Abstract
Aging results in progressive deteriorations in the structure and function of the heart and is a dominant risk factor for cardiovascular diseases, the leading cause of death in Western populations. Although the phenotypes of cardiac aging have been well characterized, the molecular mechanisms of cardiac aging are just beginning to be revealed. With the continuously growing elderly population, there is a great need for interventions in cardiac aging. This article will provide an overview of the phenotypic changes of cardiac aging, the molecular mechanisms underlying these changes, and will present some of the recent advances in the development of interventions to delay or reverse cardiac aging.
The molecular mechanisms underlying cardiac aging (e.g., altered mTOR signaling) are beginning to be understood. This knowledge may greatly advance the development of interventions (e.g., pharmacological inhibitors of mTOR).
Cardiovascular diseases are the leading cause of death in most developed nations. Although it has received the least public attention, aging is by far the dominant risk factor for development cardiovascular diseases, as the prevalence of cardiovascular diseases increases dramatically with increasing age. The prevalence rate of cardiovascular diseases is >70% for Americans 60 to 79 years of age and >80% for Americans >80 years of age (Go et al. 2014). Even without associated systemic risk factors, intrinsic cardiac aging leads to structural and functional deteriorations of the heart in elderly individuals. Therefore, interventions to combat cardiac aging will not only improve healthspan of the elderly but can also extend lifespan by delaying cardiovascular disease-related deaths. Although there is presently no treatment for cardiac aging, recent advances in the understanding of the mechanisms of cardiac aging have provided new insights, and we are now poised on the threshold of development of new interventions to attenuate or reverse cardiac aging.
CARDIAC AGING IN HUMAN AND ANIMAL MODELS
The Framingham Heart Study and the Baltimore Longitudinal Study on Aging (BLSA) have shown that, in healthy individuals without concomitant cardiovascular diseases, aging results in an increase in the prevalence of left ventricular (LV) hypertrophy, a decline in diastolic function, and relatively preserved systolic function at rest but a decline in exercise capacity, as well as an increase in the prevalence of atrial fibrillation (Lakatta and Levy 2003b). These changes can be independent of conventional risk factors for heart disease (smoking, hypertension, blood lipid levels, diabetes, etc.) and, thus, may be considered to be part of intrinsic cardiac aging. At rest, systolic function measured by the ejection fraction (EF) remains steady in older populations. However, on exercise, maximum heart rate and EF are lower in older populations, indicating reduced cardiac reserve (Lakatta 2002). An age-dependent increase in myocardial performance index (MPI) has also been shown (Spencer et al. 2004). An increase in MPI indicates that a greater fraction of systole is spent to cope with the pressure changes during isovolumetric phases, and has been shown to reflect both LV systolic and/or diastolic dysfunction (Tei et al. 1997). Because of impaired early diastolic filling and an increased contribution of atrial contraction to LV filling, the peak early filling velocity and the ratio of the early and late (E/A ratio) filling velocity decrease with age; the early component is larger than the late atrial component of filling in young persons, but, when this reverses, it is an indicator of diastolic dysfunction (Downes et al. 1989; Swinne et al. 1992; Kitzman 2002; Choi et al. 2009). Diastolic dysfunction is increasingly seen in the elderly in the absence of systolic heart failure, a condition that has been given the designation of heart failure with preserved ejection fraction (HFpEF). It is especially prevalent in aged women (Brouwers et al. 2012) and is an increasing cause of hospital admissions (Oktay et al. 2013).
Rodents, particularly the mouse model, are widely used in cardiac aging studies. Murine cardiac aging phenotypes closely recapitulate the phenotypes of human cardiac aging (Lakatta and Levy 2003a). Echocardiography performed on a mouse longevity cohort showed that left ventricular mass index (LVMI) and left atrial dimension significantly increased with age. Diastolic function measured by tissue Doppler declines with age, whereas systolic function showed a modest reduction from young adult to the oldest group. The MPI also worsens with age, which is consistent with the age-related declines in systolic and diastolic function (Barger et al. 2008). In addition to the similar cardiac aging phenotypes, the relatively short lifespan and the availability of genetically modified mice are the advantages of using mouse model in the study of the molecular mechanisms of cardiac aging. Despites sharing similar cardiac aging phenotypes as human, laboratory mice do not develop elevated blood pressure or adverse blood glucose and lipid profiles (Zheng et al. 2003; Dai et al. 2009). This allows the intrinsic cardiac changes of aging to be investigated without the added complications of cardiovascular risk factors, including hypertension and diabetes.
MOLECULAR MECHANISMS OF CARDIAC AGING
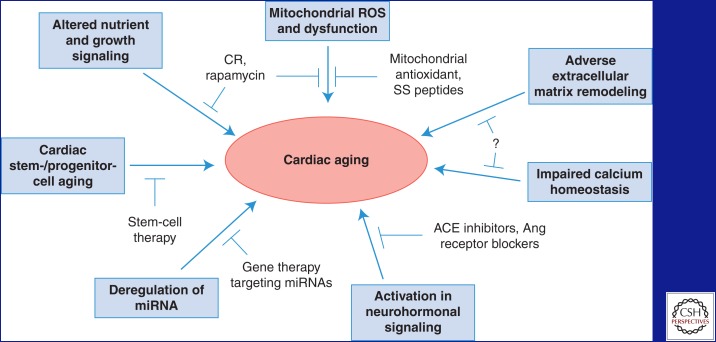
Recent studies have shown the involvements of multiple molecular mechanisms in the pathogenesis of cardiac aging. These mechanisms are summarized in Figure 1 and discussed below.
Figure 1.
A schematic summary of the molecular mechanisms of cardiac aging and potential cardiac aging interventions. ROS, Reactive oxygen species; CR, calorie restriction; SS, Szeto–Schiller; miRNA, microRNA; ACE, angiotensin-converting enzyme; Ang, angiotensin.
