Abstract
In animal models of hepatocellular carcinoma (HCC), deficiency of S-adenosylmethionine (SAMe) increased the risk of HCC while administration of SAMe reduced HCC. The aim of this trial was to determine whether oral SAMe administration to patients with hepatitis C cirrhosis would decrease serum AFP level, a biomarker of HCC risk in hepatitis C. This was a prospective, randomized, placebo-controlled, double-blind trial of SAMe, up to 2.4 grams/day, for 24 weeks as compared with placebo among subjects with hepatitis C cirrhosis and a mildly elevated serum AFP. Primary outcome was change in AFP between baseline and week 24. Secondary outcomes included changes in routine tests of liver function and injury, other biomarkers of HCC risk, SAMe metabolites, markers of oxidative stress, and quality of life. 110 subjects were randomized and 87 (44 SAMe and 43 placebo) completed treatment. There was no difference in the change in AFP during 24 weeks among subjects receiving SAMe as compared with placebo. Changes in markers of liver function, liver injury, and hepatitis C viral level were not significantly different between groups. Similarly, SAMe did not change markers of oxidative stress or serum glutathione level. SAMe blood level increased significantly among subjects receiving SAMe. Changes in quality of life did not differ between groups. Overall, this trial did not find that SAMe treatment improved serum AFP in subjects with advanced hepatitis C cirrhosis and a mildly elevated AFP. SAMe did not improve tests of liver function or injury, or markers of oxidative stress or antioxidant potential.
Keywords: S-adenosylmethionine, hepatocellular carcinoma, chemoprevention, alpha fetoprotein, hepatitis C, cirrhosis
Introduction
The incidence of hepatocellular carcinoma (HCC) in the United States increased approximately three-fold between 1980 and 2010, and is estimated to increase by another 50% between 2010 and 2020 [1, 2]. This increase is primarily due to the development of cirrhosis and HCC among Americans infected with hepatitis C between 1965 and 1990 [1]. Curing hepatitis C (HCV) decreases the incidence of HCC [3, 4]. However, the cost of HCV treatment is high and the availability of inexpensive drugs that decreased the incidence of HCC would be useful.
S-adenosylmethionine (SAMe) is synthesized from methionine and adenosine triphosphate (ATP) and is a substrate for several biochemical pathways [5, 6]. These include the aminopropylation pathway in which the aminopropyl moiety of SAMe is used to synthesize polyamines, transmethylation pathways in which the methyl group (CH3) of SAMe is transferred to an acceptor molecule such as nucleic acids, proteins, phospholipids, biologic amines or other small molecules, and the trans-sulfuration pathway in which homocysteine is converted into glutathione. Oral SAMe has been available in the United States as a nutritional supplement for more than 10 years. It has an excellent safety profile, with gastrointestinal side effects occurring in a minority of subjects [7].
Several studies suggest SAMe might be important in the development of hepatocellular carcinoma. SAMe deficiency, created by feeding a methionine-choline deficient (MCD) diet, is hepatocarcinogenic in rats and several strains of mice [8–10]. More importantly, SAMe administration reduces liver cancer in a chemical model of hepatocellular carcinoma in rats, suggesting a potential chemopreventive use [11, 12].
Alpha-fetoprotein (AFP) has been used as a serum marker for hepatocellular carcinoma for the past 40 years [13]. Although alpha-fetoprotein is not directly involved in the carcinogenesis pathway, multiple studies have shown an association between increased serum levels of AFP and increased risk for subsequent development of HCC [14, 15].
The current study evaluated the effect of oral SAMe for 24 weeks, at doses up to 2.4 grams/day, on serum AFP in patients with advanced hepatitis C and a mildly elevated serum AFP level. Secondary outcomes included the effect of SAMe on other markers of liver function/injury, markers of oxidative stress, other biomarkers of HCC risk, quality of life, and metabolites in the methionine cycle. A dose of 2.4 grams of SAMe was selected for study because it represented the highest dose that could be easily tolerable from a pill burden perspective (3 tablets twice a day).
Materials and Methods
From 2007 to 2012 we enrolled subjects 18 years of age or older who had chronic hepatitis C infection and evidence of advanced liver fibrosis based on liver biopsy or a platelet count less than 150,000/mm3 or an aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio >0.75, who also had a serum alpha-fetoprotein level at their clinical laboratory between 15 ng/mL (approximately 50% greater than the upper limit of normal) and 100 ng/mL. All patients had a recent radiologic examination of the liver that excluded a liver mass suggestive of hepatocellular carcinoma, and had not received treatment for hepatitis C in the prior four months and agreed to refrain from HCV treatment during the study period. Exclusion criteria included liver disease other than hepatitis C, mass in the liver suggestive of possible HCC within the prior six months, model for end stage liver disease (MELD) score >15, hospitalization in the prior five years for mania or bipolar disorder, use of a monoamine oxidase inhibitor, or other serious condition which interfered with patient’s ability to complete the study.
Study design and oversight
This was a randomized, double-blind, placebo-controlled clinical trial in which patients were randomized 1:1 to receive SAMe or placebo for 24 weeks. SAMe was donated by Gnosis S.p.A. (Milan, Italy) and packaged in individual blister packs by Generic Pharmaceutical Services, Inc. (Hauppauge, NY). SAMe was administered as a 400 mg tablet, taken orally twice a day. For the first 4 weeks, patients consumed 400 mg (1 tablet) twice a day (800 mg/day). The dose was increased to two tablets BID (1600 mg/day) for weeks 5 through 8, and further increased to 3 tablets BID (2400 mg/day) for weeks 9 through 24. Patients randomized to placebo consumed matching placebo at the same frequency. All patients were prescribed a multivitamin tablet (B-50) containing vitamin B6, B12, and folic acid (Pharmavite, Mission Hills, CA) to consume daily during the 24 weeks; these vitamins are required cofactors for the conversion of S-adenosylhomocysteine to methionine (by the enzyme, methionine synthase) or to cystathionine (initial step in trans-sulfuration pathway). Patients were seen in clinic every 4 weeks through week 24 (end of treatment) and again at week 30 for an end of study visit. At each clinic visit, patients were assessed for medication compliance and adverse events, and blood was obtained for safety laboratory tests and study outcomes. Quality of life was assessed at weeks 0, 12, 24, and 30 using the short form (SF)-36 and the chronic liver disease questionnaire (CLDQ) [16]. Three clinical sites started the study. Because of low enrollment, two enrollment sites were added.
