Abstract
Deficits in N-methyl-D-aspartate receptor (NMDAR) function play a critical role in the pathophysiology of schizophrenia. Animal models are needed to investigate possible mechanisms underlying NMDA dysfunction in schizophrenia as well as development of new therapeutic approaches. A major difficulty in developing animal models for schizophrenia is the identification of quantifiable measures that can be tested in a similar fashion in both humans and animals. The majority of animal models utilize analogous measures, wherein species-specific behaviors are used as presumed parallel manifestations of a common underlying construct. In vivo microdialysis and electrophysiology represent two methodologies in which homologous measures can instead be obtained in both animals and humans. In both techniques, well-validated, NMDA-sensitive measures are analyzed in rodents using probes implanted directly into cortex or subcortical structures. We discuss the currently available data from studies that used these methods in non-human primate and rodent glutamate models. In addition, we emphasize the possible relevance of the amphetamine-challenge studies to positive symptoms and of EEG measures to cognitive deficits in schizophrenia.
Keywords: PCP NMDA, Schizophrenia, Animal models, Glutamate, Glycine, Negative symptoms, Cognitive disorders, Neurophysiology, Event-related potentials
1. Introduction
Schizophrenia is a severe mental disorder that affects up to 1% of the population, and is one of the leading causes of chronic disability worldwide. Symptoms of schizophrenia are traditionally divided into three main clusters: positive, negative and cognitive (aka “disorganized”). Positive symptoms consist of items indicative of overall hyperactivity such as agitation, paranoia, and hallucinations. In contrast, negative symptoms consist of items indicative of behavioral underactivity, including emotional blunting and passive/apathetic social withdrawal [55]. Cognitive symptoms include conceptual disorganization, difficulty in abstract thinking and disorientation. In addition, schizophrenia is associated with objective neuropsychological deficits across a variety of cognitive domains [101], implicating distributed neurophysiological dysfunction.
The first effective treatments for schizophrenia were discovered fortuitously in the late 1950s, and subsequently shown to mediate their effects at dopamine D2 receptors. Since that time, dopamine has been the primary neurotransmitter implicated in schizophrenia, and the majority of neurochemical studies of schizophrenia continue to focus on dopaminergic mechanisms [20]. However, over the past half-century, limitations in the dopamine model have become increasingly apparent. First, whereas dopaminergic dysfunction models account well for positive symptoms of the disorder, they account poorly for both negative symptoms and neurocognitive dysfunction. Second, despite decades of investigation, there appear to be few intrinsic deficits that could account for the pattern of dopaminergic dysfunction observed in schizophrenia [44].
An alternative to the dopamine model was first proposed in the early 1990s, based upon the observation that phencyclidine (PCP) and similarly acting psychotomimetic compounds induce their unique behavioral effects by blocking neurotransmission at N-methyl-D-aspartate (NMDA)-type glutamate receptors [43,44]. The ability of these compounds to transiently reproduce key symptoms of schizophrenia by blocking NMDA receptors led to the concept that symptoms in schizophrenia may reflect underlying dysfunction or dysregulation of NMDA receptor-mediated neurotransmission. Over the past 15 years, convergent evidence has accumulated to support a primary role for glutamatergic dysfunction in the pathophysiology of schizophrenia [2,22,102,128]. In particular, studies have documented a close congruence between symptomatic and neurocognitive effects induced by NMDA antagonists such as PCP and the related drug ketamine, and the pattern observed in schizophrenia. Further, both genetic and neurochemical studies have begun to identify pathogenetic events that may impact upon glutamatergic neurotransmission, and provide plausible bases for underlying NMDA dysfunction. Finally, findings from both animal and human studies indicate that the hyper-dopaminergia associated with schizophrenia may, in fact, result from underlying dysfunction of NMDA-related neuromodulatory feedback mechanisms. Overall, these findings suggest new etiological and psychotherapeutic conceptualizations of schizophrenia.
Several varieties of animal behavioral models have been developed for schizophrenia, and are reviewed elsewhere in this issue. While effective, the majority of these approaches depend upon analogous models—that is, models in which behaviors in rodents are related, but not identical to, the behaviors that they are meant to model in humans. For example, rodent hyperactivity is typically used to model positive symptoms of schizophrenia. However, issues such as paranoia and hallucinations cannot be assessed in rodents, so that the quality of the model depends in large part upon the true degree of parallelism between the rodent and human conditions. In the case of hyperactivity, for example, it is noteworthy that drugs such as PCP and ketamine induce hyperactivity in rodents but withdrawal in monkeys and mixed behaviors in humans, pointing out the complexities in translating behaviorally between rodents (or even monkeys) and humans.
The present review will focus on two models in which homologous functions can be assessed in rodents and humans—that is, in which the same process can be studied in both rodents and humans. The first of these approaches uses in vivo microdialysis in awake, behaving animals coupled with NMDA blockade to recreate the dopaminergic instability associated with schizophrenia. The second uses electrophysiological recordings in both rodents and primates to investigate mechanisms underlying impaired event-related potential (ERP) generation in schizophrenia. Whereas dopaminergic hyperactivity appears to provide a good model for positive symptoms of schizophrenia, neurophysiological models may be most relevant to negative symptoms and neurocognitive dysfunction.
2. Neurochemical models of schizophrenia
As noted above, disturbances in dopaminergic function are among the best validated measures in schizophrenia. Amphetamine and other agents that stimulate dopamine release reliably induce positive symptoms when given at high dose. Further, behavioral effects of amphetamine are reliably reversed in both humans and animal models by dopamine depletion using compounds such as reserpine or by administration of dopamine antagonists.
Presumed dopaminergic hyperactivity in schizophrenia is currently addressed by blocking dopamine D2 receptors, which are the primary target of dopamine in striatum. The association between antipsychotic potency and D2 occupancy remains one of the strongest relationships in all of clinical medicine, with the majority of antipsychotics studied to date producing clinically beneficial effects at D2 occupancy levels of 60–80%. A limitation of the current antipsychotic treatment approach, however, is that such drugs do not reverse the dopaminergic instability associated with schizophrenia, but merely prevent downstream consequences. Further, many individuals show persistent positive symptoms despite adequate (or even excessive) treatment with antipsychotics, suggesting that dopaminergic hyperactivity alone is not sufficient to account for positive symptoms in all cases. Finally, dopaminergic agents such as amphetamine do not induce negative symptoms and cognitive dysfunction associated with schizophrenia. Thus, at best, models of dopaminergic instability in schizophrenia are relevant primarily to positive symptoms of the disorder.
Positive symptoms of schizophrenia have been linked most strongly to dopaminergic hyperactivity within dorsal striatal circuits in humans. Dopaminergic activity can be studied objectively in humans using PET- or SPECT-based radioreceptor imaging of dopamine receptors, particularly in striatum. In this approach, a radiolabeled D2 receptor ligand is used such as [125I]IBZM or [14C]raclopride and basal binding is obtained. Amphetamine or another dopamine-releasing agent is then administered. When dopamine is released, it competes for binding with the radiolabeled compound, leading to a decrease in effective tissue concentration of the label. The degree of reduction in radiolabel concentration thus serves as an index of stimulated dopamine release. In normal volunteers, multiple agents including amphetamine and methylphenidate induce reliable reductions in radiolabel binding in striatum, consistent with their ability to stimulate net striatal dopamine release [14,67].
Amphetamine induces DA release by reverse transport of DA from the cytoplasmic pool to the synapse through the dopamine transporter (DAT). Thus, blocking DAT with DA uptake inhibitors such as nomifensine results in a blunting of amphetamine-induced DA release. Because amphetamine releases DA from the cytoplasmic pool, treatments that deplete cytoplasmic DA also inhibit amphetamine-induced DA release. Early studies in SPECT and PET D2 receptor imaging confirmed that sensitivity of the assay to DAT blockers and DA depletion, supporting the relationship between D2 radiolabel binding and D2 release [69].
