Abstract
Epileptic seizures represent dysfunctional neural networks dominated by excessive and/or hypersynchronous activity. Recent progress in the field has outlined two concepts regarding mechanisms of seizure generation or ictogenesis. First, all seizures, even those associated with what have historically been thought of as “primary generalized” epilepsies appear to originate within local microcircuits and then propagate from that initial ictogenic zone. Second, seizures propagate through cerebral networks and engage microcircuits in distal nodes—a process that can be weakened or even interrupted by suppressing activity in such nodes. Here, we describe various microcircuit motifs, with a special emphasis on one broadly implicated in several epilepsies - feed-forward inhibition. Further, we discuss how, in the dynamic network in which seizures propagate, focusing on circuit “choke points” remote from the initiation site might be as important as that of the initial dysfunction—the seizure “focus.”
Introduction
Epilepsy research and neuroscience owe much to insights we have learned from operating on the human brain. In the first half of the last century, neurosurgeon Wilder Penfield and his colleague Herbert Jasper pioneered incredible advances, such as characterizing motor and sensory maps and describing the form of cerebral electrical activity during seizures1. Their findings have inspired a decades-long inquiry aimed at understanding and treating epilepsy. Since then, we have found many changes in structure and/or function in the epileptic brain of humans and animals, such as altered morphology and excitability of individual neurons, changes in expression of neurotransmitter receptors, astrocytic and blood-brain-barrier dysfunction, neuroinflammation, and gains or losses of individual circuit components, which would render a neural network hyperexcitable. These studies have documented molecular and/or anatomical changes associated with the epileptic brain, and have been comprehensively described elsewhere (e.g.2). Despite these insightful studies, there is still no cure for epilepsy. Existing treatments only aim at controlling seizures and have significant side effects, and more than one third of all epilepsies remain uncontrolled.
More recently, technological advances have begun to provide detailed descriptions of microcircuit function in both humans and animal models of epilepsy. The results of these state-of-the-art approaches—such as paired (or even higher order) intracellular recordings, high-density multi-site extracellular arrays, activity-dependent reporter dyes and proteins, and optogenetics—are beginning to provide unique insight into how networks at the micro scale organize and contribute to generating, propagating, and modulating seizure activity. These findings challenge the established, yet somewhat simplistic, view that epilepsy simply results from imbalances between excitation and inhibition. These advances are starting to reveal critical circuit junctures or choke points, potentially outside of the ictogenic network, that likely represent targets for highly specific and effective anti-epileptic therapies. In this review, we discuss epileptic choke points in the context of several microcircuit motifs implicated in animal models of epilepsy, as well as those that have been confirmed in humans.
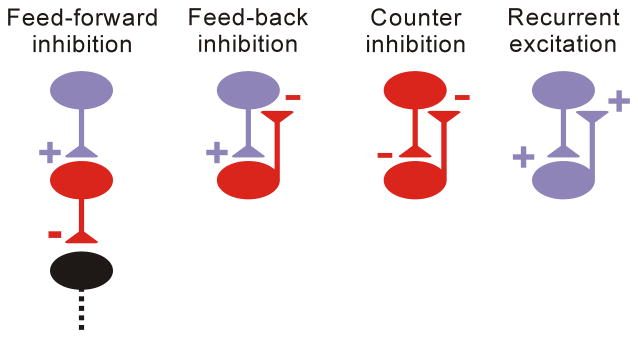
We will consider the following microcircuit motifs (Fig. 1): 1) feed-forward inhibition, in which excitatory inputs from extrinsic brain regions recruit local inhibitory networks that tune the strength and form of the efferent signal; 2) feed-back inhibition, in which locally-activated inhibitory neurons shape recurrent excitatory activity; 3) counter-inhibition, in which local connections between inhibitory neurons that, when active, can decrease output of inhibitory cells and induce disinhibition or alter oscillatory coupling; and 4) local recurrent excitatory circuits, a common motif in cortical networks in which ~80% of neurons and synapses are excitatory. We also briefly consider relevant circuits outside of the microcircuit. These considerations include longer-range excitatory, inhibitory, and neuromodulatory connections that link and influence local microcircuit activities. For each of these motifs, we will identify dysfunctions that have been described at the microcircuit level, illustrate the relevance of these defects to epileptic seizures, and highlight potential therapeutic approaches that might profitably improve treatment of persons with epilepsy. Notably, these motifs do not exist in isolation, but are embedded in larger networks; the fine balance between these motifs dictates the dynamics of large-scale networks. We focus on the concept that epileptic seizures emerge from dysfunction of specific microcircuits, which then progressively engage other microcircuits to activate the full seizure network—an overall process termed ictogenesis. In this context, ictogenic choke points are any microcircuits or bridges between microcircuits that are required for full expression of seizures.
Figure 1. Microcircuit motifs whose dysfunctions have been identified in epilepsy.
Feed-forward inhibition: excitatory inputs from remote brain regions recruit local inhibitory networks that control the strength of the efferent signal; Feed-back inhibition: local activation of inhibitory neurons creates local recurrent excitatory activity; Counter-inhibition: local connections between inhibitory neurons shape network-inhibitory output; Recurrent excitation: major mode of connectivity in cortical networks; Purple and red represent excitatory glutamatergic and inhibitory GABAergic neurons, respectively, in this and all following figures.
Feed-forward Inhibition
Within the last decade, epilepsy research has provided compelling results regarding the particular importance of feed-forward inhibition (Fig. 2a, b), which will be a major focus of this review. Feed-forward inhibition commonly occurs in several regions of the nervous system, including neocortical, hippocampal, basal ganglia, and thalamic networks. We will discuss how changes in feed-forward inhibition within different circuits can cause abnormal circuit dynamics that underlie epileptic seizures.
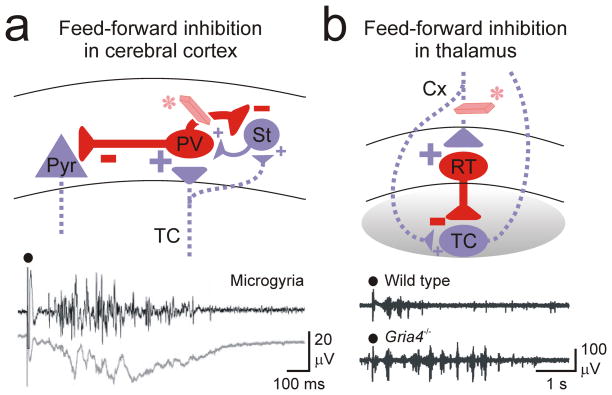
Figure 2. Feed-forward inhibition in cortical and thalamic microcircuits.
