Abstract
Background
Electroconvulsive therapy (ECT) elicits a rapid and robust clinical response in patients with refractory depression. Neuroimaging measures of structural plasticity relating to and predictive of ECT response may point to the mechanisms underlying rapid antidepressant effects and establish biomarkers to inform other treatments. Here, we determine the effects of 1) diagnosis and 2) ECT on global and local variations of hippocampal and amygdalar structure in major depression and predictors of ECT-related clinical response
Methods
Longitudinal changes in hippocampal and amygdala structure were examined in patients with major depression (N= 43, scanned thrice; prior to ECT, after the 2nd ECT session, and within one week of completing the ECT treatment series) referred for ECT as part of their standard clinical care. Cross-sectional comparisons with demographically similar controls (N= 32, scanned twice) established effects of diagnosis.
Results
Patients showed smaller hippocampal volumes compared to controls at baseline (p<.04). Both hippocampal and amygdalar volumes increased with ECT (p<.001) and in relation to symptom improvement (p<.01). Hippocampal volume at baseline predicted subsequent clinical response (p<.05). Shape analysis revealed pronounced morphometric changes in the anterior hippocampus and basolateral and centromedial amygdala. All structural measures remained stable across time in controls.
Conclusions
ECT induced neuroplasticity in the hippocampus and amygdala relates to improved clinical response and is pronounced in regions with prominent connections to ventromedial prefrontal cortex and other limbic structures. Smaller hippocampal volumes at baseline predict a more robust clinical response. Neurotrophic processes including neurogenesis shown in preclinical studies may underlie these structural changes.
Keywords: Neuroimaging, ECT, neuroplasticity, limbic, antidepressant, brain stimulation
INTRODUCTION
Major Depressive Disorder (MDD) affects millions of people each year and presents a substantial societal and economic burden (1). Though amenable to treatment, up to two-thirds of patients do not respond sufficiently to first-line therapies, which take weeks to months to exert their effect; approximately a third remain unresponsive despite further pharmacological interventions (2–5). Phenotypes relating to and/or predicting clinical response may help identify the mechanisms underlying successful treatment and inform the development of more effective or accessible fast acting therapeutic approaches (6). One currently available treatment, ECT, has a rapid onset of effect and induces a substantial clinical response (50–70%) in most eligible individuals (7, 8). Unlike medications that are first absorbed into the blood to affect monoaminergic neurotransmitter systems and a downstream antidepressant response, ECT may involve a more direct action on the central nervous system (CNS). Thus, though used mostly in refractory depression, ECT is well suited to determine the biological indicators of treatment response, not mediated or confounded by systemic effects, over relatively short time intervals.
Mounting evidence suggests that structural variations of the hippocampus reflect clinical state and relate to treatment response in MDD. Volume deficits are reported in first-episode depression, though typically after illness onset (9), appear greater during a depressive episode than in remission (10) and appear influenced by number of prior depressive episodes (11). Existing data, though often cross-sectional, also suggest that pharmacotherapy protects against disease-related volume reductions (12–16), at least in treatment responders (14, 16). Notably, preliminary evidence from two recent regions-of-interest (ROI) studies examining the effects of ECT on hippocampal (17) and amygdalar structure (18) both reported increased substructure volume with ECT. However, neither study, which included concurrent use of psychotropic medications (antidepressants and/or benzodiazepines), showed relationships with clinical response. Therefore, it remains unclear if ECT-related structural plasticity occurs solely as a consequence of seizure therapy or accounts for improvements in depressive symptoms.
To expand on these early findings (17, 18), the current study examined treatment-related changes in hippocampal and amygdalar structure in patients with experiencing a major depressive episode (n=43) followed prospectively while receiving ECT. Patients were assessed at three time points – prior to ECT, after the 2nd ECT and at completion of the ECT treatment (index) series. To establish normative values, disease effects, and the variance associated with repeated measurements, demographically similar controls were assessed at two time points; effects of diagnosis were determined by comparing patients and controls at baseline. Since regional changes in hippocampal morphology may indicate disease and/or treatment-related specificity (19), in addition to volume, local variations in hippocampal and amygdalar shape were also investigated. Considering evidence that connections of the hippocampus are organized in an anterior-posterior gradient (19, 20) and the anterior hippocampus is more densely connected to ventro-medial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC) and the ventral striatum/pallidum, changes in morphology were hypothesized to affect anterior hippocampal regions preferentially.
METHODS
Participants
Patients (N=43, 20 males and 23 females) were recruited from individuals scheduled to receive ECT as part of their routine care at the University of California, Los Angeles (UCLA) Resnick Neuropsychiatric Hospital. Patients included those with a DSM-IV TR diagnosis of MDD (n=35) or of bipolar disorder (n=7) currently experiencing a DSM-IV TR defined major depressive episode as confirmed by psychiatric evaluation and the Mini-International Neuropsychiatric Interview (M.I.N.I.) (21). All patients had experienced two or more major depressive episodes in the past and had failed to respond to a least two prior standard antidepressant treatments. Patients with comorbid psychiatric disorders, dementia, first episode depression, depression onset after 50 years of age, depression related to serious medical illness, or any neuromodulation treatment (e.g., vagal nerve stimulation, repetitive transcranial magnetic stimulation) within 6 months of the ECT index series, were excluded. All patients were tapered off all psychotropic medications including antidepressants and benzodiazepines (over a period of 48–72 hours) before enrollment and ECT treatment.
