Abstract
The Tohoku Medical Megabank Organization reports the whole-genome sequences of 1,070 healthy Japanese individuals and construction of a Japanese population reference panel (1KJPN). Here we identify through this high-coverage sequencing (32.4 × on average), 21.2 million, including 12 million novel, single-nucleotide variants (SNVs) at an estimated false discovery rate of <1.0%. This detailed analysis detected signatures for purifying selection on regulatory elements as well as coding regions. We also catalogue structural variants, including 3.4 million insertions and deletions, and 25,923 genic copy-number variants. The 1KJPN was effective for imputing genotypes of the Japanese population genome wide. These data demonstrate the value of high-coverage sequencing for constructing population-specific variant panels, which covers 99.0% SNVs of minor allele frequency ≥0.1%, and its value for identifying causal rare variants of complex human disease phenotypes in genetic association studies.
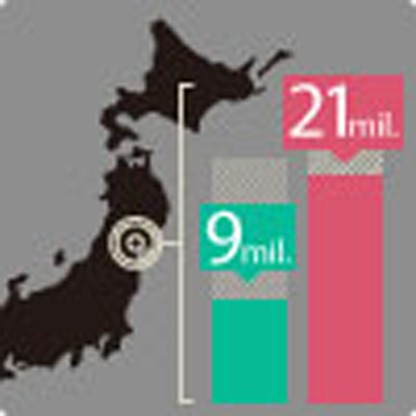 The Tohoku Medical Megabank Organization establishes a biobank with detailed patient health care and genome information. Here the authors analyse whole-genome sequences of 1,070 Japanese individuals, allowing them to catalogue 21 million single-nucleotide variants including 12 million novel ones.
The Tohoku Medical Megabank Organization establishes a biobank with detailed patient health care and genome information. Here the authors analyse whole-genome sequences of 1,070 Japanese individuals, allowing them to catalogue 21 million single-nucleotide variants including 12 million novel ones.
Tohoku Medical Megabank Organization (ToMMo) established a biobank that combines medical and genome information for the community medical system in the Tohoku region, located in the northeast part of Japan. We initiated the prospective genome cohort study in the region to identify genetic and environmental factors in diseases, and to enable personalized medicine based on an individual's genomic information. In the experimental design, we performed whole-genome sequencing (WGS) of 1,070 samples by PCR-free sequencing with more than 30 × coverage genome wide. This enabled us to identify very rare as well as novel single-nucleotide variants (SNVs), which was impossible to find by single-nucleotide polymorphism (SNP) microarrays or are difficult to find by low coverage sequencing on the same sample size. Notably, all sequencing and bioinformatics analyses were conducted using the same protocols in a single institute, allowing stringent control over systematic errors that might arise from using different equipment, protocols or bioinformatics pipelines.
Since the International Human Genome Project was completed1, a great deal of effort has been devoted to discovering, cataloguing and haplotyping common nucleotide sequence variants in populations by targeted sequencing and SNP arrays, such as in the International HapMap Project2. The knowledge of these variants enabled genome-wide association studies (GWASs), through which many SNPs associated with human traits and diseases have been discovered3,4 under the assumption of common-disease/common-variants hypothesis5. However, these identified SNPs can only explain a small fraction of genotype–phenotype relationships underlying the problem of ‘missing heritability6'. Recent GWAS conducted on a large number of case and control samples revealed that lower-frequency variants contribute to a substantial fraction of the heritability of common diseases, such as type 2 diabetes7 and cancers8. The 1000 Genomes Project (1KGP) has catalogued human genetic variations at minor allele frequency (MAF) >1% through WGS from multiple ethnic groups9. However, low frequency (0.5%<MAF≤5%), rare (0.1%<MAF≤0.5%) or very-rare (MAF≤0.1%) variants have not been thoroughly catalogued, in comparison with common variants (MAF>5%) because the existence of lower-frequency variants is population-specific9. Targeted high-coverage sequencing has been conducted to detect very-rare variants in the coding sequences of European Americans and African Americans10,11, while, in contrast, the majority of rare and very-rare variants in intergenic regions have not yet been discovered. Since the ENCODE project showed that a substantial fraction of noncoding regions (80.4%) are biochemically active12, these data suggest that these regions may also be associated with phenotypes or diseases.
Structural variants (SVs), including copy-number variants (CNVs), have also been catalogued in several studies13,14. In addition, a number of studies have revealed associations of SVs with occurrence of disease, including autism15, schizophrenia16 and Crohn's disease17. CNVs may also explain traits of populations, such as dietary habits of agricultural societies and hunter–gatherers18, or drug responses19. For identifying SVs, a middle-coverage sequencing strategy (∼13 × ) with 250 parent–offspring families by the Genome of the Netherlands succeeded in extending the catalogue of deletions from 20 to 100 bp compared with the 1KGP data set20. As high-coverage WGS is becoming less expensive, it is now feasible to detect lower-frequency SVs as well as SNVs21,22,23 in specific target populations24.
From the identified SNVs, we construct a reference panel of 1,070 Japanese individuals (1KJPN), including some very-rare SNVs. The 1KJPN cohort provides unique insights into the landscape of functional variations, especially in noncoding regions. We demonstrate here that 1KJPN is useful for genotype imputation for the Japanese population. In this analysis, a functional variant associated with Moyamoya disease (MMD) was identified through the imputed genotypes based on 1KJPN.
Results
Data processing and variant discovery
From the collected DNA samples in the ToMMo biobank, we selected 1,344 candidates for constructing 1KJPN, considering the traceability of participants' information, and the quality and abundance of DNA samples for SNP array genotyping and WGS analysis. All participants provided written informed consent, and all DNA samples and personal information were analysed anonymously. All the DNA samples were genotyped with Illumina HumanOmni2.5–8 BeadChip (Omni2.5). Among the genotyped samples, 1,070 samples were then selected by filtering out close relatives and outliers (Supplementary Fig. 1).
PCR bias is one of the major sources of sequencing error25. Hence, the selected 1,070 samples were sequenced by Illumina HiSeq 2500 using the latest PCR-free protocol (162 bp paired-end reads and 550 bp insert size, improving the accuracy for detecting SVs26; Fig. 1a and Methods). An in-house density check protocol was employed before sequencing27, and subsequent data quality-control (QC) was performed with originally developed software28 named SUGAR to maximize the quality and throughput of each experiment. In total, 100.4 trillion bases of DNA sequence reads were generated (Table 1). The sequence reads were aligned to the human reference genome (GRCh37/hg19) with decoy sequences (hs37d5), and then variants were called by several computational algorithms (see Methods). This strategy led to the discovery of 29.6 million SNVs (the high-sensitive SNVs), 1.97 million short deletions (72.6% novel), 1.38 million short insertions (<100 bp; 75.0% novel), 47,343 large deletions and 9,354 large insertions (equal to or longer than 100 bp) in autosomes (Table 1).
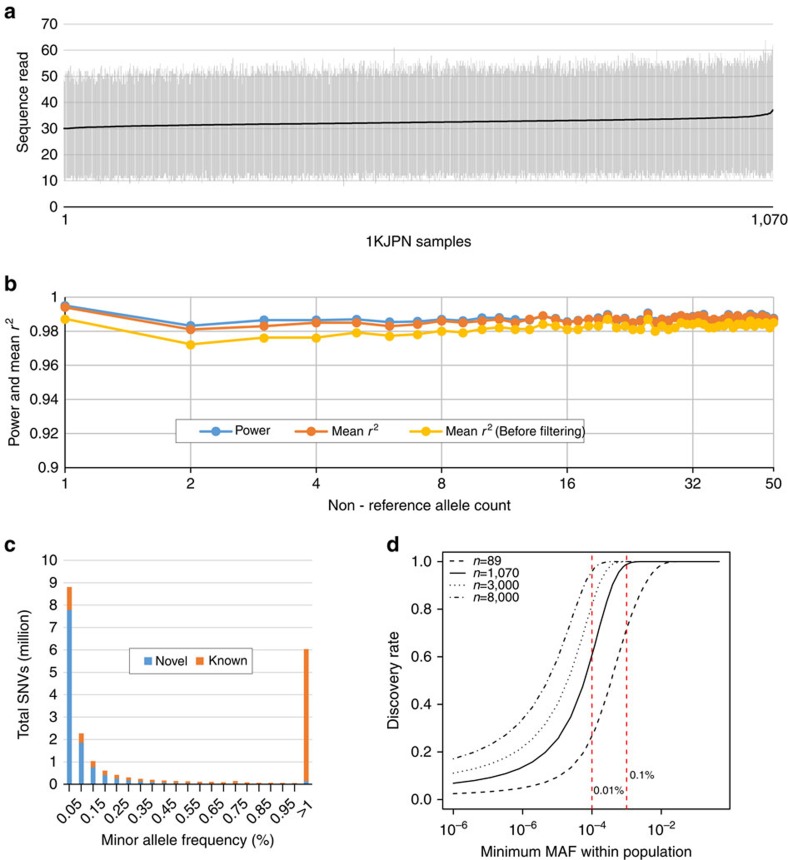
Figure 1. SNVs in 1KJPN.
