivorans grew poorly with the LasR− mutant compared with the protease-producing triple-antimicrobial P. aeruginosa strain (Table 1).
FIG 4.
Competitions in casein medium. Wild-type B. multivorans was inoculated at various frequencies with either wild-type P. aeruginosa (A and C) or the antimicrobial-defective (hcnC phzA1 rhlB) mutant (B and D). Competitions were carried out in nutrient-limited casein medium as described in Materials and Methods. In panels A and B, the data are plotted as the frequency of B. multivorans relative to P. aeruginosa (log10 final/initial frequency). In panels C and D, the same data are plotted as the fitness of B. multivorans alone (log10 final/initial CFU). Some of these data are also shown in Table 1. Lines indicate a nonlinear regression fit using an ordinary fit model.
TABLE 1.
Final yields of P. aeruginosa and B. multivorans in casein coculture
| P. aeruginosa strain cocultured with wild-type B. multivorans | Final growth yield (CFU/ml)a |
|
|---|---|---|
| P. aeruginosa | B. multivorans | |
| Wild type | 9 (±7) × 109 | <1 × 102 |
| LasR− mutant | 1 (±1) × 108 | 5 (±0.3) × 107 |
| Antimicrobial mutantb | 5 (±4) × 109 | 4 (±3) × 108 |
The values are the means from at least three independent experiments, with ranges indicated in parentheses. The starting ratio of B. multivorans to P. aeruginosa was 1:1,000 (see Materials and Methods).
The P. aeruginosa antimicrobial mutant has disruptions in three antimicrobial genes (hcnC, phzA1, and rhlB).
The results demonstrate a negative frequency-dependent selection of B. multivorans when grown in coculture with the P. aeruginosa triple-antimicrobial mutant in casein medium (Fig. 4B and D). This finding is consistent with B. multivorans cheating on the P. aeruginosa-produced public good elastase (28) because the benefit of cheating decreases as the frequency and number of cooperators decline. That is, as cheaters increase in frequency, they “cheat” less effectively because there are fewer cooperators to provide the public good. There are other potential explanations for negative frequency dependence that would be consistent with the observed results, including niche construction (29). If the observed selection were not a consequence of a cooperator-cheater dynamic, we would expect negative frequency dependence under other conditions where B. multivorans and the antimicrobial-deficient P. aeruginosa are grown together. However, we observed that in LB, B. multivorans had no fitness benefit at any starting frequency (see Fig. S1 in the supplemental material). This further supports the idea that the invasion of P. aeruginosa by B. multivorans in casein medium reflects B. multivorans cheating on the public good elastase.
DISCUSSION
Social cheating poses a threat to cooperating populations because cheaters have the potential to overrun the population and cause the cooperative behavior to be lost (30, 31). We previously described mechanisms by which quorum-sensing mutants are restrained in populations (25, 30). However, in many environmental situations, cooperating populations of bacteria may coexist with other species that could also avail themselves of the products of public goods. This is a form of cheating by other species. Here we used a laboratory coculture model to provide evidence that supports the idea that coregulation of antimicrobials with public goods can prevent a competitor from cheating.
In this light, B. multivorans may be viewed as a type of cheater that can utilize the nutrients liberated by P. aeruginosa proteases without incurring any costs. By definition, a cheater must engage in rivalrous competition for public goods with other members of the population. Our evidence indicates that P. aeruginosa and B. multivorans may compete for the same nutrients (Fig. 3), demonstrating that B. multivorans can act as a cheater in cooperating populations of P. aeruginosa. Previous studies have shown that LasR mutants can also act as cheaters (7, 8). LasR mutants are more classical cheaters in that they arise from the wild type and therefore have metabolism and fitness similar to those of the wild type. However, the B. multivorans cheater is fundamentally different from the LasR mutant cheaters, and B. multivorans and P. aeruginosa are not equal competitors. For example, P. aeruginosa may inhibit growth of B. multivorans by using intoxicants, and some of these may be QS independent. This may explain why, in our experiment, we observed that B. multivorans invaded the population and reached 10% of the total population (Fig. 4), whereas LasR mutants reach 30% or more of the total population and can, under certain circumstances, cause the population to collapse (8, 25, 30).
Many antimicrobials have more than one function. Hydrogen cyanide, for example, is classically thought of as a poison that can promote virulence in a host or competitiveness with other species (32, 33). The latter is supported by results in the present study indicating that hydrogen cyanide, with several other antimicrobials, promotes the ability of P. aeruginosa to compete with B. multivorans (Fig. 2A). However, hydrogen cyanide is also important for controlling intraspecies cheating in P. aeruginosa. Hydrogen cyanide was recently shown to be important for policing cooperating P. aeruginosa populations against runaway cheating (25). This is because hydrogen cyanide produced by cooperators inhibits growth of LasR mutant cheaters and prevents them from invading to a high frequency (25). Hydrogen cyanide production by natural populations of P. aeruginosa may have different roles dependent on environmental conditions.