(a) Extrinsic excitatory projections from regions outside of local cortical networks recruit feed-forward inhibition. Cortical inter-areal or thalamic inputs to the cortex result in stronger activation of FS parv cells than excitatory stellate and pyramidal cells, thus causing a robust feed-forward inhibition of excitatory cells. In case of a loss of this feed-forward inhibition (eraser*), thalamic inputs to the cortex recruit epileptiform activity in a neocortical microgyrus model of focal neocortical epilepsy (bottom multi-unit and local field recordings7). (b) Excitatory inputs from the cortex to the thalamus results in stronger activation of the inhibitory interneurons, which causes a strong feed-forward inhibition of relay excitatory neurons. Loss of feed-forward inhibition (eraser*) has been implicated in the gria4−/− mouse model of absence epilepsy (multi-unit recordings21) Black circle: electrical stimulation of excitatory afferents. Cx, cortex; parv, parvalbumin-positive interneuron; Pyr, pyramidal neuron; RT, reticular thalamic neuron; St, stellate; TC, thalamocortical neuron.
Feed-forward inhibition in neocortex and hippocampus
Incoming sensory signals traveling from the periphery to the cortex arise from the thalamus in the form of glutamatergic excitation that is largely focused on the sensory receptive zone in the cortical layer 43. In turn, intracortical circuits are composed largely of excitatory neurons that are recurrently connected4,5. These neurons amplify and process incoming signals by propagating through a canonical microcircuit to superficial and then deeper cortical layers. While incoming sensory signals are excitatory, a prominent feature of neocortical microcircuits is feed-forward inhibition mediated predominantly by fast-spiking (FS) basket cells containing the calcium binding protein parvalbumin (parv). Thus, incoming sensory signals directly and potently excite parv cells in layer 4, causing them to fire and release the inhibitory neurotransmitter GABA onto excitatory neurons in this layer. This causes a powerful feed-forward inhibition that sets a brief window for temporal synaptic integration in which spikes can be generated6, and an overall limit for overexcitation in the neocortex5–8. Similar circuitry exists in the other cortical regions, including, for example, hippocampal dentate gyrus9. Notably, individual parv cells have potent output, mainly onto cell bodies and proximal dendrites, through convergent input to individual pyramidal cells10,11. This feature positions parv cells to powerfully suppress output of pyramidal and other principal cells. Note that while feed-forward inhibition generally suppresses activity, under some conditions, feed-forward activation of inhibitory neurons, especially Chandelier cells, can enhance network output12. Recently, findings demonstrate connectivity rules that add a level of complexity to feedforward inhibitory circuits. Accordingly, parv basket cells in the CA1 region of hippocampus do not indiscriminately target all CA1 pyramidal neurons within the domain of their axonal arbor, but specifically target subsets of pyramidal neurons with their own specific output projections13. Thus, this represents another potential choke point, as targeted excitation of relevant parv cells that suppress output to a specific region could prevent propagation to that region.
The powerful nature of feed-forward inhibition in thalamocortical (and other) circuits results from several factors, including a larger convergence of single-afferent thalamocortical axons onto individual parv–inhibitory cells that reliably generate spikes8,14–16; divergence of output from such parv cells17,18; and the strength of unitary connections from individual parv cells8,11. These observations support the hypothesis that the nervous system operationally requires adequate feed-forward inhibition, and failure of this key microcircuit leads to over-excitation of cortical networks and seizures. This hypothesis is supported by evidence in several models of epilepsy, including those induced by neonatal cortical freeze lesions that result in focal cortical dysplasia7, and in the stargazer19, tottering20 and gria4−/−, 21 models of generalized-absence epilepsy.
Losing feed-forward inhibition is consistent with the “dormant basket-cell” hypothesis of epilepsy 22,23 – that inhibitory neurons would lose so much connectivity that they would begin to fail in their necessary role of providing timely feed-forward inhibition. While the dormant basket cell theory considers both feed-forward and feedback inhibition (discussed in the next section), the former has often been shown to play a major role in studies with in vitro slice or whole hippocampal models that acutely induce epileptiform activity with chemoconvulsants24–26. Indeed, Cammarota et al. found that parv cells are primarily involved in feed-forward inhibition, much greater than the second-largest population of interneurons, somatostatin-positive (SOM) interneurons, which appear to significantly effect feed-back inhibition. The dormant basket–cell hypothesis has been controversial in terms of the actual circuit changes that might cause dormancy; however, it remains critical, because loss of feed-forward inhibition, with its powerful effects on the function of local excitatory neurons, causes potent dysfunction of circuits. Importantly, feed-forward inhibition has been shown to prevent seizures from developing. Indeed, selectively impairing Ca2+ channels in neocortical parv interneurons27, which would cause a loss of feed-forward inhibition, produces generalized-absence seizures. Similarly, specific reduction of the intrinsic excitability or synaptic excitation of parv inhibitory interneurons, but not of excitatory cells, decreases feed-forward inhibition. In recent studies, reduced function of Nav1.1 sodium channels in parv FS interneurons was implicated in epileptic seizures in a mouse model of severe Dravet syndrome28–30. Additionally, deficits in Nav1.1 in parv neurons contribute to epileptiform hippocampal activity in mouse models of familial Alzheimer’s disease. Moreover, overexpressing Nav1.1 reduces epileptiform activity31. By considering how parv cells affect feed-forward inhibition, we propose that rescuing hypofunctional inhibition could prevent seizures by restoring feed-forward inhibition.
Can feed-forward inhibition regulate seizure propagation over long distances? According to studies with novel in vitro preparations that retain callosal or commissural connections, it can. For example, in a callosum-intact bilateral neocortical slice preparation32, chemically-induced epileptiform activity leads mainly to feed-forward inhibition in the contralateral cortex. Similar effects occurred in bilateral-intact hippocampal preparations, especially in the early phase of seizure induction in which interictal spikes were most prominent26. Thus, prominent phasic inhibition from afar can signal an impending seizure.
Feed-forward inhibition also critically regulates the dynamics of the hippocampal network, as shown in models of temporal lobe epilepsy (TLE). In this network, the tri-synaptic loop is most often discussed with regards to activity propagation from entorhinal cortex to dentate gyrus to CA3 to CA1; however, this network contains other pathways that may play key roles in seizure genesis and/or propagation. For example, in addition to the entorhinal projection to dentate, there is also a projection directly to CA1 through the temporoammonic pathway. Losing feed-forward inhibition in this pathway occurs in the pilocarpine model of TLE as a result of several factors, including cell loss in superficial neurons in layer 3 of the entorhinal cortex33, which project to hippocampal CA1 34; loss of stratum oriens-lacunosum moleculare (O-LM) interneurons35 that in addition to their major role in feed-back inhibition also mediate feed-forward inhibition in the tempero-ammonic pathway36; and distal dendritic inhibitory denervation of hippocampal CA1 cells, a region preferentially regulated by O-LM interneurons37,38. Combining these processes would produce a loss of feed-forward inhibition from the entorhinal cortex to CA1. This hypothesis is consistent with results of a voltage-imaging study in which entorhinal stimulation massively activated the pathological network in CA1 hippocampus of post-pilocarpine epileptic animals39. Interestingly, surviving O-LM cells in CA1 send aberrant fibers into dentate gyrus, which may, at least partially, compensate for the loss of local dentate inhibitory cells40.