Altered Nutrient and Growth Signaling
Cardiac hypertrophy is a hallmark of cardiac aging. Deregulation of nutrient and growth signaling pathways, including mechanistic target of rapamycin (mTOR) and insulin-like growth factor-1 (IGF-1) signaling, have been implicated in cardiac hypertrophy and aging. mTOR integrates nutrient and hormonal signals to regulate growth and is a major modulator of aging and age-related disease (Kennedy et al. 2007). Previous studies in Drosophila and mouse models have shown that increased mTOR signaling impairs and reduced mTOR signaling improves resistance to cardiac aging. Bodmer’s laboratory initially showed that inhibition of the mTOR pathway could attenuate the age-related decline in cardiac function in Drosophila (Luong et al. 2006). They later showed that eukaryotic translation-initiation factor 4E (eIF4E)-binding protein (4EBP) overexpression attenuates the age-related decline to a similar extent as overexpression of the TOR antagonist tuberous sclerosis complex (TSC), and overexpression of eIF4E leads to an accelerated decline of myocardial function (Wessells et al. 2009). These findings implicate a major role of mTOR/eIF4E signaling in cardiac aging in Drosophila. In addition, Meikle et al. (2005) showed that mice with cardiac-specific deletion of TSC1, a model of increased mTOR signaling, develop dilated cardiomyopathy and have a median lifespan of 6 mo. Although there is no evidence yet on the beneficial effects of genetic manipulation to decrease mTOR activity in the aging mammalian heart, inhibition of mTOR signaling by caloric restriction (CR) or rapamycin (see below) has been shown to protect against cardiac aging.
Insulin/IGF-1 signaling is one of the best-characterized pathways of lifespan regulation in animal models. Deficiency in insulin/IGF-1 signaling improves cardiac performance at advanced age in Drosophila and attenuates age-related cardiomyocyte dysfunction in mice (Wessells et al. 2004; Li et al. 2008). However, in humans, an age-dependent decline in serum IGF-1 levels (Corpas et al. 1993) correlates with an increased risk of heart failure among elderly patients without prior history of heart disease (Vasan et al. 2003), and interventions that increase IGF-1 signaling, such as growth hormone therapy, may be beneficial in heart failure (Broglio et al. 1999; Khan et al. 2002). The beneficial effects of IGF-1 on cardiovascular disease may be conferred by mitochondrial protection mechanisms. One study showed that in vitro treatment of endothelial cells and cardiomyocytes with IGF-1 decreased mitochondrial superoxide production (Csiszar et al. 2008). Furthermore, low-plasma levels of growth hormone (GH) and IGF-1 in Ames dwarf mice are associated with increased mitochondrial oxidative stress in the vasculature and the heart, which is responsible for the impaired contractile function (Ren and Brown-Borg 2002). Recent studies show that IGF-1 treatment of aged rats protects against mitochondrial oxidative stress (Puche et al. 2008), and other studies suggest that interventions that increase circulating IGF-1 levels exert cardiovascular protective effects in aging (Rivera et al. 2005; Groban et al. 2006; Lopez-Lopez et al. 2007). The roles of mitochondrial oxidative stress in cardiac aging are discussed further below.
Mitochondrial Oxidative Damage and Mitochondrial Dysfunction
The mitochondrial free-radical theory of aging proposes that excessive mitochondrial reactive oxygen species (ROS) damages mitochondrial DNA and redox-sensitive mitochondrial proteins, causing mitochondrial dysfunction and further increase in ROS production (the “vicious cycle” of ROS-induced ROS release), and that this oxidative damage leads to cellular and organ functional declines that limit lifespan and healthspan (Harman 1972).
Cardiomyocytes, being postmitotic, are highly susceptible to age-related mitochondrial damage. Mitochondria in aged cardiomyocytes are usually enlarged with swelling, loss of cristae, and even destruction of inner membranes and are deficient in ATP production (Terman and Brunk 2004). A previous study has shown that mitochondrial production of ROS significantly increases in the heart with advanced age (Judge et al. 2005). Also, increasing evidence suggests that abnormal mitochondrial ROS production and impaired ROS detoxification contribute to mitochondrial dysfunction and cardiomyopathy in old age (reviewed in Terzioglu and Larsson 2007; Trifunovic and Larsson 2008; Mammucari and Rizzuto 2010).
Direct evidence supporting the role of mitochondrial oxidative damage in cardiac aging was provided by mice overexpressing catalase targeted to the mitochondria (mCAT) (Schriner et al. 2005; Dai et al. 2009). In addition to an extension of median and maximum lifespan, mCAT mice displayed greatly attenuated cardiac aging phenotypes, including reduced cardiac hypertrophy and improved diastolic function and myocardial performance (Dai et al. 2009). The attenuated cardiac aging phenotypes in mCAT mice were accompanied by significantly reduced mitochondrial protein oxidative damage and mitochondrial DNA mutation and deletion frequencies, suggesting prevention of mitochondrial oxidative damage as a strategy for protection from cardiac aging. Additional evidence is provided by mice with homozygous mutation of mitochondrial polymerase γ (Polgm/m), which have substantial increases in mtDNA mutations and deletions with age (Trifunovic et al. 2004; Kujoth et al. 2005). Polgm/m mice have a shortened lifespan and develop cardiomyopathy in middle age (13–14 mo) (Trifunovic et al. 2004; Dai et al. 2010). Interestingly, mCAT partially rescues the mitochondrial damage and cardiomyopathy in Polgm/m mice, further supporting the role of mitochondrial ROS in cardiac aging (Dai et al. 2010).
Peroxisome proliferator-activated receptor γ coactivator 1α (PCG-1α) is the key regulator of mitochondrial biogenesis, and PCG-1α enhances mitochondrial function in the heart (Wenz 2011). PCG-1α expression is repressed in the failing heart, and PCG-1α knockout mice have reduced mitochondrial gene expression and develop cardiac dysfunction at 7 mo of age (Arany et al. 2005). Cardiac-specific overexpression of PCG-1α in adult mice nevertheless leads to cardiomyopathy (Russell et al. 2004).
Given the complexity of the systems involved, mitochondrial dysfunction and aberrant ROS production likely contribute to aging through both direct damage to cellular macromolecules and interference with normal signaling and energetics. The effect of mitochondrial ROS in signaling and energetics in cardiac aging was previously reviewed (Dai et al. 2014a).
Adverse Extracellular Matrix (ECM) Remodeling
ECM is a complex collection of proteins located outside the cells and provides structural and biological supports to the surrounding cells. Cardiac fibroblasts are the primary sources of cardiac ECM proteins, including collagen types I, II, III, IV, V, and VI, elastin, fibronectin, laminin, and fibrinogen (DeQuach et al. 2010). Cardiac ECM aligns cardiomyocytes and provides structural support to the heart; however, excessive ECM deposition increases the stiffness of the myocardium and mediates diastolic dysfunction (Ouzounian et al. 2008). ECM composition is dynamically remodeled by the balance of the synthesis and degradation of ECM proteins by matrix metalloproteinases (MMPs) and other proteases. Cardiac aging is associated with myocardial fibrosis, and deregulation of ECM synthesis and degradation has both been observed in aging hearts.
