Abstract
Here we report the direct asymmetric amination of α-substituted cyclic ketones catalyzed by a chiral phosphoric acid, yielding products with a N-containing quaternary stereocenter in high yields and excellent enantioselectivities. Kinetic resolution of the starting ketone was also found to occur on some of the substrates under milder conditions, providing enantio-enriched α-branched ketones, another important building block in organic synthesis. The utility of this methodology was demonstrated in the short synthesis of (S)-ketamine, the more active enantiomer of this versatile pharmaceutical.
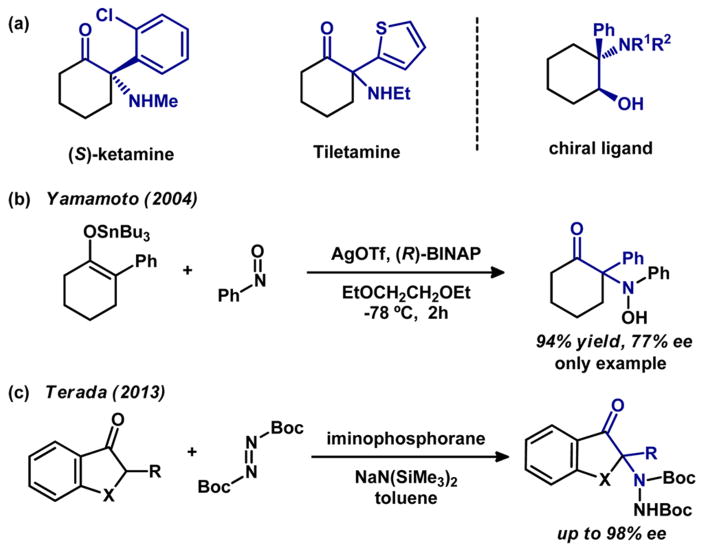
α-Amino cyclic ketones with a N-containing quaternary stereocenter are important building blocks in organic synthesis and versatile precursors to physiologically active compounds such as ketamine1 and tiletamine (Figure 1a).2 Additionally, β-amino alcohols, which are derivatives of α-amino cyclic ketones, are widely used as chiral ligands in asymmetric catalysis.3 The past decade has witnessed the development of direct asymmetric α-amination of carbonyl compounds as a useful method for the preparation of chiral amine-containing structures.4 Despite these advances, the majority of reported reactions rely on activated carbonyl compounds, such as 1,3-dicarbonyls, 2-oxindoles, α-cyanoacetates, and other reactive substrates.4,5 For carbonyl compounds with lower reactivity, enamine catalysis has been employed in the highly enantioselective amination of aldehydes6,7 and ketones8 without substitution at the α-position.9 Alternatively, chiral α-amino ketones have been accessed via asymmetric amination of preactivated unsubstituted ketone equivalents, such as silyl enol ethers,10 metal enolates,11 and enamides.12 Terada’s chiral organosuperbase-catalyzed amination of 2-alkyl-substituted cyclic aromatic ketones (Figure 1c)13 and Yamamoto’s silver-catalyzed amination of the tin enolate of 2-phenylcyclohexanone (Figure 1b)11b stand as rare example of enantioselective amination to produce N-containing quaternary stereocenters.
Figure 1.
Previous work on the catalytic asymmetric synthesis of 2-aminocyclohexanone bearing a quaternary stereocenter.