Feed-forward inhibition can also be relevant to intra-areal cortical excitation. It is largely responsible for surround inhibition, which was documented decades ago in pioneering studies of acute neocortical or hippocampal seizures in felines41,42. Recently, both feed-forward and surround inhibition have been investigated with optical and electrophysiological methods to study the spread of seizures from a focal zone that initiates epileptic seizures—the “ictogenic” zone. These results, obtained largely in rodent models in which epileptic seizures were induced by chemoconvulsants, show that the earliest forms of peri-ictal synaptic activity are multiphasic, repetitive, and create potent inhibitory signals. This early activity is associated with normal (non-ictal) background behavior in the network, but is followed by a sudden collapse of inhibition, such that strong excitatory signals dominate individual cellular responses. As a result, these signals produce precipitous step-like waves of local excitation at the network level, as observed with Ca2+ imaging43. This cycle then repeats to propagate seizure activity to the next microcircuit. Recently, analogous neural activities have been revealed from intra-operative intracranial electrical recordings obtained from the cerebral cortex of epilepsy patients being evaluated for neurosurgical resections44. These recordings suggest that during clinical seizures, feed-forward inhibition fails through mechanisms similar to those observed in experimental animals.
Feed-forward inhibition in thalamus
Circuit motifs differ between brain regions, especially between cortical and subcortical microcircuits. The thalamus—as a sensory relay station—shapes incoming peripheral information through three inhibitory pathways: 1) feed-forward dendro-dendritic inhibition mediated by local circuit interneurons that sculpt packets of primary afferent signals to delay firing45; 2) direct feed-back inhibition driven by triggering thalamocortical (TC) drive of inhibitory thalamic reticular (RT) neurons; and 3) inhibition via the RT nucleus triggered by cortical feedback. The latter form can be confusing, because recurring excitatory signals from cortex to thalamus would normally be considered feed-back. Yet, from a microcircuit perspective, output from cortex triggers feed-forward inhibition, because the major effect of cortical output is preferred recruitment of inhibitory cells in the RT nucleus21,46. Thus, RT cells provide powerful inhibitory output onto excitatory TC relay cells.
Recent studies have suggested that loss of feed-forward inhibition in the cortico-thalamic pathway can be epileptogenic. For example, studies revealed that inhibitory RT neurons lose AMPA-mediated excitation in two genetic models of generalized-absence epilepsy: stargazer and gria4−/− mice21,47,48. In the latter model, the synaptic defects within the cortico-thalamic microcircuit were deconstructed with optogenetics—a promising new approach to studying epileptogenetic pathways. This approach revealed how loss of a specific microcircuit component—synaptic excitatory drive from neocortex onto inhibitory RT cells—can cause a deficit in feed-forward, but not feed-back, inhibition21 (Fig. 2b). These findings suggest that even though cortical efferents are largely, if not exclusively excitatory, their primary effects on thalamic activity can be inhibitory (for discussion of the potential physiological roles for such feed-forward inhibition, see49). These results further suggest that specifically restoring excitatory inputs from the cortex onto RT cells would rescue feed-forward inhibition and suppress absence seizures that would otherwise develop in the thalamocortical network.
Feed-forward inhibition: a potential target of anti-epileptic drugs?
Feed-forward inhibition is critical for normal circuit function yet is also paradoxically fragile because of several factors, including intracellular Cl− accumulation, GABA depletion, and presynaptic inhibition50–53. Altering these factors with drugs may create restorative treatments against epilepsy. Further, if a loss of feed-forward inhibition is a cause of epilepsy, then anti-epileptic drugs (AEDs) should in principle re-establish it, and in no case should they suppress it. However, several AEDs, including phenytoin, carbamazepine54,55, and lamotrigine56, may work through a mechanism that blocks Na+ channels, especially in the context of action potentials that fire at high-frequency. Parv cells, which largely mediate feed-forward inhibition and fire at high frequencies, may be susceptible to reduced firing by AEDs. Thus, AEDs could potentially worsen seizures. To resolve this paradox, a study recently addressed the effects of Na+ channel blockers (e.g., the anti-convulsant drugs carbamazepine, phenytoin, and lamotrigine) on different cell types. These compounds specifically reduced repetitive firing in pyramidal neurons, but not in FS or other interneurons57. The AEDs also did not affect recruitment of inhibition during repetitive activity. Thus, AEDs reduce action potential firing primarily in excitatory neurons and spare interneurons to maintain feed-forward and other forms of inhibition.
To conclude, the anatomical connectivity and functional features of parv basket cells in cortex and hippocampus and parv RT cells in the thalamus enable them to serve as central players in feed-forward inhibition. Furthermore, this inhibition is well-positioned to prevent epileptic activity from bridging between microcircuits, and its failure could readily propagate seizures. Thus, mediators of feed-forward inhibition—mainly parv cells—could serve as potential seizure choke points.
Feed-back Inhibition
In contrast with feed-forward inhibition, which is a microcircuit motif engaged by extrinsic sources, feed-back inhibition generally results from excitation within local circuit elements (Fig. 3a, b). Like feed-forward, feed-back inhibition is a common theme in cerebral circuits. While different classes of inhibitory cells can mediate both forms of inhibition, their relative roles differ. Indeed, the parv cells described above appear to play a major role in feed-forward inhibition, while a second major class of inhibitory cells, SOM-containing interneurons, appear to play a more important role in feed-back inhibition.
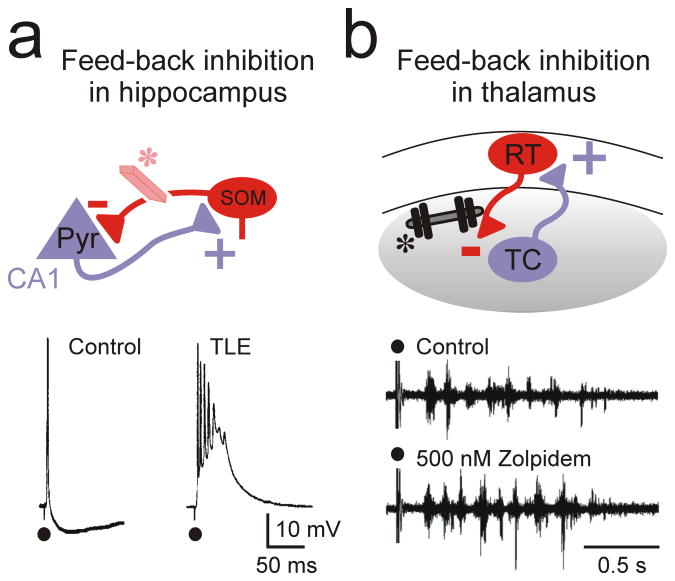
Figure 3. Feed-back inhibition in cortical and thalamic microcircuits.