Transforming growth factor-β (TGF-β) is a profibrotic factor that has been shown to induce the expression of ECM proteins and inhibit matrix degradation by MMPs (Bujak and Frangogiannis 2007). Reduced TGF-β1 expression results in reduced myocardial fibrosis and improved LV compliance in 24-mo-old TGF-β1 heterozygous mice (Brooks and Conrad 2000). Connective tissue growth factor (CTGF), another profibrotic factor, is a downstream mediator from TGF-β and its expression increases with age (Wang et al. 2010). Mice overexpressing CTGF in a cardiomyocyte-specific manner show accelerated cardiac aging and begin to develop age-related cardiac dysfunction at 7 mo of age (Panek et al. 2009). The role of ECM synthesis in cardiac aging is also implicated by the accelerated myocardial fibrosis that accompanies higher TGF-β and CTGF levels in senescence-accelerated mice that display diastolic dysfunction at 6 mo of age (Reed et al. 2011). In another study, Bradshaw et al. (2010) showed that expression of a matricellular protein, secreted protein acidic and rich in cysteine (SPARC), increased with age, and that deletion of SPARC resulted in reduced fibrillar collagen content in the LV and decreased LV diastolic stiffness. Together, this evidence suggests that increased ECM synthesis is an important mediator of diastolic dysfunction with age and that reduced ECM synthesis can improve cardiac aging.
MMPs are a family of 25 zinc-dependent enzymes that regulate ECM degradation; tissue inhibitors of matrix metalloproteinase (TIMPs) are a family composed of TIMP-1, -2, -3, and -4, which regulate MMP proteolytic activity in the tissue (Tayebjee et al. 2005). The expression levels of MMPs and TIMPs are differentially regulated by age (Lindsey et al. 2005; Bonnema et al. 2007), but the roles of most MMPs and TIMPs in cardiac aging have not been established. Spinale and colleagues showed that cardiac-specific MT1-MMP overexpression accelerated cardiac aging responses in mice and that MT1-MMP-overexpressing mice have increased myocardial fibrosis and LV dysfunction at middle age (Spinale et al. 2009). More recently, Chiao et al. (2011) showed that MMP-9 levels increase in the LV and plasma of aged C57Bl6 mice, and that aged MMP-9 knockout mice display attenuated cardiac aging phenotypes, including reduced collagen deposition and preserved diastolic function (Chiao et al. 2012). The attenuated cardiac aging phenotypes in MMP-9 knockout mice are accompanied by reduced expression of profibrotic proteins, periostin, and CTGF and a compensatory increase in MMP-8 levels in the LV (Chiao et al. 2012). Together, these findings suggest a role of ECM degradation that is under complex regulation by MMPs in cardiac aging.
Impaired Calcium Homeostasis
One mechanism underlying age-dependent diastolic dysfunction is impaired active relaxation of cardiomyocytes (Zile and Brutsaert 2002; Kass et al. 2004). During relaxation, calcium ions dissociate from the actin–myosin complex and are taken up into the sarcoplasmic reticulum (SR) or extruded outside the cardiomyocyte. Impaired Ca2+ cycling, increased myofilament stiffness, reduced Ca2+ sensitivity of myofilament proteins, and alterations in actin or myosin properties can lead to impaired cardiomyocyte relaxation (Zile and Brutsaert 2002; Kass et al. 2004; Borlaug and Kass 2006). Aged mouse hearts have reduced sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) expression (Dai et al. 2009) and activity (Janczewski and Lakatta 2010), with compensatory increase in the levels of the Na+/Ca2+ exchanger (Koban et al. 1998). Studies suggest that the aged heart uses the compensatory increase in the L-type Ca2+ currents (Josephson et al. 2002) and prolongation of action potential duration to preserve SR loading and to maintain intracellular Ca2+-transients and contractions in old cardiomyocytes (Janczewski et al. 2002). Posttranslational modifications of SERCA2, including age-related oxidation and nitration, have also been shown (Knyushko et al. 2005; Sharov et al. 2006), but their roles in cardiac aging are as yet unclear.
Chronic Activation in Neurohormonal Signaling
The renin angiotensin aldosterone system (RAAS) is the key endocrine system regulating hypertension and stress-induced cardiac hypertrophy. Angiotensin II (Ang II) infusion induces cardiomyocyte hypertrophy, increases cardiac fibrosis, and impairs cardiomyocyte relaxation (Domenighetti et al. 2005); these responses closely recapitulate the age-related changes in the heart (Dai et al. 2009). Studies have showed that Ang II concentration significantly increased in the aged rodent heart (Groban et al. 2006; Dai et al. 2009) potentially caused by increased tissue levels of angiotensin-converting enzyme (ACE) (Lakatta 2003). Moreover, long-term inhibition of Ang signaling by ACE inhibitors, angiotensin receptor blockers, or genetic disruption of Ang II receptor type I extend lifespan and delay age-dependent cardiac pathology in rodents (Basso et al. 2007; Benigni et al. 2009).
Activation of the β-adrenergic signaling increases heart rate, contractility, blood pressure, wall stress, and metabolic demand of the heart, and chronic stimulation of β-adrenergic signaling is deleterious to the heart. Deletion of adenylate cyclase type 5 (AC5), a key enzyme downstream from β-adrenergic signaling, extends murine lifespan and is protective against age-dependent cardiac hypertrophy, systolic dysfunction, apoptosis, and fibrosis (Yan et al. 2007).