(a) Statistics on read depth in 1KJPN. The vertical bars indicate the minimum and maximum depth of the number of sequence reads on each individual after filtering. They were sorted according to the average sequenced read depth (the black line). (b) The plot shows the power to detect SNVs (blue) of the confidence SNVs and the mean r2 values before (yellow) and after (orange) filtering with SNP array data for the same sample on non-reference allele counts ranging from 1 to 50. The r2 between genotypes from the SNVs in 1KJPN and the SNP array data is given by the squared Pearson correlation. (c) The numbers of novel and known SNVs in each MAF bin. The novel SNV frequency begins to dominate for lower MAFs. (d) The rate of variant discovery by minimum MAF in the 1KJPN population. The rates of variant discovery in our sequencing strategy were plotted against minimum MAF in the 1KJPN population by different sampling size. The distribution of population MAF was estimated on the basis of the demographic model shown in Supplementary Fig. 3.
Table 1. Summary of WGS of Japanese individuals and variant detection in autosomes.
| Info | ||
| Total samples | 1,070 | |
| Total raw bases | 100.4 trillion bases | |
| Mean sequenced depth | 32.4 × | |
| SNVs | High-sensitive SNVs | High-confidence SNVs |
| Total | 29,588,649 | 21,221,195 |
| Number of known variants* | 12,308,520 | 9,219,783 |
| Number of novel variants* | 17,280,129 | 12,001,412 |
| Novelty rate | 58.40% | 56.55% |
| Average number per sample | 3,886,081 | 2,716,853 |
| Average individual heterozygosity | 2,252,841 | 1,532,773 |
|
Length |
||
| Deletions | 1 bp≤length<100 bp | 100 bp≤length |
| Number of sites overall | 1,969,302 | 47,343 |
| Number of novel variants† | 1,429,636 | — |
| Novelty rate | 72.60% | — |
| Number of inframe/frameshift | 3,112/4,454 | — |
| Average number per sample | 190,857 | 2,654 |
|
Length |
||
| Insertions | 1 bp≤length<100 bp | 100 bp≤length |
| Number of sites overall | 1,384,230 | 9,354 |
| Number of novel variants† | 1,037,839 | 9,354 |
| Novelty rate | 74.98% | — |
| Number of inframe/frameshift | 1,577/2,506 | — |
| Average number per sample | 159,359 | 45 |
SNV, single-nucleotide variant; WGS, whole-genome sequencing.
All data listed here are limited to the autosomal genome.
*Comparison based on dbSNP build 138.
†The decision of novel sites is described in Methods.
To obtain reliable SNV calls, we applied multiple filtering steps (Supplementary Fig. 2), including the depth of coverage of reads (Fig. 1a), software-derived biases, departure from Hardy–Weinberg equilibrium (HWE) and complexity of genomic regions around variants (Methods and Supplementary Table 1). The performance of genotype calls in the high-confidence SNVs was improved after filtering (Fig. 1b). Consequently, we obtained a 21.2-million SNV call set, which hereafter referred to as the high-confidence SNVs (56.6% are novel; Table 1 and Fig. 1c). The high novel SNV ratio is consistent with a previous observation that rare variants tend to be population-specific20. The false discovery rate (FDR) of the high-confidence SNVs was confirmed using several different experimental technologies (see Methods); the FDR for SNVs, deletions and insertions were 0% (0 out of 174; confidence interval (CI) 0.0–1.10%), 0% (0 out of 32; CI 0.0–5.78%) and 3.85% (1 out of 22; CI 0.49–19.34%), respectively (Supplementary Table 2). We further conducted validation experiments for novel SNVs using a custom-designed Illumina SNP array. Combined with the genotyping results obtained with Omni2.5, the overall FDR was 0.8% with CI 0.63–0.97% (Supplementary Table 3 and Methods). It is important to note that the estimates of FDRs were not strongly affected by MAF, indicating that the discoveries of novel variants in this study are fairly robust with respect to the allele frequency.
Estimation of variant discovery rate
We estimated the rate of variant discovery with the sample size of 1,070. Because the distribution of allele frequency in a population is affected by underlying demographic history29,30, we inferred the demographic model of 1KJPN population from the site frequency spectrum (SFS) constructed from the intergenic regions (Supplementary Fig. 3a). As expected from excess in rare variants, the population of 1KJPN has experienced recent population expansion (Supplementary Fig. 3b), which is consistent with previous studies10,30. This demographic model is used for the calculation of the variant discovery rate (Fig. 1d and Supplementary Methods). According to Fig. 1d, 99.0 % of SNVs with MAF 0.1% or larger were expected to be captured by the present sampling strategy.
Functional impact of very-rare variants
The high-coverage PCR-free protocol of WGS has generated higher-power SNP discovery, especially for rare and very-rare SNVs. On comparison of the SFSs of intergenic region of 1KJPN and 1KGP SNVs, the data demonstrate a higher proportion of very-rare SNVs in the 1KJPN data set than in 1KGP phase 1 data set (Fig. 2a). In addition, although the number of SNVs in 1KGP was generally larger than that in 1KJPN, the number of very-rare variants detected in intergenic region was higher in 1KJPN than in 1KGP (Fig. 2b). These observations imply that there was less bias in discovering the very-rare variants of 1KJPN even in the intergenic region, although a possibility of faster expansion rate in 1KJPN cannot be excluded.
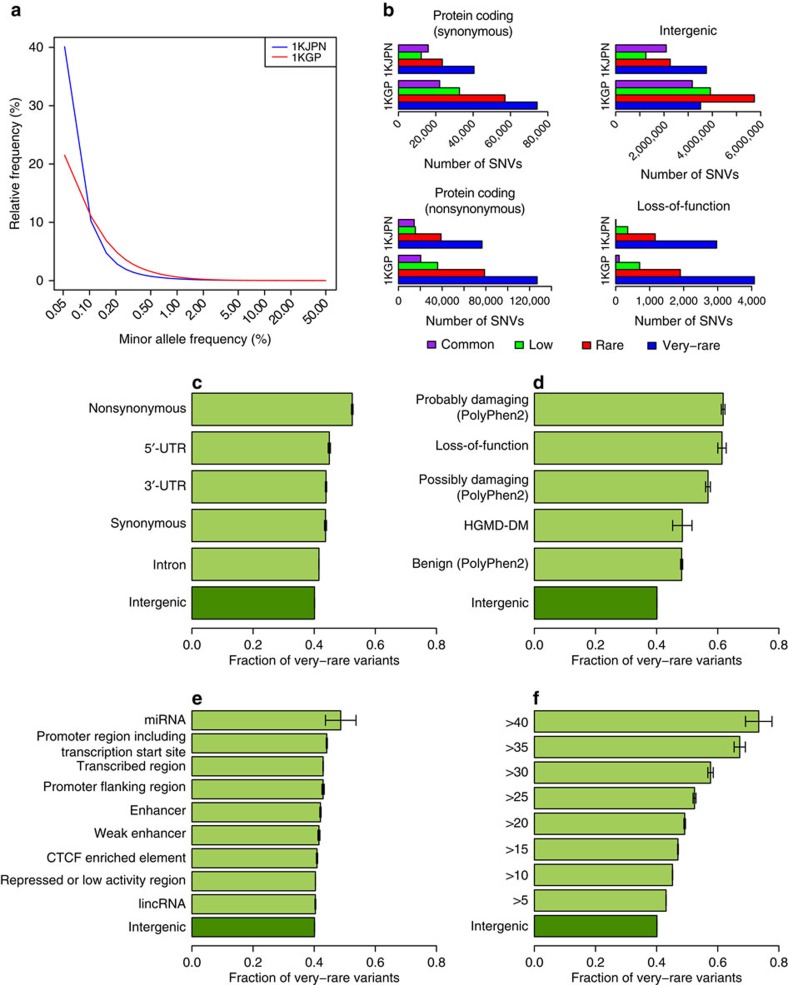
Figure 2. The impact of very-rare variants on genomic regions and functional categories.
(a) The SFSs of intergenic region for SNVs of 1KJPN (blue) and 1KGP (red). (b) The numbers of SNVs observed in 1KJPN and 1KGP are depicted as four functional categories. The fraction of very-rare variants observed in 1KJPN are depicted with 95% binomial confidence interval according to (c) genomic region, (d) probable consequences for coding regions, (e) in noncoding regions and (f) for scaled C scores. Because the number of genotyped individuals in the confidence SNVs is different among sites because of the individual depth filter, we applied a hypergeometric projection65, which subsamples each variant down to a sample size of 963 (90% of 1,070 samples) to obtain the SFSs of the confidence SNVs for a,c–f.
Because deleterious mutations are removed from populations faster than neutral mutations, SNVs observed at lower frequency in a population are indicative of purifying (negative) selection, and their selection strength differs among the various functional genomic categories. Along with this idea, the SFS has been analysed to evaluate relative influence of (mostly) negative selection on SNVs of each functional category in large sequencing projects9,10,20. Because we conducted the WGS without PCR amplification or exome capture, it is expected that there are less bias in variant detection between coding and noncoding regions. Therefore, the SFS of functional categories can be directly compared with intergenic region. We classified the high-confidence SNVs into predicted functional categories and evaluated the effect of purifying selection as a fraction of very-rare variants (FVRV; Fig. 2c–f). The FVRV of intergenic regions was 40.1%, which was the lowest among all categories (Fig. 2c), supporting the notion that most of the sites in intergenic regions are evolutionarily neutral. In contrast, FVRVs of noncoding regions other than intergenic were significantly higher than the FVRV of intergenic regions—introns (41.6%), synonymous (43.7%), 3′-untranslated region (UTR, 43.9%) and 5′-UTR (45.0%)—implying that a substantial fraction of noncoding regions are functional and under weak purifying selection. Similar tendencies were observed for insertions and deletions (Supplementary Fig. 4a). We conducted the same analysis with 1KGP phase I data set. In contrast to 1KJPN, the FVRVs of 5′-UTR, 3′-UTR and intron from 1KGP data set were lower than the FVRV of synonymous SNVs (Supplementary Fig. 4b). This might not be a signature of weak purifying selection on UTR and intron regions. This is rather due to the low power of SNP discovery of 1KGP in these regions where majority of them have been sequenced with low coverage in the project.
