Alemtuzumab has been extensively used in the salvage setting of chronic lymphocytic leukemia (CLL) resulting less effective on bulky lymphadenopathy.1 Higher response rates have been achieved when combined with fludarabine and cyclophosphamide (FC), but this regimen has led to some safety challenges both in first-line and relapsed settings.2–4 Compared to FC, bendamustine shows a better safety profile, including a lower rate of myelosuppression and infective complications,5–7 and was found to be an ideal chemotherapy approach to be investigated in combination with alemtuzumab (BenCam). We conducted an Italian multicenter, single arm, open label, phase I/II study in relapsed and refractory CLL to define the efficacy and tolerability of the combination intravenous (IV) bendamustine Days 1 and 2, and subcutaneous (SC) alemtuzumab Days 1–3, following at least one line of treatment including alkylating agents or purine analogs, alone or in combination. Refractoriness was defined as treatment failure or disease progression within six months of the last anti-leukemic therapy.
In the first phase, standard 3+3 stepwise dose-escalation design was planned to identify dose-limiting toxicity (DLT) and maximum tolerated dose (MTD). Dose level 1: bendamustine 50 mg/m2, alemtuzumab 20 mg; dose level 2: bendamustine 50 mg/m2, alemtuzumab 30 mg; dose level 3: bendamustine 70 mg/m2, alemtuzumab 30 mg. Courses were repeated every 28 days for up to four cycles.
Adverse events (AE) were reported according to the National Cancer Institute Common Toxicity Criteria version 3.0.8 DLTs was defined as: grade 3 or higher non-hematologic toxicity; platelet count less than 20×109/L persisting for more than two weeks and/or ANC less than 0.5×109/L despite G-CSF administration persisting more than two weeks; severe infection requiring more than two weeks of antibiotic therapy.
BenCam activity at MTD in terms of responses, safety profile, response duration, progression-free survival (PFS) and time-to-re-treatment (TTR) were determined in phase II. Response was determined according to the NCI Working Group 1996 criteria for CLL, including bone marrow test and adding a CT scan for confirmation of complete response (CR).9
Patients provided informed written consent and the study protocol was approved by the ethics committee of each center in accordance with the Declaration of Helsinki and Good Clinical Practices Guidelines. Subjects were aged 18 years or over with CD52+ CLL needing treatment according to International Workshop on CLL guidelines.10 Additional inclusion criteria were World Health Organization (WHO) performance status (PS) 0–2, and adequate renal, pulmonary and hepatic function. Patients were excluded if they had received previous stem cell transplantation or alemtuzumab combined with chemotherapy. Patients were not eligible if presenting an active viral hepatitis C or B infection; lamivudine was administered to prevent reactivation in patients with hepatitis B core antibody (HBcAb).
Prophylaxis with co-trimoxazole and acyclovir was administered throughout treatment and for at least three months after treatment discontinuation. Use of granulocyte colony-stimulating factor (G-CSF) and erythropoietin was allowed at the physician’s discretion. CMV DNA was monitored weekly by PCR during treatment and for the six weeks following treatment discontinuation.
From July 2008 through March 2012, 50 patients were enrolled: median age 67 years; 20 (40%) aged 70 years or over. Patients had received a median of 2 previous treatments (range 1–6); 35 (70%) had received prior monoclonal antibodies.
Twelve patients were enrolled in the dose-escalation phase. Three patients received dose levels 1 and 2 without DLT. Due to a DLT (grade 3 enteritis), 6 patients received dose level 3, with no further DLT among the remaining patients. Thus, MTD corresponded to the highest levels of bendamustine (70 mg/m2) and of alemtuzumab (30 mg).
Thirty-seven patients (74%) completed the 4 programmed courses and no dose reductions were performed during treatment. Reasons for treatment discontinuation in 13 (26%) patients included: disease progression (n=5), persistent cytopenia (n=3), major infection (n=3), cardiological problems (n=1), autoimmune hemolytic anemia (n=1).
