Abstract
Objective
Laryngotracheal stenosis is largely considered a structural entity, defined on anatomic terms (i.e. percent stenosis, distance from vocal folds, overall length). This has significant implications for identifying at-risk populations, devising systems-based preventive strategies, and promoting patient-centered treatment. The present study was undertaken to test the hypothesis that LTS is heterogeneous in regard to etiology, natural history, and clinical outcome.
Study Design
Retrospective cohort study of consecutive adult tracheal stenosis patients from 1998–2013.
Methods
Subjects diagnosed with laryngotracheal stenosis (ICD-9: 478.74, 519.19) between January 1, 1998 and January 1, 2013 were identified. Patient characteristics (age, gender, race, follow-up duration), and comorbidities were extracted. Records were reviewed for etiology of stenosis, treatment approach, and surgical dates. Stenosis morphology was derived from intraoperative measurements. The presence of tracheostomy at last follow-up was recorded.
Results
150 patients met inclusion criteria. 54.7% had an iatrogenic etiology followed by idiopathic (18.5%), autoimmune (18.5%), and traumatic (8%). Tracheostomy dependence differed based on etiology (p<0.001). Significantly more patients with iatrogenic (66%) and autoimmune (54%) etiologies remained tracheostomy dependent compared to traumatic (33%) or idiopathic (0%) groups. On multivariate regression analysis, each additional point on Charlson Comorbidity Index was associated with a 67% increased odds of tracheostomy dependence (OR 1.67, 95% CI 1.04 – 2.69; p=0.04).
Conclusions
Laryngotracheal stenosis is not a homogeneous clinical entity. It has multiple distinct etiologies that demonstrate disparate rates of long-term tracheostomy dependence. Understanding the mechanism of injury and contribution of comorbid illnesses is critical to systems-based preventive strategies and patient-centered treatment.
Keywords: Tracheal Stenosis, Subglottic Stenosis, Laryngotracheal Stenosis, Intubation, Tracheostomy
INTRODUCTION
Laryngotracheal stenosis (LTS) is a life threatening, fixed, extrathoracic restriction in pulmonary ventilation. LTS is an umbrella term, encompassing luminal compromise at the level of the larynx, subglottis or trachea, which exists in a watershed of specialty care. Diagnosis is frequently delayed as patients rapidly transition from acute inpatient care to outpatient facilities. The majority of patients are not definitively diagnosed until outpatient specialty evaluation1. Many specialists (e.g. intensivists, otolaryngologists, interventional pulmonologists, thoracic surgeons) initially interact with this population. This makes it difficult to establish the natural history of the disease, define universal predictors of disease outcome and create cogent personalized plans of care. Additionally, long-term sequelae of intensive respiratory support (endotracheal intubation and elective tracheostomy) do not develop on a timescale necessary for recognition by practitioners providing acute care, impeding quality-driven improvement efforts2.
Moreover, LTS is generally described in terms of its structural characteristics, defined on anatomic terms (i.e. percent stenosis, distance from vocal folds, overall length). This neglects the unique biology driving luminal compromise in heterogeneous patient populations and has significant implications for identifying at-risk populations, devising systems-based preventive strategies, and promoting patient-centered treatment directed at the diverse pathophysiology driving airway injury. The present study was undertaken to test the hypothesis that LTS is heterogeneous in regard to etiology, natural history, and clinical outcome.
PATIENTS & METHODS
This study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and was approved by the Baylor College of Medicine Institutional Review Board (IRB No. H33195).
Patients
Subjects diagnosed with laryngotracheal stenosis (ICD-9: 478.74, 519.19) between January 1, 1998 and January 1, 2013 were identified. Those with a history of tracheal malignancy or isolated laryngeal stenosis were excluded. Laryngeal and tracheal stenosis share an association with both prolonged endotracheal intubation, and many of the same comorbid medical risk factors. However, isolated laryngeal stenosis remains a distinct anatomic and structural injury with a unique treatment algorithm, meriting dedicated independent study, and is not discussed in the present work. Patients meeting inclusion were grouped into 4 categories based on stenosis etiology: idiopathic, iatrogenic, autoimmune, and polytrauma (Table 1).
Idiopathic: No history of significant laryngotracheal injury. No significant history of endotracheal intubation or tracheotomy within 2 years of the presentation. No thyroid or major anterior neck surgery. No neck irradiation. No caustic or thermal injuries to the laryngotracheal complex. No history of vasculitis. Negative titers for angiotensin-converting enzyme (ACE) and antinuclear cytoplasmic antibody (ANCA). The lesion must involve the subglottis.