Recently, our group reported the asymmetric fluorination of α-branched cyclic ketones enabled by a combination of chiral anion phase-transfer catalysis and enamine catalysis,14 a rare example of asymmetric functionalization of α-branched cyclic ketones to construct chiral quaternary stereocenters.15 Continuing our interest in the asymmetric functionalization of α-branched cyclic ketones, here we report the direct asymmetric amination of α-branched cyclic ketones with azodicarboxylates catalyzed by a chiral phosphoric acid.16
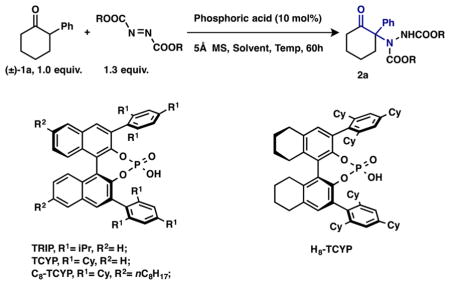
We selected 2-phenylcyclohexanone as a model substrate and di-tert-butyl azodicarboxylate as the nitrogen source, which is widely used in asymmetric amination processes (Table 1). Reaction of these two components, catalyzed by (S)-TRIP (10 mol%) in xylenes (0.1 M) at 45 °C for 60 h, produced a minor amount of the desired product; substantial starting ketone remained unreacted (entry 1). Other chiral phosphoric acids (TCYP, H8-TCYP, and C8-TCYP) were also tested, and after reaction for 60 h at 45 °C, the highest enantioselectivity (97% ee) was generated with (R)-C8-TCYP as catalyst, albeit with low conversion (38% yield, entry 4). In an attempt to improve the conversion, the reaction mixture was heated at 60 °C for 60 h; a higher yield of the product was isolated (57% yield), but with a decrease in enantioselectivity (91% ee, entry 5). A less sterically demanding electrophile, diethyl azodicarboxylate, was also employed in this reaction, providing a higher yield (60%) but a similar decrease in enantioselectivity (89% ee, entry 6). Utilizing a higher concentration (0.4 M) at 45 °C improved the conversion (52% yield) after 60 h with unchanged enantio-selectivity (entry 7). Finally, we found that running the reaction under “neat” conditions (via removal of the small amount of DCM after all the reagents were dissolved) gave almost full conversion after 60 h, and the desired product was obtained in 97% yield with 99% ee (entry 8).17 Surprisingly, the regioselectivity of this reaction was excellent; no 6-amination or 2,6-diamination products were isolated.
Table 1.
Investigation of Asymmetric Amination of 2-Phenylcyclohexanone Catalyzed by Chiral Phosphoric Acids
| |||||||
|---|---|---|---|---|---|---|---|
| entry | R | phosphoric acid | temp (°C) | solvent | conc (M) | conv (%)a | ee (%)b |
| 1 | tBu | (S)-TRIP | 45 | xylenes | 0.1 | 18 (18) | −88 |
| 2 | tBu | (R)-TCYP | 45 | xylenes | 0.1 | 31 (31) | 96 |
| 3 | tBu | (R)-H8-TCYP | 45 | xylenes | 0.1 | 26 (25) | 97 |
| 4 | tBu | (R)-C8-TCYP | 45 | xylenes | 0.1 | 39 (38) | 97 |
| 5 | tBu | (R)-C8-TCYP | 60 | xylenes | 0.1 | 70 (57) | 91 |
| 6 | Et | (R)-C8-TCYP | 45 | xylenes | 0.1 | 68 (60) | 89 |
| 7 | tBu | (R)-C8-TCYP | 45 | xylenes | 0.4 | 53 (52) | 97 |
| 8c | tBu | (R)-C8-TCYP | 45 | – | neat | 97 | 99 |
Conversions calculated based on isolated yield of recovered starting material. Number in parentheses is isolated yields after chromatography.
Determined by chiral HPLC analysis of isolated products.
Reagents were dissolved in DCM; after removal of the solvent, the mixture was heated at 45 °C for 60 h. In the absence of 5 Å MS, 2a was formed in 87% yield and 99% ee.
With the optimized conditions in hand, we explored the substrate scope of the reaction (Table 2). A range of substituted aryl groups18 were tolerated at the α-position of cyclohexanone, including electron-neutral, electron-donating, and electron-withdrawing arenes (2b–2h). Surprisingly, substrates with ortho substitution (2i, 2j), which were previously reported to be unsuitable for enantioselective construction of quaternary centers due to steric hindrance,14,15c also worked well. Additionally, the cyclopentanone analogue (2k) gave the desired amination product in high yield and with excellent enantio-selectivity. Substrates bearing heteroatoms at the 4-position of the cyclohexanone ring (2l, 2m) were also amenable to enantioselective amination, extending this methodology to potentially useful heterocyclic compounds.