Demographically similar control subjects (N=32, 14 males and 18 females) were recruited from the Los Angeles area. Controls received M.I.N.I. screening and were excluded for a history of depression, other psychiatric or medical illness, and/or a history of antidepressant use. Exclusion criteria for all subjects included history of alcohol or substance abuse within the past 6 months and/or dependence within the past 12 months, any neurological disorder, and contraindication to MRI scanning. All participants provided written informed consent approved by the UCLA Institutional Review Board.
Procedures
Figure 1 depicts the study design. Patients were assessed at 3 time points: T1: <24 hours before the first ECT treatment, T2: <24 hours after the third ECT treatment, and T3: within one week of completing the ECT index series at transition to maintenance therapy at which time medication treatment resumed if clinically indicated. Of the 43 patients examined at T1, 36 and 29 patients completed T2 and T3 assessments respectively. Attrition was mostly attributable to patient-initiated discontinuation of ECT or the inability to tolerate further MRI scanning. Healthy controls completed two testing sessions (C1 and C2 including 32 and 30 subjects respectively) occurring 2–5 weeks apart, approximating the time interval between the patient T1 and T3 assessments.
Figure 1.
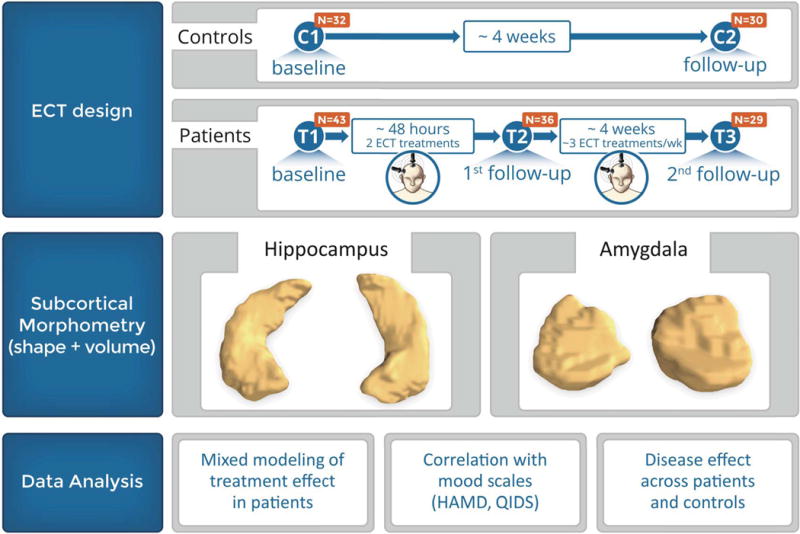
Schematic showing the study research design and types of analyses conducted on the hippocampus and amygdala.
ECT treatment
During the index series, ECT (5000Q MECTA Corp.) was administered three times a week, using a standard protocol for general anesthesia (methohexital at 1mg/kg dosage) and for paralysis (succinylcholine at 1mg/kg dosage). ECT followed the seizure threshold (ST) titration method where after establishing the ST at the first ECT session, subsequent treatments were delivered at 5× ST for right unilateral (RUL) d’Elia lead placement, using an ultrabrief pulse-width (0.3msec), and at 1.5× ST for bilateral placement, using a brief pulse-width (0.5msec). Thirty-two patients received RUL lead placement, 9 patients received mixed RUL and bilateral lead placement and 2 patients received bilateral lead placement during the treatment index series. The number of index ECT sessions was individually determined (mean number of ECT treatments for patients completing the ECT index treatment series: 11.45 sessions, 3.21 SD, range 7–22).
Clinical assessments
Patients received clinical assessments at each time point using the Hamilton Depression Rating Scale (HAM-D), 17-item(22) and the Montgomery–Åsberg Depression Rating Scale (MADRS) (23). Since the HAM-D and MADRS are highly correlated, the HAM-D was used as the primary measure of response (24). The Quick Inventory of Depressive Symptomatology (QIDS) Self Report (25) determined subjective response.
Image acquisition and analysis
High-resolution motion-corrected multi-echo MPRAGE images (26, 27) were acquired on a Siemens 3T Allegra system (Erlangen, Germany) for all subjects and time points (TEs/TR= 1.74, 3.6, 5.46, 7.32/2530 ms, TI=1260 ms, FA=7°, FOV=256 × 256 mm, 192 sagittal slices, voxel resolution = 1.3 × 1.0 × 1.0 mm3).
Image processing of structural T1 data, performed using Freesurfer (version 5.3.0) software, included removal of non-brain tissue (28), intensity normalization – i.e., correction for radio frequency inhomogeneities (29) and automated volumetric segmentation of deep gray matter structures including the hippocampus and amygdala using established workflows that use probabilistic information based on manually labeled training sets (30, 31). Hippocampal and amygdala segmentations underwent visual inspection by two independent raters. Approximately 10% of the segmentations required correction of mislabeled voxels or larger anatomical errors. To test for the reliability of segmentation and estimate the variance for repeated measurements across time within subjects, intraclass correlations for the control subjects were computed. High concordance was observed across the two separate time points (left and right hippocampus: r1=.94 and .90; left and right amygdala, r1 = .94 and .93). Concordance rates were also computed prior to manual correction of small segmentation errors to ensure reduction of variance across time after correction. As expected, intraclass correlations prior to manual correction were lower across time (left and right hippocampus: r1=.87 and .72; left and right amygdala, r1 = .71 and .76). Please see Supplement 1 to view an example of hippocampal volume extraction prior to and following ECT in a single subject.