Iatrogenic: Patients that developed subglottic or tracheal stenosis following tracheostomy. Or subglottic or tracheal stenosis developing within 2 years of intubation.
Autoimmune: Patients with documented clinical along with serologic and/or histologic diagnosis of Wegener’s (GPA), Relapsing Polychondritis (RPC), Systemic Lupus Erythematous (SLE), Rheumatoid Arthritis (RA), Epidermolysis Bullosa (EB), Sarcoidosis, or Amyloidosis
Polytrauma: Patients presenting with Laryngotracheal stenosis following documented traumatic injuries involving multiple organ systems.
Table 1.
Definitions of laryngotracheal stenosis etiology of injury utilized in this study.
| Idiopathic | No history of significant laryngotracheal injury. No significant history of endotracheal intubation or tracheotomy within 2 years of the presentation. No thyroid or major anterior neck surgery. No neck irradiation. No caustic or thermal injuries to the laryngotracheal complex. No history of vasculitis. Negative titres for angiotensin-converting enzyme (ACE) and antinuclear cytoplasmic antibody (ANCA). The lesion must involve the subglottis. |
| Autoimmune | Patients with documented clinical along with serologic and/or histologic diagnosis of Wegeners (GPA), Relapsing Polychondritis (RPC), Systemic Lupus Erythematous (SLE), Rheumatoid Arthritis (RA), Epidermolysis Bullosa (EB), Sarcoidosis, or Amyloidosis |
| Polytrauma | Patients presenting with Laryngotracheal stenosis following documented traumatic injuries involving multiple organ systems. |
| Iatrogenic | Patients that developed subglottic or tracheal stenosis following tracheostomy. Or subglottic or tracheal stenosis developing within 2 years of intubation. |
Data Collected
Patient characteristics (age, gender, race, follow-up duration), and comorbidities were extracted. Records were reviewed for etiology of stenosis, treatment approach (i.e. endoscopic, open), and surgical dates. Stenosis morphology [% luminal obstruction, distance from glottis (cm), and overall length (cm)] and tracheomalacia were derived from intraoperative findings. Patients were staged with the established Cotton-Myer, Lano, & McCaffrey classification systems as previously described3–5 (Table 2). The number and frequency between repeat procedures were captured.
Table 2.
Definitions of Clinical Laryngotracheal Stenosis Classification Systems.
| Cotton-Myer | I | < 50% obstruction |
| II | 51–70% obstruction | |
| III | 71–99% obstruction | |
| IV | Complete obstruction | |
|
| ||
| Lano | I | One subsite involvement |
| II | Two subsite involvement | |
| III | Three subsite involvement | |
| * subsites defined as glottis, subglottis, & trachea | ||
|
| ||
| McCaffrey | I | Subglottis or trachea < 1cm |
| II | Subglottis > 1cm | |
| III | Subglottis & trachea >1cm | |
| IV | Any lesion involving glottis | |
Procedures
Treatments for tracheal stenosis included: 1) endoscopic dilations of the stenotic trachea6, 2) open surgical resection of the diseased tracheal segment with end-to-end anastomosis7, and 3) permanent tracheostomy. The treatment algorithm consisted of initial endoscopic dilation for all patients. In patients who required multiple dilation procedures rigorous selection criteria were applied for consideration of open surgical reconstruction. Patients less than 45 years old, without type II diabetes or connective tissue disease, and with stenosis 2cm or more below the glottis and less then 2cm in length were offered open surgical reconstruction.
Outcomes
Presence of a tracheostomy at last follow-up was the primary outcome. This represented failure of surgical management to correct airway narrowing.
Statistical Analysis
All data management and analysis were done using STATA/MP version 12.1 software (STATACorp, College Station, Texas). Univariate analyses were performed using ANOVA, Pearson’s chi-squared tests, and Fisher’s exact tests as appropriate. Stepwise multivariate logistic regression analysis was used to identify independent risk factors for tracheostomy. A significance level of p<0.20 on univariate analysis was used as the criterion for inclusion in multivariate model. As convention, p<0.05 was required for statistical significance in the model.