Table 2.
Substrate Scope of the Asymmetric Amination of 2-Aryl Cyclic Ketonesa
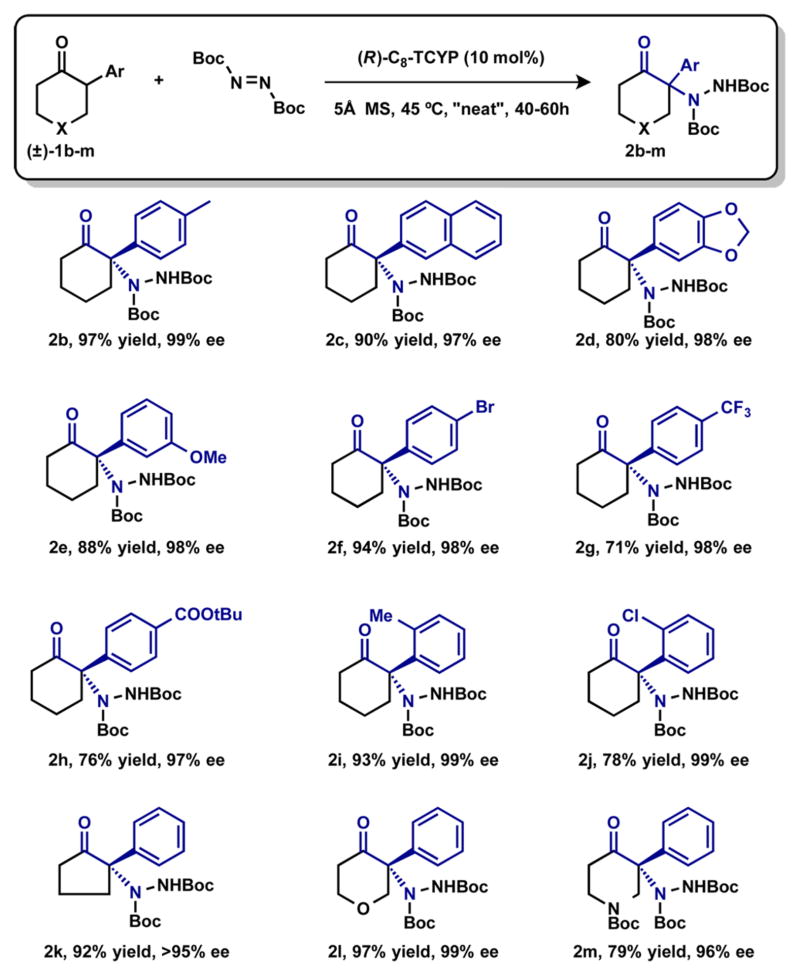
|
Reactions were carried out with ketone (0.3 mmol), BocN=NBoc (0.39 mmol), (R)-C8-TCYP (0.03 mmol), and 5 Å MS (50 mg) in DCM (0.3 mL). After removal of the solvent, the mixture was heated at 45 °C for 40–60 h. Yields are isolated yields after chromatography. Absolute configurations assigned by analogy with 2v (determined by X-ray crystallography) and (S)-ketimine.