Shape analysis
The manually edited Freesurfer segmentations for the hippocampus and amygdala were smoothed (FWHM=1.0 mm3) and rigidly registered (32) to the first time point within each subject. The resulting transformation was then applied to high-resolution surface meshes obtained using conformal parameterization (33) for both sub-structures. The surface meshes were cut along automatically defined landmark points corresponding to the anterior and posterior coordinates, and were flattened and aligned to a rectangular coordinate system (33, 34) and brought into correspondence across subjects and time points. Radial distance (35) was computed for each spatially aligned mesh as the distance from each parametric surface point to the central core of the substructure and used as the feature for statistical analysis to estimate local surface deformations inferring local changes in volume.
Statistical analysis
The general linear model (GLM) established cross-sectional effects of diagnosis for hippocampal and amygdala volumes at baseline (comparing T1 and C1) controlling for age, sex and total brain volume.
The general linear mixed model (GLMM) tested for longitudinal effects of ECT within patients and effects of time within controls including sex and age as covariates. The GLMM, which is robust to randomly missing data points, allowed for the inclusion of all available data despite the absence of follow-up assessments for some subjects. Time point (T1, T2 and T3 or C1 and C2) was used as a continuous variable for longitudinal analysis with random intercepts and slopes to account for within-subject correlations for repeated measurements. Dependent variables included hippocampal and amygdalar volume for volumetric analysis performed using IBM SPSS Statistics, Version 22.0. Radial distances were used as dependent measures for shape analysis executed in R (http://www.R-project.org) using statistical models identical to those used for volume. Follow-up analyses compared each of the 3 time points pairwise (in patients).
To determine ECT-related clinical response and relationships between clinical response and change in substructure volume across time, mood scores (HAM-D and QIDS ratings) were included as covariates of interest in the GLMMs. Though the majority of patients (74%) received RUL ECT for the duration of the ECT index series, to establish possible effects of lead placement on change in sub-structure volume, interactions between time point and the percentage of RUL ECT sessions received by each subject were also examined. Further, since a prior study suggests differential effect of ECT in patients with unipolar and bipolar depression (36) on brain morphology, including of the hippocampus, we also examined whether treatment-related changes in substructure volume varied in patients similarly diagnosed in the current study. Finally, repeated measures ANOVA was used to determine if volume measures at baseline predicted ECT-related change in clinical response. Only patients completing all three-time points (n=29) were included in this statistical test.
RESULTS
Baseline characteristics
Patient and control groups did not differ in age F(1, 74) = 1.34, p = .25, gender χ2(1, 74) = .05, p= .81 or education F(1,73) = 3.73, p = .06. Age of onset, duration of current episode and duration of illness (controlling for age) did not associate with hippocampal or amygdalar volumes at baseline. Test distributions did not deviate from normality, Kolmogorov-Smirnov tests all p > .05.
Cross sectional effects of diagnosis on volume
Significantly smaller right, F(1,74) = 4.14, p < .05, and left hippocampal volumes, F(1,74) = 15.62, p < .000001, were observed in patients compared to controls at baseline. These effects trended towards significance for the left, F(1,74) = 3.63, p < .07, and right amygdala, F(1, 74) = 3.20, p < .08 [Table 1, Fig 2].
Table 1.
Baseline demographics and mean mood and volume measures at each study time point.
| Patients with MDD, N = 43 | Controls, N = 32 | ||||
|---|---|---|---|---|---|
| Baseline Demographics | |||||
|
| |||||
| Age, mean (SD), y | 42.0 (13) | 39.70 (12) | |||
| Gender (M/F) | 20/23 | 14/18 | |||
| Race/ethnicity | |||||
| African American | 2 | 4 | |||
| Asian | 4 | 3 | |||
| Hispanic | 5 | 1 | |||
| White | 30 | 23 | |||
| Multi-ethnic | 2 | 1 | |||
| Adjusted education, years | 15.76 (2.79) | 16.93 (2.29) | |||
| Dextral/non-dextrala | 32/11 | 28/4 | |||
| Clinical Information | |||||
| Unipolar/bipolar | 35/7 | – | |||
| Subjects receiving RUL, mean (SD), % | 86 (27.43) | ||||
| Responders/remitters, % | 68/31 | ||||
| Age at onsetb, mean (SD), y | 29.63 (13.03) | – | |||
| Current episodeb, mean (SD), y | 1.41 (2.21) | – | |||
| Lifetime illnessb, mean (SD), y | 16.71 (12.98) | – | |||
|
| |||||
| Time point | T1, N = 43 | T2, N = 36 | T3, N = 29 | C1, N = 32 | C2, N = 30 |
|
| |||||
| HAM-D | 25.64 (6.12)* | 20.74 (6.40)† | 11.28 (8.11)‡ | – | – |
| QIDS-SR | 20.81 (4.21)* | 16.97 (4.88)† | 9.00 (5.57)‡ | – | – |
| MADRS | 39.61 (9.80)* | 33.43 (8.65)† | 14.21 (10.73)‡ | – | – |
| Brain volumec, mean (SD), cm3 | 1264.23 (140.93) | 1252.71 (144.13) | 1241.61 (133.91) | 1240.91 (167.79) | 1250.03 (167.47) |
| L hippo volumec, mean (SD), mm3 | 3609.95 (359.54)* | 3761.13 (476.05)† | 3978.10 (463.67)‡ | 3967.01 (419.73) | 3991.01 (373.35) |
| R hippo volumec, mean (SD), mm3 | 3683.69 (384.03)* | 3838.91 (375.18)† | 4075.06 (401.45)‡ | 3890.63 (444.34) | 3931.45 (455.76) |
| L amyg volumec, mean (SD), mm3 | 1440.63 (181.83)* | 1478.37 (198.53)† | 1574.89 (214.31)‡ | 1525.82 (214.34) | 1532.27 (201.10) |
| R amyg volumec, mean (SD), mm3 | 1451.08 (185.04)* | 1505.94 (226.63)† | 1586.96 (224.86)‡ | 1530.25 (221.69) | 1526.09 (192.16) |
Abbreviations: T1: Patient baseline; T2: After the 2nd ECT; T3: After the ECT index series; C1: Control baseline; C2: Control follow-up; RUL: Right unilateral lead placement; HAM-D: Hamilton Rating Scale for Depression; QIDS-SR: Quick Inventory of Depressive Symptomatology – Self-Report; MADRS: Montgomery – Åsberg Depression Rating Scale, Responders defined as patients with a >50% improvement in HAM-D scores over the course of treatment, Remitters defined as patients with HAMD < 7 at the end of ECT, L: left, R: right, hippo: hippocampus, amyg: amygdala.
