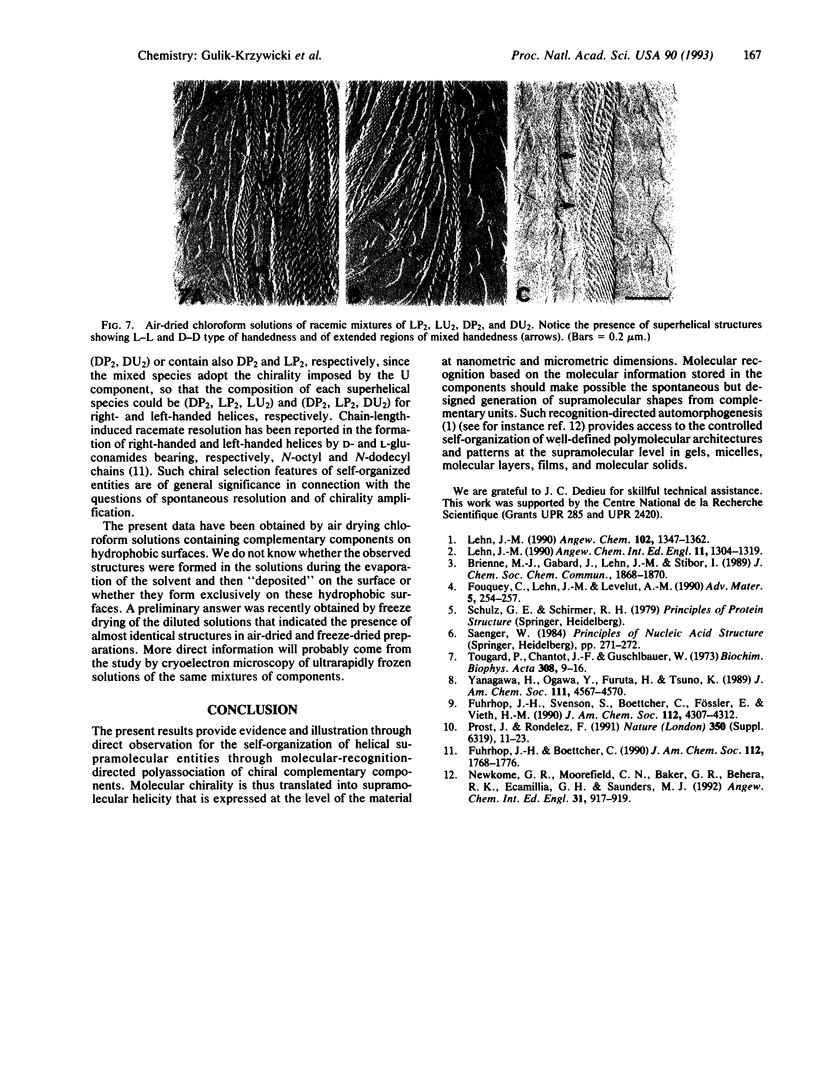
Abstract
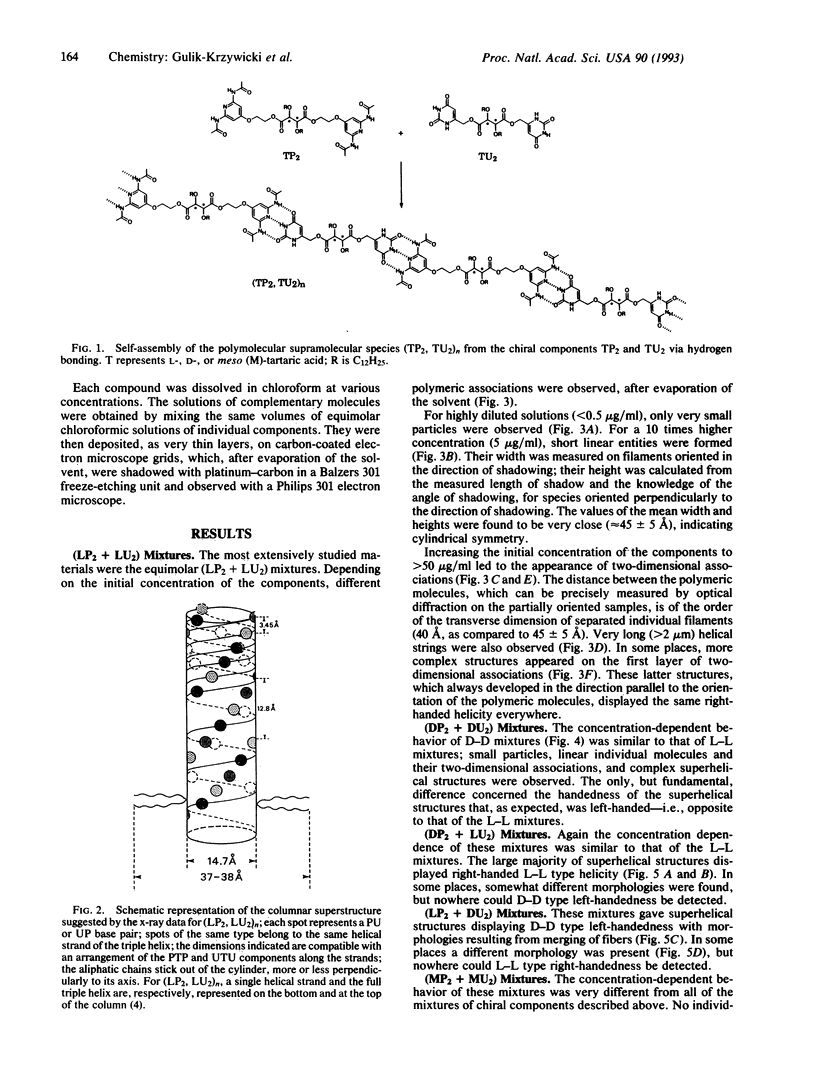
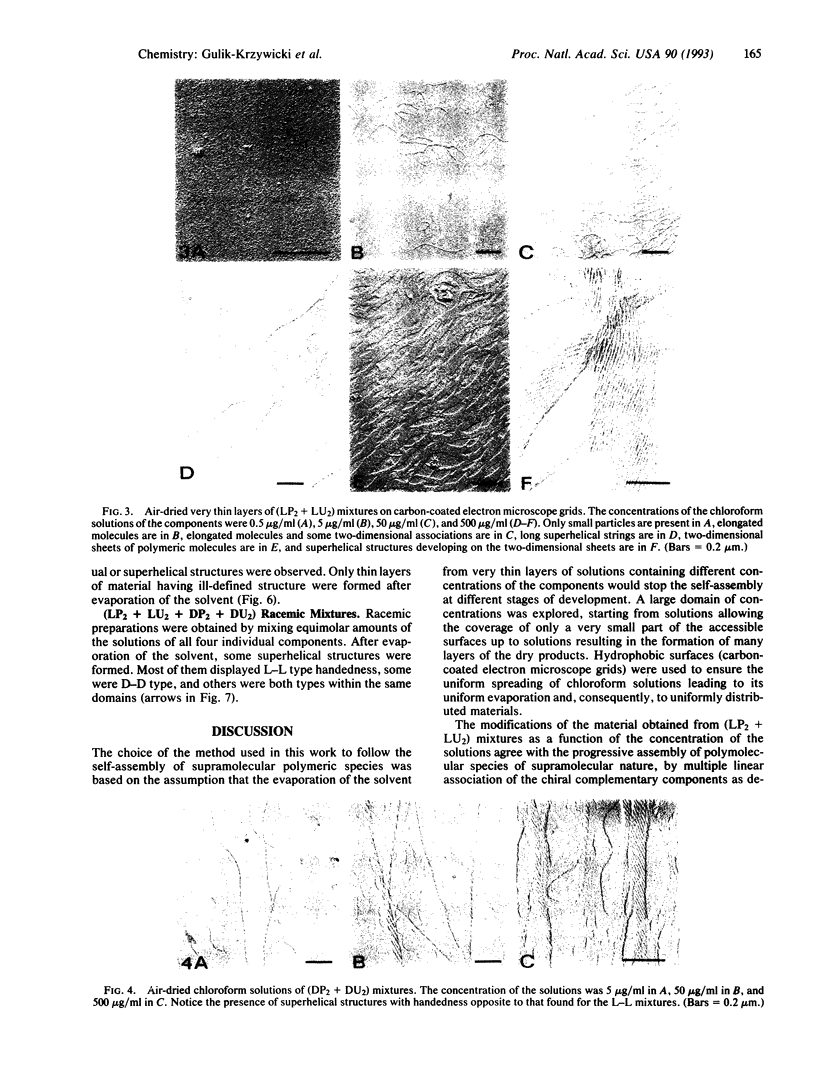
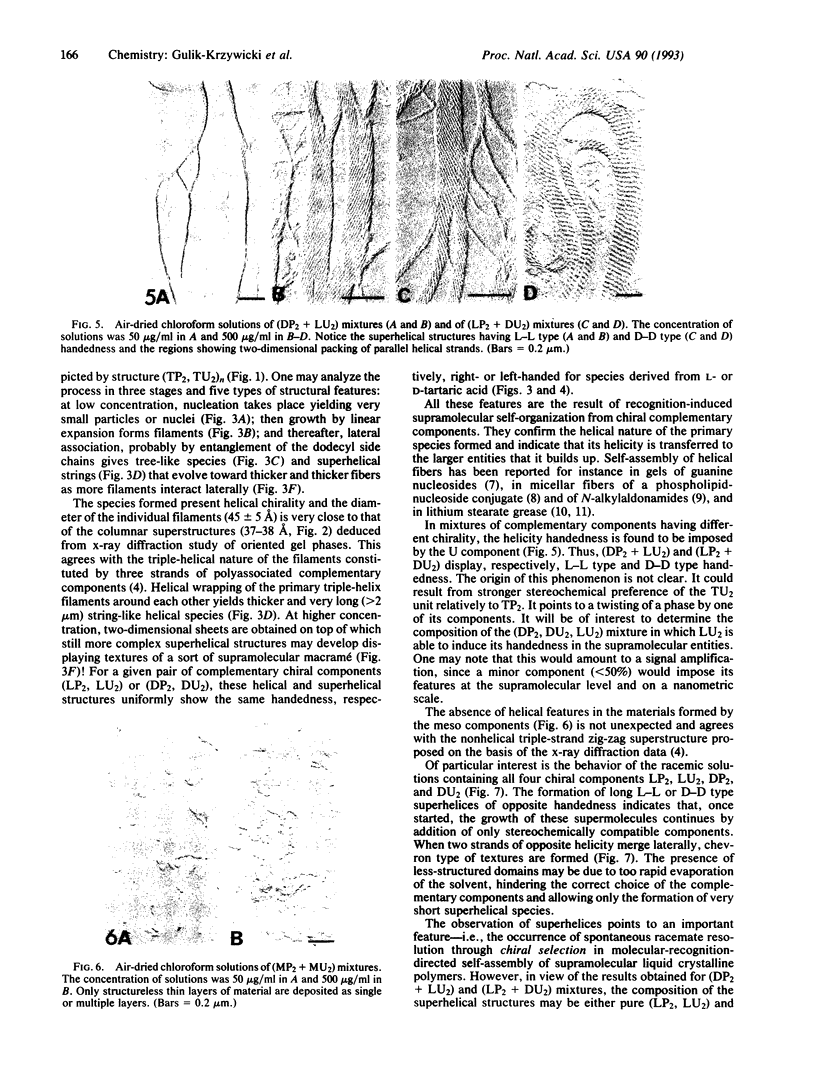
Electron microscopic observation provides insight into the nature of the polymeric supramolecular liquid crystalline species (TP2, TU2)n formed by polyassociation of the complementary components TP2 and TU2 derived from D-, L-, or meso-tartaric acid (where T is any form of tartaric acid, D is the D species, and L is the L species) and from pyridine (P) and uracil (U) derivatives. Increasing the concentration of equimolecular solutions of (LP2 + LU2) mixtures leads to the progressive assembly of very long supramolecular-polymolecular entities. The process involves successively nucleation to give small nuclei, growth to filaments, and lateral association to tree-like