The study was designed by the primary author in consultation with the University of California-Irvine (UCI) Chao Family Comprehensive Cancer Center (CFCCC) and the study sponsor (Division of Chemoprevention, NCI) in accordance with Good Clinical Practice guidelines, the principles of the declaration of Helsinki and applicable regulations. The Data Safety Monitoring Board for the UCI CFCCC reviewed the conduct of the study annually. The study was approved by the Institutional Review Board (IRB) at each study site prior to patient enrollment. All patients provided written informed consent. The site investigators gathered the data and the UCI CFCCC conducted the data analysis. All authors participated in the development of the manuscript.
Efficacy and Safety Assessments
Blood for liver function tests and for safety laboratories (CBC, BMP) were analyzed at the clinical laboratory at each participating hospital. Serum was also frozen and assayed subsequently in batches for AFP, AFP lectin-3 (AFP-L3), and des-gamma-carboxy prothrombin (DCP) (by Wako Laboratories, Richmond, VA), for SAMe, S-adenosylhomocysteine (SAH) and methionine [17], for total homocysteine (tHcy) and glutathione (GSH) [18] for serum markers of oxidative stress (malondialdehyde [MDA] and, 4-hydroxynonenal [4-HNE]) (Cell Biolabs Inc., San Diego, CA), and serum level of HCV ribonucleic acid (RNA) (COBAS TaqMan HCV test [Roche Molecular Systems]). SF-36 and CLDQ were performed and scored as recommended. The site investigator classified the severity of adverse events as mild, moderate or severe, and determined the relationship to the study medicine. Adverse events (AE) were categorized for severity and body site in accordance with the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
End points
The primary efficacy endpoint was change in serum AFP between start of treatment (W0) and end of treatment (W24). Secondary outcomes included change in routine liver blood tests, HCV RNA level, other serum markers of hepatocellular carcinoma (AFP-L3, DCP), SAMe metabolites, serum markers of oxidative stress, and quality of life.
Statistical analysis
The primary statistical hypothesis of the study was that SAMe treatment for 24 weeks would decrease the serum AFP level. The sample size was based on preliminary data showing an expected mean AFP level in the study group of 35 ng/mL (SD=20 ng/mL). An effect size of 12 ng/mL (approximately 33% reduction) was selected and the correlation between the pre- and post-intervention measurements was assumed to be 0.5. With 40 subjects per treatment arm, power was estimated at 80% to detect with significance level of 0.05 a reduction in serum AFP of 11.7 ng/mL, assuming no change in the control group, using a univariate, two group repeated measures analysis of variance model. We anticipated a 10% drop out rate and therefore proposed 45 patients/group (total study size 90 subjects).
All subjects who completed the week 24 visit as planned were included in the data analysis. The number of subjects in each analysis differed slightly because the number of subjects with data at both time points (i.e., week 0 and week 24) varied depending on the outcome measured. Changes in serum AFP and other laboratory values from week 0 to week 24 were compared between treatment arms using two-group t-tests with two-sided significance level of 0.05. For any measures that were not normally distributed (SAMe, SAH and SAMe/SAH), a Mann-Whitney nonparametric test was used. Repeated measures analysis of variance methods were used to compare trends over time between treatment arms for those with complete data at all visits. Change in quality of life domains and subdomains were compared using two-group t-tests. No adjustments were made for multiple comparisons.
Results
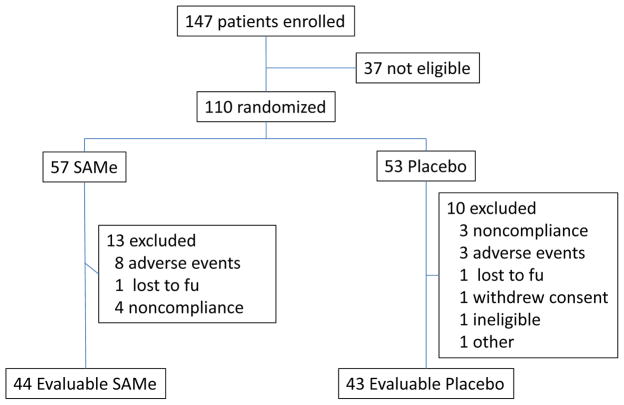
A total of 147 patients were screened and 110 were randomized, 57 to the treatment group and 53 to placebo control (Figure 1). The baseline demographic and laboratory data were similar in the two treatment groups with no significant differences between arms (Table 1). 23 subjects were excluded after randomization, 13 receiving SAMe (8 adverse events, 4 non-compliant, and 1 lost to follow-up) and 10 receiving placebo (3 non-compliant, 3 adverse events, 1 lost to follow-up, 1 withdrew consent, 1 ineligible and 1 other). A total of 87 patients (43 receiving placebo and 44 receiving SAMe) completed the 24 week treatment (Figure 1).
Figure 1.
Flow chart for patient enrollment, randomization, and completion of the study.
Table 1.