(a) In the cortex, inhibitory SOM interneurons provide a feed-back inhibition to pyramidal neurons that excite them. Loss of this inhibition (eraser*) has been implicated in temporal lobe epilepsy (TLE)37. (b) In the somatosensory thalamus, inhibitory interneurons provide a robust feed-back inhibition to TC neurons that excite them. Increasing this feed-back inhibition (dumbbell weight *) by Zolpidem, or by clonazepam in α3H126R mice (not shown69), which specifically affects RT-TC but not RT-RT connections, enhances epileptiform oscillations. Pyr, pyramidal; SOM, somatostatin-positive; RT, reticular thalamic neuron; TC, thalamocortical neuron.
Although diverse subclasses of SOM cells can be involved in epilepsy, we will focus our discussion mainly on one subclass of SOM cells—Martinotti neurons—which target distal dendrites of pyramidal neurons10,58,59. Compared with parv-mediated inhibition, Martinotti-mediated inhibition is weaker at baseline because post-synaptic cells have fewer synapses11. However, Martinotti-dependent inhibition is progressively recruited by simultaneous repetitive activity in multiple pre-synaptic pyramidal cells, as would happen, for example, during intense activation of local microcircuits in seizures. Such recruitment results from facilitating short-term synapses of both the excitatory inputs onto and the inhibitory outputs from neocortical Martinotti cells and related neurons of the hippocampus60–62. In contrast, inhibition from parv basket cells is initially robust because of convergent input coupled with high-probability sites of release onto pyramidal cells. However, due to short-term synaptic depression, the efficacy of parv-mediated inhibition rapidly drops during repetitive activation61.
The progressive nature of Martinotti-cell recruitment could be important for dampening activity to locally suppress seizures in the microcircuit. Consistent with this, mice deficient in the transcription factor DLX1 show reduced SOM cells and a mild epilepsy phenotype63. Furthermore, in a murine model of Dravet syndrome, SOM-mediated inhibition is also reduced28.
In addition to SOM/Martinotti cells, other neurons may contribute to feed-back inhibition in epileptic microcircuits. For example, neocortical chandelier cells, which target the initial axon segments of pyramidal neurons, may prevent hyperexcitation related to epilepsy. In an in vivo study that examined the spontaneous and whisker-evoked activity of a variety of neuronal types in the barrel cortex, chandelier cells only responded weakly to whisker stimulation; only small synaptic potentials were observed and they rarely evoked action potentials64. However, disinhibition induced by local cortical application of the GABA-receptor antagonist bicuculline caused a 20-fold increase in the spontaneous firing rate of chandelier cells, which exceeded that of any other cells recorded. This finding suggests that chandelier cells may be specifically recruited by epileptic activity, and that by vetoing spike output via shut-down of pyramidal cell axons, may serve as a microcircuit emergency brake. Although the specific excitatory versus inhibitory effects of activating chandelier cells remain controversial12,65–67, their activation potentially represents another seizure choke point.
Another example of the role of feed-back inhibition in epilepsy comes from studies of thalamocortical circuits primarily implicated in generalized-absence epilepsy. Here, feedback inhibition has a powerful seizure-promoting role, especially within the thalamus. The thalamic network is composed of topographically related, reciprocally connected inhibitory neurons in RT and excitatory TC cells located in specific relay nuclei within dorsal thalamus68 (Fig. 3b). Activity of the excitatory TC cells activates synapses of RT neurons to cause recurrent feed-back inhibition in the same TC cells. Such inhibition promotes activity of the oscillatory network within the thalamus, because TC cells exhibit a form of paradoxical activation—they fire post-inhibitory rebound bursts of action potentials when strongly inhibited by synchronized output of RT neurons. At the microcircuit level, enhancing feed-back inhibition with pharmacological interventions, such as those that block uptake of the inhibitory neurotransmitter GABA, or pharmacological treatments that specifically target RT-TC synapses, exacerbate epileptiform activity in vitro69,70 (see also Fig. 3b) and worsen generalized-absence seizures in epilepsy patients71.
In the thalamus, TC-RT-TC feed-back inhibition can promote seizure responses, whereas in the cortex, feed-back inhibition largely suppresses seizure activities. Thus, caution is required when interpreting results from global gene knock–out models that generally affect microcircuits, such as those that enhance feed-back inhibition. Similarly, treatments that non-specifically target feed-back inhibition through the brain might not only be ineffective, but might also exacerbate seizures.
To conclude, feed-back inhibition can engage specific microcircuits to either stimulate or inhibit seizure activity. Accordingly, we need to dissect relevant microcircuits involved in ictogenesis to identify specific seizure choke points in different types of epilepsies.
Counter-Inhibition
The nervous system makes its own, sometimes inscrutable, rules about the type and strength of connections made by any individual cell type. In some cases, the synaptic output of a particular neuronal class is quite promiscuous, as it couples indiscriminately to any nearby neurons that fall within its range of efferent axonal output72; however, in other cases, it exclusively targets either neurons of its own or other subclasses73. Inhibitory neurons have unique connectivity rules that seem to take this idea to the extreme. In addition to their potent inhibitory output to pyramidal neurons, parv basket cells form powerful autaptic connections (i.e., they synapse onto themselves)74,75—a relatively rare form of connectivity in the nervous system.
Along these lines, many classes of inhibitory interneurons make chemical and/or electrical synaptic connections with other interneurons within or outside their own class72,76,77, and some inhibitory cell classes (in cortical layer I and/or expressing the peptide vasoactive intestinal peptide, VIP) have been shown to specifically mediate disinhibitory effects through inhibition of SOM and parv cells 78–80. Thus, stimulation of a given set of inhibitory neurons could cause a specific disinhibitory effect, perhaps promoting overexcitation, while inhibition of Layer I/VIP cells might produce an increase in SOM/parv output and result in a seizure choke point. Given the diversity of inhibitory motifs in microcircuits described so far, blocking one of these motifs could have disparate and, perhaps, opposite consequences on the overall function of microcircuits. Thus, counter-inhibition—inhibition of inhibition—(Fig. 4a, b) is a key concept in epileptic microcircuits. For example, counter-inhibition of parv basket cells may largely suppress feed-forward inhibition (motif 1) and promote seizure propagation between regions, while counter-inhibition of Martinotti cells may promote local ictogenesis through loss of the progressively activated feed-back circuit60,62. Here, we will focus on one type of counter-inhibition: between cells of the same inhibitory class.