2.1. In vivo studies of DA release in schizophrenia
In schizophrenia, enhanced amphetamine-induced dopamine release has been demonstrated across a number of cohorts using both SPECT and PET imaging and various radiolabeled compounds [13,67,65]. However, in these studies, dopaminergic hyperactivity was observed only in individuals during the acute stage of their illness. The degree of excess dopamine release did not appear to be affected by antipsychotics, as similar deficits were observed in both medicated and unmedicated patients, supporting the contention that antipsychotics primarily affect downstream consequences of dopaminergic hyperactivity without reversing the underlying abnormality [68]. Excess dopamine release was also not observed in individuals with schizotypal personality disorder [1]. Thus, overall, striatal DA release may only serve as a model for exacerbation of positive symptoms during acute decompensation. Nevertheless, this is a critical component of schizophrenia, and treatments that prevent such decompensations would be therapeutically important in schizophrenia.
A study in schizophrenia has also evaluated the relative involvement of different striatal subdivisions. The human striatum is functionally organized into limbic, associative, and sensorimotor subdivisions, which process information related to emotional, cognitive, and motor function, respectively. In an anatomic study, amphetamine induced significantly greater stimulation of DA release in limbic and sensorimotor, compared with associative, striatal region. Further, the increase in euphoria reported by subjects after amphetamine was associated with larger dopamine release in the limbic and sensorimotor regions, but not in the associative regions. However, because of its larger size (62% of total striatal volume), the associative striatum still contributed the majority of signal following dopamine release. It was speculated therefore that the hyperactivity of dopamine release observed in schizophrenia reflects increased release primarily in the associative division [85].
2.2. Role of NMDA in DA dysregulation
Despite the reproducibility of dopaminergic hyperactivity, underlying mechanisms remain to be determined. To date, no intrinsic dopaminergic deficits have been demonstrated that could account for the abnormalities. While some studies have noted association between the val158met polymorphism of the catechol-O-methyltransferase (COMT) gene [141], COMT genotype has been shown to predict cortical, but not striatal, dopamine receptor occupancy [126]. In addition, although amphetamine induces DA release via the DAT, studies using selective DAT labels have shown normal DAT density in schizophrenia [70].
Although the mechanism underlying DA dysfunction in schizophrenia is not known, one potential mechanism is underlying dysfunction of NMDA receptor-mediated neurotransmission. In normal volunteers, administration of ketamine prior to amphetamine challenge leads to dopaminergic hyperactivity similar to that observed in schizophrenia [56]. The increased dopamine release following ketamine does not appear to reflect a direct effect of ketamine on dopamine [57], but instead must reflect effects of ketamine on feedback circuits that normally serve to limit dopamine release following ketamine administration. Thus, disturbances in dopaminergic regulation in schizophrenia, rather than reflecting endogenous dopaminergic dysfunction, may reflect failures of NMDA-mediated dopaminergic regulation, which can be demonstrated in humans by investigating glutamate/dopamine interactions.
Ketamine challenge studies are, for ethical reasons, limited to single acute doses. However, persistent schizophrenia-like alterations in cognition [24] and D1 receptor expression [100] have recently been documented in chronic ketamine abusers, suggesting that repetitive ketamine exposure may provide an even better model for schizophrenia. Schizophrenia-like hallucinations are not typically observed during acute ketamine challenge. However, in monkeys, progressive development of hallucinatory-like behavior is observed during persistent subchronic administration [79], further supporting the utility of chronic administration models.
2.3. Animal models of NMDA-induced DA dysregulation
In animal models, dopamine release can be measured directly using in vivo microdialysis, rather than indirectly through radiolabel displacement. In the microdialysis approach, a probe containing a semi-permeable membrane is inserted into the region of interest, and perfused with an artificial CSF solution. The perfusate is collected over minutes to tens of minutes. Small molecules from the brain diffuse through the membrane and are collected in the perfusate, permitting them to be assayed using techniques such as high-performance liquid chromatography (HPLC). Dopamine, along with other monoamines, is particularly amenable to electrochemical detection (HPLC-EC), with typical sensitivities in the low femtomolar range. Drugs can also be administered through the perfusate by reverse dialysis, permitting pharmacological manipulation in the immediate region surrounding the microdialysis probe with no change in general brain function. Such an approach is particularly effective with prototypic molecules that frequently have low brain penetrance.
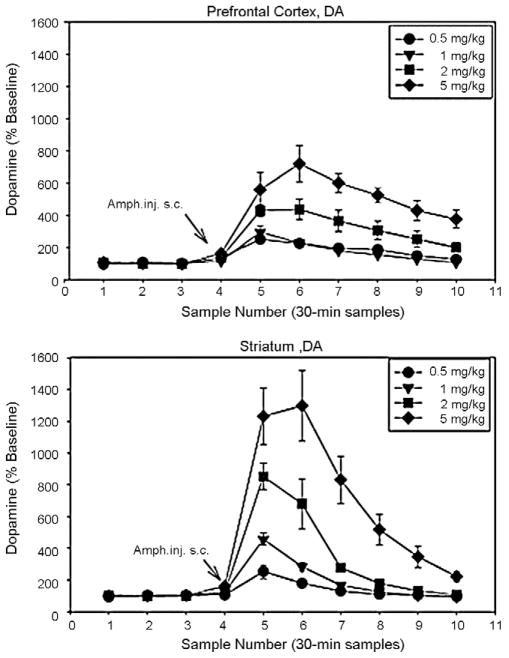
In rodents, amphetamine produces a well-described elevation in dopamine levels in striatum, nucleus accumbens and frontal cortex that persists for approximately 90 min following acute administration. Effects of amphetamine are strongly dose-dependent, permitting selection of doses in animals that closely match the dose of 0.3 mg/kg used in most clinical studies. In primate studies, the degree of radiolabel displacement observed in clinical studies – 10–38% – corresponds to increases in striatal dopamine levels of between 400 and 1500% [13,69,66]. In rodents, amphetamine doses of 1–5 mg/kg induce levels similar to those observed in clinical studies (Fig. 1), suggesting that such doses are most appropriate for animal models of striatal dopaminergic hyperactivity [120].
Fig. 1.
Effect of amphetamine on dopamine release in rat frontal cortex and striatum. In human D2 displacement studies, typical doses of amphetamine (e.g. 0.3 mg/kg) produce increases in striatal D2 release of between 400 and 1500%. In rats, doses of between 1 and 5 mg/kg produce similar levels of effect, suggesting that they may be most effectively used for animal models of dopaminergic hyper-reactivity in schizophrenia. From [120].
As in humans, NMDA antagonists potentiate amphetamine-induced DA release, suggesting that this preparation may serve as an appropriate animal model of schizophrenia [7]. Similar effects are observed in prefrontal cortex (PFC), a region implicated in cognitive dysfunction in schizophrenia [8]. Potentiation of amphetamine-induced DA release is also observed following administration of the NMDA antagonist MK-801 [90]. In both preparations, increases are seen to systemic but not local amphetamine administration, suggesting that effects are not mediated by direct interaction of NMDA antagonists with the receptors on the presynaptic terminal or by interaction with the DAT itself [6]. Rather, effects appear to depend upon more complex neural interactions.
Although microdialysis represents the most direct measure of NMDA antagonist effects amphetamine-induced DA release, other approaches also document profound effects of NMDA antagonists in DA function. In frontal cortex, ketamine induces increases in DA release that are associated with impairments in working memory function [92]. NMDA antagonist-induced deficits in working memory are associated with increased tonic glutamate release [93] and tonic neuronal firing and disruption of normal burst-firing activity [40]. Alterations in prefrontal glutamate and dopamine produce modulatory effects on working memory, with glutamate primarily affecting encoding and dopamine mediating mnemonic retention [5]. In schizophrenia, deficits are seen primarily in encoding, with secondary effects of retention [74], implicating glutamatergic dysfunction. As with microdialysis, therefore, NMDA-induced alterations in glutamatergic firing rates may provide an indirect measure of impaired PFC dopaminergic function.