Mutations that disrupt protein and/or transcript structure are highly detrimental. This analysis reconfirmed that the FVRV of nonsynonymous transcribed SNPs (52.5%) was distinctly higher than synonymous (43.7%) and intronic (41.6%) variants (Fig. 2c). Nonetheless, the FVRV of loss of function mutations (61.4%) was much higher than nonsynonymous SNVs (Fig. 2d). In addition, we detected heterogeneity in the FVRV of nonsynonymous SNVs in terms of functional consequences predicted by PolyPhen-2, as previously reported10. The FVRV of SNVs that were predicted to be ‘probably damaging' was the highest (61.8%), followed by the fraction that was ‘possibly damaging' (56.8%) and finally ‘benign' (48.2%). We can also infer the impact of purifying selection on disease-causing mutations, those categorized as ‘disease mutations' in the Human Gene Mutation Database (HGMD)31 in terms of FVRV. The FVRV of disease mutations was 48.4%, which is very close to benign SNVs.
Although the intergenic region exhibits the lowest FVRV, the ENCODE project revealed that a large proportion of intergenic regions may be associated with biochemical activity32. Thus, we inferred the influence of natural selection on intergenic regions using the predicted chromatin state33 from the chromatin immunoprecipitation-Seq data produced by the ENCODE Consortium32. Among seven categories of predicted chromatin states, the SNVs observed on genomic segments bearing some functionally predicted activity exhibited higher FVRVs than repressed or low-activity regions (Fig. 2e). The difference in FVRV among chromatin states was small, but significant. This indicates weak selection on specific intergenic regions, such as promoters and enhancers, for gene regulation. Furthermore, we observed that the FVRV of microRNAs (miRNAs), but not lincRNAs, was higher, not only than for intergenic regions but also for functionally predicted ENCODE regions.
Notably, the degree of deleterious SNVs predicted by scaled C score in Combined Annotation Dependent Depletion34, which incorporates several annotations such as conservation metrics and regulatory information, was highly correlated with the FVRV of the 1KJPN variants (Fig. 2f). These observations clearly illustrate that this reported sequencing paradigm successfully discovered biologically relevant SNVs, most of which are rare in the Japanese population.
Structural variants
High-coverage sequencing data allowed us to catalogue both novel and known deletions and insertions within 1KJPN (Fig. 3a). The size-frequency spectrum showed that larger indels were less abundant than smaller ones, consistent with observations from both 1KGP9 and Genome of the Netherlands20. Notably, most of the longer insertions (>10 bp) detected in this pipeline35 were novel, which represents the usefulness of this high-coverage sequencing strategy (see Methods).
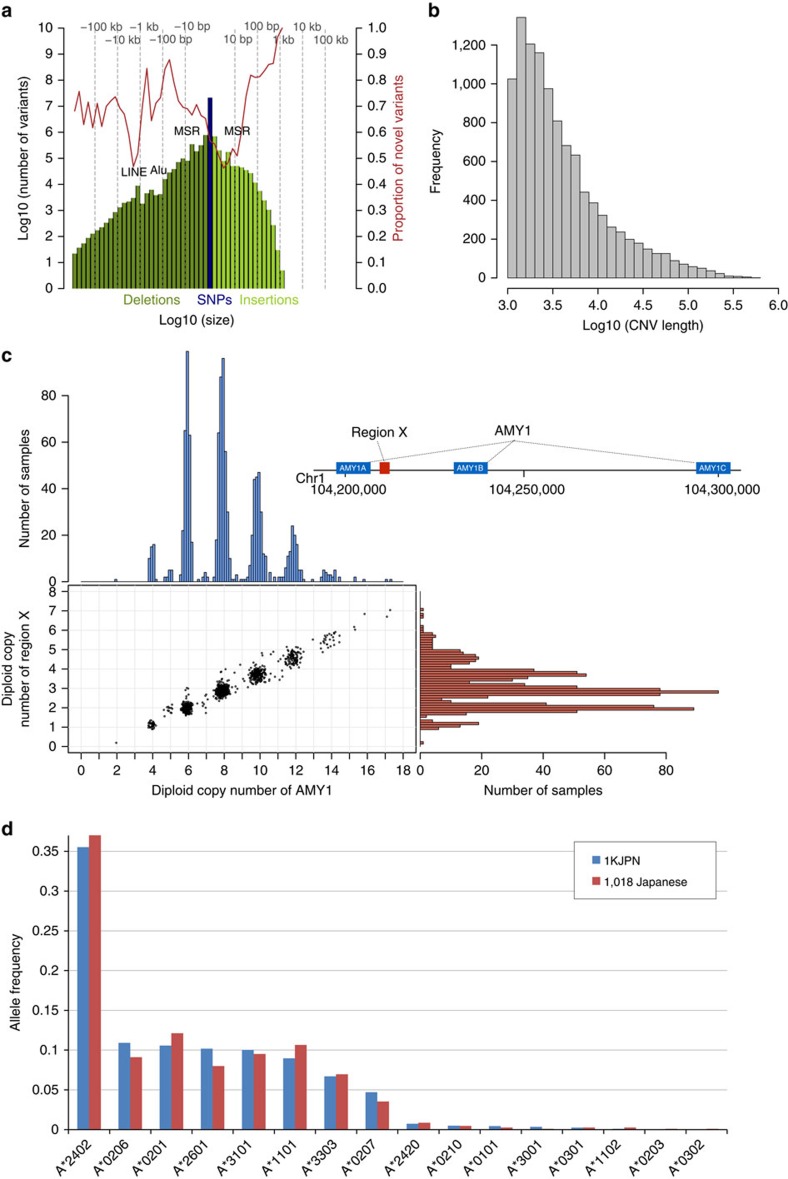
Figure 3. Properties of genomic variation discovered in 1KJPN.
(a) The size-frequency spectrum of SNVs, deletions and insertions discovered by high-coverage sequencing in 1KJPN. Novelty rates are shown by the red line. Peaks corresponding to long interspersed elements (LINE), Alu and microsatellite repeat (MSR) are shown. (b) Size-frequency spectrum of CNVs estimated from high-coverage sequencing data in the genic regions in 1KJPN. (c) Histograms and scatterplot of diploid copy numbers of AMY1 genes (blue) and region X (red) in 1KJPN. A diagram depicting the positions of AMY1A, Region X, AMY1B and AMY1C on chromosome 1 of GRCh37 is shown in the right top. (d) Allele frequencies for HLA-A in 1,070 individuals in 1KJPN estimated by high-coverage sequencing (blue), and 1,018 Japanese individuals typed by PCR-SSOP (red)42.
We also constructed a genome-wide map of CNVs overlapping with the genic region in 1KJPN, in which 25,923 CNV loci were identified; precise copy numbers were quantified on the basis of the alignment status and depth of coverage of sequence reads on individual CNV loci (Fig. 3b, Methods and Supplementary Data 1).
Among the revealed CNVs, we found that most Japanese individuals have more than two diploid copies of the salivary amylase gene (AMY1; Fig. 3c). The mean diploid copy number of AMY1 within 1KJPN was 8.27, which is significantly higher than the number reported in populations who consume low amounts of starch (mean 5.44, N=93)18. This observation is consistent with the higher copy number of AMY1 reported in high-starch populations, suggesting adaptation of an increased copy number according to dietary habits18. Interestingly, we found that the copy-number unit of AMY1 was two (that is, even AMY1 diploid copy numbers), in which the closely linked AMY1A and AMY1B loci duplicated together (Fig. 3c). Indeed, the diploid copy number of AMY1 is proportional to the copy number of intermediate region between AMY1A and AMY1B (designated by ‘Region X' in the schematic diagram of the human reference genome in Fig. 3c), in which the relationship can be described as y=2n+2, as the diplotype model, where y is the diploid copy number of AMY1, and n is the diploid copy number of Region X. We also performed the digital PCR analysis to validate the estimated copy numbers in 10 samples (Methods and Supplementary Fig. 5). This means that two AMY1 gene loci, AMY1A and AMY1B, and Region X are close to one another on a chromosome, and this region as a whole is the copy unit of the AMY1 gene. We further confirmed that there is almost no variation in copy number outside of the AMY1 genes. For example, diploid copy numbers of AMY2B and Region Y are mostly two (Supplementary Fig. 6b). This observation is consistent with the previously proposed haplotype model for the salivary amylase gene, (AMY1A-AMY1B)n-AMY1C36. Since the copy number of CNV loci present in a sample (gene dosage) is likely to affect the relative abundance of gene expression, this information will be useful for finding association of genetic variants with phenotypes or diseases in future follow-up studies. The CNVs reported here can be used to investigate associations with human disease phenotypes as previously reported37,38.
HLA types
Another important aspect of deep sequencing is the ability to determine highly polymorphic gene alleles. Human leucocyte antigen (HLA) genes are highly polymorphic in the human genome, and their haplotype structures are different among human populations39. In this study, we have typed HLA alleles of HLA-A, HLA-B and HLA-C loci of 1KJPN using newly developed software called HLA-VBSeq40. The method is based on the alignment of sequence reads to the genomic HLA allele sequences registered in the IMGT/HLA database41. We successfully typed HLA-A alleles for most of the individuals in 1KJPN (2,063 out of 2,140 alleles) at full resolution (8-digit in HLA nomenclature). We observed that the frequencies of HLA-A, -B and -C alleles at 4-digit resolution (nucleotide differences that change amino-acid sequences) estimated in our study were very similar to previously published frequencies (Fig. 3d and Supplementary Fig. 6c,d), in which HLA types were determined using PCR-SSOP among a different set of 1,018 Japanese individuals42. Because HLA genes are crucial in determining the outcome of organ transplantation and susceptibility to infectious and autoimmune diseases43,44, this HLA typing will be particularly important for GWAS45 and clinical practice, such as donor–recipient matching46.