Grade 3/4 neutropenia developed in 33% of courses (29 patients), febrile neutropenia in 15%. G-CSF was administered in 77% of cycles. CMV reactivation was observed in 14 patients (4 symptomatic). Major infections occurred in 3.8% of courses (7 patients) and included: pneumonia (n=3), sepsis (E.Coli n=2; Staph.Aureus n=1), and enteritis (n=1). The objective response rate (ORR) was 68%, with 24% of patients achieving CR. Results and pre-treatment clinical and biological parameters affecting responses are reported in Table 1. Among clinical characteristics, only disease status at the time of enrollment was significantly associated to the achievement of response. Median PFS was 17.3 months (95%CI: 12.8–28.8%; n=34) (Figure 1A). A trend towards a better PFS was observed in patients who obtained CR compared to patients who achieved PR (29.9 vs. 12.1 months; P=0.0575) and among patients lacking TP53 genetic lesions compared to patients with TP53 abnormalities (26.7 vs. 10.3 m; P=0.0682).
Table 1.
Response to BenCam according to clinical and biological disease characteristics.
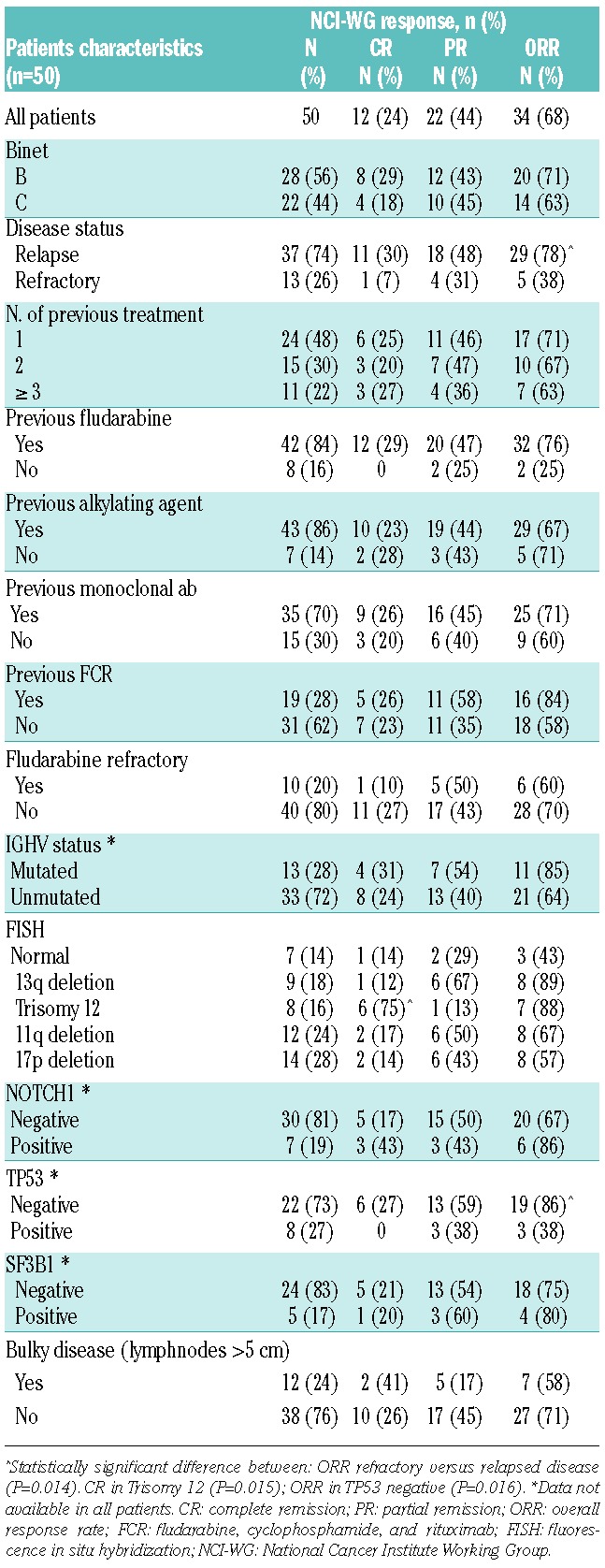
Figure 1.