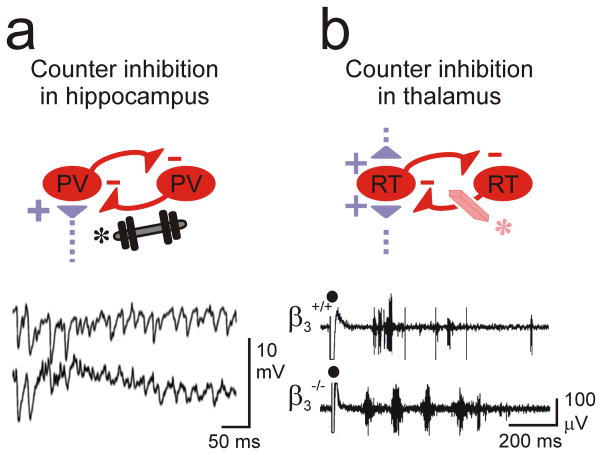
Figure 4. Counter-inhibition in hippocampal and thalamic microcircuits.
(a) Inhibition between FS parv cells in the hippocampus can enhance gamma rhythmicity81. Increasing this inhibition (weight*) has been suggested to enhance network synchrony associated with epilepsy. (b) Inhibition between RT neurons in the thalamus desynchronizes the thalamic network oscillations between TC and RT cells. Loss of RT-RT counter-inhibition (eraser*) in a β3−/− mouse enhances intra-thalamic network synchrony and has been implicated in epilepsy87. RT, reticular thalamic neuron; TC, thalamocortical neuron.
Counter-inhibition in neocortex and hippocampus
Counter-inhibition can promote activity through several mechanisms. First, among inhibitory cells, counter-inhibition can disinhibit downstream excitatory cells, leading to a general increase in firing. Alternatively, it can promote oscillatory activity in reciprocally connected networks. For example, synaptic inhibition between parv FS cells can promote oscillatory output from microcircuits to produce gamma-frequency oscillations81. Such gamma- and related higher-frequency oscillations have been implicated in ictogenesis in limbic epilepsy82 (Fig. 4a).
Counter-inhibition in thalamus
Counter-inhibition affects thalamic function and has been implicated in ictogenesis in absence epilepsy. In thalamic microcircuits, RT neurons mediate feed-forward and feedback inhibition (as described above). Additionally, RT neurons are locally interconnected by both chemical-inhibitory83 and electrical synapses83,84. Chemical inhibition between RT cells is potent and characterized by long-lasting synaptic responses85, and also can limit the synchronous activation of RT cells during epileptiform oscillatory responses in the network86. Hence, specific loss of RT-RT counter-inhibition by deleting a critical, nucleus-specific, GABAA-receptor β3-subunit, is associated with enhanced emergent hypersynchrony and the development of epilepsy87 (Fig. 4b). Accordingly, targeting hypersynchrony and epilepsy in thalamic networks with pharmacotherapies will need to cause a greater net effect on RT-RT inhibition (anti-oscillatory) versus TC-RT-TC feedback inhibition (pro-oscillatory)69. Indeed, the anti-epileptic drug clonazapam decreases output of RT neurons by specifically enhancing RT-RT counter-inhibition88.
Thus, in contrast to the generally suppressive effects on target excitatory cells described above for feed-back and feed-forward inhibition, counter-inhibition can promote or reorganize the excitatory activity of microcircuits, respectively. These effects can occur either through disinhibition or entrainment of recurrent inhibitory networks that produce periodic-phased synaptic inhibition to control the timing of excitatory cells.
Recurrent Excitation
This recurrent excitation microcircuit motif (Fig. 5a, b) falls well within the context of the excitation/inhibition discussions of epileptogenic mechanisms—and for good reason. Recurrent excitation is enhanced in most experimental epilepsies. Yet, modern approaches are now promoting identification of specific, and sometimes de novo, changes in excitatory circuits. One powerful approach is photostimulation, often with photo-labile ligands such as caged-glutamate89. With this approach, originally reported over a decade ago, light can be focally delivered to specific locations in a brain circuit, most commonly in an acute brain slice. This light activates neurons in that region and generates synaptic excitatory signals in neurons post-synaptic to the stimulated cells. This approach showed that recurrent excitation within the dentate gyrus commonly occurred in a limbic epilepsy model90. More recently, this approach revealed intricate changes in dentate connectivity, with notable increases in inputs to dentate gyrus granule cells from not only other granule cells, but also hilar excitatory neurons and CA3 pyramidal neurons91 (Fig. 5b). Such changes can create a strong basis for a hyperconnected, epileptic network, if the reorganization follows the principles of hub-cell connectivity, in which a small number of well-connected neurons help develop complex network activity such as seizures92.
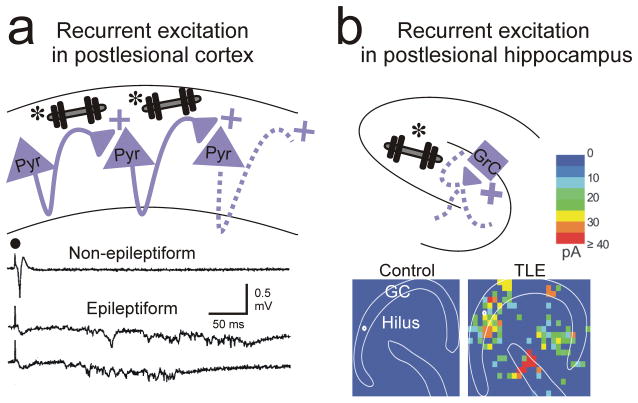
Figure 5. Recurrent excitation in cortex and hippocampus.
(a) Recurrent excitation between pyramidal excitatory cells (weights*) develops after neocortical lesions and has been implicated in epileptiform activities in the undercut model of focal neocortical epilepsy 107. Bottom traces: local recordings of epileptiform field potentials from the injured neocortex evoked by electrical stimulation (black circle). (b) Ectopic recurrent excitation (weight*) between presynaptic excitatory neurons in dentate, hilus, and CA3 and post-synaptic granule cells in the hippocampus develops in the pilocarpine model of temporal lobe epilepsy. Bottom: Connectivity maps based on glutamate photo-uncaging evoked excitatory postsynaptic currents in slices from control and epileptic (TLE) mice91.
In neocortex, recurrent excitatory connections are enhanced following cortical injury and are notably precise. For example, in the isolated cortical slab, which produces epileptogenic insult (Fig. 5a), enhanced connectivity was restricted to infragranular layers, especially layer 593; however, in a model of focal cortical dysplasia, enhanced connectivity to layer 5 cells was seen from both infra and supra-granular regions94. These findings suggest that lesion-specific reorganization occurs in different injury models.