Cyclic ketone substrates containing alkenyl substitution at the α-position were also explored (Table 3).19 The reaction proceeded well with several types of substrates, including trans-1,2 (2n), cis-1,2 (2p, 2q), cyclic alkenyl (2r), and simple vinyl substitution (2o). Substitution at the 4-positon of the cyclo-hexanone ring also gave high yield and excellent enantio-selectivity for products 2s and 2t. A cyclopentanone analogue yielded product 2v, whose absolute (S)-configuration was determined by X-ray crystallography. Seven-membered analogue 2u formed slowly under the conditions and was obtained with lower enantioselectivity (72% ee). α-Phenylethynyl-substituted 1w gave the product with lower yield (33% yield, 93% ee) due to some decomposition of substrate under the standard conditions. Surprisingly, 2-methylcyclohexanone (1x) also exclusively yielded the more substituted addition product, albeit with a slightly diminished enantioselectivity (86% ee). Increasing the size of the alkyl substituent (1y) furnished the adduct with high enantioselectivity (99% ee).
Table 3.
Substrate Scope of the Asymmetric Amination of 2-Substituted Cyclic Ketonesa
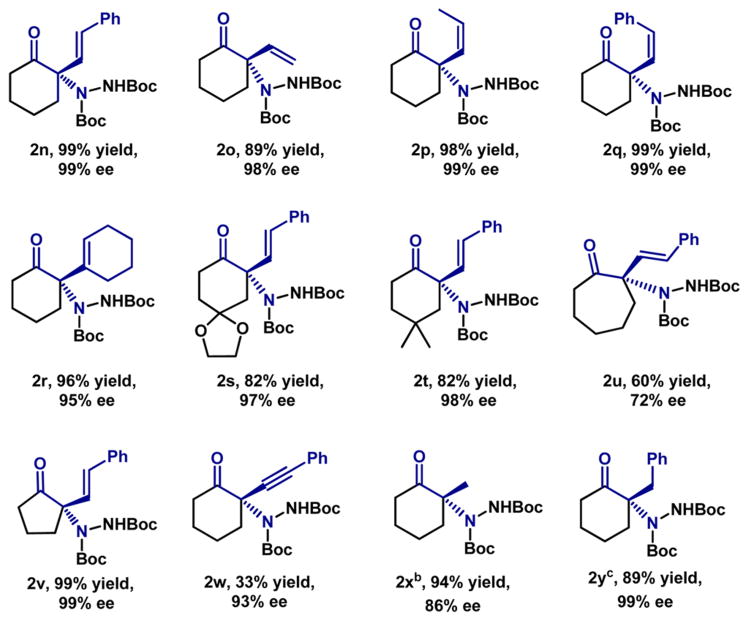
|
Conditions as indicated in Table 2, except 5 mol% (R)-C8-TCYP was used.
ee was determined after conversion to the benzamide derivative (see Supporting Information).
10% catalyst was used.
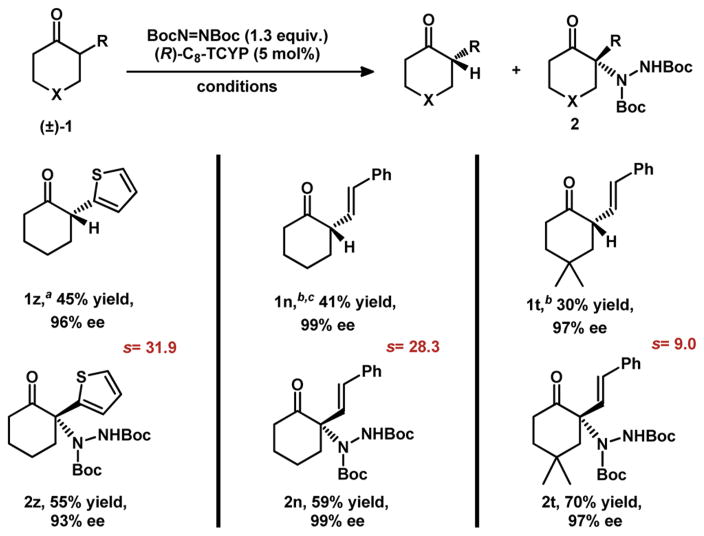
Initially, we envisioned a mechanism in which the phosphoric acid only mediated the enantioselective amination of the enol form of the ketone. Given that the chiral center of the starting ketone is destroyed in the keto/enol tautomerization, it seemed likely that this reaction was a simple dynamic kinetic asymmetric transformation; however, careful monitoring of the reaction showed that, in some cases, the reaction rate slowed when the conversion reached >50%. Therefore, milder reaction conditions (e.g., room temperature and lower concentration of 0.1 or 1 M) resulted in a good to excellent kinetic resolution of the starting ketones (Table 4).20 For example, using ketone 1z, the desired product was obtained in 55% yield with 93% ee, and the ketone was recovered in 45% yield with 96% ee after 60 h stirring at rt (s-factor of 32).21 trans-Styrene-substituted substrates 1n and 1t also gave good to excellent kinetic resolution, affording the recovered ketones with 99% ee and 97% ee, respectively. This method provides an entry to chiral α-heteroaryl- and alkenyl-substituted cyclic ketones, which are very useful building blocks in organic synthesis but difficult to prepare enantiomerically enriched.22
Table 4.