Haplotyping and imputation
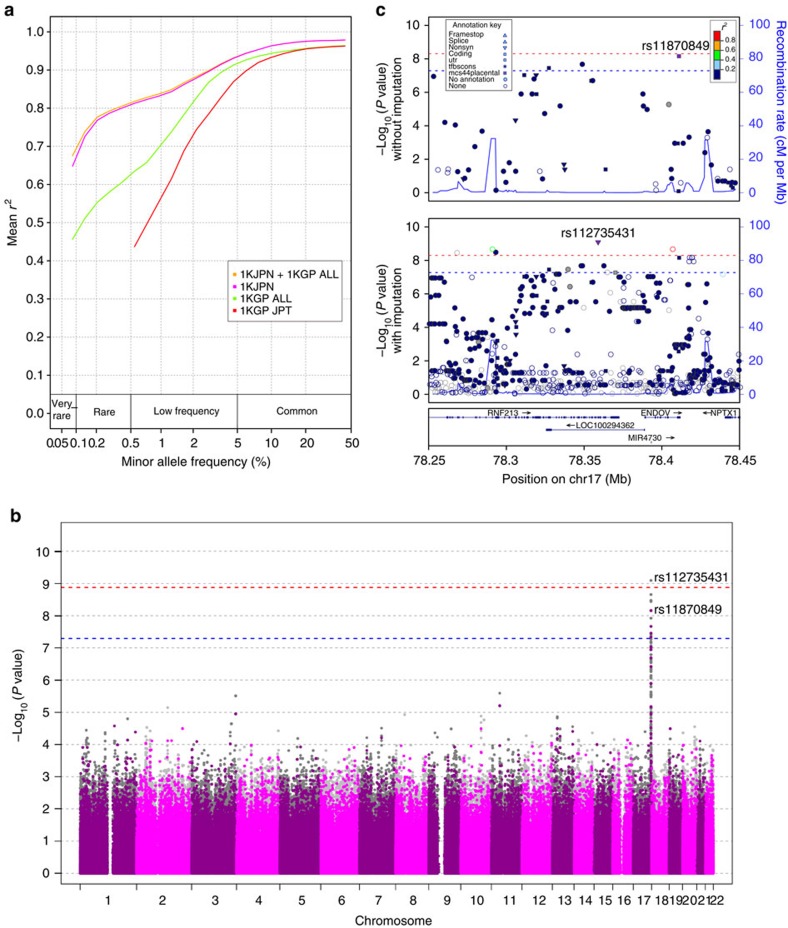
Genome-wide genotype imputation is a statistical technique to estimate untyped genotypes from known haplotype information, which is cost-effective for GWAS with SNP arrays when compared with exome sequencing and WGS. We constructed a phased reference panel with high-sensitive SNVs plus short insertions and deletions in 1KJPN using HapMonster47 and ShapeIT2 (ref. 48). The performance of genotype imputation with the reference panel for 131 Japanese individuals (who were not among the individuals used to compile 1KJPN and not cryptic relatives to them; estimated identity-by-descent <0.125) was assessed by comparing their imputed genotypes and those obtained with the same sequencing protocol and variant calling pipeline that was used to generate 1KJPN. For the imputation, genotypes at sites designed in Omni2.5 were used (Supplementary Methods). We compared 1KJPN and the following three types of reference panels for genotype imputation: (i) the 1,092 cosmopolitan samples in 1KGP, (ii) the 89 JPT samples in 1KGP and (iii) the 1KJPN plus 1KGP. From the comparison, the highest r2 value (the measure of imputation accuracy) was achieved with 1KJPN plus 1KGP, especially in variants with MAF≤5%; the mean r2 values were 0.47 for very-rare SNVs, 0.66 for rare SNVs and 0.78 for low frequency SNVs (Fig. 4a). The significant improvement of imputation accuracy using the 1KJPN data suggests the importance of construction and examination of a population-specific reference panel.
Figure 4. Imputation with the Japanese reference panel.
(a) Comparison of imputation performance (r2) for four reference panels: 1,070 individuals in 1KJPN (1KJPN), 1,092 cosmopolitan samples in 1KGP (1KGP ALL), 1KJPN plus 1KGP ALL (1KJPN+1KGP ALL) and 89 Japanese individuals in 1KGP (1KGP JPT). The x axis represents the MAF of each panel. The y axis represents the averaged r2 at SNV sites that exist in both the cosmopolitan samples of 1KGP and 1KJPN. (b) A Manhattan plot of P values from GWAS of MMD. The SNV sites from the original data set and imputed markers are plotted as dots in magenta and grey, respectively. Blue and red lines display the significance threshold of the original and imputed results, respectively. Only one significant signal was identified on chromosome 17. (c) A plot of P values from GWAS of MMD with the original (non-imputed; upper panel) and imputed (lower panel) data set around the SNP exhibiting the significant signal in b. In the imputed result, the SNP with the highest association is a nonsynonymous variant of RNF213, and was reported as one of the MMD-causing variants in the original study. In contrast, from the non-imputed result the SNP with the highest association is located in the coding region of ENDOV.
To illustrate the effectiveness of the genotype imputation strategy in GWAS, we performed imputation from genotyped data set in a case–control study of MMD49 with 1KJPN (Supplementary Methods). MMD is a progressive cerebrovascular disorder caused by blocked arteries at the base of the brain and has unusually high prevalence among the Japanese. The data set contains the genotypes with Illumina HumanOmni1-Quad BeadChip for 72 Japanese MMD patients and 45 healthy controls from HapMap JPT collection. We performed GWAS for the imputed data sets with the same statistical parameters used in the original study49. A synonymous SNP rs11870849 located at the coding region of ENDOV (chr17:78,411,073) with a P value of 6.95 × 10−9 (χ2-test) was identified as the highest association SNP (Fig. 4c top) from the original (non-imputed) data set (Fig. 4b). In contrast, by the imputation employing 1KJPN (Fig. 4c bottom), a nonsynonymous SNP rs112735431 located in RNF213 (chr17:78,358,945; MAF=0.0089 in 1KJPN) was identified as the highest association SNP with a P value of 8.07 × 10−10 over a significance threshold of a P value <5.06 × 10−9. This SNP was confirmed by fine mapping of chromosome 17q ter locus with another set of SNPs followed by targeted sequencing49, which was suggested to be a major MMD causal variant49,50. Thus, we have directly inferred the causal SNP with our 1KJPN reference panel.
Functional variant load in individuals
We summarized the number of disease-causing variants in autosomes per individual for each derived allele frequency (Table 2 and Supplementary Methods). On average, one individual has 11.2 disease-causing alleles in terms of disease-causing mutation (DM) category of HGMD31 (9.6 as heterozygous and 1.6 as homozygous in the high-confidence SNVs). Similarly, we calculated the number of nonsense (stop-gained) variants per individual on the high-confidence SNVs. Each individual has on average 41.6 heterozygous and 12.1 homozygous stop-gained SNVs. These estimates are reasonably consistent with those in East Asian populations in 1KGP (JPT, CHB and CHS). Although there are reports on higher mutational loads in founding populations13,21,51, our analysis of mutational load did not show clear difference between Japanese and other East Asian populations. In terms of nonsense mutation, the estimate of individual load in 1KJPN is consistent with those in the analysis of the Dutch population20. In 1KJPN, 3,505 SNVs were annotated as stop-gained, while among them only 4.5% were annotated as phenotypic variants in HGMD. This low proportion suggests that the biological or pathological effects of most stop-gained SNVs are yet to be revealed.
Table 2. Individual variant load in coding regions.