species, strings, and fibers. The species formed are helical; their helicity is right-handed, induced by the chirality of the components and transferred to the larger entities. The data agree with the formulation of the primary filament as a triple-helical species formed by three helically wound supramolecular strands. (DP2 + DU2) mixtures yield left-handed helical species. The helicity of the materials obtained from complementary components having different chirality is imposed by the U component, being, respectively, right- and left-handed for (DP2 + LU2) and (LP2 + DU2). No helicity is found for the meso compounds. The racemic mixture of all four L and D components yields long superhelices of opposite handedness that coexist in the same sample. This points to the occurrence of spontaneous racemate resolution by chiral selection of the components in the self-assembly of these supramolecular liquid crystalline species. The present results illustrate how extended supramolecular-polymolecular entities build up through molecular-recognition-directed polyassociation of complementary components. They also show that molecular chirality is translated into supramolecular helicity that is expressed at the level of the material on nanometric and micrometric scales.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Prost J., Rondelez F. Structures in colloidal physical chemistry. Nature. 1991 Apr 18;350(6319 Suppl):11–23. doi: 10.1038/350011a0. [DOI] [PubMed] [Google Scholar]
- Tougard P., Chantot J. F., Guschlbauer W. Nucleoside conformations. X. An X-ray fiber diffraction study of the gels of guanine nucleosides. Biochim Biophys Acta. 1973 Apr 21;308(7):9–16. [PubMed] [Google Scholar]