Handedness was estimated using the modified Edinburgh Handedness lnventory(77) where a laterality quotient of < .7 was used to define non-dextrals.
Data for 1 patient each was missing for education, age of onset, duration of current episode and duration of lifetime illness (with lifetime illness estimated from patient self-report).
Means for MRI measures are adjusted for covariates in the statistical model including age and gender and/or brain volume.
Significant effect between T1 and T2,
significant effect between T2 and T3;
significant effect between T1 and T3.
Figure 2.
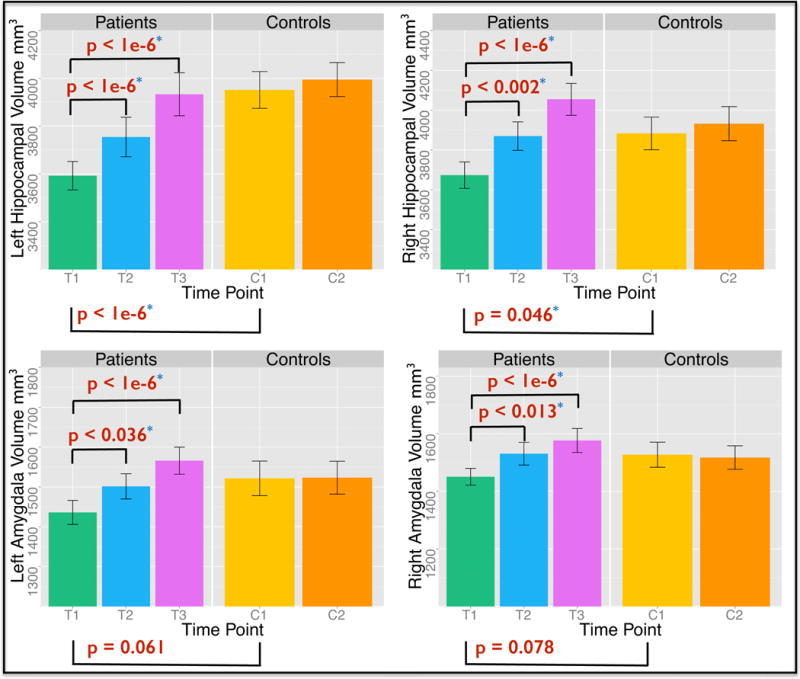
Cross-sectional effects (after controlling for age, gender and total brain volume) of diagnosis and longitudinal effects of ECT in patients and time in controls on hippocampal (top) and amygdala (bottom) volume (mm3).
Longitudinal effects of ECT and time on volume
Significant longitudinal effects of ECT were observed for all substructures: left hippocampus, F(2, 31.09) = 36.73; right hippocampus, F(2, 32.68) = 48.82; left amygdala, F(2, 31.16) = 18.65 and right amygdala, F(2, 32.04) = 14.88, all p < .000001. Significant increases in volume were also present between each time point when compared pairwise within patients. Volumes remained stable across time in controls [Table 1 and 2; Fig 2]. Brain volume did not vary significantly across time points in patients or controls, all p > .05.
Table 2.
Pairwise comparisons between study time points.
| HAM-D | QIDS-SR | Left Hippocampus | Right Hippocampus | Left Amygdala | Right Amygdala | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | Mean Diff | Sig. | Mean Diff | Sig. | Mean Diff | Sig. | Mean Diff | Sig. | Mean Diff | Sig. | Mean Diff | Sig. |
| T1 vs. T2 | 5.14 | .0001 | 4.08 | .0001 | 151.17 | .002 | 155.21 | .0001 | 37.73 | .036 | 54.85 | .013 |
| T1 vs. T3 | 14.19 | .0001 | 11.83 | .0001 | 368.14 | .0001 | 391.36 | .0001 | 134.25 | .0001 | 135.8 | .0001 |
| T2 vs. T3 | 9.05 | .0001 | 7.75 | .0001 | 216.97 | .0001 | 236.15 | .0001 | 96.52 | .0001 | 81.02 | .0001 |
| C1 vs. C2 | – | – | – | – | 24.08 | N.S. | 40.83 | N.S. | 6.94 | N.S. | −4.15 | N.S. |
Abbreviations: T1: Patient baseline; T2: After the 2nd ECT; T3: After the ECT index series; C1: Control baseline; C2: Control follow-up; HAM-D: Hamilton Rating Scale for Depression; QIDS-SR: Quick Inventory of Depressive Symptomatology – Self Report, Mean Diff: difference in means between time points, N.S.: not significant – all p > 20.