Other Potential Mechanisms
Increasing evidence suggests that microRNAs (miRNAs) are important regulators of aging and cardiovascular diseases (Smith-Vikos and Slack 2012; Quiat and Olson 2013), and several recent studies implicated the roles of miRNAs in cardiac aging. van Almen et al. (2011) showed that the expression of the miR-17-92 cluster (consisting of miR-18a, miR19a, and miR-19b) decreases, whereas the expression of their targets, CTGF and ECM protein thrombospondin-1 (TSP-1) increases, in heart-failure-prone C57Bl6 × 129Sv mice. In aged cardiomyocyte cultures, these investigators showed that miR-18a and miR-19b regulated expression of CTGF, TSP-1, and collagen, suggesting that these miRNAs mediate age-related ECM remodeling in the hearts. In C57Bl6 mice, Jazbutyte et al. (2013) detected an age-related increase in miR-22 in hearts and showed that miR-22 regulates cardiac fibroblast senescence. In a recent study, Boon et al. (2013) showed that expression of miR-34a increased in aged mouse hearts, and in vivo silencing of miR-34a for 1 wk rescued the increase in cardiomyocyte cell death in aged mice. They also showed that aged miR-34a knockout mice had improved contractile function and reduced cardiac hypertrophy compared to wild-type littermates. This evidence suggests that increased miR-34a expression in the aged heart contributes to cardiac aging.
Previous studies have shown that cardiac stem cells and progenitor cells may regenerate the adult heart to some extent (Beltrami et al. 2003; Hsieh et al. 2007). Cardiac stem cells in the aged heart may have impaired regenerative capacity, either by senescence intrinsic to the stem cells or by an extrinsic hostile microenvironment associated with advanced age (reviewed in Anversa et al. 2005; Ballard and Edelberg 2007). Torella and colleagues (2004) observed an increased proportion of senescent cardiac stem cells (which express senescent marker p16ink4a and have reduced telomere length) in old wild-type mice and showed that IGF-1 overexpression can prevent senescence of cardiac stem cells. A recent study also showed that attenuation of the IGF-1/IGF-1-receptor and hepatocyte growth factor/mesenchymal-epithelial transition factor (c-Met) systems mediates aging of cardiac progenitor cells (Gonzalez et al. 2008). In another study, Bergmann et al. (2009) measured 14C from nuclear bomb tests in genomic DNA of human myocardial cells and used this method to show that the turnover or renewal rate of cardiomyocytes is reduced from 1% in young adult hearts to 0.45% in the hearts of the elderly. These results suggest that the regenerative capacity of cardiac stem cells declines with aging and that such declines may mediate the impaired myocardial repair in aged hearts.
RECENT ADVANCES IN INTERVENTIONS FOR CARDIAC AGING
The improved understanding of the pathogenesis of cardiac aging may greatly advance the development of interventions that target specific mechanisms to delay or treat cardiac aging (Fig. 1).
Calorie Restriction (CR)
CR is the most well-studied longevity intervention and has been shown to increase lifespan in a wide array of model organisms, from yeast and nematodes to mice, rats, and (perhaps) rhesus monkeys (Colman et al. 2009; Cruzen and Colman 2009; Fowler et al. 2010; Kastman et al. 2010; McKiernan et al. 2011). CR is protective against a variety of age-related pathologies, including cardiovascular disease, in rodents and nonhuman primates (Cruzen and Colman 2009; Niemann et al. 2010; Shinmura et al. 2011a). An early study by Taffett et al. (1997) showed that CR had a large positive effect on age-related impairment of diastolic function in mice. Kemi et al. (2000) then found that moderate dietary restriction (35% reduction in calorie intake) can attenuate age-related cardiomyopathy in male Sprague–Dawley rats. A later study showed that human volunteers undertaking CR for a mean of 6.5 yr had reduced blood pressure and systemic oxidative stress, and improved diastolic function (Meyer et al. 2006). Similar improvements in diastolic function have been reproduced in individuals maintained on 1-yr CR (Riordan et al. 2008). In addition to the protective effects of long-term CR, our laboratory recently showed that CR for 10 wk was able to reverse the preexisting cardiac hypertrophy and diastolic dysfunction in old mice, and that this was accompanied by proteomic and metabolomic remodeling to a more youthful state (Dai et al. 2014b).
Multiple mechanisms have been implicated in the beneficial effects of CR including inhibition of mTOR signaling, normalization of mitochondrial biogenesis (Lopez-Lluch et al. 2006), attenuation of mitochondrial ROS production and the subsequent ROS-induced signaling (Nisoli et al. 2005; Ungvari et al. 2008; Csiszar et al. 2009; Shinmura et al. 2011b), and increased SIRT1 signaling (Lopez-Lluch et al. 2006, 2008). These have proven to be fertile areas for the study of pharmacologic interventions to enhance healthspan.
Rapamycin
Although a large body of evidence supports the protective effects of CR in age-related pathologies including cardiac aging, the use of CR in humans would be challenging. Developing CR mimetics that mimic the beneficial effects of CR by targeting cellular metabolic and stress response pathways without actual restriction on calorie intake has been of great interest to the aging research community. mTOR plays a principle role in nutrient signaling and rapamycin is a well-established inhibitor of mTOR. The National Institute on Aging (NIA) Intervention Testing Program has recently shown that long-term rapamycin treatment initiated at 9 or 18 mo of age extended lifespan in mice with a mixed genetic background; these results were reproducible in three independent laboratories (Harrison et al. 2009; Miller et al. 2011). Subsequent studies have confirmed these results and have extended the studies to other measures of healthspan (Wilkinson et al. 2012).
With increasing evidence supporting the role of mTOR in aging and healthspan, the effects of rapamycin on cardiac aging are undoubtedly of interest to the aging research community. Rapamycin treatment for 1 yr initiated at mid-life attenuated the increased LV dimensions in aged hearts, but failed to show any effect on systolic function in male mice (Neff et al. 2013). Flynn et al. (2013) showed that rapamycin treatment for 12 wk initiated at late life can attenuate age-related cardiac hypertrophy and marginally improve systolic function in female mice, accompanied by a reduction in age-related inflammation. Recently, our laboratory showed that short-term rapamycin (10 wk) recapitulated the effect of CR and substantially improved both diastolic function and LV hypertrophy in old mice (Dai et al. 2014b). The reversal of cardiac aging phenotypes appeared to be mechanistically linked to proteomic and metabolic remodeling to increase mitochondrial protein content and reverse the age-related metabolic shift from fatty acid oxidation (FAO) to glycolysis and gluconeogenesis.
Further investigations on the mechanisms and kinetics of rapamycin benefits will be required to evaluate the potential of rapamycin as a pharmacological intervention to prevent, or even reverse, cardiac aging and its concomitant negative physiological consequences.