Baseline Characteristics and Lab Values
| Baseline Lab Values | Placebo | SAMe | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| AFP (ng/mL) | 48 | 34.10 | 27.28 | 55 | 34.57 | 23.85 |
| DCP (ng/mL) | 33 | 1.04 | 1.78 | 35 | 1.40 | 3.77 |
| AFP-L3 (%) | 32 | 7.77 | 5.50 | 38 | 7.75 | 2.23 |
| SAMe (nmol/L) | 49 | 121.54 | 50.10 | 55 | 109.72 | 61.93 |
| SAH (nmol/L) | 49 | 36.14 | 11.11 | 55 | 34.33 | 13.33 |
| SAM/SAH | 49 | 3.48 | 1.19 | 55 | 3.33 | 1.30 |
| Methionine (umol/L) | 49 | 40.78 | 21.34 | 55 | 40.83 | 21.08 |
| tHcy (umol/L) | 49 | 9.68 | 3.12 | 55 | 9.57 | 2.80 |
| tGSH (umol/L) | 49 | 2.08 | 1.08 | 55 | 2.10 | 0.92 |
| MDA (umol/L) | 49 | 3.42 | 1.68 | 55 | 2.94 | 1.16 |
| 4-HNE (mg/mL) | 49 | 1.09 | 0.48 | 55 | 1.08 | 0.70 |
| Platelets (103/mm3) | 52 | 121.67 | 48.17 | 56 | 127.75 | 57.28 |
| Albumin (g/dL) | 53 | 3.48 | 0.45 | 56 | 3.54 | 0.40 |
| Alk Phosphatase (IU/L) | 52 | 99.64 | 47.60 | 56 | 93.89 | 29.69 |
| Bilirubin (mg/dL) | 53 | 1.24 | 0.66 | 56 | 1.06 | 0.45 |
| AST (IU/L) | 53 | 115.78 | 61.42 | 56 | 112.59 | 60.55 |
| ALT (IU/L) | 53 | 112.91 | 77.32 | 56 | 112.52 | 55.91 |
| AST/ALT | 53 | 1.11 | 0.31 | 56 | 1.05 | 0.30 |
| INR | 49 | 1.13 | 0.15 | 52 | 1.15 | 0.15 |
| HCV RNA (IU/mL) | 48 | 2835799 | 3296975 | 49 | 3373670 | 3862249 |
| Patient characteristics | ||||||
| Age (years) | 53 | 57.25 | 5.75 | 57 | 58.47 | 4.88 |
| BMI | 53 | 29.67 | 5.60 | 56 | 30.15 | 5.78 |
| N | % | N | % | |||
|
|
||||||
| Gender -Male | 47 | 88.7 | 48 | 84.2 | ||
| Ethnicity-Hispanic | 10 | 18.9 | 14 | 24.6 | ||
| Race - Caucasian | 30 | 56.6 | 33 | 57.9 | ||
| Black | 17 | 32.1 | 16 | 28.1 | ||
| Asian/other/unk | 6 | 11.3 | 8 | 14.0 | ||
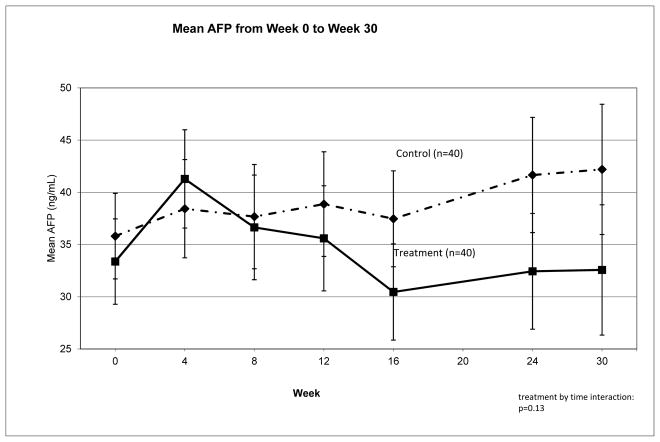
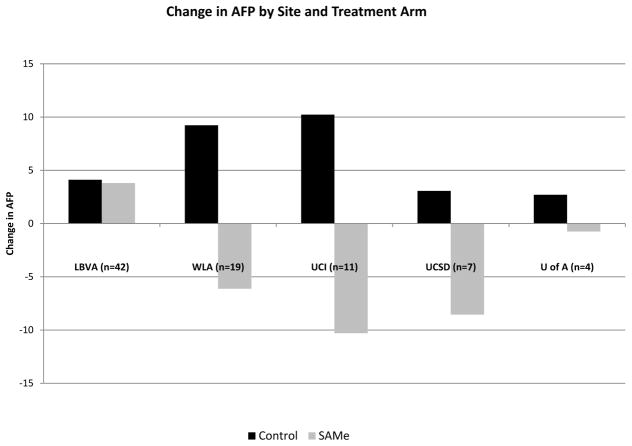
Between week 0 and week 24, serum AFP as measured by Wako laboratories decreased from 34.6 ng/mL to 32.7 ng/mL in subjects receiving SAMe but increased from 35.8 to 41.7 ng/mL in subjects receiving placebo (difference between arms=7.78, p=0.16) (Table 2). When data were analyzed including only subjects with complete data at all visits (n=83), a similar non-significant difference between arms was observed for change over time in serum AFP (p=0.13) (Figure 2). Results did not change when data from local laboratories were used or when restricted to good compliers with no dose reduction (data not shown). There was considerable variation across sites, with AFP decreasing following SAMe treatment at four of the five sites (mean difference between arms equal to 15.18 ng/mL for 41 subjects, p=0.057). However, at the site with the largest patient enrollment (n=42), there was no difference between treatment arms (Figure 3). Adjustment for multiple baseline and treatment variables (e.g., SAMe blood levels, compliance, baseline tests of liver disease severity, etc.) did not explain the difference in response to SAMe among the study sites.
Table 2.