Effects of lead placement or diagnosis of bipolar disorder on change in volume
Lead placement (quantified as the percentage of sessions performed with RUL lead placement) was not shown to significantly influence change in volume occurring with ECT for the left or right hippocampus or amygdala, all p > .30. Change in substructure volume with ECT was not shown to differ between patients diagnosed with unipolar (n=32) or bipolar depression (n=7), all p > .82.
Longitudinal effects of ECT on shape
Local ECT-related deformations in surface structure were pronounced in anterior hippocampal regions (head and anterior body), particularly in the right hemisphere, but were more regionally pervasive when comparing T1 and T3 only (p < .05, corrected (37), Fig 3). Deformations in amygdala surface structure were prominent in the vicinity of the basolateral and centromedial nuclei (p < .05, corrected (37), Fig 4)
Figure 3.
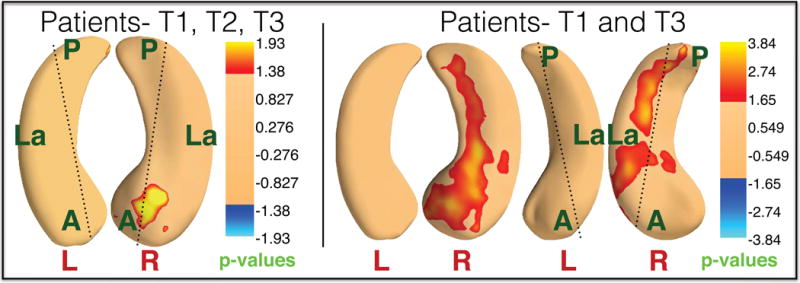
Longitudinal effect of ECT on local hippocampal shape. Left panel shows significant local expansion in the right anterior hippocampus in patients across all time points (T1, T2, and T3). Right panel shows significant expansions in the right anterior hippocampus that extend into more posterior CA2 and C3 regions in patients at T3 compared to T1. P-values are corrected for FDR (37) (q=0.05).
Figure 4.
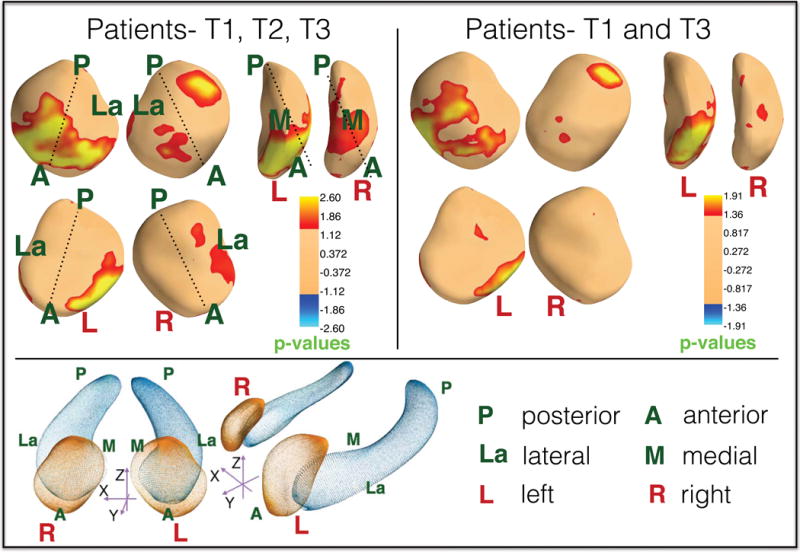
Longitudinal effect of ECT on local amygdalar shape. Top left panel shows significant local bilateral expansion in the dorsomedial amygdala as well as the left basolateral amygdala in patients across treatment time points. Top right panel shows significant expansions in the left basolateral amygdala in patients at T3 compared to T1. P-values are corrected for FDR (37) (q=0.05). The bottom panel shows the surface anatomy of the amygdala and hippocampus along with labeled orientations.
Effects of ECT on clinical response
Highly significant effects of ECT were observed for HAM-D, F(2, 29.62) = 35.99, p < .0001 and QIDS-SR scores, F(2, 29.44) = 50.03, p < .0001 [Fig 5A]. Pairwise comparisons showed symptom improvement early after the initiation of ECT (T1 vs. T2) and further improvements by the end of ECT index (T1 vs. T3 and T2 vs. T3) [Table 1 and 2]. Since ECT-related clinical response was a factor for manipulating lead placement, as expected, relationships with HAM-D, Pearson’s r = −.24, p = .013 and QIDS-SR scores, r = −.25, p <. 011 indicated that patients with lower mood scores were initially prescribed or switched to bilateral ECT. However, position of lead placement did not significantly impact change in HAMD or QIDS-SR scores over the course of the ECT index series, both p >. 05.
Figure 5.
