Abstract
Kawasaki disease (KD) is an acute, self-limited vasculitis that occurs in young children and was first described by Japanese pediatrician Tomisaku Kawasaki in 1967. Although originally thought to be a rare condition, KD has become the most common cause of acquired heart disease in the pediatric population in developed countries. The majority of patients with KD appear to have a benign prognosis, but a subset of patients with coronary artery aneurysms are at risk for ischemic events and require lifelong treatment. In the 4 decades since the initial recognition of KD, the number of patients reaching adulthood has continued to grow. Adult cardiologists will be increasingly involved in the management of these patients. Currently, there are no established guidelines for the evaluation and treatment of adult patients who have had KD. We report 4 most probable cases of KD missed in childhood and presented as acute coronary syndrome in adulthood.
Keywords: Kawasaki, Coronary aneurysm
1. Case 1
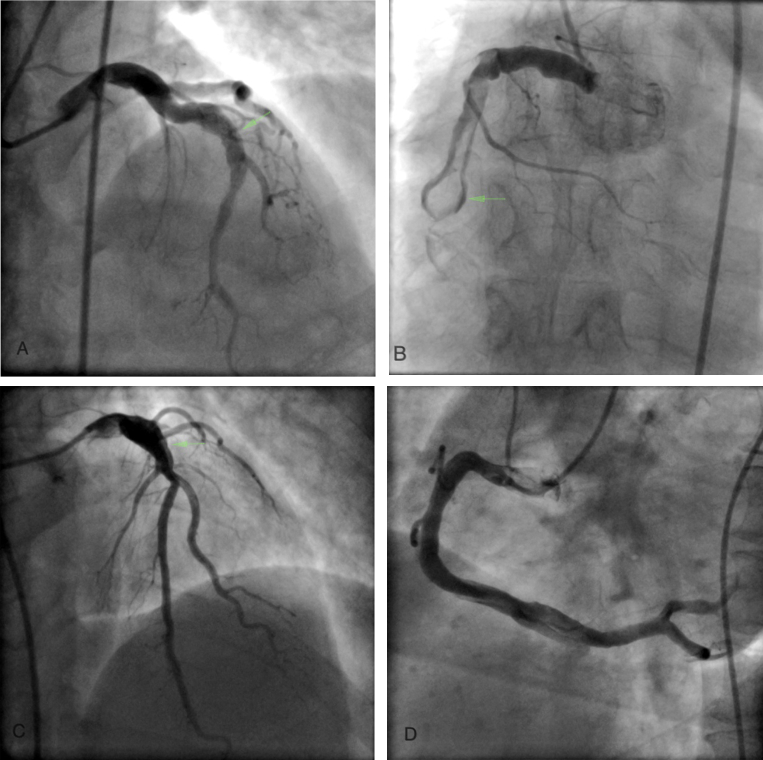
A 35 y male presented with post infarction angina after being thrombolysed for inferior wall infarction. He had no modifiable risk factors for coronary artery disease (CAD) viz. hypertension, diabetes, tobacco abuse, smoking or dyslipidemia. His coronary angiogram revealed presence of a markedly ectatic (6 mm) proximal LAD with sudden transition to a normal appearing distal segment (Fig. 1A). The right coronary artery (RCA) was severely ectatic (Fig. 1B) with presence of thrombus. An aneurysm was clearly visible in mid RCA as it was outlined by the passage of contrast around the thrombus (Fig. 1B, arrow). He has been on oral anticoagulants for last 2 years.
Fig. 1.
Panel A shows aneurysmal proximal LAD (arrow), Panel B shows RCA thrombus surrounded by contrast in the aneurysm (arrow), Panel C shows severely ectatic proximal LAD with sudden transition to normal, Panel D shows diffuse ectasia of RCA.
2. Case 2
A 40 y male with no risk factors for CAD presented with effort angina. His coronary angiogram revealed presence of a markedly ectatic proximal LAD (6 mm) followed by sudden transition to a normal segment. (Fig. 1C). The RCA also showed marked ectasia (Fig. 1D). There were no obstructive lesions in his coronary tree. Echo showed normal LV systolic function. While on a statin and antiplatelet agents, he died suddenly in a grocery store 1 year after the angiogram.
3. Case 3
A 30 y male with mild hypertension presented with class III dyspnea on effort. ECG revealed nonspecific “T” wave abnormality in anterior chest leads. Echocardiogram showed mildly hypokinetic anterior wall with normal LV systolic function. Coronary angiography revealed presence of marked ectasia of proximal LAD with sudden transition to normal sized segment (Fig. 2A). The circumflex and RCA were normal.
Fig. 2.