2.4. Rodent DA function as a model for drug development in schizophrenia
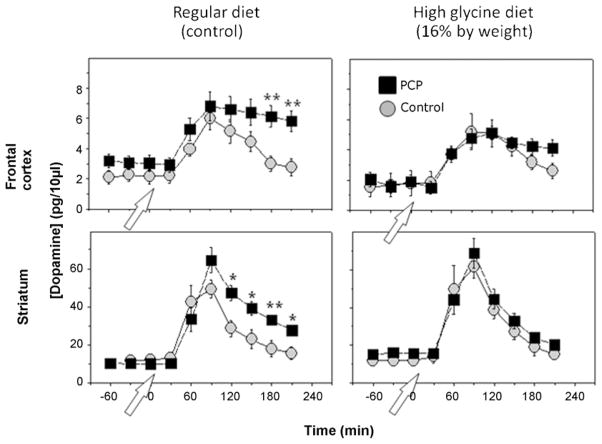
An important role of animal models in schizophrenia is the evaluation of potential new treatment modalities. One direct prediction of the PCP/NMDA model of schizophrenia is that agents which stimulate NMDA function should be beneficial in schizophrenia. Several small scale clinical studies have evaluated the role of compounds that target the NMDA modulatory site of the NMDA receptor complex, including glycine, D-serine, D-alanine and the naturally occurring glycine transport inhibitor sarcosine [52]. These compounds have been evaluated as well in animal dopaminergic models. Both glycine and glycine transport inhibitors significantly reverse the effects of PCP on amphetamine-induced DA release [50], suggesting that this model is sensitive to potential NMDA enhancing agents (Fig. 2).
Fig. 2.
Effect of glycine on PCP-induced dysregulation of amphetamine-induced dopamine release. Hyperreactivity of amphetamine-induced dopamine release similar to that observed in schizophrenia can be induced in rodents by subchronic PCP administration. Simultaneous treatment with the NMDA modulator glycine reversed the effect of PCP on amphetamine-induced dopamine release. Similar effects were observed with the prototypic glycine transport inhibitor ALX-5407, suggesting that this model shows sensitivity to putative NMDA enhancing agents. From [50].
Assays of prefrontal response to NMDA antagonists have also proven useful in the identification of potential novel agents in the treatment of schizophrenia. An early study showed that effects of NMDA on prefrontal glutamatergic activity could be reversed by a metabotropic glutamate receptor (mGluR) 2/3 agonist [93], providing an alternative approach for reversal of primary NMDA dysfunction. This approach has received significant recent report from a clinical study showing effectiveness of the novel mGluR2/3 agonist LY2140023 equivalent to that of olanzapine in the treatment of an acute schizophrenia exacerbation [106]. mGlu2/3 agonists also modulate amphetamine-induced DA release in striatum, supporting striatal dopaminergic models [140]. More recent studies support the utility of mGluR5 agonists, which potentiate NMDA receptor-mediated neurotransmission [40,73]. Such studies strongly support the potential of glutamate-based treatment approaches for reversal of both negative and cognitive deficits associated with schizophrenia.
2.5. Molecular mechanisms underlying NMDA agonist effects
A final series of studies has investigated the mechanism by which NMDA agonists modulate striatal DA release. Local circuit effects of NMDA agonists can be studied in isolated rodent striatum using in vitro [3H]DA release assays, which permit assessment of local striatal mechanisms. In striatum, inhibitory effects of NMDA agonists on striatal DA release were associated with increased GABA overflow, suggesting that a critical target of NMDA receptors is on local inhibitory GABAergic interneurons. Significant effects were observed for both glycine and prototypic glycine transport inhibitors, supporting the utility of this model in early stage drug development. Finally, inhibitory effects of NMDA agonists on striatal dopamine release was blocked by inhibitors of GABAB-, but not GABAA-, type GABA receptors [51], suggesting that presynaptic GABAB receptors on presynaptic DA terminals may be a critical site for future NMDA-based drug development.
As in striatum, glutamatergic hyperactivity in prefrontal cortex may also reflect impaired function of NMDA receptors on local GABAergic interneurons [41]. Further, in prefrontal cortex, NMDA antagonists induce reductions in parvalbumin and GAD67 expression similar to those observed in schizophrenia [37,58], suggesting that GABAergic deficits may be downstream to underlying NMDA dysfunction. In both striatum and prefrontal cortex, a critical issue may be the restoration of glutamate/GABA balance. In stiatum, GABAB receptors appear to play a critical role in maintaining glutamate/GABA balance [51]. In contrast, in prefrontal cortex, encouraging effects have recently been reported for the selective GABAA agonist MK-0777 [76].
3. Neurophysiological models of schizophrenia
Similar to the discussed micro-dialysis studies, homologous functions in humans and animal models can also be assessed with neurophysiological methods, which measure electrical activity of neuronal ensembles within the brain. In clinical studies, activity is obtained using scalp recorded electroencephalogy (EEG) and event-related potentials (ERP), or related magnetoencephalographic (MEG) techniques. In animals, activity can be recorded using either scalp recordings analogous to those used clinically, or via electrodes laying on top of the cortex or implanted directly into cortex or subcortical structures.
Intracranial recording studies provide critical insights into neural mechanisms underlying neurophysiological impairments in schizophrenia. In intractranial recordings, local field potentials (LFP) can be recorded, which represent the local correlate of surface potentials. In addition, however, measures such as current-source density (CSD) and multiunit activity (MUA) can be used to determine underlying generator mechanisms [64]. A variety of synaptic and non-synaptic potentials, including slow neural activity such as spike afterpotentials and voltage-dependent membrane potentials may contribute to intra-cortical and surface neurophysiological activity [18,54,80,91].
3.1. Sensory measures in schizophrenia research
Deficits in cognitive processes represent core features of schizophrenia and constitute a major factor determining functional outcome and rehabilitation in schizophrenia and a critical target for pharmacological development [38]. Patients with schizophrenia show deficits in numerous cognitive processes that can be evaluated using EEG- and MEG-based techniques [53]. Of these, however, sensory-based measures provide the easiest approach to cross-species modeling, as they can be recorded even from untrained rodents and primates, and take advantage of conserved auditory and visual anatomy across species. An increasing body of evidence indicates that neural deficits in patients affect not only higher cognitive functions, such as memory, executive functions, and attention, but also early sensory processes within the auditory and visual (among other sensory) modalities [17,49]. Further, impairments at these early stages of information processing may contribute significantly to higher order cognitive deficits in schizophrenia. Current treatments offer only limited, if any improvement. Thus, a detailed understanding of the basis of these deficits is needed for the development of effective treatments.
To date, several electrophysiological measures have been identified that are both reliably affected in schizophrenia and amenable to translational studies in animal models. In the auditory system, these include mismatch negativity (MMN) and auditory N1. Both of these measures represent automatic, pre-attentive processes that can be tested in passive stimulation paradigms and are thus well suited for assessment in animal models [48,114,137,133]. Furthermore, both measures appear to depend upon NMDA-dependent processes, suggesting that they can be used to investigate mechanisms and new treatment approaches for NMDA deficits in schizophrenia [53]. In the visual system, impaired magnocellular function and impaired generation of the P1 component particularly to magnocellular stimuli may also serve as an etiological model to investigate mechanisms underlying cognitive impairment in schizophrenia [16].
3.2. Mismatch negativity (MMN)
Mismatch negativity (MMN) is an ERP component that is triggered whenever a stimulus feature of an infrequently presented sound (‘deviant’) violates the regularity established by repeatedly presented sounds (‘standards’). MMN is considered to be an automatic pre-attentive process since it was elicited in sleep, under anesthesia, in comatose patients, and with attention deployed to another modality [33]. Thus, MMN is thought to reflect an automatic novelty detection mechanism that may serve to trigger an involuntary switch of attention towards the deviant stimulus.