Interventions that counteract or reverse such enhanced reorganization of excitatory microcircuits may yield novel therapeutic approaches. Note that these approaches would be most effective if they specifically targeted maladaptive reorganizations in excitatory networks and maintained normal function of recurrent excitatory networks.
Microcircuit interactions
So far, we have reviewed the properties of isolated microcircuits relevant to ictogenesis, including the important features of connection sign (inhibitory/excitatory), spatial pattern (convergence/divergence), and target region (soma/dendrite/axon). These features are all relatively static in microcircuits, yet many synaptic and cellular components of the circuits can be dynamically modulated to create a stable microcircuit that could, under the right (or wrong!) conditions, progressively shift to an ictogenic form. Further, as indicated at the outset of this review, individual microcircuits do not exist in isolation, and epilepsy results from propagation of ictal activity through the distributed microcircuits. Furthermore, we suggested the novel concept that an imbalance between diverse microcircuit motifs—such as between feed-back and feed-forward inhibition—can be ictogenic. As mentioned above with regards to gria4−/− mice, absence epilepsy results from lack of feed-forward, but unaffected feed-back, inhibition. In this case, a specific defect at the cortico-RT synapse results in lack of cortico-RT-TC feed-forward inhibition, which causes abnormal recruitment of TC cells by afferent excitatory inputs (i.e., multiple TC cells are concurrently activated by cortical output), while the intact TC-RT pathway results in powerful TC-RT-TC synchronized feed-back inhibition. Thus, an imbalance between feed-forward and feed-back inhibition enables normal excitatory inputs to recruit seizures21.
To conclude, this case, in particular, supports the emerging concept that the field needs to expand beyond the historical view that epilepsy simply results from an imbalance between excitation and inhibition and consider that epilepsy can also result from an imbalance between different microcircuit motifs.
In this next and last section, we briefly discuss two issues relevant to seizure choke-points: internal dynamics and external influences on microcircuits.
Dynamics in microcircuits
As indicated above, synaptic connections are considerably heterogeneous, not only in targets and connection strength, but also in short-term dynamics. For example, basket-cell output synapses show short-term depression and lose efficacy over time, and SOM/Martinotti cells show the opposite by augmenting synapses that increase in efficacy over time. Such dynamic changes will inevitably alter the balance between different forms of inhibition. Thus, the normally high ratio of inhibitory output of basket cells (mainly parv to somatic targets) to Martinotti and related cells (SOM to dendritic targets) observed during physiological activity will be replaced by an inverted ratio in which Martinotti-cell output predominates61. This effect may suppress abnormal activity within an ictogenic microcircuit, but leave that same microcircuit vulnerable to additional extrinsic ictogenic signals caused by a loss of feed-forward inhibition.
External influences on microcircuits
Activity can be propagated between microcircuits through efferent projections to circuit elements outside of the microcircuit. Indeed, long-range excitatory projections connect distal cerebral areas. For example, the corpus callosum is composed largely of axons of excitatory cortical neurons95, and this major commissural tract is responsible, in large part, for propagation of seizures96. In recent work, certain classes of inhibitory neurons also made long-range connections that would influence local and global epileptic networks. These findings have recently been reviewed elsewhere97 and will not be further discussed here, except to highlight that this theme is emerging with potential relevance to the motifs described above and their ictal choke points.
As with intra-hemispheric cerebrocortical networks, corticothalamocortical networks are connected through long-range, reciprocal excitatory projections. Sensory regions of dorsal thalamic nuclei are composed largely of excitatory feed-forward TC excitatory neurons that transfer peripheral sensory information to the cortex via projections primarily to cortical layer 4. There, activity reverberates and propagates between cortical layers4 to, ultimately, end up in deep cortical layers, including layer 6. Layer 6 neurons then emit axons back to thalamus to re-excite the TC neurons. In sensory thalamus and cortex, this synaptic relationship is topographic in both directions, leading to a highly localized, but long-loop, excitatory recurrent network. Interposed on this, and indeed embedded within it, is the intrathalamic loop between TC neurons and inhibitory RT neurons. As we described above, this embedded reciprocal relationship between circuits is kept in check by powerful feed-forward inhibition from the cortex that prevents significant excitation of relay neurons that might lead to runaway excitation and seizures.
An additional consideration regarding extrinsic influences on microcircuits is the effect of neuromodulatory pathways, which can selectively and specifically act on individual microcircuit components. For example, cholinergic modulation disparately inhibits basket cells and activates presumed SOM cells10. Of note, recent studies have shown that a subset of narrow spiking neurons, presumed basket cells, is negatively modulated by attendance to a visual task. This finding suggests that attentional states can lead to disinhibition through specific changes in inhibitory microcircuits98.
Circuit therapy: where are the choke points?
While the process of developing epilepsy—epileptogenesis—likely entails multiple adaptive and maladaptive circuit changes, here we have addressed several simple microcircuit motifs in which dysfunction in one element (e.g., a synapse or neuron) either through gain- or loss-of-function (e.g., change in synaptic strength or intrinsic excitability), can effectively entrain local network activity. The build-up of such local activity to the point of initiating a seizure is an ictogenesis. Thus, in each of the four different cases of maladaptive circuit motifs, restorative treatments that would reverse or counteract the specific dysfunction (or perhaps prevent the dynamic recruitment of that dysfunctional element during ictogenesis) could create an effective anti-seizure therapy. By extending this approach, some regions other than the point of maximal dysfunction might be targeted (Fig. 6a–d). Distal targeting might be more efficient because the distal sites are either critical in global ictogenesis and/or are more spatially restricted and thus easier to maximally target. If the cells in distal sites are only modestly involved in global ictogenesis, then reducing the activity of only some of them will not be effective. However, if they are concentrated in a region such that the bulk of relevant cells in the distal subnetwork can be effectively targeted, then great efficacy would be gained. For example, a rat model of cortical photothrombotic stroke developed epilepsy over time (Fig. 6a). Here, specifically inhibiting the portion of thalamus projecting to the surviving peri-infarct cortex was sufficient to abort, in real-time, automatically detected seizures99. Because of extensive long recurrent excitatory connections with cortex, these results suggest that thalamus could be an important target in epilepsies resulting from cortical lesions other than stroke.