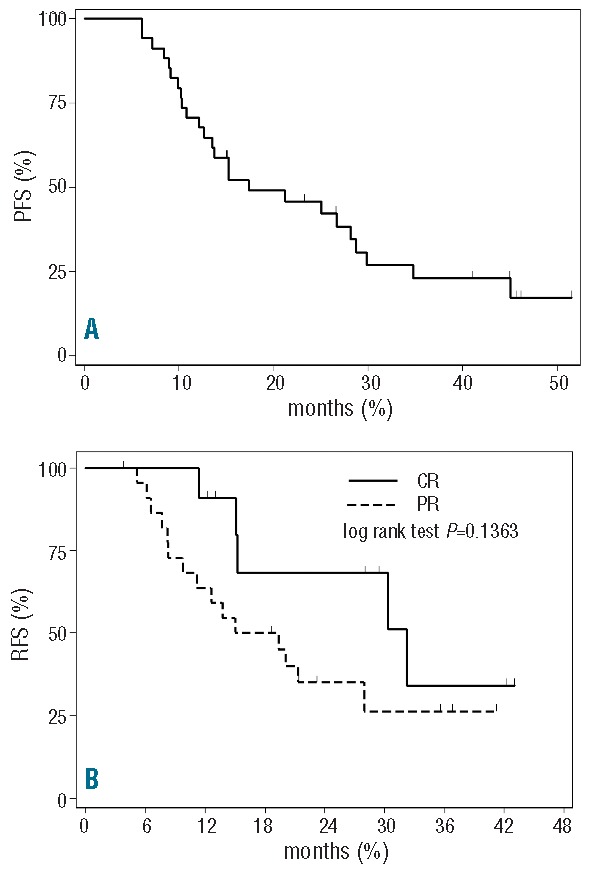
Progression-free survival (PFS) after BenCam (A); Re-treatment-free survival according to response (B).
Median TTR in responding patients was 20.1 months (CR 32.2 months vs. PR 15 months) (Figure 1B). After a median follow up of 31 months (range 10.5–51 months) median OS was 37 months for the entire cohort and “not reached” in responding patients.
BenCam combination treatment in a heavily pre-treated CLL population led to a high rate of good quality responses translating into a prolonged PFS and TTR. Responses after BenCam were independent of previous type and lines of treatment, and it is worth noting that most of the patients had previously received combination treatment with monoclonal antibodies. Furthermore, the ORR was independent of IGHV gene mutational status, NOTCH1 or SF3B1 mutations, though for the latter only a few positive cases were found in our series. The absence of any prognostic impact of these mutations could depend on alemtuzumab activity, as recently reported in the CLL2H trial.11
Responses in patients with 17pdel/TP53 mutation were consistent (57%), comparing favorably to regimens including anti-CD20 monoclonal antibody therapy either with FC (35%)12 or B (rituximab 7.1%, ofatumumab 37%).6,13
BenCam was well tolerated without unexpected toxicities and was manageable even during dose-escalation phase as MTD corresponded to the highest level. Interestingly, only 16% of patients discontinued treatment due to toxicity and no dose reductions were made during treatment. The programmed courses were administered in comparable percentages (70%) in patients aged 70 years or under or in patients aged over 70 years, and this may explain the similar outcome between the two populations.
It is difficult to compare the tolerability and activity of different regimens in relapsed and refractory CLL as characteristics of enrolled patients differ widely across studies (median number of prior treatments, percentage of patients with adverse prognostic factors, etc). Considering different alemtuzumab combination treatments, apparently fludarabine plus alemtuzumab (FluCam) led to higher ORR (82%) and longer PFS.14 However, in contrast to our series, those results were achieved in patients pre-treated with only one line of therapy (fludarabine in only 15% of cases). Furthermore, BenCam exerted a lower toxic profile in terms of myelotoxicity even considering the more heavily pre-treated patients included in our series. Lower toxicity did not translate into reduced efficacy as ORR reached after BenCam was comparable to that observed after the FC plus alemtuzumab approach in a series of patients with similar biological characteristics and median prior treatments.2
FC plus rituximab (FCR) has been extensively tested in relapsed/refractory CLL patients. In the MD Anderson Cancer Center experience responses after FCR were affected by the type of previous treatment.12 As mentioned above, number and quality of responses were unaffected by previous type and number of lines of therapy, although 38% patients had previously received FCR. Furthermore, after BenCam, compared to FCR we recorded similar responses in 11q del cases and confirmed the favorable impact of alemtuzumab combining treatment in patients showing trisomy 12.