| Variant type | Population |
Very-rare (<0.1%) |
Rare (0.1–0.5%) |
Low (0.5–5%) |
Common (>5%) |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Heterozygous | |||||||||||
| HGMD-DM* | 1KJPN (1,070) high confidence | 0.640 | 0.814 | 1.039 | 1.016 | 4.757 | 2.246 | 3.179 | 1.604 | 9.619 | 3.032 |
| 1KJPN (1,070) high-sensitive | 0.675 | 0.848 | 1.107 | 1.051 | 4.905 | 2.261 | 4.388 | 1.787 | 11.074 | 3.136 | |
| 1KGP JPT (89) | NA | NA | NA | NA | 6.270 | 2.503 | 4.169 | 1.829 | 10.438 | 2.969 | |
| 1KGP CHB (97) | NA | NA | NA | NA | 5.536 | 2.381 | 4.464 | 1.921 | 10.000 | 3.218 | |
| 1KGP CHS (100) | NA | NA | 1.470 | 1.359 | 4.320 | 2.049 | 3.680 | 1.803 | 9.470 | 2.798 | |
| Stop-gained† | 1KJPN (1,070) high-confidence | 2.385 | 1.550 | 1.563 | 1.294 | 8.679 | 2.486 | 29.017 | 4.327 | 41.644 | 5.358 |
| 1KJPN (1,070) high-sensitive | 2.624 | 1.616 | 1.777 | 1.376 | 6.008 | 2.402 | 42.125 | 4.878 | 52.535 | 5.795 | |
| 1KGP JPT (89) | NA | NA | NA | NA | 8.685 | 2.987 | 39.337 | 5.261 | 48.022 | 6.166 | |
| 1KGP CHB (97) | NA | NA | NA | NA | 9.742 | 3.215 | 37.845 | 5.593 | 47.588 | 6.777 | |
| 1KGP CHS (100) | NA | NA | 3.860 | 2.433 | 6.580 | 3.085 | 36.070 | 4.860 | 46.510 | 5.947 | |
| Homozygous | |||||||||||
| HGMD-DM* | 1KJPN (1070) high-confidence | 0.001 | 0.031 | 0.003 | 0.053 | 0.048 | 0.230 | 1.570 | 1.126 | 1.621 | 1.145 |
| 1KJPN (1,070) high-sensitive | 0.000 | 0.000 | 0.003 | 0.053 | 0.050 | 0.234 | 1.862 | 1.235 | 1.914 | 1.251 | |
| 1KGP JPT (89) | NA | NA | NA | NA | 0.022 | 0.149 | 1.899 | 1.244 | 1.921 | 1.227 | |
| 1KGP CHB (97) | NA | NA | NA | NA | 0.052 | 0.222 | 2.021 | 0.989 | 2.072 | 1.003 | |
| 1KGP CHS (100) | NA | NA | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 2.110 | 1.118 | |
| Stop-gained† | 1KJPN (1,070) high-confidence | 0.005 | 0.081 | 0.004 | 0.061 | 0.753 | 0.747 | 11.303 | 2.713 | 12.064 | 2.813 |
| 1KJPN (1,070) high-sensitive | 0.008 | 0.101 | 0.008 | 0.101 | 0.099 | 0.302 | 12.101 | 2.853 | 12.217 | 2.851 | |
| 1KGP JPT (89) | NA | NA | NA | NA | 0.067 | 0.252 | 11.315 | 2.898 | 11.382 | 2.914 | |
| 1KGP CHB (97) | NA | NA | NA | NA | 0.052 | 0.222 | 12.093 | 2.758 | 12.144 | 2.769 | |
| 1KGP CHS (100) | NA | NA | 0.000 | 0.000 | 0.070 | 0.326 | 12.900 | 3.047 | 12.970 | 3.096 | |
CHB, Han Chinese in Beijing, China; CHS , Han Chinese South, China; HGMD, Human Gene Mutation Database; JPT, Japanese in Tokyo, Japan; 1KGP, 1000 Genomes Project; 1KJPN, reference panel of 1,070 Japanese individual; NA, not available; ORF, open reading frame; SNV, single-nucleotide variant.
SNV sites with reliable ancestral states were used.
*HGMD-DM (disease-causing) alleles were analysed if they are derived alleles and alternative (non-reference) alleles.
†We selected stop-gained alleles if they are derived alleles and alternative (non-reference) alleles. We discarded stop-gained SNVs if the proportion of truncated ORF is less than 5%.
Incidence rates for inherited diseases vary among populations52. For example, the incidence of inherited metabolic diseases was found to be 1 in 9,330 in Japan, while among those of European ancestry, metabolic diseases were more prevalent (1 in 4,000–5,000)53. Information of risk allele frequencies among populations will be useful for estimating penetrance and identifying additional causes of disease, such as other pathogenic loci or environmental factors. Indeed, we found significant differences of allele frequency for HGMD variants between 1KJPN and each of 14 populations in 1KGP. The 2,638 SNVs in HGMD were significantly different (P value<10−5; Fisher's exact test) in at least one population of the 1KGP. Among them, 36 SNVs were annotated as disease-causing mutations. For example, SNP rs1047781 at chromosome 19:49,206,631 (causing an Ile129Phe change) in the FUT2 gene (encoding fucosyltransferase 2) showed a drastic difference in the non-reference allele frequency between the 1KJPN (0.38) and 1KGP CEU (0.00). The FUT2 gene encodes an enzyme for secreting ABH antigens, and the locus is known as a classic human secretor locus54. The frequency of homozygotes of this variant was 0.141 in 1KJPN, and this is consistent with the fact that ∼15% of Japanese individuals are FUT2 nonsecretors55. Recent studies showed that this SNP rs1047781 was associated with the levels of tumour biomarker and vitamin B12 (refs 56, 57).
Discussion
From the high-coverage WGS data collected in the ToMMo cohort project, we have constructed 1KJPN, a reference panel of 1,070 Japanese individuals. This panel includes a highly accurate and comprehensive collection of 21.5 million SNVs ranging from very-rare (observed MAF≤0.1%) to common (>5%) variants. Considering the demographic history of 1KJPN inferred from the SFS of intergenic region, the data cover almost all SNVs (99.0%) that exist in the Japanese population having a minor frequency of 0.1% or greater (Fig. 1d). Furthermore, we would find rarer variants if additional samples will be sequenced. For instance, while we have detected 60.5 % of SNVs with MAF>0.01% in this study, it is expected that the detection rate will rise to 81.6 % and 96.2 % for the sample size 3,000 and 8,000, respectively (Fig. 1d). Since most of very-rare variants likely arose within 5,000–10,000 years10, they are essentially population-specific. Hence, most of them would be difficult to be imputed from a reference panel that was constructed from diverse genetic backgrounds. Indeed, we showed here significantly improved imputation accuracy from 1KJPN data when compared with 1KGP (Fig. 4 and Supplementary Fig. 7).
In 1KJPN, we found the signature of purifying selection on SNVs in regulatory elements located in both coding and noncoding sequences. This observation illustrates that this SNV set is expected to contain many very-rare variants that can be associated with diseases, and thus should be useful in future GWASs to fully capture causal variants.
This high-coverage strategy has also defined fine characteristics of SVs, CNVs and HLA types in a population scale. Although a substantial number of novel SVs was discovered by the high-coverage sequencing, we still had difficulty in discovering insertions longer than 100 bp because of the limitation of sequence read lengths (162 bp paired-end). In addition, simple repeat regions and structurally complex regions (for example, centromeric and telomeric sequences) were not covered in our SV discovery. To build a more comprehensive reference panel with SVs, alternative approaches, such as the use of longer sequence reads, would be necessary.
Since the cost of WGS is gradually decreasing, population-wide sequencing, as in the present study, will become a reasonable approach to discover the full spectrum of SNVs, SVs and CNVs in a reliable manner. However, it remains challenging to sequence more than tens of thousands of samples in cohort projects even with the sophistication of current sequencing technologies. Thus, a hybrid strategy that combines both sequencing and genotyping with a customized SNP array optimized to a specific population background, for example, Japonica Array58, becomes highly desirable. Our hope is that 1KJPN will foster basic research by amalgamating more accurate genotype imputation in cohort studies with medical information, and thereby aid in constructing an advanced medical system to improve the quality of health-care services.
Methods
The Supplementary Note in this manuscript lists details of samples, data generation, selection protocols with the SNP array and also informs the bioinformatics not covered in this section, for example, the estimation of HLA types, imputation and simulation of population genetics.
Sample information
This project was performed as part of the prospective cohort study at the ToMMo, with the approval of the ethical committee of the Tohoku University School of Medicine. The samples used here were from the cohort participants, all of whom gave their written consent. In our informed consent to the participants to our cohort project, whole-genome data including ‘sequenced data, variant calls and inferred genotypes' are securely controlled under the Materials and Information Distribution Review Committee of Tohoku Medical Megabank Project and the data sharing to researchers are discussed in each research proposal by the review committee.
The current status of our committee is to put the part of the SNP frequency data as open data in the National Bioscience Database Center website (http://humandbs.biosciencedbc.jp/en/). Every year, the data availability policy will be updated with the review committee.
Whole-genome sequencing
To prevent sample mix-up, samples were handled in 96-well plates during the course of the library construction. The genomic DNA samples were diluted to a concentration of 10 ng μl−1 using a laboratory automation system (Biomek NXP, Beckman), and fragmented using a 96-well plate using the DNA sonication system (Covaris LE220, Covaris) to a target size of 550 bp, on average. The sheared DNA was subjected to library construction with the TruSeq DNA PCR-Free HT sample prep kit (Illumina) using a Bravo liquid-handling instrument (Agilent Technologies). Finally, the completed libraries were transferred into 1.5 ml tubes that were labelled with a barcode, and were then denatured and neutralized, and processed for library QC.
The library quantitation and QC were performed with quantitative MiSeq (qMiSeq), a newly developed method for library quantitation. In this protocol, 8 or 10 μl of prepared libraries was denatured with an equal volume of 0.1 N NaOH for 5 min at room temperature, and diluted with a 49-fold volume of ice-cold Illumina HT1 buffer. Overall, 50 μl of 96 denatured libraries including three control samples (those examined earlier on HiSeq) were pooled. Then, 60 μl of the pooled library was diluted with 540 μl of ice-cold Illumina HT1 buffer and analysed with MiSeq using a 25-bp, paired-end protocol. We utilized the index ratio determined by the MiSeq sequencer, as a relative concentration to determine the run condition for the HiSeq run. To share the details of qMiSeq, we have published a methodology paper27. In addition to qMiSeq, an electrophoretic analysis using the Fragment Analyzer (Advanced Analytical) software was performed as a part of the library QC.
DNA libraries were analysed using the HiSeq 2500-sequencing system, according to the manufacturer's protocol. A TruSeq Rapid PE Cluster Kit (Illumina), and one-and-a-half TruSeq Rapid SBS Kit (200 cycles, Illumina), were used to perform a 162-bp, paired-end read in Rapid-Run Mode. On the basis of the qMiSeq results, the libraries had been diluted to the appropriate concentrations and were used for on-board cluster generation (Illumina). We routinely checked the cluster density at the first base report and decided whether to continue the analysis depending on the density (∼550–650 K mm−2).