Mitochondrial Intervention
Mitochondrial dysfunction and mitochondrial ROS are critical mechanisms in the age-dependent decline in cardiac function; therefore, interventions combating mitochondrial ROS and improving mitochondrial function are attractive targets for interventions in cardiac aging. The success of mCAT protection in cardiac aging, but not of peroxisomal catalase or the nontargeted antioxidant N-acetylcysteine, underscored the importance of mitochondrial specificity in antioxidant intervention (Dai et al. 2011). One approach for targeting antioxidants to mitochondria is to use the negative potential gradient across the inner mitochondrial membrane (IMM). The negative potential gradient across IMM allows lipophilic cations to penetrate the IMM and accumulate in the mitochondrial matrix. Triphenylalkylphosphonium ions (TPP+) have been conjugated to coenzyme Q (MitoQ) and plastoquinone (SkQ1) (Skulachev et al. 2009; Smith et al. 2012) to deliver these redox-active compounds into the mitochondrial matrix. Although their effects on cardiac aging have not been established, MitoQ and SkQ1 have been shown to have beneficial effects in models of ischemia reperfusion and cardiac hypertrophy (Adlam et al. 2005; Bakeeva et al. 2008; Graham et al. 2009; Dikalova et al. 2010). A major limitation of these TPP+-conjugated antioxidants is their dependence on mitochondrial potential, which is often compromised in pathological conditions. MitoQ and SkQ have also been shown to inhibit respiration and disrupt mitochondrial potential at concentrations above 25 µM, which limits their uptake (Kelso et al. 2001; Antonenko et al. 2008). Moreover, MitoQ is reduced to a semiquinone radical at complex I and can increase superoxide production (O’Malley et al. 2006; Murphy and Smith 2007; Scatena et al. 2007); this pro-oxidant activity of MitoQ must be carefully evaluated when considering this intervention.
Another approach for targeting an intervention to mitochondria can be performed by utilizing an affinity to a mitochondrial component. The Szeto–Schiller (SS) compounds are tetrapeptides with an alternating aromatic-cationic amino acids motif. Studies have shown that SS peptides preferentially concentrate in the IMM over 1000-fold compared with the cytosolic concentration (Zhao et al. 2004; Doughan and Dikalov 2007; Bakeeva et al. 2008). In contrast to MitoQ and SkQ1, the mitochondrial uptake of SS peptides is not dependent on mitochondrial potential, and they can concentrate even in depolarized mitochondria (Zhao et al. 2004; Doughan and Dikalov 2007). The most-studied SS peptide, SS-31 (D-Arg-2′, 6′-dimethyltyrosine-Lys-Phe-NH2), was originally thought to exert its beneficial effect by the free radical scavenging activity of dimethyltyrosine (Graham et al. 2009). However, recent studies have revealed that SS-31 selectively binds to cardiolipin on the inner mitochondrial (Birk et al. 2013a,b; Szeto 2014). The binding of SS-31 to cardiolipin alters the interaction of cardiolipin with cytochrome c, and favors its electron carrier function while inhibiting its peroxidase activity (Birk et al. 2013a; Szeto 2014). SS-31 treatment increases ATP production, inhibits ROS generation, and prevents cardiolipin peroxidation and loss of cristae (Birk et al. 2013a). These findings suggest that the mitochondrial protective properties of SS-31 may be attributed to ROS-independent mechanisms, such as improved energetics, with reduction of ROS production as a secondary benefit. Our laboratory showed that the mitochondrial protective peptide SS-31 prevents pressure overload-induced cardiac hypertrophy as well as failure in a highly parallel manner to mCAT overexpression (Dai et al. 2011, 2012, 2013). Although the effects of SS-31 on cardiac aging have not been reported, recent studies from our laboratory have shown that 8-wk treatments of SS-31 can reverse age-related diastolic dysfunction in old mice (Chiao and PS Rabinovitch, unpubl.), supporting the therapeutic potential of SS-31 in cardiac aging.
Inhibition of Renin Angiotensin Aldosterone Signaling
As noted above, Ang II concentrations increase in aged hearts, and Ang II infusion induces structural, functional, and molecular changes similar to cardiac aging (Groban et al. 2006; Dai et al. 2009), highlighting the therapeutic potential of inhibition of Ang II signaling in cardiac aging. Basso et al. (2007) showed that long-term blockade of Ang II signaling by the angiotensin-converting enzyme inhibitor enalapril or by the angiotensin receptor type I inhibitor losartan can extend the lifespan of male Wistar rats and substantially attenuate age-related cardiovascular pathologies. In an earlier study, Inserra and colleagues (1995) showed that life-long treatment of enalapril can attenuate cardiac hypertrophy and interstitial fibrosis in hearts of 24-mo-old mice without significant changes in blood pressure. A later study by Stein et al. (2010) showed that long-term (10-mo) treatment with losartan, beginning at 12 mo of age, can also reduce myocardial fibrosis and fibrosis-related arrhythmias in aged mice. Groban et al. (2012) recently compared the effects of low-dose (non-blood-pressure lowering) enalapril and losartan for 6 mo initiated at 24 mo of age in male Fischer 344 × Brown Norway rats. They showed that, although low-dose enalapril and losartan both reduced cardiac oxidative stress, only enalapril was able to mitigate diastolic dysfunction, and they suggested that this may be mediated by a lowered ratio of phospholamban to SERCA2.
GDF-11
Recently, Loffredo et al. (2013) showed, by heterochronic parabiosis, that the circulation of young mice can regress cardiac hypertrophy in aged mouse hearts. By proteomic analysis of plasma samples from young and old mice, they identified that growth differentiation factor 11 (GDF11) is a circulating factor that declines with age and may be responsible for the reversal of age-related hypertrophy in heterochronic parabiosis. Importantly, restoring circulating GDF11 levels of old mice to young levels, by daily intraperitoneal injection of recombinant GDF11 (rGDF11) for 30 days can also reverse age-related hypertrophy. Treatment with rGDF11 reduced hypertrophic markers (ANP and BNP) expression and increased SERCA-2 expression, recapitulating the molecular changes mediated by parabiosis (Loffredo et al. 2013). The precise mechanism of GDF11 action, its effect on age-related diastolic dysfunction, and the role of GDF11 in human cardiac aging remain to be investigated; however, the results from the mouse model suggest an exciting therapeutic potential of GDF11 in cardiac aging.