RESULTS
A total of 340 patients with a diagnosis of tracheal or laryngeal stenosis were identified. Excluded were those with tracheal malignancy (N=9), isolated bilateral vocal fold immobility (N=181). In all, 150 patients met inclusion criteria. The most common etiology was iatrogenic (54.7%) followed by idiopathic (18.5%), autoimmune (18.5%), and traumatic (8%; Table 3). Mean follow-up was 39.3 months (95% Confidence Interval (CI) 31.9 – 46.6), but varied significantly by etiology (p<0.001; Table 3).
Table 3.
Demographics, Stenosis characteristics, Comorbidities, and Treatment of LTS grouped by etiology of injury.
| Idiopathic (n=28) | Polytrauma (n=12) | Autoimmune (n=28) | Iatrogenic (n=82) | Significance (p) | |
|---|---|---|---|---|---|
|
Demographics
| |||||
| Follow up (Mean months, 95% CI) | 56.07 (41.5 – 70.6) | 12.3 (7.2 – 17.5) | 69.1 (39.7 – 98.6) | 27.05 (20.9 – 33.1) | <0.001 |
| Age (Mean years, 95% CI) | 50.35 (45.9 – 54.8) | 35.7 (24.1 – 47.4) | 45.1 (39.7 – 50.4) | 51 (48.0 – 54.7) | 0.002 |
| Sex (% Female) | 93 | 33 | 68 | 62 | 0.002 |
| Race (%) | |||||
| Caucasian | 89 | 50 | 71 | 63 | 0.330 |
| African American | 7 | 17 | 14 | 16 | |
| Asian | 0 | 8 | 0 | 2 | |
| Hispanic | 4 | 17 | 14 | 17 | |
| Disease Morphology | |||||
| % Stenosis (Mean %, 95% CI) | 57.86 (52.3 – 63.4) | 69.6 (55.1– 84.1) | 68.5 (60.6 – 76.4) | 72.8 (68.1 – 77.6) | 0.010 |
| Distance below glottis (Mean cm, 95% CI) | 1.289 (1.0 –1.6) | 2.17 (1.29 – 3.05) | 1.94 (1.38 – 2.51) | 1.77 (1.5 – 2.02) | 0.110 |
| Stenosis Length (Mean cm, 95% CI) | 1.657 (1.3 –1.99) | 1.95 (0.99 – 2.91) | 2.12 (1.62 – 2.62) | 2.167 (1.91 – 2.42) | 0.440 |
|
Comorbidities
| |||||
| Charlson Index (Mean, 95% CI) | 0.07 (0 –0.16) | 0.00 (0) | 1.28 (0.99 –1.58) | 1.32 (0.94 – 1.7) | <0.001 |
| DMII (%) | 0 | 0 | 11 | 39 | <0.001 |
| MI (%) | 0 | 0 | 3.6 | 28 | <0.001 |
| CHF (%) | 0 | 0 | 0 | 13 | 0.027 |
| CVA (%) | 0 | 0 | 0 | 7 | 0.008 |
| COPD (%) | 4 | 0 | 7 | 13 | 0.390 |
| Connective tissue (%) | 0 | 0 | 100 | 0 | <0.001 |
| * GERD (%) | 18 | 8 | 21 | 20 | 0.859 |
|
Treatment
| |||||
| # of Procedures / year (Mean, 95% CI) | 1.75 (0.8 – 2.6) | 3.41 (1.6 – 5.2) | 1.8 (0.9 – 2.7) | 2.65 (1.7 – 3.6) | 0.490 |
Univariate Analysis
Patient Characteristics
Age at presentation differed significantly by strata (p=0.002) with those in the traumatic group being significantly younger than all others (34.4 years, CI 23.5 – 45.3; Table 3). Gender distribution also differed based on etiology (p<0.002; Table 3). In order, the idiopathic group had a significantly higher percentage of females (93%) than autoimmune (68%) iatrogenic (62%), or traumatic (33%) LTS patients. Charlson Comorbidity Index (CCI) varied between groups (p<0.001). Iatrogenic and autoimmune strata had significantly higher indices than either idiopathic or traumatic strata (Table 3).