Change from Week 0 to Week 24 for HCC biomarkers, SAMe metabolites and markers of oxidative stress.
| Control | SAMe | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Week0 | Week24 | Week24- Week0 | SDD | N | Week0 | Week24 | Week24- Week0 | SDD | Difference between arms | p-value | |
| AFP | 40 | 35.81 | 41.66 | 5.86 | 29.21 | 43 | 34.62 | 32.69 | − 1.93 | 20.30 | 7.78 | 0.160 |
| DCP | 26 | 0.98 | 1.62 | 0.65 | 1.49 | 27 | 0.96 | 1.22 | 0.26 | 0.98 | 0.39 | 0.266 |
| AFP-L3 | 26 | 7.87 | 8.20 | 0.34 | 3.49 | 27 | 7.57 | 7.71 | 0.14 | 1.15 | 0.19 | 0.789 |
| SAMe | 41 | 121.47 | 112.38 | − 9.09 | 54.62 | 43 | 102.43 | 516.67 | 414.24 | 744.37 | − 423.33 | <0.001* |
| SAH | 41 | 36.36 | 32.90 | − 3.46 | 13.10 | 43 | 32.36 | 48.68 | 16.33 | 28.21 | − 19.78 | <0.001* |
| SAM/SAH | 41 | 3.45 | 3.54 | 0.09 | 1.71 | 43 | 3.31 | 8.62 | 5.31 | 9.06 | − 5.22 | <0.001* |
| Methionine | 41 | 41.21 | 38.31 | − 2.89 | 23.67 | 43 | 39.71 | 40.13 | 0.42 | 18.79 | − 3.32 | 0.478 |
| tHcy | 41 | 9.74 | 9.07 | − 0.68 | 2.48 | 43 | 8.95 | 9.22 | 0.27 | 2.39 | − 0.94 | 0.080 |
| tGSH | 41 | 1.97 | 2.26 | 0.29 | 1.23 | 43 | 2.26 | 2.71 | 0.46 | 1.43 | − 0.17 | 0.563 |
| MDA | 41 | 3.38 | 3.38 | 0.00 | 1.16 | 43 | 2.76 | 2.75 | − 0.01 | 0.85 | 0.01 | 0.983 |
| 4-HNE | 41 | 1.03 | 1.00 | − 0.03 | 0.45 | 43 | 1.04 | 0.85 | − 0.19 | 0.72 | 0.15 | 0.248 |
Mann-Whitney test
Figure 2.
AFP levels between Week 0 and Week 30. Serum alpha-fetoprotein (AFP), as reported by Wako Laboratories, for subjects who had AFP at every time point from baseline (Week 30) to end of follow-up (Week 30). Error bars represent standard errors.
Figure 3.
Change in AFP by site and treatment arm. Serum AFP increased between Week 0 and Week 24 among subjects randomized to placebo (control) at all sites. At four of the five sites, serum AFP decreased among subjects randomized to SAMe. However, AFP did not decrease among subjects receiving SAMe at the site with the largest number of subjects enrolled (LBVA).
AFP-L3 and DCP were available in a subset of patients (26 placebo and 27 SAMe). Changes in DCP and AFP-L3 between week 0 and week 24 did not differ significantly between the SAMe and the placebo arms (Table 2). Similarly, blood levels of the oxidative stress markers MDA and 4-HNE did not change with SAMe treatment (Table 2). Treatment and control groups did not differ significantly with respect to change over time for levels of routine blood tests for liver function and liver injury, or in HCV RNA level (supplemental Table 1).
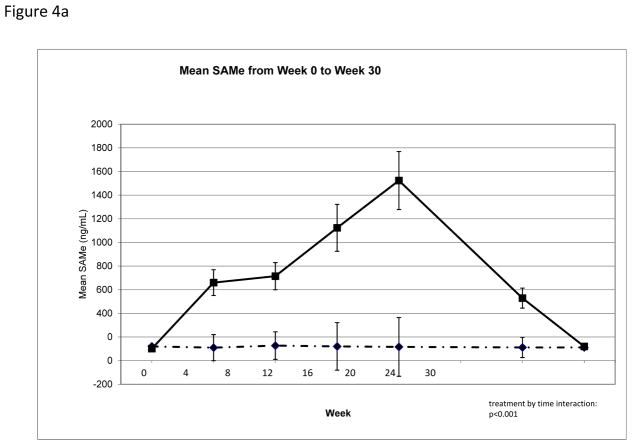
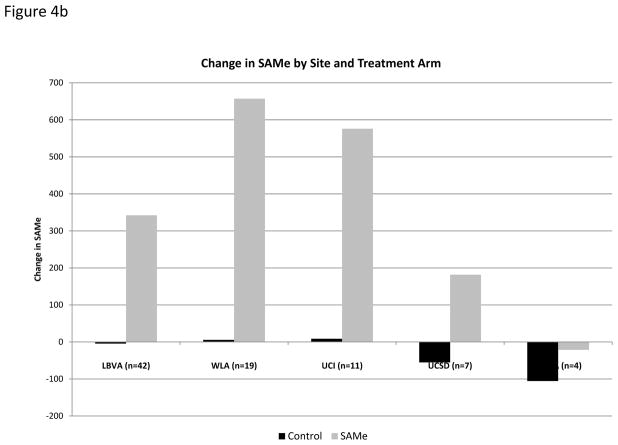
Compliance with medication consumption, as assessed by pill count at each clinic visit, was excellent among the 87 subjects who completed the week 24 visit (supplemental Table 2). Mean SAMe plasma level increased over time among subjects randomized to SAMe as compared to placebo (Table 2, Figure 4a). Although change in SAMe plasma levels from week 0 to week 24 demonstrated variability by site (Figure 4b), these differences were not statistically significant (p=0.65) and did not explain site differences in AFP change over time. When AFP change was analyzed by strata, defined by quartile of increase in SAMe, there was no clear trend to suggest that subjects with larger change in plasma SAMe level had more significant decline in AFP level (data not shown).
Figure 4.
Serum SAMe. (a) Serum SAMe at all study time points among subjects randomized to SAMe or placebo shows that SAMe increased significantly among subject receiving SAMe, but not among subjects receiving placebo, and that serum SAMe level returned to baseline by Week 30. (b) Serum SAMe levels increased between Week 0 and Week 24 among subject randomized to SAMe at all study sites, but did not increase among subjects randomized to placebo.