Social cheating has generally been described as defection from cooperation by individuals within a population. Examples of social cheaters include QS mutants in the case of P. aeruginosa or tax avoiders in the case of humans (34). However, bacteria commonly live in environments where many species may be present. These other species represent a unique threat to cooperating populations because they are not necessarily amenable to species-specific restraints on cheating. Our laboratory coculture experiments describe how, by coproducing antimicrobials with cheatable public goods, cooperating populations are able to protect themselves against such interlopers. In this case, cheating is not defection from cooperation per se but the use of a public resource provided by another species to the detriment of the providing species (through resource consumption). It is difficult to speculate whether this type of cheating may occur in the context of a cooperating bacterial community; however, recent work on interspecies cooperation suggests that it might be possible (35–37). Our data also add to the growing body of evidence suggesting that the large size of the quorum-sensing regulon of P. aeruginosa reflects multiple environmental pressures on quorum sensing, including competition with other species.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Brook Peterson, Maureen Thomason, and Sudha Chugani for helpful discussion and technical assistance. Colin Manoil and Aaron Hinz supplied transposon mutants. Jane Burns provided B. multivorans strain AMT0468-1.
N.E.S., J.R.C., and A.A.D. are supported by grant R565 CR11 from the Cystic Fibrosis Foundation and grant DBI 0929454 from the NSF BEACON Evolution in Action program. A.A.D. is also supported by a Career Award for Medical Scientists from the Burroughs-Wellcome Fund. PHS award P30 DK089507 supported the transposon mutant library and the B. multivorans strain collection of Jane Burns.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00482-15.
REFERENCES
- 1.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson JP, Passador L, Iglewski BH, Greenberg EP. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster M, Greenberg EP. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol 296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 8.Sandoz KM, Mitzimberg SM, Schuster M. 2007. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A 104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat Rev Microbiol 4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 10.Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill ME, Parsek MR, Nierman WC, Greenberg EP. 2009. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J Bacteriol 191:3909–3918. doi: 10.1128/JB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 12.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessi G, Haas D. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol 182:6940–6949. doi: 10.1128/JB.182.24.6940-6949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An D, Danhorn T, Fuqua C, Parsek MR. 2006. Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proc Natl Acad Sci U S A 103:3828–3833. doi: 10.1073/pnas.0511323103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler JR, Heilmann S, Mittler JE, Greenberg EP. 5 July 2012. Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. ISME J doi: 10.1038/ismej.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello A, Reen FJ, O'Gara F, Callaghan M, McClean S. 2014. Inhibition of co-colonizing cystic fibrosis-associated pathogens by Pseudomonas aeruginosa and Burkholderia multivorans. Microbiology 160:1474–1487. doi: 10.1099/mic.0.074203-0. [DOI] [PubMed] [Google Scholar]
- 17.Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS III. 1992. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Engelborghs Y, Michiels CW. 2006. Role of quorum sensing and antimicrobial component production by Serratia plymuthica in formation of biofilms, including mixed biofilms with Escherichia coli. Appl Environ Microbiol 72:7294–7300. doi: 10.1128/AEM.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Michiels CW. 2005. Quorum sensing dependent production of antimicrobial component influences establishment of E. coli in dual species biofilms with Serratia plymuthica. Commun Agric Appl Biol Sci 70:195–198. [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 21.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer HP. 2003. Applications of the Saccharomyces cerevisiae Flp-FRT system in bacterial genetics. J Mol Microbiol Biotechnol 5:67–77. doi: 10.1159/000069976. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. 2015. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci U S A 112:2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 27.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am Nat 170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 29.Laland KN, Odling-Smee FJ, Feldman MW. 1996. The evolutionary consequences of niche construction: a theoretical investigation using two-locus theory. J Evolution Biol 9:293–316. doi: 10.1046/j.1420-9101.1996.9030293.x. [DOI] [Google Scholar]
- 30.Dandekar AA, Chugani S, Greenberg EP. 2012. Bacterial quorum sensing and metabolic incentives to cooperate. Science 338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rainey PB, Rainey K. 2003. Evolution of cooperation and conflict in experimental bacterial populations. Nature 425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 32.Denervaud V, TuQuoc P, Blanc D, Favre-Bonte S, Krishnapillai V, Reimmann C, Haas D, van Delden C. 2004. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J Clin Microbiol 42:554–562. doi: 10.1128/JCM.42.2.554-562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher LA, Manoil C. 2001. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol 183:6207–6214. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slemrod J. 2007. Cheating ourselves: the economics of tax evasion. J Econ Perspect 21:25–48. doi: 10.1257/jep.21.1.25.19728420 [DOI] [Google Scholar]
- 35.Morris JJ, Lenski RE, Zinser ER. 2012. The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio 3(2):e00036-12. doi: 10.1128/mBio.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JJ, Papoulis SE, Lenski RE. 2014. Coexistence of evolving bacteria stabilized by a shared Black Queen function. Evolution 68:2960–2971. doi: 10.1111/evo.12485. [DOI] [PubMed] [Google Scholar]
- 37.Sachs JL, Hollowell AC. 2012. The origins of cooperative bacterial communities. mBio 3(3):e00099-12. doi: 10.1128/mBio.00099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.