The degree to which the MMN process succeeds in capturing attention depends in part upon the degree to which attentional resources are otherwise engaged. Under all conditions, MMN overlaps temporally with sensory measures including the auditory N1 potential. A technical issue, therefore, is that deviance-related activity must be differentiated from differential refractoriness of response to standard and deviant stimuli. MMN is not elicited by the first stimulus in a sequence, indicating that it reflects an active disinhibition process induced by the repetitive standards [142].
MMN is one of the most investigated human cognitive brain potentials. The extensive literature regarding its role in cognitive neuroscience is discussed in detail in other recent reviews [63,98]. Importantly, MMN is reduced in schizophrenia [78,99,123]. Indeed, since deficits in MMN generation were first demonstrated in the early 1990s, these findings have been replicated more than 40 times with minimal failures to replicate [137], making reduced MMN one of the most robust neurophysiological findings in schizophrenia.
3.2.1. MMN generators
Primary generators for MMN are localized to auditory cortex based upon intracranial recording, lesion, fMRI, and EEG/MEG source localization studies [3,95,119]. Activity in this region appears to be driven primarily by the sensory mismatch between standard and deviant stimuli, as it is heavily influenced by the degree of stimulus deviance in both intracranial and fMRI studies [119]. In addition, later frontal activity in observed within ventrolateral frontal cortex [35,119], which may reflect successful attentional capture [113,119]. However, given the long latency to activation and lateral location of the frontal generator, the degree to which it contributes to MMN activity recorded at mid-frontal electrodes during standard MMN epoch windows remains to be determined. In schizophrenia, deficits in MMN generation are observed both in MEG, which is insensitive to the putative frontal generator [61,108,129] and in fMRI, which shows activation deficits specifically within auditory cortex in schizophrenia [86]. Thus, temporal regions represent well-validated targets for study in animal models of schizophrenia.
Several pharmacological studies in humans indicate that impaired NMDA function may underlie reduced MMN generation in schizophrenia. Of four ketamine challenge studies that evaluated ketamine effects on MMN generation or its MEG equivalent, three showed significant ketamine effects [39,62,103,133]. In addition, MMN predicted the degree of psychotic response elicited in normal volunteers [134]. Treatment with N-acetyl-cysteine, a precursor of glutathione and therefore an indirect modulator of NMDA function, increases MMN in patients with schizophrenia [71]. In contrast, high doses of glycine, a substance that enhances NMDA receptor functioning via binding to the glycine modulatory site on the NMDA receptor, decreases MMN [75]. As opposed to NMDA antagonists, deficits in MMN generation are not induced by either D1 or D2 antagonists, or by psilocybin, a psychotomimetic that targets primarily serotonin 5-HT2A receptors [53].
3.2.2. Animal models
Given the importance of MMN for schizophrenia as well as auditory attention research in general, many efforts have been made to investigate mechanisms underlying normal and pathological MMN generation in animal models (Table 1). Shortly following the first demonstration of reduced MMN in schizophrenia, MMN was demonstrated in macaques using reduced-intensity deviants coupled with both epidural and intracranial recordings within the auditory cortex [45,47,46]. Furthermore, MMN-like activity was also reported in both cat [23,110,111] and guinea pig [60] model systems. In line with human studies, intracranial recordings in animals indicate that primary MMN sources are located in auditory cortex, but are separate from the generators of response to repetitive standards, such as the P1–N1 potentials. For example, epidural grid recordings in cats showed maximal MMN amplitude in a rostro-ventral regions of secondary auditory cortex, separate from the more caudal maximal amplitudes of the P1 and N1 potentials [110]. Intra-cortical recordings in macaques localized MMN generation to a source in superficial layers, whereas auditory N1 appeared to reflect activity primarily within deeper cortical laminae [47].
Table 1.
This table lists the human ketamine challenge MMN studies and the animal MMN studies cited in this paper.
| Reference | Method | NMDA antagonist | Drug effect on MMN | Comment | |
|---|---|---|---|---|---|
| Human | Heekeren et al. [39] | EEG | Ketamine | ↓ | – |
| Human | Kreitschmann-Andermahr et al. [62] | MEG | Ketamine | ↓ | – |
| Human | Oranje et al. [103] | EEG | Ketamine | = | – |
| Human | Umbricht et al. [133] | EEG | Ketamine | ↓ | – |
| Cynomolguos monkeys | Javitt et al. [45] | Epidural | – | – | |
| Macaques | Javitt et al. [46] | Intra-cortical | Systemic PCP | ↓ | – |
| Macaques | Javitt et al. [47] | Intra-cortical | Local PCP | ↓ | – |
| Cat | Csepe et al. [23] | Intra-cortical | – | – | |
| Cat | Pincze et al. [110,111] | Epidural | – | – | |
| Guinea pig | Kraus et al. [60] | Epidural/intra-thalamic | – | – | |
| Rat | Astikainen et al. [4] | Epidural, anesthetized | – | Late positivity | |
| Rat | Lazar and Metherate [72] | Epidural, anesthetized | – | No MMN | |
| Rat | Ruusuvirta et al. [115,116] | Epidural, anesthetized | – | Late positivity | |
| Rat | Sambeth et al. [117] | Epidural | – | P3, but no MMN | |
| Rat | Tikhonravov et al. [130] | Epidural, anesthetized | MK-801 | ↓ | Late positivity |
| Mouse | Ehlers and Somes [29] | Epidural, awake | – | – | |
| Mouse | Ehrlichman et al. [30] | Intra-cortical, awake | Ketamine | ↓ | – |
| Mouse | Siegel et al. [125] | Intra-cortical, awake | – | – | |
| Mouse | Umbricht et al. [135] | Epidural, awake | – | – |
Although MMN may be readily modeled in primates, the advent of genetic engineering technology in mice and the common use of rats as animal models has encouraged MMN studies in this species. Indeed, this is an emerging area of research and an increasing body of evidence indicates the feasibility of conducting MMN studies in rodents. For example, Ruusuvirta and coworkers reported MMN-like activity to frequency deviants of positive polarity in urethane-xylazine anesthetized rats between 60 and 190 ms [4,115]. A general concern with regard to intra-cortical assessment of MMN, however, is that true deviance-related activity must be differentiated from cross-refractoriness of deviant and standard stimuli [72].
A potential control condition is use of deviant-only stimuli in the absence of intervening standards vs. the same stimuli presented in an oddball sequence [72]. If a difference is seen even when deviants are compared to rate-matched control stimuli, it is strong presumptive evidence for an MMN effect. Nevertheless, the use of a stimulus rate control may mask true MMN generation, necessitating development of more complex models [116].
In a recent study Tikhonravov et al. also included a control condition consisting of frequency deviants presented at the same presentation rate as the deviants within standards condition. In this study the deviant within standards condition showed enhanced activity compared to both deviants alone, as well as compared to standards. This positivity was observed relatively late and in a short time window between 150 and 180 ms [130]. In awake rats, Sambeth et al. did not find deviance-related activity in an active auditory oddball paradigm in the expected latency range for MMN [117]. Overall, while encouraging, these results suggest the need for further optimization of rat MMN paradigms.
In mice, two studies found a late (>120 ms) deviance-related positivity in passive frequency-deviance paradigms, but no MMN-like activity during earlier time windows [29,125]. Ehrlichman et al. observed enhanced negativity to frequency deviants in the time window of N1 [30]. In a similar mouse study, Umbricht et al. found an additional negative potential to duration deviants 50 ms following onset of stimulus difference. However, they found no MMN to frequency deviants when the deviant presentation rate was controlled for [138]. Thus, similar as in the rat MMN studies, there is ample evidence for the presence of deviance-related activity in mice. However, further studies would welcome to investigate possible confounds by refractoriness issues in MMN studies in rodents.