SAMe is metabolized to SAH and subsequently to homocysteine. The mean SAH level was significantly increased among subjects randomized to receive SAMe as compared with placebo (Table 2), with stepwise increase in SAH with each increase in SAMe dose (data not shown). SAM/SAH ratio regulates the activity of enzymes that utilize SAMe in methylation reactions, with a lower ratio signifying slower reactions. Among subjects receiving SAMe, the SAMe/SAH ratio increased from 3.31 at week 0 to 8.62 at week 24, but remained unchanged among subjects receiving placebo (3.45 at W0 and 3.54 at W24) (Mann-Whitney test, p<0.001)(Table 2). Change in plasma levels of homocysteine, methionine, and total glutathione between week 0 and week 24 did not differ between subjects receiving SAMe and subjects receiving placebo (Table 2).
Quality of life was assessed using the SF-36 and CLDQ questionnaires. All 110 subjects completed baseline questionnaires. While there were no significant differences between treatment arms in change from week 0 to week 24 for either the SF-36 Physical or Mental Component Scores (p=0.88 and p=0.21, supplemental Table 2 and supplemental Figure 1), data suggest an improvement in mental well-being in the treatment arm. Subjects treated with SAMe reported a non-significant improvement over 24 weeks in the Mental Component Score relative to controls and a significant improvement in the Mental Health subdomain (treatment by time interaction: p=0.24 and p=0.04 respectively; supplemental Table 2). 106 subjects (52 placebo and 54 SAMe) completed the baseline CLDQ. There were no significant differences between treatment arms in change over time for the CLDQ Summary score or in subdomains (supplemental Table 2). Trends over the 24 weeks showed a non-significant improvement in the CLDQ for the treatment arm relative to controls (p=0.18 for treatment by time interaction; supplemental Figure 2).
Table 3 lists treatment emergent adverse events, including those that were assessed as related or not related to the study medicine. Inclusion of a placebo arm allows for comparison of frequency of adverse events between groups. Approximately 88% (97/110) of subjects had one or more grade 1 or higher adverse event, 42% (46/110) had grade 2 or higher and 9% (10/110) had grade 3 or higher, with no difference in frequency or severity of adverse events between treatment groups. Nausea was significantly more common among subjects receiving SAMe, while constipation, diarrhea, fatigue and abdominal cramps/pain were numerically more frequent. Eight subjects receiving SAMe discontinued treatment because of adverse events, five of which were gastrointestinal (nausea (2), diarrhea (2), abdominal discomfort (1)). Three subjects receiving placebo discontinued treatment, two due to gastrointestinal symptoms (gas (1), flatulence (1)). There were seven serious adverse events among five subjects receiving SAMe and four serious adverse events among three subjects receiving placebo (not significant; Table 3). None was assessed by the investigator to be related to study drug treatment. Thirteen subjects in the SAMe group reduced their medications because of adverse events as compared with five subjects receiving placebo. Adverse events occurred at all three dose levels of SAMe (i.e., 800 mg, 1600 mg and 2400 mg/day).
Table 3.
Number of patients with adverse events after randomization, by category
| Control (n-=53) | Treatment (n=57) | ||||
|---|---|---|---|---|---|
| Adverse Event | N | Percent | N | Percent | p-value* |
| Grade 1 | 45 | 85 | 52 | 91 | |
| Grade 2 | 20 | 38 | 26 | 46 | |
| Grade 3 | 5 | 9 | 5 | 9 | |
| Gastrointestinal | |||||
| Abdominal Cramps/Pain | 11 | 21 | 22 | 39 | 0.060 |
| Constipation | 5 | 9 | 13 | 23 | 0.073 |
| Diarrhea | 14 | 26 | 20 | 35 | 0.410 |
| Nausea | 7 | 13 | 17 | 30 | 0.040 |
| Heartburn | 3 | 6 | 6 | 11 | 0.492 |
| Gas | 24 | 45 | 17 | 30 | 0.116 |
| Vomiting | 3 | 6 | 5 | 9 | 0.718 |
| Mood Change | |||||
| Mood alteration negative | 2 | 4 | 5 | 9 | 0.440 |
| Mood alteration positive | 1 | 2 | 2 | 4 | 1.000 |
| Insomnia | 7 | 13 | 8 | 14 | 1.000 |
| Fatigue | 9 | 17 | 14 | 25 | 0.358 |
| Headache | 12 | 23 | 13 | 23 | 1.000 |
| Pain | 8 | 15 | 6 | 11 | 0.572 |
| Increased urination | 10 | 19 | 13 | 23 | 0.646 |
| Dry mouth | 10 | 19 | 11 | 19 | 1.000 |
| Patients who had ≥ 1 Serious Adverse Events | 3 | 6 | 5 | 9 | 0.718 |
| Serious Adverse Events | 4 | 8 | 7 | 12 | 0.530 |
| Left Leg Vascular Claudication | Abdominal Discomfort | ||||
| Hepatocellular Carcinoma | Pneumonia | ||||
| Left Thigh Abscess | Small Bowel Inflammation | ||||
| Abdominal Cellulitis | Portal Hypertension | ||||
| Abdominal Pain | |||||
| Nephrotic Syndrome | |||||
| Non-ST Elevated MI | |||||
Includes subjects with at least one follow-up visit after randomization. Rows are not mutually exclusive.
The p-value of Fisher’s exact test
Discussion
This study found a non-significant improvement in AFP among subjects with hepatitis C and advanced liver disease who received SAMe for 24 weeks as compared with subjects who received placebo. The choice of the maximal dose of SAMe, 2.4 grams per day, was empiric. The typical dose of SAMe is between 400 and 1600 mg/day when prescribed as a medicine in other countries; the daily dose in the US is unknown but is likely less than 1000 mg/day given that the typical tablet size is 200–400 mg. We elected to give as high a dose of SAMe as reasonably tolerable and within safety limitations. A dose of 2.4 grams per day was selected because it corresponded with three tablets twice a day, an amount that was thought to be generally tolerable, without causing “pill burden.”
SAMe treatment was associated with a decrease in serum AFP at week 24, when compared with placebo treatment, at four of the five treatment sites. However, SAMe treatment did not decrease serum AFP at the site with the largest subject enrollment (approximately 50% of enrolled subjects). We could not identify differences in patient characteristics or laboratory data of liver disease injury or severity, or in SAMe blood levels (medicine compliance or absorption) that could explain the different AFP responses to SAMe treatment. The apparent discordant results between the highest enrolling site and the aggregate of the other sites could reflect an underlying, but not easily determined, difference in conditions that affected the metabolism of SAMe.