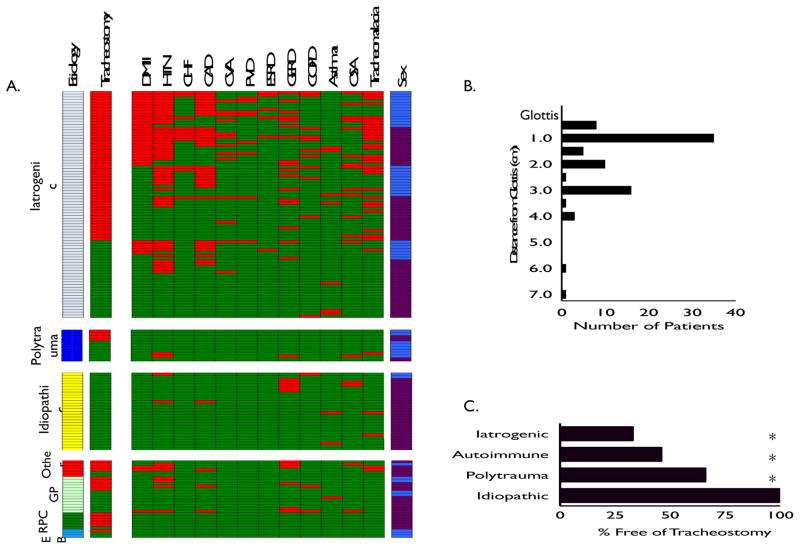
Examination of the individual components of the CCI showed cardiovascular comorbidities (i.e. myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease) and type II diabetes were significantly more prevalent in the iatrogenic strata than in other etiologies (Figure 1A and Table 3). There were not significant differences in the rate of gastroesophageal reflux disease (GERD) between strata (Table 3).
Figure 1.
Heatmap grouped by different etiologies of stenosis. Each line represents an individual patient. Tracheostomy status (red indicating trach), medical comorbidities (presence highlighted in red), and sex (blue indicating male, purple indicating female). In Autoimmune subgroup: GPA (granulomatosis with polyangitis, i.e. Wegener’s granulomatosis), RPC (relapsing polychondritis), EB (epidermolysis bullosa) (A.). Location of tracheal stenosis in iatrogenic injuries. Histogram showing location of stenotic lesion in iatrogenic subgroup in relation to distance from glottis (B.). Tracheostomy status of different etiologies at last f/u. Asterisk denotes statistical significance from idiopathic group (C.)
Disease Morphology
Degree of stenosis differed between etiologic strata (p=0.01). Idiopathic LTS involved less of the tracheal lumen (mean 57%; CI 52 – 63%) than those in the autoimmune or iatrogenic groups (Table 3). There were no differences in the mean distance from the glottis (p=0.11) or the length of stenoses between strata (p=0.44). In the iatrogenic group, LTS occurred in the subglottis (1.5cm from the glottis) in 59% of patients (49/82) (Figure 1B). Even in those patients presenting with iatrogenic LTS following tracheostomy, 41% (16/39) had subglottic injuries on intra-operative examination.
Treatment
There was no difference in number of surgeries per year of follow-up (p=0.49) or the types of surgeries performed by etiologic strata (p=0.14; Table 3). Most patients were treated with tracheal dilation (84%), followed by T-tube placement (8%), resection (6%), and no treatment (2%).
Tracheostomy Dependence
Tracheostomy dependence differed based on etiologic strata (p<0.001) (Figure 1C). Significantly more patients in the iatrogenic (66%) and autoimmune (54%) groups were tracheostomy dependent at last follow-up compared to those in either the traumatic (33%) or idiopathic (0%) groups. Tracheostomy dependence also differed based on established staging systems (Figure 2A). When stratified via Cotton-Myer staging (based on the degree of luminal obstruction), significantly more patients with grade III (90%) and grade IV (90%) lesions were tracheostomy dependent at last follow-up compared to those in either the grade II (38%) or grade I (32%) groups. (p<0.001; Figure 2A). When stratified by the Lano classification (based on the stenosis location), increasing subsite involvement was significantly associated with a higher rate of tracheostomy (p<0.001; Figure 2A). When staged according to the McCaffrey classification system (based on both stenosis location and length), increased stage was associated with progressively increased risk of tracheostomy (p<0.01) (Figure 2A).
Figure 2.