SNV step 1 alignment and variant call for each sample
The sequence reads from each sample were aligned to the reference human genome (GRCh37/hg19) with the decoy sequence (hs37d5). Two aligners, Bowtie2 (version 2.1.0) with the ‘-X 2000' option, and BWA-MEM (ver. 0.7.5a-r405) with the default option, were used. To call the SNVs, the Bcftools software (ver. 0.1.17-dev) and the Genome Analysis Toolkit (GATK version 2.5–2) were, respectively, applied to each aligned result (Step 1 in Supplementary Fig. 2). For each sample, the read depth of each SNV position was calculated for the downstream filtering steps (Supplementary Fig. 2; middle-bottom in Step 1). Here the read depth represents the total number of sequence reads aligned to the SNV position, with the mapping quality being more than or equal to 5.
We compared the SNV calls and the genotyping results from the HumanOmni2.5–8 BeadChip for the same samples to evaluate the precision and the power (=recall) at designed positions in the SNP array. The precision, as calculated using Bowtie2 with the Bcftools variant caller (Bowtie2+Bcftools), was always better than that obtained using BWA-MEM with GATK. On the contrary, the power obtained using BWA-MEM+GATK was always better than that obtained with Bowtie2+Bcftools (Supplementary Data 2). These results are consistent with a previously conducted analysis59. Thus, the SNVs obtained using BWA−MEM+GATK were considered the result of highly sensitive SNV detection (trying to find as many SNVs as possible), whereas the SNVs that were obtained using bowtie2+Bcftools were used as the candidate of the high-confidence SNVs to apply to the downstream filtering steps. Hereafter, the term ‘SNVs' will refer to variants called using Bowtie2+bcftools.
SNV step 2 genotype depth filter for each individual
We filtered out the genotype calls that had an extraordinarily low or high read depth. For each sample, we determined the range of the read depth on the SNVs to be retained, according to the precision and the recall from the comparison with the genotyping results of the HumanOmni2.5–8 BeadChip. The SNVs were grouped by read depth of next generation sequencing (NGS) (we only used the sequenced reads with a mapping quality more than or equal to 5, to avoid the reads being aligned to multiple chromosomal locations), and then precision and recall were calculated for each group (Fig. 1a and Supplementary Fig. 2; the middle plot in Step 2). The SNVs in the groups with a precision value >0.998 were extracted as reliable SNVs (Supplementary Fig. 2 SNV Filter; the left plot and figure in Step 2). In this step, 2.0% of the segregating sites were filtered out (Step 2 in Supplementary Table 1).
SNV step 3 SNV depth filter for each locus
We then filtered out the SNV loci in which more than 10 % of the samples were not genotyped because of the depth filter applied in the previous step. For example, the SNV calls in such loci might suffer from an extraordinarily high or low depth, because of the read alignment to repetitive sequences. The pass ratio of the SNV sites was calculated (Supplementary Fig. 2; the middle figure in Step 3). In this step, 4.99% of the SNV sites were filtered out (Step 3 in Supplementary Table 1).
SNV step 4 genome complexity filter
It is difficult to call SNVs in low-complexity genomic regions, such as short tandem repeats. The RepeatMasker programme annotated 56% of genomic regions as low-complexity regions. The precision of each annotated group was calculated and compared with the genotyped SNP array of the same sample (Supplementary Fig. 2; the left table in Step 4). The precision of the SNV calls was calculated for each genomic region by comparing them with the genotype calls obtained with the HumanOmni2.5–8 BeadChip. Then, the SNVs in the genomic regions whose precision was ≤0.997 were removed (Supplementary Fig. 2; the right figure in Step 4), that is, Alu, ERVK, Low_complexity, Satellite, Simple_repeat, TcMar-Mariner and others (more detail in Supplementary Data 3). In this step, 14.22% of SNVs were filtered out (Step 4 in Supplementary Table 1).
SNV step 5 tool bias filter
To control the bias of the tool used in Step 1, we only retained the SNV sites that were also discovered using the other method. Thus, we removed the SNVs that were not detected by BWA-MEM+GATK, that is, the highly sensitive SNVs. At the top of the figure in Step 5 (Supplementary Fig. 2), the leftmost variant was not detected using the other tool, and thus those SNVs were not used in the downstream analysis. In this step, 0.57% of the SNVs were filtered out (Step 5 in Supplementary Table 1).
SNV step 6 population genetics filter
Finally, we filtered out the SNVs with a HWE test P value<10−5, using the genotype frequencies of the SNVs determined in Step 5. This filter removed the SNV-genotype frequencies that are not consistent with the HWE. Most of the SNVs that were filtered out might be artefacts from the incompleteness of the reference genome, or from systematic alignment errors. In this step, 1.03% of the SNVs were filtered out, and in the end, 77.20% of the raw SNVs were mined through these filtering steps (Step 6 in Supplementary Table 1).
SNV validation
The autosomal SNVs with a MAF≤0.5%, a MAF of 0.5–1%, a MAF of 1–5% and a MAF >5% were subjected to validation. For each frequency, the MassARRAY design (Sequenom) was applied to 1,000 potential variants called in at least one of the 12 representative samples, which were randomly selected from 1,070 samples (in the MassArray experiments, as maximum 36 SNVs can be evaluated at once. There are some limitations for the combination of SNVs. Thus, we have started from enough 1,000 SNV candidates in the SNV validation). Among the SNPs that passed an assay design step (809, 813, 832 and 819 sites for each frequency, respectively), 322 sites (108, 70, 72 and 72 sites for each frequency, respectively) were selected and analysed using the Sequenom MassARRAY (Sequenom). The same sites were also analysed using amplicon-sequencing.
The MassARRAY analyses were performed according to the manufacturer's protocol. The design of the PCR and the extension primers was performed using the Assay Design suite (Sequenom). The genomic regions containing potential variants were amplified by multiplex PCR (35 or 36 sites for each region). The single-base extension reaction was followed by alkaline phosphatase treatment, and reaction mixtures were purified with resin and spotted on 96-well SpectroCHIPs using a Nanodispenser. SpectroCHIPs were analysed using the MassARRAY system. Variant calls were performed using the SpectroTyper software (ver. 4.0). The Sequenom validation was applied to 94 samples (including the 12 validation samples). If a variant was confirmed in at least one of the twelve validation samples, the variant was marked as being true. In the event that the call rate was less than 80%, or that a nonspecific, single-base extension was observed in the no-template control, the assay was considered unreliable and was marked as a no-call.
The genomic regions examined using the MassARRAY were amplified using a two-step PCR to add Illumina sequencing adaptors. In total, 322 regions of the 12 samples were sequenced with 76 bp, paired-end reads using a MiSeq sequencer with the MiSeq Reagent Kit v3. Index sequences (Illumina D701-D712) were used to distinguish the 12 validation samples. To validate the SNVs, we performed the following data analysis. (1) For each SNV site, the nucleotide sequences comprising both the 300 bp upstream and downstream of the reference allele, and of the alternative allele, were prepared, (2) the sequence reads were aligned to these reference sequences using BWA-MEM60 and (3) based on the depth of coverage at each site, the SNV call was validated. The regions of low coverage (less than 30 × ) were marked as a no-call. If a variant was confirmed in at least one of the 12 validation samples, it was marked as being true.
Sanger sequencing was performed using the BigDye terminator cycle sequencing kit, v.3.1 (Applied Biosystems), in accordance with the manufacturer's instructions. The SNVs that had discordant genotype calls between the HiSeq and the MassARRAY analyses, or between the HiSeq and the amplicon-sequencing analyses, were further validated using Sanger sequencing. The genomic DNA fragments containing one of the SNVs requiring validation were amplified by PCR, and these fragments were then sequenced in both the 5′ and the 3′ directions. The oligonucleotide sequences used for PCR and for sequencing are available on request.
From the validated 322 sites, 282 sites were remained in the high-confidence SNVs set. Finally, 62, 124 and 96 SNVs were the validation targets in rare and very-rare, low and common MAF class, respectively. If either the MassARRAY or the amplicon-sequencing analysis turned out to yield a no-call, the SNV site was also treated as a no-call. The SNV sites that were validated by both methods were treated as true positives. If the two methods produced inconsistent genotypes, we assumed that the genotypes determined by Sanger sequencing are true. The FDR was calculated by dividing the number of false variants by the sum of the true and false variants (Supplementary Table 2).
To validate the newly discovered SNVs in this study, we also conducted the validation experiment using a custom Illumina BeadChip array. The custom array contained probes of 25,317 novel SNVs that were arbitrarily picked from the novel variants in the high-confidence SNV set. These SNVs include SNVs in intergenic regions as well as in coding regions, some of which were of biological interest (for example, nonsynonymous or stop-gained variants).
The genotyping of 854 samples, which are part of the 1KJPN, was conducted using this custom array with the same protocol used for the HumanOmni2.5–8 BeadChip genotyping described above. We validated the SNV discovery at the designed sites, and calculated per site FDR of novel SNVs. If a SNV is discovered in several samples and more than one samples could detect the same SNV among these samples by using the SNP array, then the SNV is considered to be correctly discovered and the SNV is counted as true. Otherwise, the SNV is counted as false. Finally, the total FDR for each category (common, low, rare and very-rare) in Supplementary Table 3 was calculated as follows: overall per site FDR=(FDR of known SNV) × fknown+(FDR of novel SNV) × (1−fknown), where fknown is the fraction of SNVs listed in the single nucleotide polymorphism database (dbSNP) and FDR of known SNV as 0.