Substrate Scope of Kinetic Resolution and Asymmetric Amination of α-Branched Cyclic Ketones
|
Reactions were carried out with ketone (0.3 mmol), BocN=NBoc (0.39 mmol), (R)-C8-TCYP (0.03 mmol), and 5 Å MS (50 mg) in DCM (0.3 mL) for 60 h at rt.
Conditions as indicated as above except 5 mol% (R)-C8-TCYP and DCM (3 mL) were used for 40 h at rt.
Absolute configurations of recovered ketones was assigned by comparison of optical rotation of hydrogenated 1n with the literature (see Supporting Information).
On the basis of these observations, we propose that, in addition to mediating the enantioselective amination, the phosphoric acid also catalyzes the enantioselective activation (enolization) of chiral ketones.23 While phosphoric acid protonation of carbonyl groups is a well-established mode of activation toward nucleophilic addition,24 enolization has rarely been considered. These observations suggest that it is possible to achieve the kinetic resolution of the ketone substrates with phosphoric acid catalysts, provided the rate of the electrophilic addition (amination) step is faster than that of the enolization process.
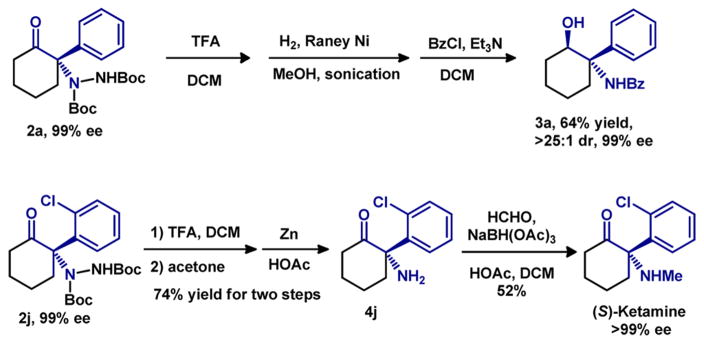
Derivatization of the enantioenriched amination products was also investigated (Scheme 1). Deprotection of Boc groups with TFA, followed by cleavage of the hydrazine bond using Raney Ni and H2, afforded the β-amino alcohol, which was then protected to afford benzamide 3a in 64% yield, preserving the enantio-purity, over three steps. To further demonstrate the utility of this method, a short synthetic route to (S)-ketamine was developed. Ketamine, which is on the World Health Organization’s List of Essential Medicines, is a drug with multiple applications, including for general anesthesia, as a pain killer, and for treatment of bronchospasm and bipolar depression.1 Normally pharmaceutical preparations of ketamine are racemic, although reports indicate that (S)-ketamine is 4 times more active than its (R) isomer.25 Recently, commercial preparations obtain (S)-ketamine via resolution of the tartaric acid salt, leaving the undesired (R) isomer as the byproduct. To our knowledge, only one asymmetric synthesis of (S)-ketamine, requiring nine steps, has been reported.26 After deprotection of the Boc of 2j, cleavage of the hydrazine bond with Zn/HOAc furnished norketamine (4j) in 74% yield; monomethylation under reductive amination conditions provided the (S)-ketamine in 52% yield with >99% ee.