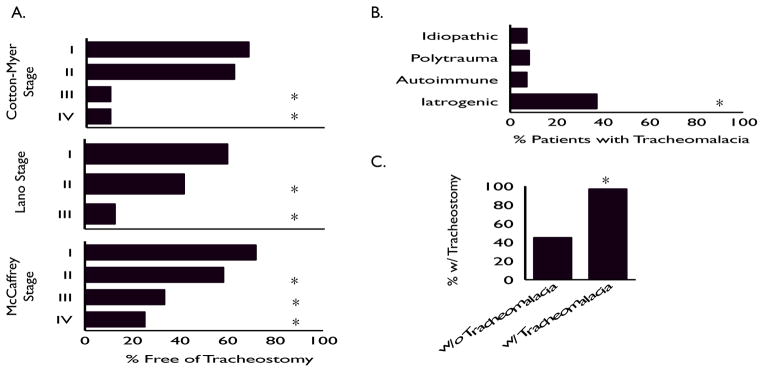
Tracheostomy status of different Cotton-Myer, Lano, & McCaffrey grades at last f/u. For Cotton-Myer staging, asterisk denotes statistical significance between Grade I&II vs. III&IV (A.). Diagnosis of tracheomalacia stratified by etiology. Asterisk denotes statistical significance between iatrogenic etiology and all other groups (B.). Rate of tracheostomy in iatrogenic etiology patients with and without a diagnosis of tracheomalacia. Asterisk denotes statistical significance (C.).
All 3 established adult LTS staging systems accurately stratified patients’ outcomes based on the severity of their structural injury. Overall (consistent with prior reports), patients in our series with more severe luminal compromise, those with longer stenosis, and those with lesions spanning multiple subsites (glottis, subglottis &/or trachea) had a much higher incidence of tracheostomy. However, this observation did not hold when patients were stratified by etiology of injury (Table 4.) No patients in the idiopathic group required tracheostomy (even those with lengthy, severe stenosis, involving multiple subsites). Conversely, patients with iatrogenic injuries had a significantly higher rate of tracheostomy even when matched at lower stenosis grades when compared with the other etiologic strata. The non-uniform rate of tracheostomy observed in different etiologic groups was seen in all three established LTS classification systems (Table 4).
Table 4.
Percentage of LTS patients with tracheostomy by Cotton-Myer, Lano, & McCaffrey stage, grouped by etiology of injury (n/a refers to an absence of patients within a given stage).
| Cotton-Myer | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| Idiopathic | 0 | 0 | 0 | n/a |
| Polytrauma | 0 | 25 | 100 | 100 |
| Autoimmune | 36 | 50 | 100 | 100 |
| Iatrogenic | 57 | 44 | 92 | 88 |
|
| ||||
| Lano | ||||
| I | II | III | ||
|
| ||||
| Idiopathic | 0 | 0 | n/a | |
| Polytrauma | 27 | 100 | n/a | |
| Autoimmune | 50 | 42 | 83 | |
| Iatrogenic | 60 | 75 | 100 | |
|
| ||||
| McCaffrey | ||||
| I | II | III | IV | |
|
| ||||
| Idiopathic | 0 | 0 | 0 | 0 |
| Polytrauma | 0 | 60 | 50 | n/a |
| Autoimmune | 63 | 50 | 50 | n/a |
| Iatrogenic | 36 | 65 | 82 | 80 |
Tracheal Structural Instability
Patients with iatrogenic injuries had a significantly higher rate of tracheomalacia seen on bronchoscopic evaluation (37% vs. 8%, p<0.001; Figure 2B). Given the retrospective nature of this work, it is not possible to establish a causative relationship between the initial injury and the loss of structural integrity associated with tracheomalacia. However, it is interesting that among the iatrogenic group, 45% of patients without malacia required tracheostomy, while 97% of those with malacia necessitated long-term tracheostomy (p<0.001; Figure 2C).
Multivariate Analysis
Multivariate regression analysis was performed to determine independent predictors of ultimate tracheostomy dependence. Each additional point on CCI was associated with a 67% increased odds of tracheostomy dependence (OR 1.67, 95% CI 1.04 – 2.69; p=0.04). Moreover, there was a 3% increased odds of tracheostomy dependence for each additional percentage of airway compromise (OR 1.03, 95% CI 1.01 – 1.06; p=0.001). LTS patient characteristics (etiology, age, sex, race) were not significantly associated with odds of tracheostomy dependency.