The role of NMDA receptors has been evaluated in both primate and rodent models. In macaques, intra-cortical infusion as well as systemic application of PCP reduced MMN, while amplitude of the preceding P1 and N1 potentials was not affected [47]. In rats, the NMDA receptor antagonist MK-801 reduced the enhanced positivity to deviants compared to standards [130]. In mice, ketamine reduced the observed deviant-related late positivity as well as the enhanced negativity in the time window of N1 [30,125]. Interestingly, in the more recent of these studies [30] ketamine significantly increased the amplitude of the N1 to standards while N1 amplitudes to deviants remained unchanged. Thus, alteration in response to standard stimuli may contribute as well to the reported NMDA-induced changes in deviance-related activity in rodents.
3.3. Auditory P1/N1
As opposed to MMN, which reflects a comparison between stimuli, auditory P1 and N1 potentials are elicited even by repetitive standards. P1 refers to a frontal positivity that occurs approximately 50 ms following stimulus presentation, whereas N1 refers to a frontal negativity that occurs at approximately 100 ms. Source localization studies in humans using high density EEG and MEG [12,42,105,112], as well as intracranial recordings [36,77] have localized the main contributors to the auditory P1 and N1 components to auditory cortex and surrounding regions of the superior temporal plane. Although P1 and N1 components are usually discussed in the EEG literature as distinct entities, in surface recordings these potentials reflect sequential as well as overlapping activation within these brain areas [36,83,96,109,144].
Although primary generators for P1 and N1 are within auditory brain regions, some studies have also observed frontal contributions [34,109]. However, sources outside the auditory cortex seem to be mainly modulated by different task requirements in active paradigms [109]. Temporal, but not frontal cortical lesions abolish generation of mid- and late-auditory event-related potentials (AEPs) [59,118], supporting the primary role of auditory cortex in N1 generation
3.3.1. Peak refractoriness
A characteristic feature of the human auditory P1 and N1 is their recovery function, describing the exponential increase of peak amplitude with increasing ISI [97]. The refractoriness period is thought to reflect the operation of an inhibitory trace on the level of the auditory cortex [15]. Indeed, the slope of the human N1 recovery function predicts the behavioral lifetime of the memory for loudness [82,81]. Thus, the recovery function may provide a neuro-physiological index for the decay of the auditory sensory memory trace. However, refractoriness seems to differ between P1 and N1. The P1 shows a relatively rapid recovery function; it reaches half-maximal amplitude at interstimulus intervals (ISIs) of about 450 ms and near maximal amplitude at an ISI of 2 s in most, but not all studies [19,31,136,143]. The N1, in contrast, does not reach half-maximal amplitude until an ISI of about 2 s, followed by a gradual rise to the plateau at an ISI of approximately 8 s [89,97]. Best fitting results for both the P1 and N1 recovery curves are usually obtained using an exponential function, with substantially longer time constants for the N1, than P1, potential [81,136].
Abnormal recovery curves for both P1 and N1 have been reported in schizophrenia [32,124]. For example, Erwin et al. showed decreased P1 amplitudes in patients at ISIs exceeding 1 s, but not at shorter repetitions [31]. This reduction was most pronounced in neuroleptics naïve patients [32]. However, currently only few studies are available that explicitly investigated the effects of ISI on P1 amplitudes in repetitive stimulation paradigms in schizophrenia. Far more studies used paired stimulus paradigms, in which pairs of clicks with a short ISI (usually 500 ms) are presented with a long inter-trial interval of several seconds. Compared to healthy controls, in the majority of studies patients show smaller P1 responses to the first, but enhanced responses to the second click [107]. More studies are available investigating N1 amplitudes in schizophrenia. The majority of studies observed reduced N1 amplitudes in patients to tones with ISIs above 1 s and preserved amplitudes to shorter ISIs [114,124]. Furthermore, in patients reduced auditory N1 amplitudes were observed independent of medication status as well as in their first degree relatives. Thus, N1 amplitudes are currently discussed as a possible endophenotype of schizophrenia [131].
Few studies are available investigating effects of the NMDA receptor antagonist ketamine on P1 and N1 amplitude in healthy subjects. Only one study specifically looked at the effect of ISI on N1 amplitudes and found a trend to a similar N1 recovery profile with ketamine as in patients, thus, reduced amplitudes to long but not short ISIs [135]. In the context of MMN studies, the N1 amplitude to standards were either unchanged at 1–2 s ISI, or increased to very short ISI of 300 ms [62,103,133]. Although no P1 amplitudes were reported in these studies, there are two reports investigating ketamine effects in a paired stimulus paradigm. Similar to findings in schizophrenia, one showed reduced P1 amplitudes with ketamine in response to the first click (particularly in combination with haloperidol) the other observed no effect [104,139]. Although, the authors from the latter study state that the missing effect on P1 may have been due to the small dose of ketamine used. Thus, from studies in humans there is modest but ample evidence that NMDA receptor block may induce a schizophrenia-like peak recovery pattern.
3.3.2. Animal models
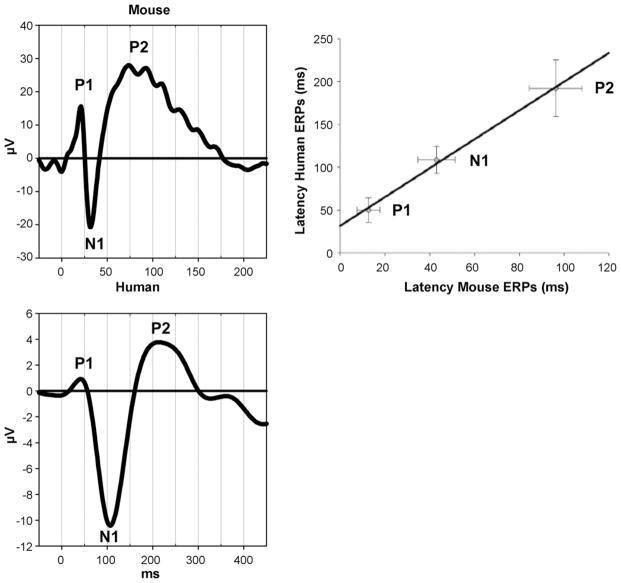
Morphologically, the waveforms of AEPs obtained in animal and human surface recordings share a similar sequence of positive and negative components across species. Namely, this remarkably stereotyped sequence of a fast biphasic positive–negative deflection (P1 and N1) followed by a slow positive component (P2) is observed in non-human primates, cats, guinea pigs, rats, and mice (Fig. 3) [27,45,60,110,117,122,136]. The main differences between species are the absolute peak latencies, most likely due to mere differences in brain sizes. For example, the components in mice and rats occur with 30–40% latency to corresponding human AEP peaks [11,27,117,125,136], whereas in monkeys they occur with approximately 70% latency. Thus, P1, N1 and P2 in rodents are observed at approximately 20, 40, and 80 ms respectively; whereas in macaques that are observed at approximately 30, 70 and 120 ms [48].
Fig. 3.
Comparison of auditory evoked potentials in humans and mice. A similar sequence of components is observed in the waveforms in humans (electrode Fz) as in mice (surface electrode over auditory cortex). The components differ in absolute latencies between species. Peaks in mice occur earlier with a ratio of approximately 0.4 compared to humans (regression from [136]).
In animal models, both the generators and temporal sensitivities of P1 and N1 appear to be similar to those of humans. For example, rats show a biphasic P1–N1 complex over primary auditory cortex, along with a monophasic positive component over secondary auditory cortex, in line with human ERP source localization studies showing contributions of generators within auditory cortex to surface P1/N1 [27]. Further, as in humans, the degree to which different generators contribute to recorded activity depends upon the location of the recording electrode relative to the generator locations. In both humans and animals, P1 and N1 invert from surface to deep cortical layers when recorded using a linear multicontact electrode [9,127].