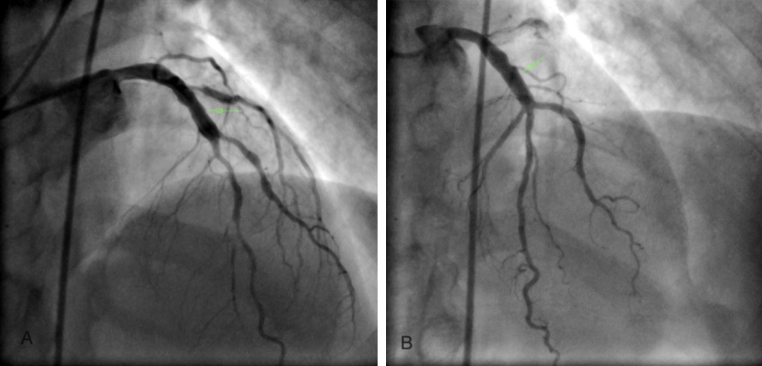
Panels A and B show ectatic proximal segments of LAD with sudden transition to normal segment.
4. Case 4
A 38 y male without any risk factors for CAD presented with effort angina and a positive treadmill test. His coronary angiogram revealed presence of proximal ectasia of LAD (Fig. 2B) with sudden transition to normal segment.
Kawasaki disease (KD) is an acute vasculitis of unknown etiology that predominantly affects children less than 5 years of age.1 Limited data on follow-up of children with KD has revealed that a subset of these patients go on to develop permanent changes in coronary arteries and less commonly in cardiac valves and the myocardium resulting in clinical sequelae in adulthood. In a series of 74 adult patients with coronary artery disease attributed to KD by Burns et al2 the mean age at presentation was 24.7 ± 8.4 years with male preponderance (77%). More than half of the patients presented with chest pain due to myocardial infarction. Ten patients (13.5%) presented with symptoms related to decreased cardiac output and arrhythmia. The event was triggered by vigorous exercise in the majority (82%) patients. Six patients (8.1%) were asymptomatic and were identified during work-up of abnormal stress test or chest radiograph. Eighteen patients (24%) died. Thirteen (72.2%) of 18 patients died during strenuous physical exercise. Aneurysm or marked ectasia of at least one coronary artery was noted at autopsy in all patients. Angiographic findings revealed presence of aneurysms or marked ectasia in 90% of patients and the lesion was located in all three major segments of left coronary artery (Left main, LAD and circumflex). The RCA was involved in 62% patients. Occlusive disease of a coronary artery segment was observed in 66% patients and prominent collateral vessels were seen in approximately 50% patients. Most frequently involved arteries in occlusive disease were LAD (53%) and RCA (61%). Out of 45 patients in who cardiovascular risk factors were specifically mentioned, about 50% had no other risk factor than antecedent KD. These data suggest that the inflammatory insult during acute phase of KD is sufficient to cause clinically significant coronary artery disease in adulthood in the absence of other risk factors.
KD as the cause of coronary artery disease should be suspected when marked ectasia or aneurysm of proximal coronary arteries (particularly LAD and RCA) is seen in relatively younger patients with none or minimal risk factors for atherosclerotic coronary artery disease. Characteristics of the coronary arteries that should prompt questioning about KD include marked proximal ectasia with or without calcification followed by sudden transition to an angiographically normal distal segment. Amongst the four cases described by us, cases 3 and 4 probably represent either transient ectasia during acute phase that did not reverse or partially regressed aneurysmal segments. Proximal ectasia with sudden tapering to a normal segment and absence of significant disease in other areas of coronary tree in young patients without other cardiovascular risk factors makes KD a very strong possibility in these two cases.
The cardiologist specializing in adult patients should be familiar with the signs and symptoms of acute KD to allow questioning of the patient or parent about an antecedent KD-compatible illness that was not diagnosed. Features of the illness that are frequently recalled by patients and parents are prolonged fever, rash, “bloodshot” eyes in acute phase, and peeling of the fingers and toes in the convalescent phase.3 History of “occurrence of measles twice” can be a strong clue to the possibility of KD. Because the etiology remains unclear, there is no specific diagnostic test that can be used to make a retrospective diagnosis.