Therapies Targeting miRNAs
As discussed above, recent studies suggest that miRNAs are important regulators of cardiac aging. With age, the expression of the miR-17-92 cluster (miR-18a, miR19a, and miR-19b) decreases, whereas the expressions miR-22 and miR-34a increase in hearts (Boon et al. 2013). Boon et al. (2013) showed that in vivo silencing of miR-34a by injection of antisense oligonucleotides (antagomirs) or locked nucleic acid (LNA)-based anti-miRs can reduce expression of miR-34a and partially rescue cardiac phenotypes in mice. This finding supports the potential of gene therapy to reverse the age-related changes in miRNAs to treat cardiac aging. However, as one miRNA is likely to have multiple targets, gene therapy targeting miRNA may trigger undesirable side effects. An alternative approach is to identify the miRNA targets that mediate cardiac aging responses and manipulate the specific target genes as treatment strategy.
Cardiac Stem-Cell or Progenitor-Cell Therapy
The recent discovery that the heart is able to regenerate although cardiac stem cells and cardiac progenitor cells has attracted enormous attention to the potential of stem-cell therapy for cardiovascular diseases and aging. Two approaches for stem-cell therapy are (1) direct delivery of cardiac stem cells/cardiac progenitor cells (with or without treatment to enhance cardiac differentiation or regenerative capacity) to the heart, and (2) delivery of agents that enhance the function of endogenous cardiac stem cells or progenitor cells (Ballard and Edelberg 2007). Potential therapeutic agents for enhancing endogenous stem-cell or progenitor-cell function include stromal-cell-derived factor (SDF)-1, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and IFG-1 (Ballard and Edelberg 2007). For direct stem-cell or progenitor-cell delivery, the therapeutic effects are limited by the proliferation, engraftment, survival, and persistence of the transplanted cells. In a recent study, Mohsin et al. (2012) used lentivirus transduction to overexpress Pim-1 kinase in cardiac progenitor cells isolated from a 68-yr-old heart failure patient, and showed enhanced survival, proliferation, differentiation, and persistence of the cardiac progenitor cells after transplanted into an immunocompromised mouse model of myocardial infarction. Strikingly, transplant of Pim-1-expressing progenitor cells significantly improved myocardial healing and function of the infarcted heart in 8 wk. The ability to rejuvenate human cardiac progenitor cells ex vivo by Pim-1 modification is highly encouraging to the development of stem-cell therapy for cardiac aging (Mohsin et al. 2013).
FUTURE PERSPECTIVES ON CARDIAC AGING INTERVENTIONS
The improved understanding on the molecular mechanisms of cardiac aging has led to promising advancements in the development of cardiac aging interventions. Recent studies have shown the potential of different therapeutic approaches to delay or treat cardiac aging, ranging from CR to pharmacologic interventions (rapamycin, enalapril, and SS-31), recombinant protein therapy (IGF-1 and GDF-11), gene therapy (miRNAs), and cardiac stem-cell therapy. However, future studies will be required to evaluate the translational potentials of these interventions.
As a general rule for cardiac aging interventions, short-term treatment(s) beginning at late life will have higher translational potential compared with long-term or life-long treatments. This is particularly relevant to systemic treatments or treatments that target multiple pathways, as a briefer treatment will lower the chances of irreversible side effects. Therefore, it is important to study the kinetics and pharmacodynamics of the treatment to determine the minimal effective dose and duration, as well as the persistence of the treatment to determine the optimum therapeutic regimen.
Another issue for consideration is that there may be gender-specific differences in therapeutic responses. Many of the potential interventions noted above have been tested in only one gender of animals, and, therefore, potential gender-specific differences in beneficial effects remain unknown. For instance, rapamycin provided a greater lifespan extension in female mice at the initial dose (14 ppm) studied by the NIA Intervention Testing Program (Harrison et al. 2009), but a later study showed an improved effect in males at a higher dose, although the lifespan extension benefit was greater in females than males at each dose. This gender difference was associated with a sexual dimorphism of rapamycin levels in blood (Miller et al. 2014).
CONCLUDING REMARKS
As the elderly population in developed countries is expected to double in the next 25 years, there will be an urgent need for interventions to attenuate or reverse cardiac impairment and the concomitant negative physiological consequences in the elderly. Recent studies show promising results of multiple novel interventions to delay or reverse cardiac aging. More in-depth understanding of the molecular mechanisms of intrinsic cardiac aging and the mechanistic effects of these interventions will be required to guide the development and future translation of these novel therapies to clinical application. Mechanistic insights may also identify other more specific therapeutic targets and provide guidance toward interventions for other age-related pathologies.
ACKNOWLEDGMENTS
We acknowledge support from the Ellison Medical Foundation and the American Federation for Aging Research, as well as National Institutes of Health (NIH) Grants AG001751, AG038550, and HL101186. Y.A.C is a Glenn/AFAR postdoctoral fellow for the Translational Research on Aging.