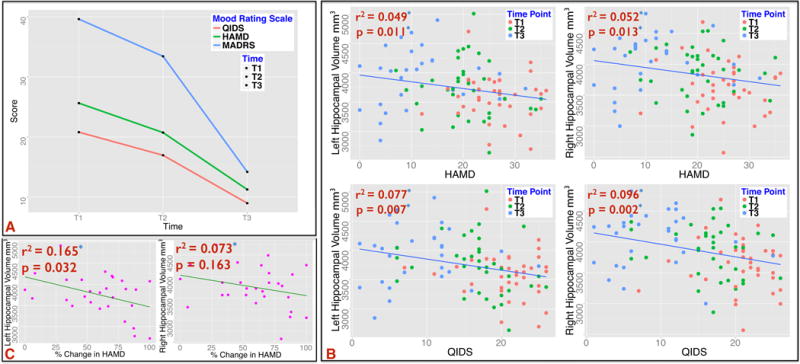
A: Treatment effect of ECT on mood scores; HAM-D, F(2, 29.62) = 35.99, p < .0001, QIDS-SR scores, F(2, 29.44) = 50.03, p < .0001 and MADRS, F(2, 29.08) = 47.5, p < .0001. B: Significant associations of hippocampal (top) and amygdala (bottom) volumes with improvement in mood scores across all time points T1, T2 and T3. C: Association of baseline volumes of the hippocampus and change in clinical response over the course of ECT. The left hippocampus is significantly correlated with improvement in percentage change in HAMD between T1 and T3.
Associations between hippocampal and amygdalar volume and clinical response
Hippocampal volumes increased in association with improved (decreasing) HAM-D, left, F(1, 43.28) = 7.12, p = .01, and right, F(1, 46.55) = 6.72, p = .013, and QIDS ratings, left, F(1, 3.61) = 8.29, p = .007, and right, F(34.71) = 11.06, p = .002. Improved QIDS ratings associated with increased left and right amygdala volume, F(1, 43.36) = 4.161, p = .047 and F(1, 37.55) = 4.01, p = .05, respectively [Fig 5B].
Predictors of clinical response
Smaller hippocampal volumes at baseline indicated greater treatment-related change in HAM-D scores, F(1,28) = 3.76, p = .038. Though interactions with hemisphere were absent, when examined separately effects exceeded the threshold of significance for the left hippocampus only, F(1,28) = 5.43, p = .032 [Fig 5C].
DISCUSSION
Several key findings emerged from this longitudinal study assessing the effects of ECT on hippocampal and amygdala structure in MDD. Study results demonstrated that 1) ECT induces structural plasticity in the hippocampus and amygdala; 2) volume deficits linked with MDD normalize towards control values; 3) regional differences in hippocampal and amygdalar morphology are more pronounced in areas with greater connections to limbic structures involved in the expression and regulation of emotion and mood; 4) the extent of ECT-related hippocampal and amygdalar plasticity relates to the extent of clinical response; and 5) the volume of the hippocampus at baseline is predictive of subsequent clinical outcome, i.e., patients with smaller hippocampal volumes prior to the start of ECT are more likely to show treatment-related clinical improvement after the initial phase of treatment.
Reductions of hippocampal volume are widely reported in patients with MDD in prior studies, particularly in patients with a more chronic course of illness (11,12, 38–41). The hippocampus is centrally involved in learning and memory and in the regulation of 1) emotion, 2) responses to emotion and 3) susceptibility to chronic stress via it’s connections with the amygdala, the limbic hypothalamic-pituitary-adrenal (HPA) axis and ventral striatal loop and dopaminergic mesolimbic system (42), all systems that appear central the pathophysiology of the disorder (43–45). The amygdala, also widely implicated in neuroimaging studies of MDD (46, 47), plays a pivotal role in emotional memory (particularly for negatively valenced stimuli), in the regulation of emotion and in modulating autonomic responses to emotion (42, 44, 48). In line with our findings, some earlier evidence suggests effects of ECT on hippocampal volume in MDD; only one prior study has simultaneously investigated ECT-related changes in amygdala volume. Specifically, bilateral increases in hippocampal volume have been reported to occur between baseline and the end of the ECT treatment series in a sample of 12 MDD patients receiving concurrent antidepressant treatment (17). An independent pilot study (18) similarly showed ECT-related increases in hippocampal as well as in amygdalar volume in 15 antidepressant-free MDD patients. However, in this study, benzodiazepines, which may interact with treatment (49, 50) and potentially also with biological processes, were permitted during ECT. As possibly attributable to the heterogeneity of major depression and the study of small samples, in contrast to our findings, neither of the studies above could demonstrate significant relationships between change in substructure volume and ECT-related clinical response.
Using a whole-brain voxel-based rather than a ROI approach, a third investigation comparing 10 ECT patients (5 unipolar/5 bipolar) undergoing variable pharmacological treatments staged with ECT, also reported a significant effect of ECT (yes, if a patient received ECT within 3 or 6-months after beginning medication treatment, or no, if the patient did not receive ECT) in right hippocampal gray matter (36). Though symptom improvement was shown to relate to regional gray matter change in post-hoc analyses, investigators also noted differential effects in the 5 unipolar and 5 bipolar patients examined. Since ECT was preceded by different medication treatments, patients were scanned at variable time points during the course of medication and ECT treatment, and statistical contrasts in this small sample were collapsed into ECT versus no-ECT groups to investigate interactions, the nature of these relationships remain opaque. Our findings more clearly demonstrate that ECT-induced structural changes in the hippocampus and amygdalar are not solely the consequence of successive seizure therapy, but rather reflect neuroplasticity associated with therapeutic effects. Further, our results also show that changes in volume occur in patients tapered off psychotropic medications prior to the initiation of ECT and thus confirm that the observed neuroplastic processes are independent of pharmacotherapy. Contrasting with the prior report mentioned above (36), the present results also do not support that the therapeutic effects of ECT on brain structure vary in patients diagnosed with unipolar and bipolar depression. Since DSM categorizations of depression may reflect both overlapping and differing biological bases (51, 52), as for this prior study (36), the current investigation may not adequately address such factors with the current sample size.