This method also allows identification computation of current source density (CSD), which serves as an index of transmembrane current flow within the different cortical layers. The laminar CSD pattern helps identifying neural populations most likely contributing to the summed surface AEP [91]. In both rats and macaques, CSD studies have localized the P1 generators to middle and superficial layers of auditory cortex, suggesting that it reflects the initial activation of the auditory cortex by inputs from the medial geniculate body of the thalamus and subsequent supragranular activation [9,46,64]. This initial activation is followed by an activity in the supragranular and infragranular layers that coincides with generation of the surface N1 potential.
In both rodents and primates, the recovery function of P1 and N1 also closely models the recovery function of human P1/N1, although some between-species difference may exist. In three mouse studies the N1 (at approximately 35 ms) showed a comparable exponential peak recovery as observed in humans [10,87,136]. Indeed, best curve fitting results for the mouse N1 recovery were obtained with the same exponential function as used in humans [136]. However, the slope of the recovery curve indicated a faster rise to plateau for the N1 in mice than has typically been observed in humans, which the authors interpreted as reflecting a faster decay of the auditory sensory memory trace in mice. In the two studies conducted by Umbricht and coworkers, the mouse P1 showed a similar refractoriness profile to the N1, whereas no ISI effect on P1 amplitude was observed by Maxwell et al. [87]. However, in this study P1 amplitudes were close to noise level. Thus, the unfavorable signal to noise ratio could have potentially prevented detection of an ISI effect. Altogether, these findings suggest that P1 refractoriness functions may be less well preserved in rodents, and reflect the complexity of modeling human ERP in rodent models.
Effects of NMDA antagonists have also been explored in rodents. For example, in rats acute pharmacological NMDA receptor block reduced P1 and N1 amplitudes to tones presented at ISIs of 1 and 2.5 s, although no ketamine effects were observed in an alternative study that used clicks presented at 9 s ISI [25,26,28]. In mice, acute ketamine application decreased N1 amplitude in most strains at 9 s ISIs in a paired click paradigm, but P1 amplitudes tended to be increased [21,88]. Thus, although still a matter of investigation, NMDA antagonists tend to reduce N1 amplitudes at long ISIs in rodent studies.
In mice the effect of reduced NMDA receptor signaling on peak recovery curves has also been investigated in a genetic model. NR1 hypomorphic mice express only 5–10% of normal levels of the NMDA receptor subunit NR1 subunit, a subunit crucial for functioning of the receptor [94]. Against expectation, the NR1 mutants showed increased P1 and N1 amplitudes to relatively short ISIs, but no differences at long ISIs (4 sec) compared to their wild type littermates [10]. Thus, patterns of activity observed in the hypomorph diverge from those observed following acute ketamine administration. Further studies are needed to determine the basis for the differential results.
In monkeys, similar recovery functions to humans are observed. In one epidural study P1 increased steeply at ISIs of between 150 and 450 ms, followed by a gradual rise to plateau. N1 also showed a non-linear increase, but did not reach maximal amplitude until 4.5 s ISI [48]. Following systemic application of the NMDA receptor antagonist PCP, the refractoriness curves modeled the peak recovery behavior observed in schizophrenia. Both peaks showed the same initial steep increase as without the drug, but maximal amplitudes were significantly reduced. Unfortunately, to our knowledge, this is the only published study investigating AEP refractoriness in non-human primates. Thus, further studies are warranted to reproduce this finding.
3.4. Frequency domain analysis
Traditionally, as in the studies discussed above, neurophysiological activity has been measured within the “time domain”, in which ERP components are characterized by both their amplitude and their latency following stimulus presentation. More recent approaches, further evaluate activity within the “frequency domain,” in which activity is analyzed separately within discrete frequency bands (e.g. gamma, theta, delta) [84,121]. While more complex, frequency domain analyses potentially provide greater information regarding underlying brain processes [53]. Different frequency bands may also be hierarchically organized [64], permitting dissection of inner cortical organization. Indeed, several studies showed that stimulus related phase alignment of neural oscillations underlie at least partially the generation of ERPs [121]. Currently, frequency domain analysis is one of the most active fields in neurophysiological schizophrenia research [53,132]. Animal studies will hopefully help elucidating mechanisms underlying alterations in neural synchrony in schizophrenia.
4. Conclusions
Overall, there is a pressing need for animal models, especially involving rodents, that can reproduce objective neurochemical and neurophysiological aspects of schizophrenia. Whereas rodents will never be able to express all aspects of schizophrenia, nevertheless we have been able to identify multiple phenomena in rodents that may reproduce critical features of schizophrenia. Most importantly, these measures show sensitivity to NMDA antagonists in both humans and animals, and have animal homologues closely resembling the human situation. In the case of neurochemistry, complementary techniques are used for human/primate vs. rodent investigation, but nevertheless the underlying phenomenon – increased striatal dopamine release – appears to be preserved. In the case of neurophysiology, the same surface recording techniques can be applied to both humans and animals, although more invasive approaches are available for animal studies as well. Invasive recording permits the use of animal models to investigate circuit- and receptor-level mechanisms underlying disturbed ERP generation in schizophrenia. Although human-like ERP are well described in monkeys, adapting these procedures to rodents, and especially mice, remains at an early stage. Nevertheless, the increasing availability of genetically engineered mouse strains provides unparalleled opportunity for development of increasingly refined animal models of schizophrenia. Development of improved recording and stimulation paradigms will permit animal modelers to take full advantage of advances in genetic and pharmacological approaches to schizophrenia as they become available.
Footnotes
Supported by grants R01 DA03383 and R37 MH49334 to DCJ and a grant from the Swiss National Science Foundation to SB.
Conflict of interest
Dr. Javitt-intellectual property rights for use of NMDA agonists and glycine transport inhibitors in treatment of schizophrenia. Dr. Bickel-none.