Imaging studies may be helpful in identifying patients with antecedent KD. Calcification of arterial wall where former aneurysms have remodeled are hallmarks of KD and are seen in 94% of patients whose ectatic segments are at least 6 mm in size.4 Use of T2-weighted magnetic resonance (MR) imaging is emerging as a modality of choice to assess structural damage (myocardial inflammation, scarring & fibrosis) late after KD.5
The management of patients with adult KD must be guided by common sense and an appreciation of uncertainty about the cardiovascular outcomes in adults with antecedent KD. The guidelines published by Japanese Circulation Society for management of adult KD are given in Table 1. The guidelines state that cardiovascular symptoms in KD patients only begin to appear 2 decades after the onset of the acute disease, so only now are patients beginning to present with sequelae. Apart from antiplatelet therapy, use of statins and renin-angiotensin axis blockade may help to promote vascular remodeling. In patients with large aneurysms (>8 mm) with sluggish blood flow, the risk of thrombosis is high and oral anticoagulation with target INR of 2.0–2.5 has been associated with improved survival in small series from Canada & Japan.6,7 Angioplasty for stenotic lesions in KD is likely to give suboptimal results due to calcific nature of lesions and techniques like rotational atherectomy may be required to treat them. There is limited experience of covered and drug eluting stents in KD.8 The surgical risk and graft survival after bypass surgery is similar to patients with atherosclerotic disease.9 Cardiac transplantation has been successfully performed for KD patients with end stage cardiomyopathy, severe ventricular arrhythmias and inoperable multivessel stenotic coronary artery disease. There has been no recurrence of KD or coronary aneurysms in transplanted hearts.10
Table 1.
American Heart Association risk stratification for Kawasaki disease.
| Risk level | Recommendation | |
|---|---|---|
| I (No coronary artery changes at any stage of illness) | Consider noninvasive testing every 3–4 years. No Medical therapy | |
| II (Transient coronary artery ectasia disappears within first 6–8 weeks) | ||
| III (One small to medium coronary artery aneurysm) | Without Symptoms | With Symptoms |
| IV (More than 1 large or giant coronary artery aneurysm or multiple aneurysms in the same coronary artery without stenosis) | Evaluate every 4–6 months with noninvasive testing and angiography every 2–3 years. Treat with low dose aspirin | Evaluate every 3–4 months with noninvasive testing and angiography as needed. Treat with low dose aspirin; other medications as dictated by the cardiovascular status |
| V (Coronary artery obstruction) | ||
• Adapted from Newburger JW et al.11
It is likely that in India the diagnosis of KD is frequently missed in the childhood and probably labeled as viral exathematous fever. Moreover, only a few of the diagnosed cases are probably receiving intravenous immunoglobulins due to financial reasons. Therefore an adult cardiologist in India is more likely to see a large number of KD in adults and will have to be prepared to care for them.
Conflicts of interest
The authors have none to declare.
References
- 1.Kawasaki T., Kosaki F., Okawa S. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 2.Burns J.C., Shike H., Gordon J.B. Sequele of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 3.Burns J.C., Glode M.P. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 4.Gordon J.B., Kahn A.M., Burns J.C. When children with Kawasaki disease grow up. J Am Coll Cardiol. 2009;54:1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaichi S., Tsuda E., Fujita H. Acute coronary artery dilation due to Kawasaki disease and subsequent late calcification as detected by electron beam computed tomography. Pediatr Cardiol. 2008;29:568–573. doi: 10.1007/s00246-007-9144-5. [DOI] [PubMed] [Google Scholar]
- 6.Arnold R., Ley S., Ley-Zaporozhan Visualization of coronary arteries in patients after childhood Kawasaki syndrome: value of multidetector CT and MR imaging in comparison to conventional coronary catheterization. Pediatr Radiol. 2007;37:998–1006. doi: 10.1007/s00247-007-0566-2. [DOI] [PubMed] [Google Scholar]
- 7.Sugahara Y., Ishii M., Muta H. Warfarin therapy for giant aneurysm prevents myocardial infarction in Kawasaki disease. Pediatr Cardiol. 2008;29:398–440. doi: 10.1007/s00246-007-9132-9. [DOI] [PubMed] [Google Scholar]
- 8.Kwon H.S., Shin J.I., Choi J.Y. Polytetrafluroethylene-covered stent deployment in the setting of Kawasaki disease. Catheter Cardiovasc Interv. 2007;69:1075–1076. doi: 10.1002/ccd.21002. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura A., Mukohara N., Ozaki N. Two adult cases of coronary artery aneurysms secondary to Kawasaki disease. Thorac Cardiovasc Surg. 2008;56:57–59. doi: 10.1055/s-2007-965056. [DOI] [PubMed] [Google Scholar]
- 10.Checchia P.A., Pahl E., Shaddy R.E. Cardiac transplantation for Kawasaki disease. Pediatrics. 1997;100:695–699. doi: 10.1542/peds.100.4.695. [DOI] [PubMed] [Google Scholar]
- 11.Newburger J.W., Takahashi M., Gerber M.A. Diagnosis and long term management of Kawasaki disease: a statement for health professionals from Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease. Council of Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2247–2271. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]