Notably, our findings newly show that hippocampal structure prior to ECT may be an important indicator of treatment outcome. That is, patients with smaller hippocampal volumes at baseline are shown more likely to exhibit increases in volume with ECT and to show concomitant improvements in clinical symptoms. Results further indicate that both clinical response to ECT and ECT-induced changes in volume occur rapidly. That is, significant changes in both clinical scores and hippocampal and amygdalar volume are observable after the 2nd ECT session (within ~72 hours of treatment initiation). These novel findings thus add to the existing literature to demonstrate that ECT-induced structural plasticity in the hippocampus both relates to and may predict therapeutic response to ECT. Though showing the same pattern, variations in size of the amygdala at baseline did not significantly relate to overall clinical outcome. Increased variability in this region due to its smaller size and/or the limited spatial resolution of structural imaging data may account for our failure to observe significant relationships. However, it is also possible that the hippocampus is more central to the mechanisms underlying positive treatment response and that changes in amygdala structure relate more closely to specific clinical symptoms.
Neuroplasticity, including processes of synaptogenesis, dendrogenesis, angiogenesis, or neurogenesis or other changes in the structure of neurons and glial cells and their processes may contribute to ECT-related changes in hippocampal and amygdala volume (53–55). Neurogenesis, the process by which neurons are generated from neural progenitor cells, is shown to occur in the hippocampal dentate subgranular zone throughout life (56, 57). Preclinical data has previously demonstrated links between adult neurogenesis, neurotrophic factors and depression (54). For example, observations that electroconvulsive shock (ECS) (58), the animal model of ECT, and antidepressants to a lesser extent (59), increase neurogenesis in the dentate gyrus support that neurogenesis may be a mechanism contributing to treatment efficacy. Further, stress, regulated via the HPA axis, can suppress ongoing neurogenesis involved in fear-related learning and memory via connections between the hippocampal dentate gyrus and amygdala (43, 60). Adult neurogenesis as well as the growth of neural processes may therefore underlie successful treatment response and contribute to structural plasticity of the hippocampus and consequently, to structural changes of the amygdala and other limbic regions.
Neurotrophic factors (proteins supporting the growth, survival and maintenance of neurons), that could precede, follow or act independently of neurogenesis, also exhibit convincing links with MDD pathophysiology and antidepressant response. Brain-derived neurotrophic factor (BDNF), for example, is shown as decreased in animal models of depression, and increases after ECS and modulates limbic circuits to promote treatment-related neuroplasticity (61–66). Other neurotrophic factors implicated in MDD and in antidepressant response include vascular endothelial growth factor (VEGF), (67–69) fibroblast growth factor-2 (FGF-2), (70, 71) and nerve growth factor (NGF) (72). Since the hippocampus appears particularly vulnerable to stress, processes that mediate immune system response (73) may also account for changes in the structure of the hippocampus and related antidepressant response.
Though the structure of the hippocampus is uniform along its extent, afferents, transmitted mostly via the entorhinal cortex (EC), and efferents are organized according to the cortical and subcortical regions to which they connect in an anterior-posterior gradient (19, 20). Findings from this study showed anterior aspects of the hippocampus exhibit pronounced volume changes with ECT, particularly in the right hemisphere. The topographic organization of the EC-hippocampal perforant path leads to a convergence of exteroceptive sensory information on the posterior hippocampal formation; information from subcortical centers, including the septum, thalamic midline nuclei and amygdala, reflecting intrinsic state, transmit more anteriorly. For the amygdala, the basolateral nuclear group receives inputs from the temporal and the orbitofrontal cortex, vmPFC cortex and the hippocampus as are involved in determining the relative value of reward; the hippocampus is vital for encoding and recalling stimuli. These connections are thus thought central to integrating complex sensory data, with respect to emotional salience. Since the anterior hippocampus has more prominent connections with the amygdala, vmPFC, ACC, as well as the medial thalamus and ventral basal ganglia, these results support the involvement of circuits relating to mood regulation and emotion (74).
Although it is not possible to distinguish amygdala cytoarchitecture and thus delineate specific amygdala nuclei with structural MRI, statistical shape maps of the amygdala structure visually identify significant ECT-related volume changes in the vicinity of the basolateral nuclei as well as the centromedial nuclei that together act to regulate emotional learning and emotional arousal (75, 76).
CONCLUSION
ECT elicits neuroplastic processes associated with clinical response that act to normalize MDD-related reductions in hippocampal and amygdalar structure. Patients with smaller relative hippocampal volumes are most likely to show volume increases and improved clinical response. The mechanisms underlying the beneficial effects of ECT are expected to overlap with those of other forms of successful treatment. Thus, variations in hippocampal and amygdalar structure may serve as potential biomarkers for the development of other fast acting treatments. Future research should address the longer-term effects of ECT in relation to structural plasticity, especially in regard to maintenance of treatment response and relapse.