Scheme 1.
Further Transformations of the α-Amino Ketone Products and Facile Synthesis of (S)-Ketamine
In summary, we have developed a chiral phosphoric acid-catalyzed asymmetric amination of α-substituted cyclic ketones which generates a N-containing quaternary stereocenter. The reaction tolerates a range of aryl, alkenyl, alkynyl, and alkyl substitutions at the α-position, different ring sizes, and modifications of the cyclohexanone ring. Kinetic resolution of the starting ketone was observed for some substrates under milder conditions, providing enantioenriched α-branched ketones. A short synthetic route to enantioenriched (S)-ketamine was developed starting with amination product 2j, demonstrating the power of this methodology. More encouragingly, using similar conditions, a highly enantioselective Mannich reaction of α-branched cyclic ketone was also achieved, creating an all-carbon quaternary center, which would broaden the application of this strategy.27
Supplementary Material
Acknowledgments
We gratefully acknowledge NIHGMS (R01 GM104534) for financial support. X.Y. acknowledges SIOC, Zhejiang Medicine, and Pharmaron for a fellowship. We thank William Wolf for obtaining the X-ray crystallographic data for 2v using the College of Chemistry CheXray (NIH Shared Instrumentation Grant S10-RR027172).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Experimental details and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ketamine is included as a general medicine in the 14th WHO model list of essential medicines: WHO Expert Committee on Drug Dependence. Critical review of ketamine, ECDD 34th report, WHO Technical Report Series 942, 2006.
- 2.Klockgether T, Turski L, Schwarz M, Sontag K-H, Lehmann J. Brain Res. 1988;461:343. doi: 10.1016/0006-8993(88)90265-x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ager DJ, Prakash I, Schaad DR. Chem Rev. 1996;96:835. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]; (b) Ianni JC, Annamalai V, Phuan PW, Panda M, Kozlowski MC. Angew Chem, Int Ed. 2006;45:5502. doi: 10.1002/anie.200600329. [DOI] [PubMed] [Google Scholar]
- 4.For reviews of asymmetric amination of carbonyl compounds, see: Vallribera A, Sebastián RM, Shafir A. Curr Org Chem. 2011;15:2539.Vilaivan T, Bhanthumnavin W. Molecules. 2010;15:917. doi: 10.3390/molecules15020917.Smith AMR, Hii KK. Chem Rev. 2010;111:1637. doi: 10.1021/cr100197z.Zhou F, Liao F-M, Yu J-S, Zhou J. Synthesis. 2014:2983.