Footnotes
Editors: S. Jay Olshansky, George M. Martin, and James L. Kirkland
Additional Perspectives on Aging available at www.perspectivesinmedicine.org
REFERENCES
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. 2005. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095. [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, Ivanova OY, Izyumov DS, Khailova LS, Klishin SS, et al. 2008. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1: Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry (Mosc) 73: 1273–1287. [DOI] [PubMed] [Google Scholar]
- Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, Bolli R. 2005. Myocardial aging—A stem cell problem. Basic Res Cardiol 100: 482–493. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. 2005. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271. [DOI] [PubMed] [Google Scholar]
- Bakeeva LE, Barskov IV, Egorov MV, Isaev NK, Kapelko VI, Kazachenko AV, Kirpatovsky VI, Kozlovsky SV, Lakomkin VL, Levina SB, et al. 2008. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2: Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc) 73: 1288–1299. [DOI] [PubMed] [Google Scholar]
- Ballard VL, Edelberg JM. 2007. Stem cells and the regeneration of the aging cardiovascular system. Circ Res 100: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, et al. 2008. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3: e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores Arnaiz M, Inserra F. 2005. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept 128: 247–252. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. 2007. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol 293: H1351–H1358. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776. [DOI] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, et al. 2009. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 119: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. 2009. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk AV, Chao WM, Bracken WC, Warren JD, Szeto HH. 2013a. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. 2013b. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. 2007. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail 13: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, et al. 2013. MicroRNA-34a regulates cardiac ageing and function. Nature 495: 107–110. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. 2006. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16: 273–279. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. 2010. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: Role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol 298: H614–H622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio F, Fubini A, Morello M, Arvat E, Aimaretti G, Gianotti L, Boghen MF, Deghenghi R, Mangiardi L, Ghigo E. 1999. Activity of GH/IGF-I axis in patients with dilated cardiomyopathy. Clin Endocrinol (Oxf) 50: 417–430. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH. 2000. Myocardial fibrosis in transforming growth factor β1 heterozygous mice. J Mol Cell Cardiol 32: 187–195. [DOI] [PubMed] [Google Scholar]
- Brouwers FP, Hillege HL, van Gilst WH, van Veldhuisen DJ. 2012. Comparing new onset heart failure with reduced ejection fraction and new onset heart failure with preserved ejection fraction: An epidemiologic perspective. Curr Heart Fail Rep 9: 363–368. [DOI] [PubMed] [Google Scholar]
- Bujak M, Frangogiannis NG. 2007. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, Dai Q, Zhang J, Lin J, Lopez EF, Ahuja SS, Chou YM, Lindsey ML, Jin YF. 2011. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet 4: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. 2012. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 96: 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Chang HJ, Choi SI, Kim KI, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Kim HS, et al. 2009. Long-term exercise training attenuates age-related diastolic dysfunction: Association of myocardial collagen cross-linking. J Korean Med Sci 24: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. 1993. Human growth hormone and human aging. Endocr Rev 14: 20–39. [DOI] [PubMed] [Google Scholar]
- Cruzen C, Colman RJ. 2009. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med 25: 733–743, ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, et al. 2008. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. 2009. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, et al. 2009. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119: 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. 2010. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. 2011. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. 2012. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc Res 93: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, et al. 2013. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail 6: 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. 2014a. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, et al. 2014b. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. 2010. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 5: e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. 2010. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. 2005. Angiotensin II–mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension 46: 426–432. [DOI] [PubMed] [Google Scholar]
- Doughan AK, Dikalov SI. 2007. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836. [DOI] [PubMed] [Google Scholar]
- Downes TR, Nomeir AM, Smith KM, Stewart KP, Little WC. 1989. Mechanism of altered pattern of left ventricular filling with aging in subjects without cardiac disease. Am J Cardiol 64: 523–527. [DOI] [PubMed] [Google Scholar]
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, et al. 2013. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CG, Chiasson KB, Leslie TH, Thomas D, Beasley TM, Kemnitz JW, Weindruch R. 2010. Auditory function in rhesus monkeys: Effects of aging and caloric restriction in the Wisconsin monkeys five years later. Hear Res 261: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. 2014. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, et al. 2008. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res 102: 597–606. [DOI] [PubMed] [Google Scholar]
- Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. 2009. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54: 322–328. [DOI] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. 2006. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci 61: 28–35. [DOI] [PubMed] [Google Scholar]
- Groban L, Lindsey S, Wang H, Lin MS, Kassik KA, Machado FS, Carter CS. 2012. Differential effects of late-life initiation of low-dose enalapril and losartan on diastolic function in senescent Fischer 344 × Brown Norway male rats. Age (Dordr) 34: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. 1972. The biologic clock: The mitochondria? J Am Geriatr Soc 20: 145–147. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. 2007. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13: 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra F, Romano L, Ercole L, de Cavanagh EM, Ferder L. 1995. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension 25: 437–442. [DOI] [PubMed] [Google Scholar]
- Janczewski AM, Lakatta EG. 2010. Modulation of sarcoplasmic reticulum Ca2+ cycling in systolic and diastolic heart failure associated with aging. Heart Fail Rev 15: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski AM, Spurgeon HA, Lakatta EG. 2002. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol 34: 641–648. [DOI] [PubMed] [Google Scholar]
- Jazbutyte V, Fiedler J, Kneitz S, Galuppo P, Just A, Holzmann A, Bauersachs J, Thum T. 2013. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr) 35: 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson IR, Guia A, Stern MD, Lakatta EG. 2002. Alterations in properties of l-type Ca channels in aging rat heart. J Mol Cell Cardiol 34: 297–308. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. 2005. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: Implications for the mitochondrial theory of aging. FASEB J 19: 419–421. [DOI] [PubMed] [Google Scholar]
- Kass DA, Bronzwaer JG, Paulus WJ. 2004. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94: 1533–1542. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, et al. 2010. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci 30: 7940–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. 2001. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596. [DOI] [PubMed] [Google Scholar]
- Kemi M, Keenan KP, McCoy C, Hoe CM, Soper KA, Ballam GC, van Zwieten MJ. 2000. The relative protective effects of moderate dietary restriction versus dietary modification on spontaneous cardiomyopathy in male Sprague–Dawley rats. Toxicol Pathol 28: 285–296. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. 2007. Ruminations on dietary restriction and aging. Cell Mol Life Sci 64: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Sane DC, Wannenburg T, Sonntag WE. 2002. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res 54: 25–35. [DOI] [PubMed] [Google Scholar]
- Kitzman DW. 2002. Diastolic heart failure in the elderly. Heart Fail Rev 7: 17–27. [DOI] [PubMed] [Google Scholar]
- Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 2005. 3-Nitrotyrosine modification of SERCA2a in the aging heart: A distinct signature of the cellular redox environment. Biochemistry 44: 13071–13081. [DOI] [PubMed] [Google Scholar]
- Koban MU, Moorman AF, Holtz J, Yacoub MH, Boheler KR. 1998. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res 37: 405–423. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. 2002. Age-associated cardiovascular changes in health: Impact on cardiovascular disease in older persons. Heart Fail Rev 7: 29–49. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part III: Cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. 2003a. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: Aging arteries—A “set up” for vascular disease. Circulation 107: 139–146. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. 2003b. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Part II: The aging heart in health: Links to heart disease. Circulation 107: 346–354. [DOI] [PubMed] [Google Scholar]
