Abstract
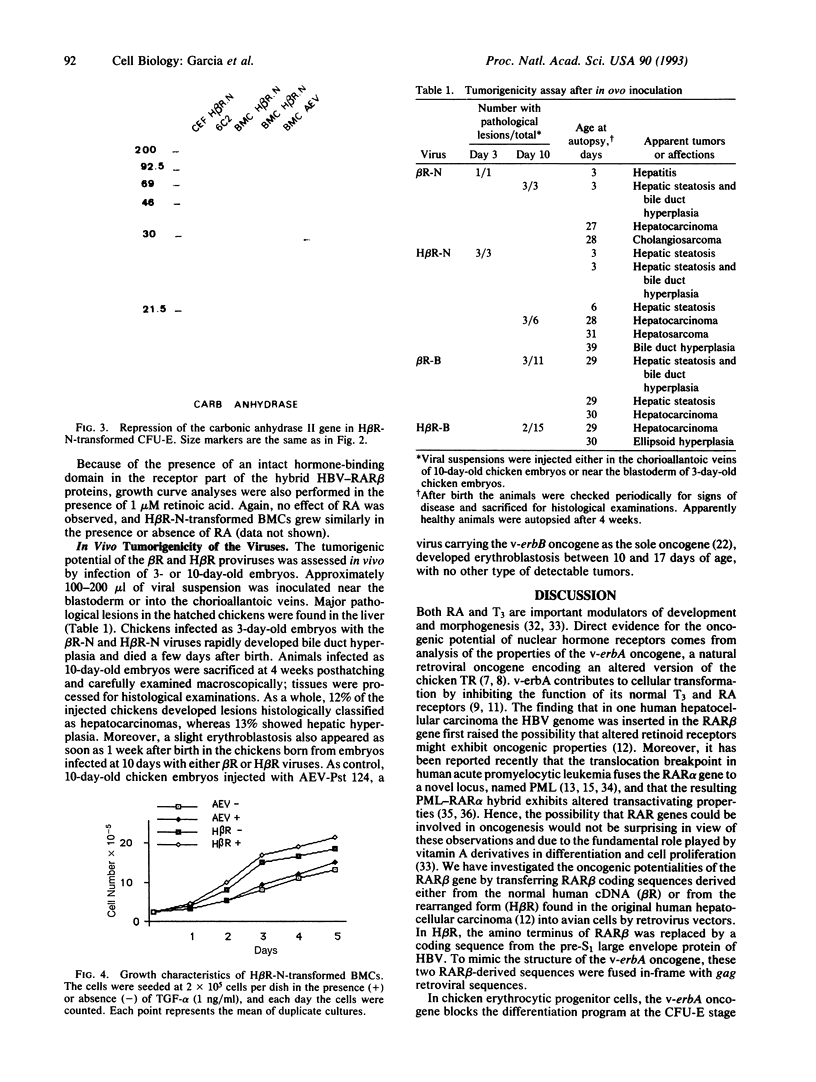
In this report, we investigated the transforming properties of retinoic acid receptor beta (RAR beta). The v-erbA protein, which is the viral oncogenic homologue of the thyroid hormone receptor, was replaced by either the complete RAR beta (beta R) or a hepatitis B virus pre-S-RAR beta (H beta R) hybrid product in an avian erythroblastosis virus-based vector. In chicken hematopoietic cells, the H beta R protein was able to transform erythroid progenitor cells, whereas no such transformation was observed with the wild-type beta R protein. Moreover, the fully transformed phenotype was observed even in the absence of v-erbB, and H beta R-transformed erythroid cells grew independently of growth factors and transforming growth factor alpha. The analysis of erythrocytic-specific proteins revealed that the transformed cells were blocked at the colony-forming unit-erythroid stage and that the expression of the carbonic anhydrase II gene, a gene normally regulated by thyroid hormones, was repressed by the H beta R protein. Finally, hepatocarcinomas rapidly developed in some chickens infected in ovo with viruses encoding either the normal or the hybrid H beta R, suggesting that an inappropriate expression of the RAR beta gene may represent an important event in oncogenesis.