The lack of effect of SAMe in reducing AFP was not due to inability of SAMe to be absorbed. SAMe blood levels increased markedly among subjects randomized to SAMe, and the increase persisted throughout the 24 week treatment duration. The elevated SAMe levels returned to baseline by six weeks after stopping the SAMe.
The reason for lack of effectiveness of SAMe in this study, as compared with its efficacy in decreasing HCC in chemical-induced carcinogenesis in animal models, is unknown although several explanations are possible. Chemical carcinogens in animal models of HCC directly interfere with SAMe metabolism, resulting in significant decreases in hepatic SAMe and in SAM/SAH ratio -- and the decrease in SAMe levels in the dysplastic nodules and in HCC continues after the carcinogen administration is discontinued [12,19–22]. Administration of SAMe to the mice that received chemical carcinogens restores hepatic SAMe level and hepatic SAM/SAH ratio, which is associated with a decrease in the number of preneoplastic liver lesions and the prevention of dysplastic nodules and HCC, and is associated with a decrease in labeling index and an increase in apoptosis in preneoplastic cells [12,20–23]. The mechanism by which chemical carcinogens are hypothesized to promote hepatocellular carcinoma is through global DNA hypomethylation, especially hypomethylation (activation) of oncogenes. In an animal model of transplantation of human HCC cell lines into mice liver, SAMe administration decreased the growth of transplanted HCC by increasing apoptosis and reducing angiogenesis [24]. Thus, in animal models of chemical hepatocarcinogenesis, hepatic SAMe level is decreased and this decrease is believed to contribute to carcinogenesis through increased oncogene expression, increased hepatocyte proliferation and increased hepatocyte survival; SAMe administration restores hepatic SAMe level and reverses these changes.
The mechanisms of hepatocellular development in chronic hepatitis C are incompletely understood but are believed to involve different, as well as more complex, pathways than those described for chemical carcinogenesis. Deficiency of SAMe has been described in the liver in chronic hepatitis C, although the magnitude of deficiency, as compared with the deficiency in chemical-induced carcinogenesis in animal models, is unclear [25]. There appear to be several additional carcinogenic pathways in hepatitis C, including oxidative stress, growth factor activation, and direct binding of HCV proteins to retinoblastoma protein as well as effects of HCV proteins on p53 function and other cell signaling cascades [26–28]. In addition, hepatitis C is a chronic inflammatory disease and cirrhosis (fibrosis) is present in the majority of patients who develop HCC [29]. Thus, the direct role of SAMe deficiency in contributing to HCC in the setting of chronic hepatitis C is less clear. Finally, this study measured change in alpha-fetoprotein, which is an indirect marker of hepatocellular carcinoma; measurement of HCC development would have required a large, long study and would have raised ethical and funding concerns. In summary, the different pathophysiology of HCC development in chemical hepatocarcinogenesis, including the different roles that SAMe deficiency may play in the two types of HCC, the different outcome measures (HCC vs. AFP), and the lack of data on SAMe in the liver prior to and during this study (i.e., SAMe administration) may help explain why SAMe was not effective in this human clinical trial as compared with its effectiveness in chemical hepatocarcinogenesis in rodents.
SAMe administration increased the blood level of S-adenosylhomocysteine (SAH), the product of SAMe dependent methyltransfer reactions. The increase in SAMe and SAH, as expected, led to an increase in the SAMe/SAH ratio in serum. We do not have biochemical measures of SAMe metabolites other than circulating serum levels of those in the methionine cycle. Thus, we cannot assess whether oral SAMe altered DNA methylation or other intracellular biochemical methylation dependent pathways that utilize SAMe and are regulated by SAH.
SAMe did not alter other downstream metabolites of the methionine cycle. In particular, homocysteine, which is the immediate downstream metabolite of SAH, was not significantly elevated in subjects receiving SAMe, possibly due to a high rate of turnover in the metabolism of homocysteine. However this finding is theoretically advantageous since elevated homocysteine levels have been associated with increased risk for atherosclerosis, stroke and cardiovascular events such as cardiac ischemia and myocardial infarctions [31–32]. Methionine, which is the downstream metabolite of homocysteine, was also not increased. This is also somewhat unexpected since cirrhotics are reported to not metabolize methionine as well as control subjects [33, 34] and the cirrhotic subjects in this study were receiving approximately 2 grams of extra SAMe per day, of which approximately two thirds is metabolized via transmethylation and a portion recycled to methionine [35]. Our findings that SAMe increased plasma SAMe and SAH, without increasing homocysteine or methionine levels is similar to a previous report of a 6 week study of oral SAMe, 1600 mg/day, in patients with depression [36].
SAMe is also the precursor of glutathione via trans-sulfuration of homocysteine [5, 37]. Patients with cirrhosis are reported to have low glutathione levels [37], and a prior study of SAMe administration in cirrhosis reported increased plasma glutathione levels with SAMe administration [38]. However, we were unable to confirm that SAMe increased plasma glutathione levels. The effect of SAMe on intracellular glutathione levels, and on mitochondrial glutathione levels in hepatocytes, were not investigated in this study.
One mechanism by which SAMe is hypothesized to be beneficial is by reducing oxidative stress. We could not demonstrate that SAMe altered plasma level of 4-HNE, a marker of protein oxidative stress, or MDA, a measure of lipid oxidative stress (lipid peroxidation). SAMe’s lack of effect on oxidative stress is consistent with the lack of detectable change in plasma glutathione level. However, these findings are limited because we measured serum levels of oxidative stress, not levels of intracellular oxidative stress.