DISCUSSION
Although most airway stenosis appears similar on anatomic imaging and clinical examination, we present data supporting the hypothesis that different mechanisms of injury are associated with differing rates of long-term tracheostomy dependence. The relationships between the anatomic stenosis characteristics (% stenosis, location, & length) and endoscopic or open surgical “success” have been established through pioneering work in both children8,9 and adults7. In advanced centers, procedural intervention for LTS offers a high rate of long-term tracheostomy free survival4,10,11. However, success in these large published series remains critically dependent on patient selection. With our consecutive series of both inpatient and outpatient consultations, we believe this study captured a more representative cross-section of symptomatic LTS patients than many prior adult surgical case series. In the “real world”, those patients deemed poor operative candidates (e.g. sicker patients) are often left with limited therapeutic options regardless of the structural morphology of their stenosis.
Endotracheal intubation and tracheostomy can be lifesaving, but should not be considered benign procedures. They harbor significant long-term risks to communication12, swallowing13, and breathing 14, particularly in the subset of patients with comorbid illness15. Ironically, this is also the population that more frequently requires intensive respiratory support. In our series each additional point on CCI was associated with a 67% increased odds of tracheostomy dependence. Although this association doesn’t appear surprising, we believe it is powerful. It demonstrates the suitability of the CCI to serve as a systems-based protocol to identify patients who mandate a heightened awareness of complication from these procedures.
Consistent with previously published series4,16,17, despite many risk factors for iatrogenic injury being clarified over the past 40 years15,18–20, more than half the LTS burden in our cohort was potentially preventable. Overall, 59% of iatrogenic injuries occurred within the subglottis and, therefore, are attributable to intubation. In a post hoc analysis, 83% (15/18) “healthy” patients (those without DMII or cardiovascular disease) with iatrogenic LTS were women. This previously reported observation21 suggests that endotracheal tube size may contribute to tracheal injury and should be carefully considered in the smaller female trachea22.
As has been consistently shown across other large series15, patients with DMII are particularly vulnerable to airway injury and have a higher likelihood of long-term tracheostomy dependence when injury occurs. Interestingly, the rate of gastroesophageal reflux disease (GERD) was not significantly different between the etiologic subgroups. Although other investigators have suggested a tight relationship between GERD and adult idiopathic LTS, this was not seen in our patient population. The limits of retrospective review prevent us from direct comparison of the objective data on the frequency and severity of reflux episodes between individuals and subgroups. Increased body mass index (BMI) also has a suggested association with increased risk of tracheal injury with intubation and worse response to procedural intervention. Our series lacked the biometric data to address this concept. Additionally, the limits of our tertiary care referral center (with limited out-of-network medical records) prevented us from exploring the relationship between the length of intubation, or type of tracheostomy procedure (open vs. percutaneous) and the ultimate injury severity or treatment outcome.
A strong association between the degree of stenosis and ultimate decannulation has previously been reported in children23. Our series supports these prior observations in the pediatric population and now extends them to adults. As previously reported in adults, the location of injury and the length of stenosis are also essential components to predict long-term tracheostomy dependence. Critically, we now also offer data supporting an additional relationship between the cause of upper airway injury and its ultimate response to therapy. This relationship had been assumed; we offer the first formal demonstration.
Anatomic staging systems are numerous3–5,24–28 yet, the ideal system in adult LTS remains unresolved. The most established allow some degree of prognosis, promote individualized treatment planning, and facilitate multi-institutional comparison. In this work we utilized three separate, established LTS classification systems. As expected, they all effectively stratify patient’s risk of long-term tracheostomy. However, interestingly, in adult LTS it appears the McCaffrey and Lano systems offer more precision than does the Cotton-Myer scale.
While in general, patients in our series with more severe luminal compromise, longer stenoses, and lesions spanning multiple subsites had a much higher incidence of tracheostomy, this observation did not hold in the idiopathic group (whom never required tracheostomy), suggesting a unique injury. Conversely, while lower LTS stages (in all 3 systems) overall had a lower rate of tracheostomy, patients with iatrogenic injuries had a significantly higher rate even when matched at lower stenosis grades (identically in all 3 systems). Grouping LTS patients solely by an anatomic classification of their injury neglects a critical component of the heterogeneous biology responsible for tracheal scar. Patients with iatrogenic stenosis appear to possess unique medical comorbidities. Their disease ultimately behaves differently as evidenced by their disparate rate of long-term tracheostomy dependence even when matched for similar degree of luminal compromise. These separate subgroups likely merit tailored treatment strategies.
The finding of the high rate of tracheomalacia in the subgroup with iatrogenic injuries, and the significant association between tracheomalacia and long-term tracheostomy dependence in this subgroup, raises questions regarding the relative contributions of mucosal injury and cartilaginous injury in LTS. Ultimately, we believe the degree of tracheal wall injury (what we term “superstructure instability”) may have significant prognostic power for overall response to therapy. However, this is difficult to quantify at present with our current diagnostic modalities and remains an area of active research.