Full text
PDF
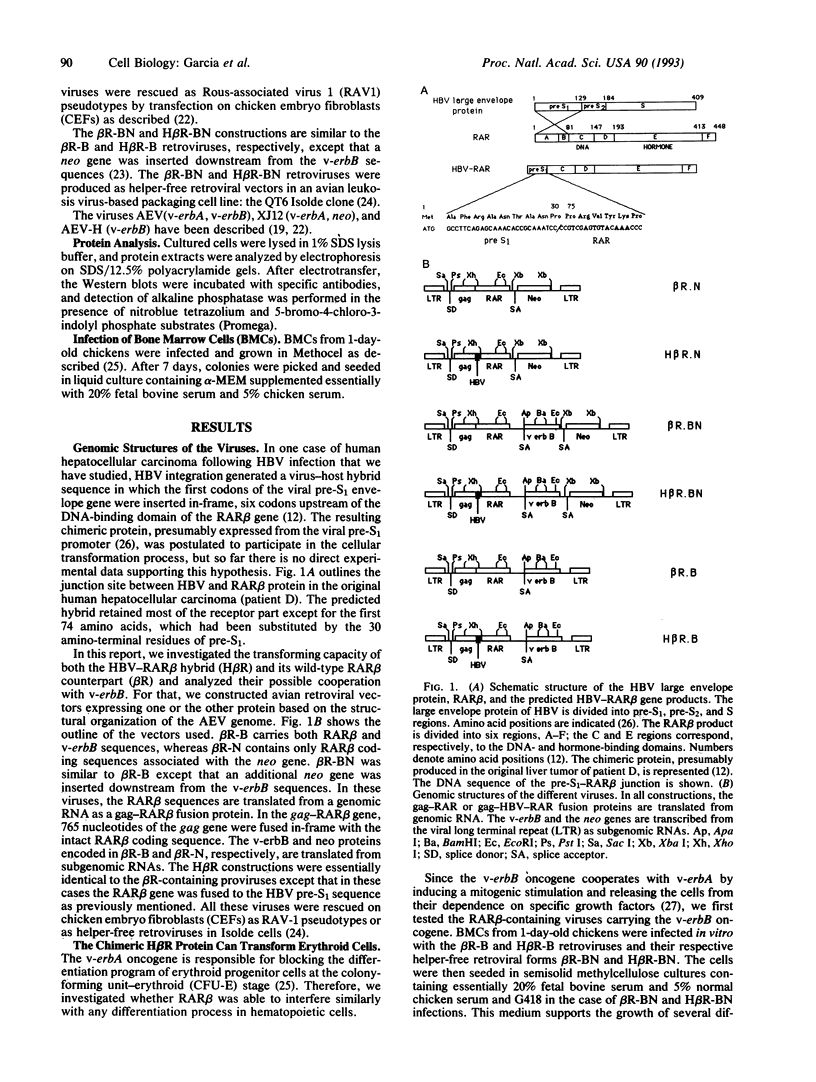


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcalay M., Zangrilli D., Pandolfi P. P., Longo L., Mencarelli A., Giacomucci A., Rocchi M., Biondi A., Rambaldi A., Lo Coco F. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor alpha locus. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Benchaibi M., Mallet F., Thoraval P., Savatier P., Xiao J. H., Verdier G., Samarut J., Nigon V. Avian retroviral vectors derived from avian defective leukemia virus: role of the translational context of the inserted gene on efficiency of the vectors. Virology. 1989 Mar;169(1):15–26. doi: 10.1016/0042-6822(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Borrow J., Goddard A. D., Sheer D., Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990 Sep 28;249(4976):1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- Cosset F. L., Legras C., Chebloune Y., Savatier P., Thoraval P., Thomas J. L., Samarut J., Nigon V. M., Verdier G. A new avian leukosis virus-based packaging cell line that uses two separate transcomplementing helper genomes. J Virol. 1990 Mar;64(3):1070–1078. doi: 10.1128/jvi.64.3.1070-1078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dejean A., Sonigo P., Wain-Hobson S., Tiollais P. Specific hepatitis B virus integration in hepatocellular carcinoma DNA through a viral 11-base-pair direct repeat. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5350–5354. doi: 10.1073/pnas.81.17.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois C., Pain B., Guilhot C., Benchaibi M., Ffrench M., Ghysdael J., Madjar J. J., Samarut J. v-erbA oncogene abrogates growth inhibition of chicken embryo fibroblasts induced by retinoic acid. Oncogene. 1991 Nov;6(11):2129–2135. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Pain B., Desbois C., Madjar J. J., Moscovici M. G., Moscovici C., Samarut J. Expression of the v-erbA product, an altered nuclear hormone receptor, is sufficient to transform erythrocytic cells in vitro. Cell. 1989 Jul 14;58(1):115–121. doi: 10.1016/0092-8674(89)90408-x. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Samarut J., Bouabdelli M., Blanchet J. P. Early precursors in the erythroid lineage are the specific target cells of avian erythroblastosis virus in vitro. Cell. 1980 Dec;22(3):683–691. doi: 10.1016/0092-8674(80)90544-9. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Pain B., Melet F., Jurdic P., Samarut J. The carbonic anhydrase II gene, a gene regulated by thyroid hormone and erythropoietin, is repressed by the v-erbA oncogene in erythrocytic cells. New Biol. 1990 Mar;2(3):284–294. [PubMed] [Google Scholar]
- Pain B., Woods C. M., Saez J., Flickinger T., Raines M., Peyrol S., Moscovici C., Moscovici M. G., Kung H. J., Jurdic P. EGF-R as a hemopoietic growth factor receptor: the c-erbB product is present in chicken erythrocytic progenitors and controls their self-renewal. Cell. 1991 Apr 5;65(1):37–46. doi: 10.1016/0092-8674(91)90405-n. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sharif M., Privalsky M. L. v-erbA oncogene function in neoplasia correlates with its ability to repress retinoic acid receptor action. Cell. 1991 Sep 6;66(5):885–893. doi: 10.1016/0092-8674(91)90435-2. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990 Oct 11;347(6293):558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]