References
- 1.Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55:1001–6. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 1998;31(Suppl 2):104–9. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- 3.Alain C, Woods DL, Knight RT. A distributed cortical network for auditory sensory memory in humans. Brain Res. 1998;812:23–37. doi: 10.1016/s0006-8993(98)00851-8. [DOI] [PubMed] [Google Scholar]
- 4.Astikainen P, Ruusuvirta T, Wikgren J, Penttonen M. Memory-based detection of rare sound feature combinations in anesthetized rats. Neuroreport. 2006;17:1561–4. doi: 10.1097/01.wnr.0000233097.13032.7d. [DOI] [PubMed] [Google Scholar]
- 5.Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–64. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- 6.Balla A, Hashim A, Burch S, Javitt DC, Lajtha A, Sershen H. Phencyclidine-induced dysregulation of dopamine response to amphetamine in prefrontal cortex and striatum. Neurochem Res. 2001;26:1001–6. doi: 10.1023/a:1012396820510. [DOI] [PubMed] [Google Scholar]
- 7.Balla A, Koneru R, Smiley J, Sershen H, Javitt DC. Continuous phencyclidine treatment induces schizophrenia-like hyperreactivity of striatal dopamine release. Neuropsychopharmacology. 2001;25:157–64. doi: 10.1016/S0893-133X(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 8.Balla A, Sershen H, Serra M, Koneru R, Javitt DC. Subchronic continuous phencyclidine administration potentiates amphetamine-induced frontal cortex dopamine release. Neuropsychopharmacology. 2003;28:34–44. doi: 10.1038/sj.npp.1300019. [DOI] [PubMed] [Google Scholar]
- 9.Barth DS, Di S. Three-dimensional analysis of auditory-evoked potentials in rat neocortex. J Neurophysiol. 1990;64:1527–36. doi: 10.1152/jn.1990.64.5.1527. [DOI] [PubMed] [Google Scholar]
- 10.Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301536. [DOI] [PubMed] [Google Scholar]
- 11.Bickel S, Lipp HP, Umbricht D. Impaired attentional modulation of auditory evoked potentials in N-methyl-D-aspartate NR1 hypomorphic mice. Genes Brain Behav. 2007;6:558–68. doi: 10.1111/j.1601-183X.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 12.Borgmann C, Ross B, Draganova R, Pantev C. Human auditory middle latency responses: influence of stimulus type and intensity. Hear Res. 2001;158:57–64. doi: 10.1016/s0378-5955(01)00292-1. [DOI] [PubMed] [Google Scholar]
- 13.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, et al. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29:142–7. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Budd TW, Barry RJ, Gordon E, Rennie C, Michie PT. Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int J Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 16.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–7. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2006 doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 19.Cardenas VA, McCallin K, Hopkins R, Fein G. A comparison of the repetitive click and conditioning-testing P50 paradigms. Electroencephalogr Clin Neurophysiol. 1997;104:157–64. doi: 10.1016/s0168-5597(97)96093-7. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–86. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 21.Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, et al. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–88. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- 22.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harvard Rev Psychiatry. 1996;3:241–53. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 23.Csepe V, Karmos G, Molnar M. Effects of signal probability on sensory evoked potentials in cats. Int J Neurosci. 1987;33:61–71. doi: 10.3109/00207458708985929. [DOI] [PubMed] [Google Scholar]
- 24.Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction (Abingdon, England) 2001;96:749–60. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- 25.Dafny N, Rigor BM. Dose effects of ketamine on photic and acoustic field potentials. Neuropharmacology. 1978;17:851–62. doi: 10.1016/0028-3908(78)90073-4. [DOI] [PubMed] [Google Scholar]
- 26.de Bruin NM, Ellenbroek BA, Cools AR, Coenen AM, van Luijtelaar EL. Differential effects of ketamine on gating of auditory evoked potentials and prepulse inhibition in rats. Psychopharmacology (Berl) 1999;142:9–17. doi: 10.1007/s002130050856. [DOI] [PubMed] [Google Scholar]
- 27.Di S, Barth DS. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol. 1992;68:425–31. doi: 10.1152/jn.1992.68.2.425. [DOI] [PubMed] [Google Scholar]
- 28.Ehlers CL, Kaneko WM, Wall TL, Chaplin RI. Effects of dizocilpine (MK-801) and ethanol on the EEG and event-related potentials (ERPS) in rats. Neuropharmacology. 1992;31:369–78. doi: 10.1016/0028-3908(92)90069-2. [DOI] [PubMed] [Google Scholar]
- 29.Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–28. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–14. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- 31.Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE. Midlatency auditory evoked responses in schizophrenia. Biol Psychiatry. 1991;30:430–42. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- 32.Erwin RJ, Shtasel D, Gur RE. Effects of medication history on midlatency auditory evoked responses in schizophrenia. Schizophr Res. 1994;11:251–8. doi: 10.1016/0920-9964(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 33.Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention—focusing the searchlight on sound. Curr Opin Neurobiol. 2007;17:437–55. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Gallinat J, Mulert C, Bajbouj M, Herrmann WM, Schunter J, Senkowski D, et al. Frontal and temporal dysfunction of auditory stimulus processing in schizophrenia. Neuroimage. 2002;17:110–27. doi: 10.1006/nimg.2002.1213. [DOI] [PubMed] [Google Scholar]
- 35.Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–40. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 36.Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112:1850–9. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–61. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 39.Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- 41.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, et al. Combined mapping of human auditory EEG and MEG responses. Electroencephalogr Clin Neurophysiol. 1998;108:370–9. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 43.Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- 44.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 45.Javitt DC, Schroeder CE, Steinschneider M, Arezzo JC, Vaughan HG., Jr Demonstration of mismatch negativity in the monkey. Electroencephalogr Clin Neurophysiol. 1992;83:87–90. doi: 10.1016/0013-4694(92)90137-7. [DOI] [PubMed] [Google Scholar]
- 46.Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 47.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci USA. 1996;93:11962–7. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Javitt DC, Jayachandra M, Lindsley RW, Specht CM, Schroeder CE. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP) Clin Neurophysiol. 2000;111:833–6. doi: 10.1016/s1388-2457(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 49.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–7. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 50.Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–7. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- 51.Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–56. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 52.Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19:151–7. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- 53.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–61. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 56.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–40. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 57.Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O, et al. NMDA antagonist effects on striatal dopamine release: positron emission tomography studies in humans. Synapse. 2002;43:19–29. doi: 10.1002/syn.10010. [DOI] [PubMed] [Google Scholar]
- 58.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–15. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal and temporal-parietal lesions on the auditory evoked potential in man. Electroencephalogr Clin Neurophysiol. 1980;50:112–24. doi: 10.1016/0013-4694(80)90328-4. [DOI] [PubMed] [Google Scholar]
- 60.Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change. J Neurophysiol. 1994;72:1270–7. doi: 10.1152/jn.1994.72.3.1270. [DOI] [PubMed] [Google Scholar]
- 61.Kreitschmann-Andermahr I, Rosburg T, Meier T, Volz HP, Nowak H, Sauer H. Impaired sensory processing in male patients with schizophrenia: a magnetoencephalographic study of auditory mismatch detection. Schizophr Res. 1999;35:121–9. doi: 10.1016/s0920-9964(98)00115-7. [DOI] [PubMed] [Google Scholar]
- 62.Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–16. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- 63.Kujala T, Tervaniemi M, Schroger E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol. 2007;74:1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–11. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 65.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 67.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–21. [PubMed] [Google Scholar]
- 68.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 69.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D’Souza DC, Krystal J, et al. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatry. 2000;47:371–9. doi: 10.1016/s0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 71.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 72.Lazar R, Metherate R. Spectral interactions, but no mismatch negativity, in auditory cortex of anesthetized rat. Hear Res. 2003;181:51–6. doi: 10.1016/s0378-5955(03)00166-7. [DOI] [PubMed] [Google Scholar]
- 73.Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62:739–46. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 75.Leung S, Croft RJ, O’Neill BV, Nathan PJ. Acute high-dose glycine attenuates mismatch negativity (MMN) in healthy human controls. Psychopharmacology (Berl) 2008;196:451–60. doi: 10.1007/s00213-007-0976-8. [DOI] [PubMed] [Google Scholar]
- 76.Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, et al. Subunit-selective modulation of GABA type a receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92:204–14. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 78.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 79.Linn GS, O’Keeffe RT, Lifshitz K, Schroeder C, Javitt DC. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology. 2007;192:27–38. doi: 10.1007/s00213-007-0771-6. [DOI] [PubMed] [Google Scholar]
- 80.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B: Biol Sci. 2002;357:1003–37. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu ZL, Williamson SJ, Kaufman L. Human auditory primary and association cortex have differing lifetimes for activation traces. Brain Res. 1992;572:236–41. doi: 10.1016/0006-8993(92)90475-o. [DOI] [PubMed] [Google Scholar]