Our study represents one of the largest published adult LTS series in the scientific literature. The data supports the hypothesis that laryngotracheal stenosis is a common endpoint to multiple pathophysiologic processes. While different mechanisms of airway injury physiologically affect the patient in similar ways, we show they occur in unique populations and have divergent responses to therapy. Management and prevention strategies should carefully consider this heterogeneous pathophysiology. This difference is not reflected in staging systems limiting themselves to an anatomic description of the tracheal scar.
CONCLUSION
Relief through endoscopic dilation, or open tracheal resection is attainable in some cases of LTS; however, treatment is not universally successful. It is incumbent on the scientific community to move beyond viewing LTS as a purely anatomic problem, remedied only through surgical reconstruction. Rather, the management of airway stenosis should transition from reactive surgical intervention to prevention through early recognition 1 of at-risk populations and increasingly personalized care through careful consideration of the pathophysiology driving airway injury in the unique subgroups affected with LTS.
Acknowledgments
Funding/Support: This work was unfunded.
Footnotes
We report no financial relationships related to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This manuscript has been read and approved by all authors, and the requirements for authorship as stated in the editorial policies have been met. This manuscript is not under consideration elsewhere.
Level of Evidence: 4
Author Contributions: Dr A. Gelbard had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No disclosures were reported.
Study concept and design: A. Gelbard, V. Sandulache.
Acquisition of data: A. Gelbard, J.C. Simmons.
Analysis and interpretation of data: A. Gelbard, D.O. Francis.
Drafting of the manuscript: A. Gelbard, D.O. Francis.
Critical revision of the manuscript: J. Ongkasuwan, D.O. Francis
Statistical analysis: D.O. Francis.
Administrative, technical, or material support: D.T. Donovan
Study supervision: D.T. Donovan, J. Ongkasuwan.
References
- 1.Nouraei SA, Singh A, Patel A, Ferguson C, Howard DJ, Sandhu GS. Early endoscopic treatment of acute inflammatory airway lesions improves the outcome of postintubation airway stenosis. The Laryngoscope. 2006;116:1417–1421. doi: 10.1097/01.mlg.0000225377.33945.14. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (U.S.). Committee on Quality of Health Care in America. Crossing the quality chasm : a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- 3.Cotton RT. Pediatric laryngotracheal stenosis. Journal of pediatric surgery. 1984;19:699–704. doi: 10.1016/s0022-3468(84)80355-3. [DOI] [PubMed] [Google Scholar]
- 4.Lano CF, Jr, Duncavage JA, Reinisch L, Ossoff RH, Courey MS, Netterville JL. Laryngotracheal reconstruction in the adult: a ten year experience. The Annals of otology, rhinology, and laryngology. 1998;107:92–97. doi: 10.1177/000348949810700202. [DOI] [PubMed] [Google Scholar]
- 5.McCaffrey TV. Classification of laryngotracheal stenosis. The Laryngoscope. 1992;102:1335–1340. doi: 10.1288/00005537-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Baugnee PE, Marquette CH, Ramon P, Darras J, Wurtz A. Endoscopic treatment of post-intubation tracheal stenosis. Apropos of 58 cases. Revue des maladies respiratoires. 1995;12:585–592. [PubMed] [Google Scholar]
- 7.Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD. Postintubation tracheal stenosis: Treatment and results. The Journal of thoracic and cardiovascular surgery. 1995;109:486–493. doi: 10.1016/S0022-5223(95)70279-2. [DOI] [PubMed] [Google Scholar]
- 8.Hartley BE, Rutter MJ, Cotton RT. Cricotracheal resection as a primary procedure for laryngotracheal stenosis in children. International journal of pediatric otorhinolaryngology. 2000;54:133–136. doi: 10.1016/s0165-5876(00)00360-8. [DOI] [PubMed] [Google Scholar]
- 9.Manning PB, Rutter MJ, Border WL. Slide tracheoplasty in infants and children: risk factors for prolonged postoperative ventilatory support. The Annals of thoracic surgery. 2008;85:1187–1191. doi: 10.1016/j.athoracsur.2007.11.019. discussion 1191–1182. [DOI] [PubMed] [Google Scholar]