Supplementary Material
Acknowledgments
Award Numbers R01MH092301 and K24MH102743 from the National Institute Of Mental Health supported this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.WHO World Health Organization. Depression. http://wwwwhoint/mental_health/management/depression/definition/en/
- 2.Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry. 2003;160:237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 4.McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163:1531–1541. doi: 10.1176/ajp.2006.163.9.1531. quiz 1666. [DOI] [PubMed] [Google Scholar]
- 5.Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–1530. doi: 10.1176/ajp.2006.163.9.1519. quiz 1665. [DOI] [PubMed] [Google Scholar]
- 6.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kho KH, van Vreeswijk MF, Simpson S, Zwinderman AH. A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT. 2003;19:139–147. doi: 10.1097/00124509-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485–491. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 10.Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 11.Videbech P, Ravnkilde B. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI, Gado MH, Kraemer HC. Untreated Depression and Hippocampal Volume Loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 14.MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:343–349. [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a metaanalysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 17.Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
- 18.Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, Voshaar RO, et al. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res. 2013;214:197–203. doi: 10.1016/j.pscychresns.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Rev Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 24.Heo M, Murphy CF, Meyers BS. Relationship between the Hamilton Depression Rating Scale and the Montgomery-Asberg Depression Rating Scale in depressed elderly: a meta-analysis. The Am J Geriatr Psychiatry. 2007;15:899–905. doi: 10.1097/JGP.0b013e318098614e. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 26.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Mag Reson Medicine. 2012;68:389–399. doi: 10.1002/mrm.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. NeuroImage. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Lui LM, Gu X, Hayashi KM, Chan TF, Toga AW, et al. Brain surface conformal parameterization using Riemann surface structure. IEEE Trans Med Imaging. 2007;26:853–865. doi: 10.1109/TMI.2007.895464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J, Thompson PM, Gutman B, Wang Y, Alzheimer’s Disease Neuroimaging I Surface fluid registration of conformal representation: application to detect disease burden and genetic influence on hippocampus. NeuroImage. 2013;78:111–134. doi: 10.1016/j.neuroimage.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. PNAS. 2014;111:1156–1161. doi: 10.1073/pnas.1321399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser (Methodological) 1995:289–300. [Google Scholar]
- 38.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. PNAS. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 40.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression Duration But Not Age Predicts Hippocampal Volume Loss in Medically Healthy Women with Recurrent Major Depression. J Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. PNAS. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duvernoy HM, Cattin F. The human hippocampus functional anatomy, vascularization and serial sections with MRI. 3. Berlin; New York: Springer; 2005. [Google Scholar]
- 43.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Rev Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience Biobehav Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg RM, Pettinati HM. Benzodiazepines and Electroconvulsive Therapy. Convulsive Therapy. 1993;9:262–273. [PubMed] [Google Scholar]
- 50.Pettinati HM, Stephens SM, Willis KM, Robin SE. Evidence for less improvement in depression in patients taking benzodiazepines during unilateral ECT. Am J Psychiatry. 1990;147:1029–1035. doi: 10.1176/ajp.147.8.1029. [DOI] [PubMed] [Google Scholar]
- 51.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 52.Keshavan MS, Ongur D. The journey from RDC/DSM diagnoses toward RDoC dimensions. World Psychiatry. 2014;13:44–46. doi: 10.1002/wps.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19:329–338. doi: 10.1016/j.euroneuro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouckaert F, Sienaert P, Obbels J, Dols A, Vandenbulcke M, Stek M, et al. ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT. 2014;30:143–151. doi: 10.1097/YCT.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 56.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 57.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Experimental Neurology. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 58.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 59.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 63.Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7:231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neuroscience. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Bioll Psychiatry. 2010;67:125–132. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Segawa M, Morinobu S, Matsumoto T, Fuchikami M, Yamawaki S. Electroconvulsive seizure, but not imipramine, rapidly up-regulates pro-BDNF and t-PA, leading to mature BDNF production, in the rat hippocampus. T Int J Neuropsychopharmacology. 2013;16:339–350. doi: 10.1017/S1461145712000053. [DOI] [PubMed] [Google Scholar]
- 67.Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. The European J Neurosc. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 69.Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. PNAS. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Gale K, Kondratyev A. Effects of repeated minimal electroshock seizures on NGF, BDNF and FGF-2 protein in the rat brain during postnatal development. Int J Dev Neurosc. 2010;28:227–232. doi: 10.1016/j.ijdevneu.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondratyev A, Ved R, Gale K. The effects of repeated minimal electroconvulsive shock exposure on levels of mRNA encoding fibroblast growth factor-2 and nerve growth factor in limbic regions. Neuroscience. 2002;114:411–416. doi: 10.1016/s0306-4522(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 72.Diniz BS, Teixeira AL, Machado-Vieira R, Talib LL, Gattaz WF, Forlenza OV. Reduced serum nerve growth factor in patients with late-life depression. Am J Geriatr Psychiatr. 2013;21:493–496. doi: 10.1016/j.jagp.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 74.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nieuwenhuys R, Voogd J, Huijzen Cv. The human central nervous system. 4. New York: Springer; 2008. [Google Scholar]
- 76.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9 doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 77.Oldfield RC. The Assessment and Analysis of Handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