SAMe administration did not affect routine blood tests of liver function (e.g., bilirubin, albumin) or liver injury (e.g., AST, ALT), nor did it affect the level of HCV RNA in the blood. SAMe has been recommended as a treatment for several types of liver disease, with reports of improvement in routine tests of liver function. SAMe administration for two years improved survival among subjects with advanced alcoholic cirrhosis, although the mechanism by which SAMe improved survival was not described [39]. Tests of liver injury (e.g., ALT and AST) in patients with hepatitis can change quickly when patients are treated with drugs that inhibit the hepatitis C virus. Consequently, it is reasonable to expect that AST or ALT would have changed during the 24 week treatment period if SAMe had an effect on liver injury or hepatitis C viral replication. Thus, our finding of lack of effect of SAMe on these measures, in more than 40 patients treated for 24 weeks, suggests that the effects of SAMe on liver function in hepatitis C cirrhosis are minimal or difficult to detect with routine blood tests.
Tolerability of SAMe was reasonable. Gastrointestinal side effects (nausea, abdominal pain, constipation, diarrhea) were more frequent among subjects receiving SAMe and several patients were unable to tolerate SAMe because of gastrointestinal symptoms. Nevertheless, the majority of patients tolerated 2.4 grams of SAMe per day, and many of the common adverse events among subjects receiving SAMe were also noted among subjects receiving placebo. Numerically more patients receiving placebo than receiving SAMe required dose reductions because of adverse events, although none of the serious adverse events was assessed by the study investigator as related to SAMe.
SAMe has been extensively evaluated as a treatment for depression with inconsistent results [40]. We did not directly measure depression, but we did measure quality of life using the SF-36, a widely used and general questionnaire of quality of life, and the CLDQ, a quality of life instrument for patients with liver disease. We were not able to demonstrate an effect of SAMe on either the Physical or Mental Component scores of the SF-36. However, SAMe did improve the mental health subdomain, suggesting a possible effect on depression. SAMe did not improve the overall CLDQ score, nor did it alter any of the subdomains when compared with placebo. Although our patients with hepatitis C had higher scores on the SF-36 than the general US population (mean score of 50), prior studies of patients with hepatitis C and advanced liver disease tend to report lower quality of life and more depression, possibly because of patients’ decreased functional status and potential limitation in life expectancy [41, 42]. Overall, SAMe does not appear to improve quality of life in patients with advanced hepatitis C. Whether SAMe is effective as a treatment for depression in patients with hepatitis C will need to be tested using depression-specific instruments.
In summary, SAMe administration, at doses up to 2.4 grams/day for 24 weeks, failed to improve the blood level of AFP, a biomarker of HCC risk, in patients with hepatitis C and advanced liver disease. SAMe administration increased blood level of SAMe and of SAH, the major metabolite of SAMe in the methionine cycle. However, oral SAMe did not alter the blood level of homocysteine, methionine, or glutathione, all of which are downstream metabolites of SAH in the methionine cycle or the trans-sulfuration pathway. Blood levels of routine biochemical tests of liver function and of liver injury were not altered by SAMe administration. Likewise, blood levels of oxidative stress were not affected by SAMe. SAMe did not change quality of life, although it did improve the mental health subdomain of the SF-36. SAMe was generally well tolerated. Overall, this study failed to suggest that SAMe should be further tested as a chemopreventive agent against hepatocellular carcinoma among patients with advanced hepatitis C. The study also suggests SAMe is unlikely to reduce liver injury or improve function in patients with HCV cirrhosis.
Supplementary Material
Acknowledgments
Supported by Contract N01-CN-35160 from the National Cancer Institute to F.L. Meyskens. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153 to D.M. Cooper.
SAMe chemoprevention study group: Aliya Asghar, Jeanette Baldonado, Jinah Chung, David Dang, Janis DeJohn, Vladimir Dranovskiy, Jerry Hernandez, Corrinne Maton, Sharon Maxwell, Deval Modi, Jason Smith, Vanessa Wong, Matthew Williams.
SAMe and matching placebo were generously donated by Gnosis S.p.A (Milan, Italy). B-50 multivitamins were kindly donated by Pharmavite (Mission Hills, CA).
ABBREVIATIONS
- 4-HNE
4-hydroxynonenal
- AE
adverse event
- AFP
alpha fetoprotein
- AFP-L3
AFP lectin-3
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- BID
twice a day
- CFCCC
Chao Family Comprehensive Cancer Center
- CLDQ
chronic liver disease questionnaire
- CTCAE
Common Terminology Criteria for Adverse Events
- DCP
des-gamma-carboxy prothrombin
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IRB
institutional review board
- MDA
malondialdehyde
- MELD
Model for end stage liver disease
- RNA
ribonucleic acid
- SAH
S-adenosylhomocysteine
- SAMe
S-adenosylmethionine
- SF-36
short form-36
- tHcy
total homocysteine
- UCI
University of California, Irvine
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure statement:
Teodoro Bottiglieri reports having been the chairman of the advisory board for Methylation Sciences Inc., holding stock options in Methylation Sciences Inc., Scientific Advisor to Gnosis S.p.A. and Nestle Health Sciences, Pamlab Inc. and having received research funding from Nestle Health Sciences, Pamlab Inc., distributor of B vitamins as a medical food.
The Principle Investigator and all other Investigators did not have financial conflicts of interest.