- Li Q, Ceylan-Isik AF, Li J, Ren J. 2008. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res 11: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. 2005. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res 66: 410–419. [DOI] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, et al. 2013. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153: 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, et al. 2006. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci 103: 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. 2008. Mitochondrial biogenesis and healthy aging. Exp Gerontol 43: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. 2007. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci 27: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. 2006. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab 4: 133–142. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Rizzuto R. 2010. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 131: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, Weindruch R. 2011. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol 46: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, McMullen JR, Sherwood MC, Lader AS, Walker V, Chan JA, Kwiatkowski DJ. 2005. A mouse model of cardiac rhabdomyoma generated by loss of Tsc1 in ventricular myocytes. Hum Mol Genet 14: 429–435. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. 2006. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47: 398–402. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. 2011. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, et al. 2014. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, et al. 2012. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol 60: 1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, et al. 2013. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res 113: 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. 2007. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656. [DOI] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, et al. 2013. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 123: 3272–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. 2010. Caloric restriction delays cardiac ageing in rats: Role of mitochondria. Cardiovasc Res 88: 267–276. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. 2005. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317. [DOI] [PubMed] [Google Scholar]
- Oktay AA, Rich JD, Shah SJ. 2013. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 10: 401–410.24078336 [Google Scholar]
- O’Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. 2006. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281: 39766–39775. [DOI] [PubMed] [Google Scholar]
- Ouzounian M, Lee DS, Liu PP. 2008. Diastolic heart failure: Mechanisms and controversies. Nat Clin Pract Cardiovasc Med 5: 375–386. [DOI] [PubMed] [Google Scholar]
- Panek AN, Posch MG, Alenina N, Ghadge SK, Erdmann B, Popova E, Perrot A, Geier C, Dietz R, Morano I, et al. 2009. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS ONE 4: e6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche JE, Garcia-Fernandez M, Muntane J, Rioja J, Gonzalez-Baron S, Castilla Cortazar I. 2008. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology 149: 2620–2627. [DOI] [PubMed] [Google Scholar]
- Quiat D, Olson EN. 2013. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J Clin Invest 123: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AL, Tanaka A, Sorescu D, Liu H, Jeong EM, Sturdy M, Walp ER, Dudley SC Jr, Sutliff RL. 2011. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol 301: H824–H831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Brown-Borg HM. 2002. Impaired cardiac excitation–contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-I deficiency. Growth Horm IGF Res 12: 99–105. [DOI] [PubMed] [Google Scholar]
- Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, Villareal DT, Fontana L, Holloszy JO, Kovacs SJ. 2008. The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function. Am J Physiol Heart Circ Physiol 294: H1174–H1182. [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. 2005. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J Alzheimers Dis 8: 247–268. [DOI] [PubMed] [Google Scholar]
- Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. 2004. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator-1α promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94: 525–533. [DOI] [PubMed] [Google Scholar]
- Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B. 2007. The role of mitochondria in pharmacotoxicology: A reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol 293: C12–C21. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. 2005. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911. [DOI] [PubMed] [Google Scholar]
- Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. 2006. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: Selective protein oxidation during biological aging. Biochem J 394: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. 2011a. Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50: 117–127. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, Ohta S, Katsumata Y, Fukuda K, Ishiwata K, et al. 2011b. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res 109: 396–406. [DOI] [PubMed] [Google Scholar]
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, et al. 2009. An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta 1787: 437–461. [DOI] [PubMed] [Google Scholar]
- Smith RA, Hartley RC, Cocheme HM, Murphy MP. 2012. Mitochondrial pharmacology. Trends Pharmacol Sci 33: 341–352. [DOI] [PubMed] [Google Scholar]
- Smith-Vikos T, Slack FJ. 2012. MicroRNAs and their roles in aging. J Cell Sci 125: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KT, Kirkpatrick JN, Mor-Avi V, Decara JM, Lang RM. 2004. Age dependency of the Tei index of myocardial performance. J Am Soc Echocardiogr 17: 350–352. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Beck C, Bouges S, Stroud RE. 2009. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: Effects on myocardial remodeling with aging. Circ Heart Fail 2: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Boulaksil M, Jansen JA, Herold E, Noorman M, Joles JA, van Veen TA, Houtman MJ, Engelen MA, Hauer RN, et al. 2010. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol 299: H310–H321. [DOI] [PubMed] [Google Scholar]
- Swinne CJ, Shapiro EP, Lima SD, Fleg JL. 1992. Age-associated changes in left ventricular diastolic performance during isometric exercise in normal subjects. Am J Cardiol 69: 823–826. [DOI] [PubMed] [Google Scholar]
- Szeto HH. 2014. First-in-class cardiolipin therapeutic to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffet GE, Pham TT, Hartley CJ. 1997. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci 52: B285–B290. [DOI] [PubMed] [Google Scholar]
- Tayebjee MH, Lip GYH, Blann AD, MacFadyen RJ. 2005. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res 115: 205–210. [DOI] [PubMed] [Google Scholar]
- Tei C, Nishimura RA, Seward JB, Tajik AJ. 1997. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 10: 169–178. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. 2004. Myocyte aging and mitochondrial turnover. Exp Gerontol 39: 701–705. [DOI] [PubMed] [Google Scholar]
- Terzioglu M, Larsson NG. 2007. Mitochondrial dysfunction in mammalian ageing. Novartis Found Symp 287: 197–208; discussion 208–2113. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, et al. 2004. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94: 514–524. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Larsson NG. 2008. Mitochondrial dysfunction as a cause of ageing. J Intern Med 263: 167–178. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, et al. 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. 2008. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ Res 102: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, et al. 2011. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging cell 10: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, Levy D, Wilson PW. 2003. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: The Framingham Heart Study. Ann Intern Med 139: 642–648. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. 2010. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol 48: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T. 2011. Mitochondria and PGC-1α in aging and age-associated diseases. J Aging Res 2011: 810619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. 2004. Insulin regulation of heart function in aging fruit flies. Nat Genet 36: 1275–1281. [DOI] [PubMed] [Google Scholar]
- Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, et al. 2009. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell 8: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. 2012. Rapamycin slows aging in mice. Aging Cell 11: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. 2007. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130: 247–258. [DOI] [PubMed] [Google Scholar]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. 2004. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690. [DOI] [PubMed] [Google Scholar]
- Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, Leclercq B, Zisman A, Striker LJ, Striker GE. 2003. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol 162: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zile MR, Brutsaert DL. 2002. New concepts in diastolic dysfunction and diastolic heart failure. Part II: Causal mechanisms and treatment. Circulation 105: 1503–1508. [DOI] [PubMed] [Google Scholar]