- 82.Lu ZL, Williamson SJ, Kaufman L. Behavioral lifetime of human auditory sensory memory predicted by physiological measures. Science. 1992;258:1668–70. doi: 10.1126/science.1455246. [DOI] [PubMed] [Google Scholar]
- 83.Lutkenhoner B, Steinstrater O. High-precision neuromagnetic study of the functional organization of the human auditory cortex. Audiol Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- 84.Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–4. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 85.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II. Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 86.Mathiak K, Rapp A, Kircher TT, Grodd W, Hertrich I, Weiskopf N, et al. Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole-head magnetoencephalography. Hum Brain Mapp. 2002;16:190–5. doi: 10.1002/hbm.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, et al. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–46. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- 88.Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–24. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- 89.McEvoy L, Levanen S, Loveless N. Temporal characteristics of auditory sensory memory: neuromagnetic evidence. Psychophysiology. 1997;34:308–16. doi: 10.1111/j.1469-8986.1997.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 90.Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- 91.Mitzdorf U. Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int J Neurosci. 1987;33:33–59. doi: 10.3109/00207458708985928. [DOI] [PubMed] [Google Scholar]
- 92.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neuro-transmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the pre-frontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 94.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 95.Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15:545–51. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- 96.Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 97.Naatanen R. Attention and brain function. NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 98.Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118:2544–90. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 99.Naatanen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2008:1–11. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 100.Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, et al. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry. 2005;162:2352–9. doi: 10.1176/appi.ajp.162.12.2352. [DOI] [PubMed] [Google Scholar]
- 101.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery. Part 1. Test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 102.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 103.Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 104.Oranje B, Gispen-de Wied CC, Verbaten MN, Kahn RS. Modulating sensory gating in healthy volunteers: the effects of ketamine and haloperidol. Biol Psychiatry. 2002;52:887–95. doi: 10.1016/s0006-3223(02)01377-x. [DOI] [PubMed] [Google Scholar]
- 105.Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, et al. Specific tonotopic organizations of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. Electroencephalogr Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
- 106.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–7. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 107.Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, et al. P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res. 2008;158:226–47. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Pekkonen E, Katila H, Ahveninen J, Karhu J, Huotilainen M, Tiihonen J. Impaired temporal lobe processing of preattentive auditory discrimination in schizophrenia. Schizophr Bull. 2002;28:467–74. doi: 10.1093/oxfordjournals.schbul.a006954. [DOI] [PubMed] [Google Scholar]
- 109.Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, et al. Intracerebral sources of human auditory-evoked potentials. Audiol Neurootol. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- 110.Pincze Z, Lakatos P, Rajkai C, Ulbert I, Karmos G. Separation of mismatch negativity and the N1 wave in the auditory cortex of the cat: a topographic study. Clin Neurophysiol. 2001;112:778–84. doi: 10.1016/s1388-2457(01)00509-0. [DOI] [PubMed] [Google Scholar]
- 111.Pincze Z, Lakatos P, Rajkai C, Ulbert I, Karmos G. Effect of deviant probability and interstimulus/interdeviant interval on the auditory N1 and mismatch negativity in the cat auditory cortex. Brain Res. 2002;13:249–53. doi: 10.1016/s0926-6410(01)00105-7. [DOI] [PubMed] [Google Scholar]
- 112.Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 ms latency auditory evoked field component. Electroencephalogr Clin Neuro-physiol. 1988;70:490–8. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- 113.Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Naatanen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–9. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- 114.Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia—a critical review. Psychiatry Res. 2008 doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 115.Ruusuvirta T, Penttonen M, Korhonen T. Auditory cortical event-related potentials to pitch deviances in rats. Neurosci Lett. 1998;248:45–8. doi: 10.1016/s0304-3940(98)00330-9. [DOI] [PubMed] [Google Scholar]
- 116.Ruusuvirta T, Koivisto K, Wikgren J, Astikainen P. Processing of melodic contours in urethane-anaesthetized rats. Eur J Neurosci. 2007;26:701–3. doi: 10.1111/j.1460-9568.2007.05687.x. [DOI] [PubMed] [Google Scholar]
- 117.Sambeth A, Maes JH, van LG, Molenkamp IB, Jongsma ML, Van Rijn CM. Auditory event-related potentials in humans and rats: effects of task manipulation. Psychophysiology. 2003;40:60–8. doi: 10.1111/1469-8986.00007. [DOI] [PubMed] [Google Scholar]
- 118.Scherg M, von CD. Psychoacoustic and electrophysiologic correlates of central hearing disorders in man. Eur Arch Psychiatry Neurol Sci. 1986;236:56–60. doi: 10.1007/BF00641060. [DOI] [PubMed] [Google Scholar]
- 119.Schonwiesner M, Novitski N, Pakarinen S, Carlson S, Tervaniemi M, Naatanen R. Heschl’s gyrus, posterior superior temporal gyrus, and mid-ventrolateral prefrontal cortex have different roles in the detection of acoustic changes. J Neurophysiol. 2007;97:2075–82. doi: 10.1152/jn.01083.2006. [DOI] [PubMed] [Google Scholar]
- 120.Sershen H, Balla A, Aspromonte JM, Xie S, Cooper TB, Javitt DC. Characterization of interactions between phencyclidine and amphetamine in rodent prefrontal cortex and striatum: Implications in NMDA/glycine-site-mediated dopaminergic dysregulation and dopamine transporter function. Neurochem Int. 2008;52:119–29. doi: 10.1016/j.neuint.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 121.Shah AS, Bressler SL, Knuth KH, Ding M, Mehta AD, Ulbert I, et al. Neural dynamics and the fundamental mechanisms of event-related brain potentials. Cereb Cortex. 2004;14:476–83. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- 122.Shaw NA. The auditory evoked potential in the rat—a review. Prog Neurobiol. 1988;31:19–45. doi: 10.1016/0301-0082(88)90021-4. [DOI] [PubMed] [Google Scholar]
- 123.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–62. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 124.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 125.Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–82. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- 126.Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13:821–7. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- 127.Steinschneider M, Arezzo J, Vaughan HG., Jr Phase-locked cortical responses to a human speech sound and low-frequency tones in the monkey. Brain Res. 1980;198:75–84. doi: 10.1016/0006-8993(80)90345-5. [DOI] [PubMed] [Google Scholar]
- 128.Tamminga CA, Holcomb HH, Gao X, Lahti AC. Glutamate pharmacology and the treatment of schizophrenia: current status and future directions. Int Clin Psychopharmacol. 1995;10(Suppl 3):29–37. [PubMed] [Google Scholar]
- 129.Thonnessen H, Zvyagintsev M, Harke KC, Boers F, Dammers J, Norra C, et al. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. A combined EEG and MEG study. Biol Psychol. 2008;77:205–16. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 130.Tikhonravov D, Neuvonen T, Pertovaara A, Savioja K, Ruusuvirta T, Naatanen R, et al. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Res. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, et al. Abnormal auditory n100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64:1051–9. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the patho-physiology of schizophrenia. Schizophr Bull. 2008;34:927–43. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–47. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 134.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–6. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 135.Umbricht D, Koller R, Bieber K. Ketamine-induced deficits in encoding in healthy volunteers: comparison to corresponding deficits in schizophrenia. Schizophr Res. 2004;67:21. [Google Scholar]
- 136.Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D’Adamo P, et al. Midlatency auditory event-related potentials in mice: comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- 137.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin Neurophysiol. 2005;116:353–63. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 139.van Berckel BN, Oranje B, van Ree JM, Verbaten MN, Kahn RS. The effects of low dose ketamine on sensory gating, neuroendocrine secretion and behavior in healthy human subjects. Psychopharmacology (Berl) 1998;137:271–81. doi: 10.1007/s002130050620. [DOI] [PubMed] [Google Scholar]
- 140.van Berckel BN, Kegeles LS, Waterhouse R, Guo N, Hwang DR, Huang Y, et al. Modulation of amphetamine-induced dopamine release by group II metabotropic glutamate receptor agonist LY354740 in non-human primates studied with positron emission tomography. Neuropsychopharmacology. 2006;31:967–77. doi: 10.1038/sj.npp.1300902. [DOI] [PubMed] [Google Scholar]
- 141.Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther. 2005;27(Suppl A):S8–15. doi: 10.1016/j.clinthera.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 142.Winkler I, Karmos G, Naatanen R. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Res. 1996;742:239–52. doi: 10.1016/s0006-8993(96)01008-6. [DOI] [PubMed] [Google Scholar]
- 143.Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response. Biol Psychiatry. 1992;32:839–41. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]
- 144.Zouridakis G, Simos PG, Papanicolaou AC. Multiple bilaterally asymmetric cortical sources account for the auditory N1m component. Brain Topogr. 1998;10:183–9. doi: 10.1023/a:1022246825461. [DOI] [PubMed] [Google Scholar]