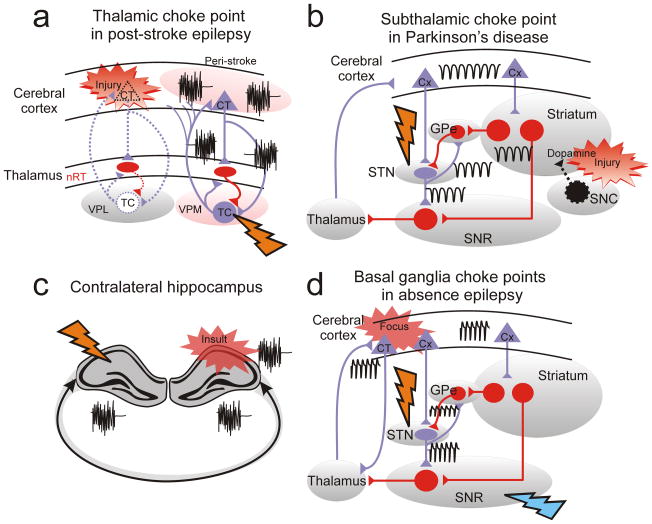
Figure 6. Circuit therapy: focus on choke points.
(a) The thalamus is a choke point for epileptic seizures in post-stroke epilepsy99. Note that the choke point (flash: thalamus) is remote from the initial dysfunction (red flash), which is a stroke in the cerebral cortex. (b) The subthalamus (STN) is an efficient choke point for pathological circuit oscillations in Parkinson’s disease. Note that the choke point (black flash: STN) is remote from the initial dysfunction (yellow flash), which results from degeneration of dopaminergic cells (Dopamine) projecting from the substantia nigra compacta (SNC) to striatum. (c) Contralateral hippocampus is a choke point for controlling ipsilateral hippocampal epileptic activity100. (d) STN and SNR are choke points for spike-and-wave discharges associated with absence epilepsy and generated in somatosensory cortex 108. Black oscillations: pathological oscillations; Red flash: initial injury or insult; Orange flash: choke point for pathological network oscillation. Other abbreviations: GPe: External globus pallidus; SNR: substantia nigra pars reticulata. Purple cells/projections: excitatory glutamatergic; Red cells/projections: inhibitory GABAergic.
Several additional examples of localized, off-site seizure control are evident and further support that remotely regulating seizures might create a generally useful concept regarding ictogenic choke points. For example, in a model of limbic epilepsy caused by unilateral intrahippocampal injection of the excitotoxin kainic acid, optogenetic excitation of inhibitory cells of either the primary ipsilateral epileptogenic zone or in the contralateral hippocampus reduced seizures100 (Fig. 6c). In another example of off-site control this same group has shown that optogenetic activation of cerebellar Purkinje neurons suppresses seizures in this animal model of epilepsy 101. Additionally, experimental seizures induced by either electrical or chemical stimulants are strongly suppressed by locally inhibiting the substantia nigra 102. Thus, targeting such subcortical structures, such as the thalamus or substantia nigra, remote from the initial cortical dysfunction, might have major advantages. For instance, targeting the thalamus in real time would be less deleterious than targeting the eloquent cortex. We propose that the thalamus could be a choke point in epileptic circuits in the same way that the subthalamus (STN) is a choke point for abnormal circuit dynamics in Parkinson’s disease. Indeed, the concept of circuit motif choke points can be broadly applied to nervous system disorders. In the case of Parkinson’s disease, the initial dysfunction results from the degeneration of neurons in the substantia nigra pars compacta and, therefore, is remote from the STN. However, targeting the STN is the major therapy used in Parkinsonian patients. Indeed, the STN is a choke point of abnormal circuits in Parkinson’s disease because of its key location within the circuit, even though the initial dysfunction is remote (Fig. 6b) 103. Of note, high-frequency stimulation of STN or inhibition of substantia nigra pars reticulata104,105 also strongly suppresses seizures in GAERS106—a model of generalized-absence epilepsy—further supporting the concept of distal epileptic choke points.
Conclusions
While we need to identify the “focus” of the initial dysfunction, we also need to look for potential control or choke points that are remote and could be distant from the “focus” of the initial dysfunction. Thus, by scanning regions outside that of the initial insult, we may find “foci” far from what has historically been considered the focus, and, in so doing, may find unique opportunities for effective therapies that target these circuits.
Acknowledgments
This work is supported by the NIH/NINDS and CURE, Citizens United Against Epilepsy. We would like to thank Christopher Makinson for critical comments.
Reference List
- 1.Avoli M. A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia. 2012;53:779–789. doi: 10.1111/j.1528-1167.2012.03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; Bethesda: 2012. [PubMed] [Google Scholar]
- 3.Jones EG. Viewpoint: The core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- 4.Douglas RJ, Martin KA. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 6.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Sun QQ, Huguenard JR, Prince DA. Reorganization of barrel circuits leads to thalamically-evoked cortical epileptiform activity. Thalamus Relat Syst. 2005;3:261–273. doi: 10.1017/S1472928807000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–12607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol. 2002;88:740–750. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- 12.Molnar G, et al. Complex events initiated by individual spikes in the human cerebral cortex. PLoS Biol. 2008;6:e222. doi: 10.1371/journal.pbio.0060222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, et al. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagnall MW, Hull C, Bushong EA, Ellisman MH, Scanziani M. Multiple clusters of release sites formed by individual thalamic afferents onto cortical interneurons ensure reliable transmission. Neuron. 2011;71:180–194. doi: 10.1016/j.neuron.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swadlow HA, Gusev AG. Receptive-field construction in cortical inhibitory interneurons. Nat Neurosci. 2002;5:403–404. doi: 10.1038/nn847. [DOI] [PubMed] [Google Scholar]
- 16.Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somogyi P, Kisvarday ZF, Martin KA, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience. 1983;10:261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- 18.Inoue T, Imoto K. Feedforward inhibitory connections from multiple thalamic cells to multiple regular-spiking cells in layer 4 of the somatosensory cortex. J Neurophysiol. 2006;96:1746–1754. doi: 10.1152/jn.00301.2006. [DOI] [PubMed] [Google Scholar]
- 19.Maheshwari A, Nahm WK, Noebels JL. Paradoxical proepileptic response to NMDA receptor blockade linked to cortical interneuron defect in stargazer mice. Front Cell Neurosci. 2013;7:156. doi: 10.3389/fncel.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki S, Huda K, Inoue T, Miyata M, Imoto K. Impaired feedforward inhibition of the thalamocortical projection in epileptic Ca2+ channel mutant mice, tottering. J Neurosci. 2006;26:3056–3065. doi: 10.1523/JNEUROSCI.5422-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paz JT, et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci. 2011;14:1167–1173. doi: 10.1038/nn.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 23.Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993;259:97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- 24.Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol. 2013;591:807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sah N, Sikdar SK. Transition in subicular burst firing neurons from epileptiform activity to suppressed state by feedforward inhibition. Eur J Neurosci. 2013;38:2542–2556. doi: 10.1111/ejn.12262. [DOI] [PubMed] [Google Scholar]
- 26.Khalilov I, Holmes GL, Ben Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 27.Rossignol E, Kruglikov I, van den Maagdenberg AM, Rudy B, Fishell G. CaV 2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann Neurol. 2013;74:209–222. doi: 10.1002/ana.23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA. 2014;111:E3139–E3148. doi: 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamakawa K. Molecular and cellular basis: insights from experimental models of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):70–71. doi: 10.1111/j.1528-1167.2011.03006.x. [DOI] [PubMed] [Google Scholar]