Indel validation
Autosomal indels ranging in size from 1 to 30 bp were subjected to validation. The 150 potential indels called in at least one of the twelve representative samples, which were randomly selected from the total 1KJPN samples, were applied to the MassARRAY design (Sequenom). Among the 98 indels that passed the assay design step, 95 sites were randomly selected and analysed using amplicon sequencing.
The 95 regions of the 12 samples were sequenced with 76-bp, paired-end reads using a MiSeq sequencer with the MiSeq Reagent Kit, v3. Index sequences (Illumina D701-D712) were used to distinguish between the 12 validation samples. To validate the indel calls, we performed the following data analysis: (i) for each indel site, the nucleotide sequences comprising both the 300 bp upstream and downstream of the reference allele, and of the alternative allele (either insertions or deletions), were prepared, (ii) the sequence reads were aligned to these reference sequences using BWA-MEM and (iii) based on the depth of coverage at each site, the indel call was validated. The regions of low coverage (less than 100 × ) were marked as no-calls. If an indel was confirmed in at least one of the twelve validation samples, it was marked as being true.
If the amplicon-sequencing analysis turned out to yield a no-call, we tried to determine the genotypes using Sanger sequencing. If Sanger sequencing could not determine the genotypes, the variants were treated as a no-calls. The FDR was calculated by dividing the number of false variants by the sum of the true and false variants (Supplementary Table 2).
Variant annotation
The effects of the coding and the intergenic variants were predicted by the SnpEff software (ver. 3.3c), based on the gene annotation model of GENCODE version 17. The functional categories of synonymous, nonsynonymous, intron, 5′-UTR and 3′-UTR were applied to the SNVs whose transcript label was ‘protein-coding'. For the insertion and the deletion variants, the variants on the protein-coding region were labelled according to the impact record of the SnpEff annotation. The category of ‘intergenic' was applied for intergenic regions when no annotation was assigned to the transcript label. The functional consequences of nonsynonymous SNVs were retrieved from the PolyPhen-2 records of the dbNSFP 2.0 database61. The scaled Combined Annotation Dependent Depletion scores (C scores)34 were added to each SNV by intersecting the list of the precomputed C scores with all possible SNVs. The predictions of the chromatin state were obtained from the ENCODE Project. The predictions were categorized into seven chromatin states—TSS: predicted promoter region including TSS; PF: promoter-flanking region; E: enhancer; WE: weak enhancer or open chromatin cis-regulatory element; CTCF: CTCF-enriched element; T: transcribed region; and R: repressed or low-activity region. The combined predictions of ChromHMM33 and Segway segmentation62 in the GM12878 cell line were assigned to the respective SNVs. The RNA regions (miRNA, lincRNA (long intergenic non-coding RNA), small nuclear RNA (snRNA) and snoRNA (small nucleolar RNA)) of the SNVs were annotated from several sources. The annotations of miRNA and lincRNA were performed according to the miRBase63 and the Human lincRNA Catalog64, respectively. Only the mature parts of the miRNAs were used for annotation (Supplementary Fig. 4). The annotation model of GENCODE17 was used for annotation of the snRNAs and of the snoRNAs.
Site frequency spectrum
The SFSs were analysed to assess the impact of natural selection on each category of SNVs. The number of genotyped individuals to obtain the high-confidence SNVs varies from one SNV to another because of the individual depth filter (Step 2 in Supplementary Fig. 2). Thus, we applied the hypergeometric projection65, in which each variant was downsampled to a uniform sample size across all SNV sites. The downsampled size was 963, which was the minimum number of genotyped individuals to obtain the high-confidence SNVs. To directly compare the SFSs in the 1KGP with those in the 1KJPN, we also downsampled the SNVs of the 1KGP to the uniform sample size of 963. The FVRV was calculated by dividing the number of very-rare SNVs by the total number of SNVs within the resampled SFS.
Structural variants
SVs were called using the BreakDancer66 software (ver. 1.1), the Pindel67 software (ver. 0.2.5a3) and the Haplotype Caller software implemented in the Genome Analysis Toolkit68 (version 2.5–2), from the binary of sequence alignment/map (BAM) files constructed with BWA-MEM (version 0.7.5a-r405). For the insertions and deletions of a length <100 bp, calls from Haplotype Caller were used. For the deletions of a length between 100 bp and 1 M bp, calls from Pindel (≥100 bp) and BreakDancer (≥1 k bp) were used, and merged into one unified call set, as described in the integrated structural variation pipeline35. SV calls that were overlapping by ≥80% were merged into one unified call. The start and end positions of the merged call were defined as the mean of the start and the end positions of the original calls, respectively. The novelty rates of SV calls were calculated based on the dbSNP 138 database and the Database of Genomic Variants, as of 23 July 2013. The insertion calls whose nearest known insertion was within 10 bp, and the deletion calls that overlapped by ≥50% with a known deletion, were regarded as known calls.
Whole-genome identification of genic CNVs
CNVs were called with the CNVnator69 software (ver. 0.3), using default options and a 100-bp bin size. We filtered out calls when 90% of the bases of the called region were ‘N' in the hg19 reference genome, or if ‘cnvnatorP1' or ‘cnvnatorP2' was ≥1. The CNV calls that overlapped by ≥80% were merged into one unified call. The start and the end positions of the merged calls were defined as the mean of the start and the end positions of the original calls, respectively. The autosomal CNVs whose biallelic MAF was ≥1%, length was ≥1,000 bp and positions were overlapping with a human gene annotation in Ensembl (release-75) were analysed. The copy numbers of each CNV call in the 1KJPN that overlapped with human gene loci are summarized in Supplementary Data 3.
Diploid copy-number estimation and validation of the AMY1 genes
First, we counted the number of reads that aligned to the following regions: AMY2B (chr1:104096436–104122156), AMY1A (chr1:104,096,436–104,207,173), AMY1B (chr1:104,230,036–104,239,302), AMY1C (chr1:104,293,027–104,301,312), Region X (chr1:104,210,000–104,211,500), Region Y (chr1:104,350,000–104,400,000) and Region Z (chr1:104,400,000–104,450,000), where the genomic coordinates (hg19 reference genome) are illustrated (see Fig. 3c and Supplementary Fig. 6a,b). Region Z was chosen on the basis of the mappability of the reference genome so that the depth of coverage in each region across the whole genome in each sample can be determined on the basis of the average depth of this region. We then normalized read counts in these regions, by dividing them by half of the counts in Region Z, so that the normalized counts became two in diploid regions. The sum of the normalized counts in AMY1A, AMY1B and AMY1C, and the normalized counts in Region X, were calculated for each sample (Fig. 3c). Region X is located between the AMY1A and AMY1B regions, and most of the reads were uniquely aligned to this region. Three AMY1 gene loci (AMY1A, AMY1B and AMY1C) in the reference genome are very similar to each other (having more than 99.9% sequence identity), and hence it is difficult to distinguish one locus from another. The normalized coverages in AMY1 and in Region X were highly correlated (Fig. 3c), which suggests that the locus that contains AMY1A and AMY1B is a copy unit of the salivary amylase gene. While all samples had two diploid copies of Region Y, 17 samples had more than two diploid copies of AMY2B.
Digital PCR was performed with the QuantStudio 3D Digital PCR System (Life Technologies) according to the manufacturer's protocol. Diploid copy numbers of the AMY1 gene in 10 samples (whose diploid copy numbers were predicted to be six, seven or eight) were determined using predesigned TaqMan Copy Number Assay (Hs07226362_cn for AMY1A, AMY1B and AMY1C, Life Technologies) and normalizing with TaqMan Copy Number Reference Assay RNase P (Life Technologies). The PCR reaction mixture contained 12 ng of MspI-digested genomic DNA, 7.5 μl of QS3D digital PCR Master Mix (Life Technologies), 0.75 μl of TaqMan Copy Number Assay and 0.75 μl of TaqMan Copy Number Reference Assay in a final volume of 15 μl. The data were analysed with QuantStudio 3D AnalysisSuite (Life Technologies), and the quantification of the target genomic region was presented as the number of copies per microlitre of PCR mixture. The estimated diploid copy numbers of AMY1 with WGS were consistent with the result obtained with the digital PCR analysis (Supplementary Fig. 5).
Additional information
Accession code: Sequence data, variant calls and inferred genotypes will be available on request after approval of the Ethical Committee and the Materials and Information Distribution Review Committee of Tohoku Medical Megabank Project. Part of the data is available as open data from the National Bioscience Database Center website (http://humandbs.biosciencedbc.jp/en/) under the accession ID hum0015.
How to cite this article: Nagasaki, M. et al. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat. Commun. 6:8018 doi: 10.1038/ncomms9018 (2015).
Supplementary Material
Supplementary Figures 1-7, Supplementary Tables 1-3, Supplementary Methods and Supplementary References
Whole-genome identification of genic CNVs
Precision and recall of SNVs
Precision and recall of SNVs of the RepeatMasker annotation
Acknowledgments
This work was supported (in part) by the Tohoku Medical Megabank Project (Special Account for reconstruction from the Great East Japan Earthquake). All computational resources were provided by the ToMMo supercomputer system (http://sc.megabank.tohoku.ac.jp/en). We are indebted to all volunteers who participated in this ToMMo project. We thank members of ToMMo at the Tohoku University for seminal contribution to the establishment of the genome cohort and biobank and for help in the genome analyses. The member list of ToMMo at the Tohoku University is available in the following website http://www.megabank.tohoku.ac.jp/english/a141201. We thank the members of Iwate ToMMo at the Iwate Medical University, especially Drs Kenji Sobue, Jiro Hitomi and Atsushi Shimizu, for discussion.