- 5.(a) Xu C, Zhang L, Luo S. Angew Chem, Int Ed. 2014;53:4149. doi: 10.1002/anie.201400776. [DOI] [PubMed] [Google Scholar]; (b) Zhang T, Cheng L, Liu L, Wang D, Chen YJ. Tetrahedron: Asymmetry. 2010;21:2800. [Google Scholar]; (c) Terada M, Amagai K, Ando K, Kwon E, Ube H. Chem—Eur J. 2011;17:9037. doi: 10.1002/chem.201101076. [DOI] [PubMed] [Google Scholar]; (d) Zhou F, Ding M, Liu YL, Wang CH, Ji CB, Zhang YY, Zhou J. Adv Syn Catal. 2011;353:2945. [Google Scholar]; (e) Yang Z, Wang Z, Bai S, Liu X, Lin L, Feng X. Org Lett. 2011;13:596. doi: 10.1021/ol102804p. [DOI] [PubMed] [Google Scholar]; (f) Nelson HM, Patel JS, Shunatona HP, Toste FD. Chem Sci. 2015;6:170. doi: 10.1039/c4sc02494j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Bøgevig A, Juhl K, Kumaragurubaran N, Zhuang W, Jørgensen KA. Angew Chem, Int Ed. 2002;41:1790. doi: 10.1002/1521-3773(20020517)41:10<1790::aid-anie1790>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]; (b) List B. J Am Chem Soc. 2002;124:5656. doi: 10.1021/ja0261325. [DOI] [PubMed] [Google Scholar]
- 7.(a) Vogt H, Vanderheiden S, Bräse S. Chem Commun. 2003:2448. doi: 10.1039/b305465a. [DOI] [PubMed] [Google Scholar]; (b) Baumann T, Vogt H, Bräse S. Eur J Org Chem. 2007:266. [Google Scholar]; (c) Desmarchelier A, Yalgin H, Coeffard V, Moreau X, Greck C. Tetrahedron Lett. 2011;52:4430. [Google Scholar]; (d) Theodorou A, Papadopoulos GN, Kokotos CG. Tetrahedron. 2013;69:5438. [Google Scholar]; (e) Fu JY, Yang QC, Wang QL, Ming JN, Wang FY, Xu XY, Wang LX. J Org Chem. 2011;76:4661. doi: 10.1021/jo102361h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kumaragurubaran N, Juhl K, Zhuang W, Bøgevig A, Jørgensen KA. J Am Chem Soc. 2002;124:6254. doi: 10.1021/ja026412k. [DOI] [PubMed] [Google Scholar]; (b) Liu TY, Cui HL, Zhang Y, Jiang K, Du W, He ZQ, Chen YC. Org Lett. 2007;9:3671. doi: 10.1021/ol701648x. [DOI] [PubMed] [Google Scholar]; (c) Hayashi Y, Aratake S, Imai Y, Hibino K, Chen QY, Yamaguchi J, Uchimaru T. Chem—Asian J. 2008;3:225. doi: 10.1002/asia.200700307. [DOI] [PubMed] [Google Scholar]
- 9.Only ketones with no α-substitution gave high enantioselectivities in the α-hydrazonation with α-diazoesters catalyzed by an N,N′-dioxide–scandium complex: Li W, Liu X, Hao X, Hu X, Chu Y, Cao W, Qin S, Hu C, Lin L, Feng X. J Am Chem Soc. 2011;133:15268. doi: 10.1021/ja2056159.
- 10.(a) Evans DA, Johnson DS. Org Lett. 1999;1:595. doi: 10.1021/ol990113r. [DOI] [PubMed] [Google Scholar]; (b) Yamashita Y, Ishitani H, Kobayashi S. Can J Chem. 2000;78:666. [Google Scholar]; (c) Liang JL, Yu XQ, Che CM. Chem Commun. 2002:124. doi: 10.1039/b109272c. [DOI] [PubMed] [Google Scholar]; (d) Mizar P, Wirth T. Angew Chem, Int Ed. 2014;53:5993. doi: 10.1002/anie.201400405. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa A, Miyake R, Yoshida K. Org Biomol Chem. 2014;12:1935. doi: 10.1039/c3ob42111b.Silver-catalyzed amination of the tin enolate of 2-phenyl cyclohexanone gave the aminated product in 77% ee: Momiyama N, Yamamoto H. J Am Chem Soc. 2004;126:5360. doi: 10.1021/ja039103i.