References
- 1.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. 521 e1–6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–44. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol. 2000;32:391–5. doi: 10.1016/s1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 6.Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92:1515–42. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger R, Nowak H. A new medical approach to the treatment of osteoarthritis. Report of an open phase IV study with ademetionine (Gumbaral) Am J Med. 1987;83:84–8. doi: 10.1016/0002-9343(87)90858-8. [DOI] [PubMed] [Google Scholar]
- 8.Ghoshal AK, Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis. 1984;5:1367–70. doi: 10.1093/carcin/5.10.1367. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi B. The choline-devoid diet model of hepatocarcinogenesis in the rat. In: Fow F, et al., editors. Chemical Carcinogenesis. Models and Mechanisms. New York: Plenum; 1988. [Google Scholar]
- 10.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res. 1992;52:2071s–2077s. [PubMed] [Google Scholar]
- 11.Pascale RM, Simile MM, De Miglio MR, Feo F. Chemoprevention of hepatocarcinogenesis: S-adenosyl-L-methionine. Alcohol. 2002;27:193–8. doi: 10.1016/s0741-8329(02)00227-6. [DOI] [PubMed] [Google Scholar]
- 12.Simile MM, Saviozzi M, De Miglio MR, Muroni MR, Nufris A, Pascale RM, et al. Persistent chemopreventive effect of S-adenosyl-L-methionine on the development of liver putative preneoplastic lesions induced by thiobenzamide in diethylnitrosamine-initiated rats. Carcinogenesis. 1996;17:1533–7. doi: 10.1093/carcin/17.7.1533. [DOI] [PubMed] [Google Scholar]
- 13.Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34:2075–91. doi: 10.1007/s13277-013-0904-y. [DOI] [PubMed] [Google Scholar]
- 14.Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410–33. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue-Choi M, Nelson HH, Robien K, Arning E, Bottiglieri T, Koh WP, et al. Plasma S-adenosylmethionine, DNMT polymorphisms, and peripheral blood LINE-1 methylation among healthy Chinese adults in Singapore. BMC Cancer. 2013;13:389. doi: 10.1186/1471-2407-13-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991;565:441–6. doi: 10.1016/0378-4347(91)80407-4. [DOI] [PubMed] [Google Scholar]
- 19.Feo F, Garcea R, Pascale R, Pirisi L, Daino L, Donaera A. The variations of S-adenosyl-L-methionine content modulate hepatocyte growth during phenobarbital promotion of diethylnitrosamine-induced rat liver carcinogenesis. Toxicol Pathol. 1987;15:109–14. doi: 10.1177/019262338701500117. [DOI] [PubMed] [Google Scholar]
- 20.Garcea R, Daino L, Pascale R, Simile MM, Puddu M, Frassetto S, et al. Inhibition of promotion and persistent nodule growth by S-adenosyl-L-methionine in rat liver carcinogenesis: role of remodeling and apoptosis. Cancer Res. 1989;49:1850–6. [PubMed] [Google Scholar]
- 21.Pascale RM, Simile MM, Satta G, Seddaiu MA, Daino L, Pinna G, et al. Comparative effects of L-methionine, S-adenosyl-L-methionine and 5′-methylthioadenosine on the growth of preneoplastic lesions and DNA methylation in rat liver during the early stages of hepatocarcinogenesis. Anticancer Res. 1991;11:1617–24. [PubMed] [Google Scholar]
- 22.Pascale RM, Simile MM, De Miglio MR, Nufris A, Daino L, Seddaiu MA, et al. Chemoprevention by S-adenosyl-L-methionine of rat liver carcinogenesis initiated by 1,2-dimethylhydrazine and promoted by orotic acid. Carcinogenesis. 1995;16:427–30. doi: 10.1093/carcin/16.2.427. [DOI] [PubMed] [Google Scholar]
- 23.Pascale RM, Simile MM, De Miglio MR, Feo F. Chemoprevention of hepatocarcinogenesis: S-adenosyl-L-methionine. Alcohol. 2002;27:193–8. doi: 10.1016/s0741-8329(02)00227-6. [DOI] [PubMed] [Google Scholar]
- 24.Lu SC, Ramani K, Ou X, Lin M, Yu V, Ko K, et al. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–71. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvisi DF, Simile MM, Ladu S, Pellegrino R, De Murtas V, Pinna F, et al. Altered methionine metabolism and global DNA methylation in liver cancer: relationship with genomic instability and prognosis. Int J Cancer. 2007;121:2410–20. doi: 10.1002/ijc.22940. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Wang H. Multiple interactive factors in hepatocarcinogenesis. Cancer Lett. 2014;346:17–23. doi: 10.1016/j.canlet.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Koike K. The oncogenic role of hepatitis C virus. Recent Results Cancer Res. 2014;193:97–111. doi: 10.1007/978-3-642-38965-8_6. [DOI] [PubMed] [Google Scholar]
- 28.Rusyn I, Lemon SM. Mechanisms of HCV-induced liver cancer: what did we learn from in vitro and animal studies? Cancer Lett. 2014;345:210–5. doi: 10.1016/j.canlet.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertoia ML, Pai JK, Cooke JP, Joosten MM, Mittleman MA, Rimm EB, et al. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235:94–101. doi: 10.1016/j.atherosclerosis.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: A meta-analysis of prospective observational studies. Nutr Metab Cardiovasc Dis. 2014 doi: 10.1016/j.numecd.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Still RA, McDowell IF. ACP Broadsheet No 152: March 1998; Clinical implications of plasma homocysteine measurement in cardiovascular disease. J Clin Pathol. 1998;51:183–8. doi: 10.1136/jcp.51.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz JH, Rypins EB, Henderson JM, Heymsfield SB, Moffitt SD, Bain RP, et al. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology. 1981;81:668–75. [PubMed] [Google Scholar]
- 34.Kinsell LW, Harper HA. Rate of disappearance from plasma of intravenously administered methionine in patients with liver damage. Science. 1947;106:589. [PubMed] [Google Scholar]
- 35.Giulidori P, Galli-Kienle M, Catto E, Stramentinoli G. Transmethylation, transsulfuration, and aminopropylation reactions of S-adenosyl-L-methionine in vivo. J Biol Chem. 1984;259:4205–11. [PubMed] [Google Scholar]
- 36.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–8. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 37.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, et al. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989;24:407–15. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- 39.Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 40.Bottiglieri T. Folate, vitamin B12, and S-adenosylmethionine. Psychiatr Clin North Am. 2013;36:1–13. doi: 10.1016/j.psc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Foster GR. Quality of life considerations for patients with chronic hepatitis C. J Viral Hepat. 2009;16:605–11. doi: 10.1111/j.1365-2893.2009.01154.x. [DOI] [PubMed] [Google Scholar]
- 42.Nelligan JA, Loftis JM, Matthews AM, Zucker BL, Linke AM, Hauser P. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: results from a retrospective chart review. J Clin Psychiatry. 2008;69:810–6. doi: 10.4088/jcp.v69n0514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.