- 30.Dutton SB, et al. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol Dis. 2013;49:211–220. doi: 10.1016/j.nbd.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verret L, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker J, Storch G, Quach-Wong B, Sonnenfeld J, Aaron G. Propagation of epileptiform events across the corpus callosum in a cingulate cortical slice preparation. PLoS One. 2012;7:e31415. doi: 10.1371/journal.pone.0031415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- 35.Dinocourt C, Petanjek Z, Freund TF, Ben Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol. 2003;459:407–425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- 36.Leao RN, et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cossart R, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 38.Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 39.Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Z, et al. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- 42.Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I Characteristics and topographical features. J Neurophysiol. 1969;32:649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- 43.Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schevon CA, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigeland LE, Contreras D, Palmer LA. Synaptic mechanisms of temporal diversity in the lateral geniculate nucleus of the thalamus. J Neurosci. 2013;33:1887–1896. doi: 10.1523/JNEUROSCI.4046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci USA. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacey CJ, Bryant A, Brill J, Huguenard JR. Enhanced NMDA receptor-dependent thalamic excitation and network oscillations in stargazer mice. J Neurosci. 2012;32:11067–11081. doi: 10.1523/JNEUROSCI.5604-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyer B, et al. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum Mol Genet. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andolina IM, Jones HE, Sillito AM. Effects of cortical feedback on the spatial properties of relay cells in the lateral geniculate nucleus. J Neurophysiol. 2013;109:889–899. doi: 10.1152/jn.00194.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarren M, Alger BE. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985;53:557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- 51.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. II Effects of extracellular potassium, furosemide, and membrane potential on ECl- in hippocampal CA3 neurons. J Neurophysiol. 1989;61:512–523. doi: 10.1152/jn.1989.61.3.512. [DOI] [PubMed] [Google Scholar]
- 52.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. I Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989;61:501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- 53.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. III Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. J Neurophysiol. 1989;61:524–533. doi: 10.1152/jn.1989.61.3.524. [DOI] [PubMed] [Google Scholar]
- 54.Willow M, Gonoi T, Catterall WA. Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol. 1985;27:549–558. [PubMed] [Google Scholar]
- 55.Yaari Y, Selzer ME, Pincus JH. Phenytoin: mechanisms of its anticonvulsant action. Ann Neurol. 1986;20:171–184. doi: 10.1002/ana.410200202. [DOI] [PubMed] [Google Scholar]
- 56.Cheung H, Kamp D, Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res. 1992;13:107–112. doi: 10.1016/0920-1211(92)90065-2. [DOI] [PubMed] [Google Scholar]
- 57.Pothmann L, et al. Function of inhibitory micronetworks is spared by Na+ channel-acting anticonvulsant drugs. J Neurosci. 2014;34:9720–9735. doi: 10.1523/JNEUROSCI.2395-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 59.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- 62.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y, Stornetta RL, Zhu JJ. Chandelier cells control excessive cortical excitation: characteristics of whisker-evoked synaptic responses of layer 2/3 nonpyramidal and pyramidal neurons. J Neurosci. 2004;24:5101–5108. doi: 10.1523/JNEUROSCI.0544-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Toprani S, Tang Y, Vrabec T, Durand DM. Mechanism of highly synchronized bilateral hippocampal activity. Exp Neurol. 2014;251:101–111. doi: 10.1016/j.expneurol.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodruff AR, et al. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31:17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabadics J, et al. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 68.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Sohal VS, Keist R, Rudolph U, Huguenard JR. Dynamic GABA(A) receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci. 2003;23:3649–3657. doi: 10.1523/JNEUROSCI.23-09-03649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sohal VS, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Intrinsic and synaptic dynamics interact to generate emergent patterns of rhythmic bursting in thalamocortical neurons. J Neurosci. 2006;26:4247–4255. doi: 10.1523/JNEUROSCI.3812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skardoutsou A, Voudris KA, Vagiakou EA. Non-convulsive status epilepticus associated with tiagabine therapy in children. Seizure. 2003;12:599–601. doi: 10.1016/s1059-1311(03)00102-x. [DOI] [PubMed] [Google Scholar]
- 72.Olah S, et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamás G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J Neurosci. 2003;23:859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galarreta W, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 77.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 78.Letzkus JJ, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- 79.Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 82.Grasse DW, Karunakaran S, Moxon KA. Neuronal synchrony and the transition to spontaneous seizures. Exp Neurol. 2013;248:72–84. doi: 10.1016/j.expneurol.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 83.Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landisman CE, et al. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl− currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78:2280–2286. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]
- 86.Sohal VS, Huguenard JR. Inhibitory interconnections control burst pattern and emergent network synchrony in reticular thalamus. J Neurosci. 2003;23:8978–8988. doi: 10.1523/JNEUROSCI.23-26-08978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 88.Huguenard JR, Prince DA. Clonazepam suppresses GABAB-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- 89.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, Huguenard JR, Buckmaster PS. Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–1196. doi: 10.1523/JNEUROSCI.5342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci USA. 2008;105:6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 2006;26:4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brill J, Huguenard JR. Enhanced infragranular and supragranular synaptic input onto layer 5 pyramidal neurons in a rat model of cortical dysplasia. Cereb Cortex. 2010;20:2926–2938. doi: 10.1093/cercor/bhq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobson S, Trojanowski JQ. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res. 1974;74:149–155. doi: 10.1016/0006-8993(74)90118-8. [DOI] [PubMed] [Google Scholar]
- 96.Wilson DH, Culver C, Waddington M, Gazzaniga M. Disconnection of the cerebral hemispheres. An alternative to hemispherectomy for the control of intractable seizures. Neurology. 1975;25:1149–1153. doi: 10.1212/wnl.25.12.1149. [DOI] [PubMed] [Google Scholar]
- 97.Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Curr Opin Neurobiol. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 98.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paz JT, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014 doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- 103.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paz JT, Chavez M, Saillet S, Deniau JM, Charpier S. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J Neurosci. 2007;27:929–941. doi: 10.1523/JNEUROSCI.4677-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- 106.Vercueil L, et al. High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res. 1998;31:39–46. doi: 10.1016/s0920-1211(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 107.Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- 108.Polack PO, et al. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]