- 12.(a) Chang L, Kuang Y, Qin B, Zhou X, Liu X, Lin L, Feng X. Org Lett. 2010;12:2214. doi: 10.1021/ol100540p. [DOI] [PubMed] [Google Scholar]; (b) Drouet F, Lalli C, Liu H, Masson G, Zhu J. Org Lett. 2011;13:94. doi: 10.1021/ol102625s. [DOI] [PubMed] [Google Scholar]
- 13.Takeda T, Terada M. J Am Chem Soc. 2013;135:15306. doi: 10.1021/ja408296h. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Phipps RJ, Toste FD. J Am Chem Soc. 2014;136:5225. doi: 10.1021/ja500882x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Dong XQ, Teng HL, Tong MC, Huang H, Tao HY, Wang CJ. Chem Commun. 2010;46:6840. doi: 10.1039/c0cc01987a. [DOI] [PubMed] [Google Scholar]; (b) Kang JY, Carter RG. Org Lett. 2012;14:3178. doi: 10.1021/ol301272r. [DOI] [PubMed] [Google Scholar]; (c) Kano T, Hayashi Y, Maruoka K. J Am Chem Soc. 2013;135:7134. doi: 10.1021/ja403340r. [DOI] [PubMed] [Google Scholar]
- 16.For chiral phosphoric acid-catalyzed asymmetric aminations, see: Zhang Z, Antilla JC. Angew Chem, Int Ed. 2012;51:11778. doi: 10.1002/anie.201203553.Wang SG, Yin Q, Zhuo C-X, You SL. Angew Chem, Int Ed. 2015;54:647. doi: 10.1002/anie.201409756.For chiral phosphoric acid-catalyzed asymmetric transformation of unactivated ketones, see: Pousse G, Le Cavelier F, Humphreys L, Rouden J, Blanchet J. Org Lett. 2010;12:3582. doi: 10.1021/ol101176j.Felker I, Pupo G, Kraft P, List B. Angew Chem, Int Ed. 2015;54:1960. doi: 10.1002/anie.201409591.
- 17.A larger scale (2.0 mmol) asymmetric amination of 1a gave the desired product in 96% yield and 99% ee, with recovery of 35% of the catalyst (see Supporting Information).
- 18.Several heteroarylketones were unstable to the reactions conditions. For example, subjecting compound 1z to the standard conditions led to partial decomposition of the starting material and therefore low yield of the desired product. On the other hand, 1z showed excellent kinetic resolution under milder conditions.
- 19.Under the standard conditions, α-tetralone gave the amination product in low yield and enantioselectivity (7% yield, 36% ee), and acyclic ketones failed to give the desired product.
- 20.For examples of kinetic resolution of α-substituted ketones, see: Zhou L, Liu X, Ji J, Zhang Y, Hu X, Lin L, Feng X. J Am Chem Soc. 2012;134:17023. doi: 10.1021/ja309262f.Reetz MT, Wu S. J Am Chem Soc. 2009;131:15424. doi: 10.1021/ja906212k.Kim H, Kawasaki H, Nakajima M, Koga K. Tetrahedron Lett. 1989;30:6537.
- 21.Kagan HB, Fiaud JC. Top Stereochem. 1988;18:249. The s factor was calculated on the basis of the ee of the recovered starting material. [Google Scholar]
- 22.(a) Enders D, Whitehouse DL. Synthesis. 1996:621. [Google Scholar]; (b) Huang Z, Lim LH, Chen Z, Li Y, Zhou F, Su H, Zhou J. Angew Chem, Int Ed. 2013;52:4906. doi: 10.1002/anie.201300621. [DOI] [PubMed] [Google Scholar]
- 23.The enantioselective enolization can be viewed as the microscopic reverse of the catalytic enantioselective protonation: Cheon CH, Yamamoto H. J Am Chem Soc. 2008;130:9246. doi: 10.1021/ja8041542.
- 24.Rueping M, Kuenkel A, Atodiresei I. Chem Soc Rev. 2011;40:4539. doi: 10.1039/c1cs15087a. [DOI] [PubMed] [Google Scholar]
- 25.Adams HA, Werner C. Anaesthesist. 1997;46:1026. doi: 10.1007/s001010050503. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama R, Matsumoto S, Nomura S, Higaki T, Yokoyama T, Kiyooka S. Tetrahedron. 2009;65:5181. [Google Scholar]
- 27.Mannich reaction of 1n and N-Boc benzaldimine gave the product in 72% yield, >25:1 dr, and 96% ee (see Supporting Information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.