Footnotes
Author contributions S.K., A.H. and N.Y. organized the cohort. H.K. and N.Y. organized the medical support team in the disaster area. Ni.M. organized the biobank. J.Y. organized the group of sequence analysis. NGS data were mainly generated and qualified by K.T., X.P, S.I., J.Yo. and F.K. SNP array and custom array experiments were generated by S.N., R.S. and I.D. Validations of SNVs, indels and CNVs were conducted by J.Y., S.S., S.I., K.T., Y.K. and F.K. M.N. organized the group of in silico analysis. K.Ki. and M.N. designed and implemented the super computer. Y.S., M.N., N.N. and K.K. checked the quality of the generated sequenced data. M.N., N.N. and K.K. mainly developed the NGS analysis pipeline on the super computer. Y.Y.-K., I.D., S.N., R.S. and M.N. chose the reference panel candidates by removing closed individuals and outliers. M.N., K.K., N.N., T.M. and Y.K. performed SNV analysis. Y.K. performed demography and very-rare variant functional analysis. N.N. and T.M. performed SV, CNV and HLA analyses. K.K. and M.N. performed imputation and GWAS analysis. Y.Y.-K. performed the annotation of pathogenic variants and the calculation of individual variant load. S.K., N.F., O.T. and J.D.E. provided the critical feedback on the manuscript. M.N., J.Y., F.K., N.N., K.K., Y.K., Y.Y.-K. and M.Y. wrote the manuscript. All authors including ToMMo Japanese Reference Panel Project have seen and approved the final manuscript.
Contributor Information
ToMMo Japanese Reference Panel Project:
Akito Tsuboi, Fuji Nagami, Hiroshi Kawame, Hiroaki Tomita, Ichiro Tsuji, Jun Nakaya, Junichi Sugawara, Kichiya Suzuki, Masahiro Kikuya, Michiaki Abe, Naoki Nakaya, Noriko Osumi, Riu Yamashita, Soichi Ogishima, Takako Takai, Teiji Tominaga, Yasuyuki Taki, and Yoichi Suzuki
References
- Lander E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001). [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium. A haplotype map of the human genome. Nature 437, 1299–1320 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K. et al. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat. Genet. 32, 650–654 (2002). [DOI] [PubMed] [Google Scholar]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and. Nature 447, 661–678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich D. E. & Lander E. S. On the allelic spectrum of human disease. Trends Genet. 17, 502–510 (2001). [DOI] [PubMed] [Google Scholar]
- Manolio T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almgren P. et al. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 54, 2811–2819 (2011). [DOI] [PubMed] [Google Scholar]
- Jiao S. et al. Estimating the heritability of colorectal cancer. Hum. Mol. Genet. 23, 3898–3905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493, 216–220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. R. et al. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science 337, 100–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encode Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler D. M. et al. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S. W. et al. Challenges and standards in integrating surveys of structural variation. Nat. Genet. 39, S7–S15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H. et al. Large recurrent microdeletions associated with schizophrenia. Nature 455, 232–236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll S. A. et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat. Genet. 40, 1107–1112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G. H. et al. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 39, 1256–1260 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsesia A., Macé A., Jacquemont S., Beckmann J. S. & Kutalik Z. The growing importance of CNVs: new insights for detection and clinical interpretation. Front. Genet. 4, 92–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Genome of the Netherlands Consortium. Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat. Genet. 46, 818–825 (2014). [DOI] [PubMed] [Google Scholar]
- Carmi S. et al. Sequencing an Ashkenazi reference panel supports population-targeted personal genomics and illuminates Jewish and European origins. Nat. Commun. 5, 4835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. P. et al. Deep whole-genome sequencing of 100 southeast Asian Malays. Am. J. Hum. Genet. 92, 52–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D. F. et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 47, 435–444 (2015). [DOI] [PubMed] [Google Scholar]
- Sims D., Sudbery I., Ilott N. E., Heger A. & Ponting C. P. Sequencing depth and coverage: key considerations in genomic analyses. Nat. Rev. Genet. 15, 121–132 (2014). [DOI] [PubMed] [Google Scholar]
- Aird D. et al. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 12, R18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W. S. et al. Long insert whole genome sequencing for copy number variant and translocation detection. Nucleic Acids Res. 42, e8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuoka F. et al. An efficient quantitation method of next-generation sequencing libraries by using MiSeq sequencer. Anal. Biochem. 466, 27–29 (2014). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. SUGAR: graphical user interface-based data refiner for high-throughput DNA sequencing. BMC Genomics 15, 664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S. H. et al. Simultaneous inference of selection and population growth from patterns of variation in the human genome. Proc. Natl Acad. Sci. USA 102, 7882–7887 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutenkunst R. N., Hernandez R. D., Williamson S. H. & Bustamante C. D. Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genet. 5, e1000695 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Ball E. V. & Krawczak M. The human gene mutation database. Nucleic Acids Res. 26, 285–287 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E. et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J. & Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods 9, 215–216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46, 310–315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T. et al. iSVP: an integrated structural variant calling pipeline from high-throughput sequencing data. BMC Syst. Biol. 7 Suppl 6, S8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot P. C. et al. The human alpha-amylase multigene family consists of haplotypes with variable numbers of genes. Genomics 5, 29–42 (1989). [DOI] [PubMed] [Google Scholar]
- Gilissen C. et al. Genome sequencing identifies major causes of severe intellectual disability. Nature 511, 344–347 (2014). [DOI] [PubMed] [Google Scholar]
- Stefansson H. et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 505, 361–366 (2014). [DOI] [PubMed] [Google Scholar]
- de Bakker P. I. W. et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat. Genet. 38, 1166–1172 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai N. et al. HLA-VBSeq: accurate HLA typing at full resolution from whole-genome sequencing data. BMC Genomics 16 Suppl 2, S7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. et al. The IMGT/HLA database. Nucleic Acids Res. 41, D1222–D1227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y. et al. High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR-SSOP-Luminex method in the Japanese population. Immunogenetics 57, 717–729 (2005). [DOI] [PubMed] [Google Scholar]
- Marks C. Immunobiological determinants in organ transplantation. Ann. R Coll. Surg. Engl. 65, 139–144 (1983). [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Hosomichi K., Inoko H. & Kulski J. K. The HLA genomic loci map: expression, interaction, diversity and disease. J. Hum. Genet. 54, 15–39 (2009). [DOI] [PubMed] [Google Scholar]
- Noguchi E. et al. Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in Asian populations. PLoS Genet. 7, e1002170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahi P. B. et al. Donor-recipient allele-level HLA matching of unrelated cord blood units reveals high degrees of mismatch and alters graft selection. Bone Marrow Transplant. 49, 1184–1186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K. et al. HapMonster: a statistically unified approach for variant calling and haplotyping based on phase-informative reads. Lect. Notes Comput. Sci. 8542, 107–118 (2014). [Google Scholar]
- Delaneau O., Zagury J.-F. & Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods 10, 5–6 (2013). [DOI] [PubMed] [Google Scholar]
- Kamada F. et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 56, 34–40 (2011). [DOI] [PubMed] [Google Scholar]
- Liu W. et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 6, e22542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E. T. et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 10, e1004494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuchtbaum L., Carter J., Dowray S., Currier R. J. & Lorey F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 14, 937–945 (2012). [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Newborn screening in Japan: restructuring for the new era. Ann. Acad. Med. Singapore 37, 13–15 (2008). [PubMed] [Google Scholar]
- Mohr J. Estimation of linkage between the Lutheran and the Lewis blood groups. Acta Pathol. Microbiol. Scand. 29, 339–344 (1951). [DOI] [PubMed] [Google Scholar]
- Kudo T. et al. Molecular genetic analysis of the human Lewis histo-blood group system. II. Secretor gene inactivation by a novel single missense mutation A385T in Japanese nonsecretor individuals. J. Biol. Chem. 271, 9830–9837 (1996). [DOI] [PubMed] [Google Scholar]
- He M. et al. A genome wide association study of genetic loci that influence tumour biomarkers cancer antigen 19-9, carcinoembryonic antigen and alpha fetoprotein and their associations with cancer risk. Gut 63, 143–151 (2014). [DOI] [PubMed] [Google Scholar]
- Lin X. et al. Genome-wide association study identifies novel loci associated with serum level of vitamin B12 in Chinese men. Hum. Mol. Genet. 21, 2610–2617 (2012). [DOI] [PubMed] [Google Scholar]
- Kawai Y. et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J. Hum. Genet (doi: 10.1038/jhg.2015.68) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30, 2843–2851 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Jian X. & Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum. Mutat. 34, E2393–E2402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. M. et al. Unsupervised pattern discovery in human chromatin structure through genomic segmentation. Nat. Methods 9, 473–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A. & Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili M. N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Hubisz M. J. & Clark A. G. Reconstituting the frequency spectrum of ascertained single-nucleotide polymorphism data. Genetics 168, 2373–2382 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. et al. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 6, 677–681 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K., Schulz M. H., Long Q., Apweiler R. & Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25, 2865–2871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abyzov A., Urban A. E., Snyder M. & Gerstein M. CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 21, 974–984 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7, Supplementary Tables 1-3, Supplementary Methods and Supplementary References
Whole-genome identification of genic CNVs
Precision and recall of SNVs
Precision and recall of SNVs of the RepeatMasker annotation