- 10.Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. The Annals of thoracic surgery. 1982;33:3–18. doi: 10.1016/s0003-4975(10)63191-8. [DOI] [PubMed] [Google Scholar]
- 11.Laccourreye O, Brasnu D, Seckin S, Hans S, Biacabe B, Laccourreye H. Cricotracheal anastomosis for assisted ventilation-induced stenosis. Archives of otolaryngology--head & neck surgery. 1997;123:1074–1077. doi: 10.1001/archotol.1997.01900100048007. [DOI] [PubMed] [Google Scholar]
- 12.Whited RE. Posterior commissure stenosis post long-term intubation. The Laryngoscope. 1983;93:1314–1318. doi: 10.1002/lary.1983.93.10.1314. [DOI] [PubMed] [Google Scholar]
- 13.Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Critical care. 2011;15:R231. doi: 10.1186/cc10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streitz JM, Jr, Shapshay SM. Airway injury after tracheotomy and endotracheal intubation. The Surgical clinics of North America. 1991;71:1211–1230. doi: 10.1016/s0039-6109(16)45586-6. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Su ZZ, Hu LJ, et al. Analysis of the risk factors causing tracheal stenosis after tracheotomy for mechanical ventilation in 560 patients. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2007;42:839–842. [PubMed] [Google Scholar]
- 16.Kleiss IJ, Verhagen AF, Honings J, Schuurbiers OC, van der Heijden HF, Marres HA. Tracheal surgery for benign tracheal stenosis: our experience in sixty three patients. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2013;38:343–347. doi: 10.1111/coa.12131. [DOI] [PubMed] [Google Scholar]
- 17.Mutrie CJ, Eldaif SM, Rutledge CW, et al. Cervical tracheal resection: new lessons learned. The Annals of thoracic surgery. 2011;91:1101–1106. doi: 10.1016/j.athoracsur.2010.11.066. discussion 1106. [DOI] [PubMed] [Google Scholar]
- 18.Mathias DB, Wedley JR. The effects of cuffed endotracheal tubes on the tracheal wall. British journal of anaesthesia. 1974;46:849–852. doi: 10.1093/bja/46.11.849. [DOI] [PubMed] [Google Scholar]
- 19.Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. British medical journal. 1984;288:965–968. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews MJ. The incidence and pathogenesis of tracheal injury following tracheostomy with cuffed tube and assisted ventilation. Analysis of a 3-year prospective study. The British journal of surgery. 1971;58:749–755. doi: 10.1002/bjs.1800581010. [DOI] [PubMed] [Google Scholar]
- 21.Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC pulmonary medicine. 2008;8:18. doi: 10.1186/1471-2466-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griscom NT, Wohl ME. Dimensions of the growing trachea related to age and gender. AJR American journal of roentgenology. 1986;146:233–237. doi: 10.2214/ajr.146.2.233. [DOI] [PubMed] [Google Scholar]
- 23.Hartnick CJ, Hartley BE, Lacy PD, et al. Surgery for pediatric subglottic stenosis: disease-specific outcomes. The Annals of otology, rhinology, and laryngology. 2001;110:1109–1113. doi: 10.1177/000348940111001204. [DOI] [PubMed] [Google Scholar]
- 24.Anand VK, Alemar G, Warren ET. Surgical considerations in tracheal stenosis. The Laryngoscope. 1992;102:237–243. doi: 10.1288/00005537-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. The European respiratory journal. 1999;13:888–893. doi: 10.1034/j.1399-3003.1999.13d32.x. [DOI] [PubMed] [Google Scholar]
- 26.Freitag L, Ernst A, Unger M, Kovitz K, Marquette CH. A proposed classification system of central airway stenosis. The European respiratory journal. 2007;30:7–12. doi: 10.1183/09031936.00132804. [DOI] [PubMed] [Google Scholar]
- 27.Grillo HC, Mark EJ, Mathisen DJ, Wain JC. Idiopathic laryngotracheal stenosis and its management. The Annals of thoracic surgery. 1993;56:80–87. doi: 10.1016/0003-4975(93)90406-8. [DOI] [PubMed] [Google Scholar]
- 28.Grundfast KM, Morris MS, Bernsley C. Subglottic stenosis: retrospective analysis and proposal for standard reporting system. The Annals of otology, rhinology, and laryngology. 1987;96:101–105. doi: 10.1177/000348948709600123. [DOI] [PubMed] [Google Scholar]