The FCR combination was shown to be poorly tolerated, as only a minority of patients (42%) completed the number of courses programmed; this was observed particularly among elderly patients.12 Better compliance was observed in the REACH trial, but patients previously treated with FC or rituximab were excluded from this study.15 Bendamustine in combination with rituximab given in the salvage setting was found to be less toxic than FCR, and ORR and CR rates of only 59% and 9% respectively, were achieved.6 The overall response and CR rates observed in our study are superior and have been obtained in patients the vast majority of whom had received immunochemotherapy.
Furthermore, in our series, the infection rate was not superior to that observed after rituximab in combination with FC or bendamustine administered in the same setting.6,12
Even if bendamustine and alemtuzumab was shown to effectively overcome the poor prognostic characteristics conferred by del11q and trisomy 12, it obtained shorter response duration in cases with 17pdel/TP53 mutation. This confirms that patients carrying this genomic aberration still remain a challenge, warranting further investigation to find the most appropriate treatment.
PFS and OS in the entire group of patients treated with BenCam were in line with those observed after BR and FCR.6,12 To explain the longer PFS observed after FCC salvage treatment, we must emphasize that only 13% of patients treated with FCC had previously received monoclonal antibodies compared to 70% of those in the BenCam trial.2
In conclusion, our data show that BenCam combination is as effective as more toxic treatments like FCC, and is safer in the setting of heavily pre-treated and elderly patients. Moreover, BenCam is a valuable option in patients who have previously received FCR. Although targeted therapies exert disease control in a high proportion of patients, few responses are complete, and risk of resistance and duration of responses are still not completely determined. In this context, the BenCam chemoimmunotherapeutic approach could form the backbone of combinations with the new molecules. Finally, in high-risk patients with CLL requiring allogeneic transplantation, BenCam might be a valuable option as a bridge to transplant.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–61. [DOI] [PubMed] [Google Scholar]
- 2.Montillo M, Tedeschi A, Petrizzi VB, Ricci F, Crugnola M, Spriano M, et al. An open-label, pilot study of fludarabine, cyclophosphamide, and alemtuzumab in relapsed/refractory patients with B-cell chronic lymphocytic leukemia. Blood. 2011;118(15):4079–85. [DOI] [PubMed] [Google Scholar]
- 3.Elter T, James R, Busch R, Winkler D, Ritgen M, Böttcher S, et al. Fludarabine and cyclophosphamide in combination with alemtuzumab in patients with primary high-risk, relapsed or refractory chronic lymphocytic leukemia. Leukemia. 2012;26(12):2549–52. [DOI] [PubMed] [Google Scholar]
- 4.Lepretre S, Aurran T, Mahé B, Cazin B, Tournilhac O, Maisonneuve H, et al. Excess mortality after treatment with fludarabine and cyclophosphamide in combination with alemtuzumab in previously untreated patients with chronic lymphocytic leukemia in a randomized phase 3 trial. Blood. 2012;119(22):5104–10. [DOI] [PubMed] [Google Scholar]
- 5.Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(26):4378–84. [DOI] [PubMed] [Google Scholar]
- 6.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29(26):3559–66. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–16. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC) v3.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- 9.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–7. [PubMed] [Google Scholar]
- 10.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnaiter A, Paschka P, Rossi M, Zenz T, Bühler A, Winkler D, et al. NOTCH1, SF3B1, end TP53 mutations in Fludarabine-refractory CLL patients treated with Alemtuzumab: results from the CLL2H trial of GCLLSG. Blood. 2013;122(7):1266–70. [DOI] [PubMed] [Google Scholar]
- 12.Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortelezzi A, Sciumè M, Liberati AM, Vincenti D, Cuneo A, Reda G, et al. Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA multicenter phase II trial. Leukemia. 2014;28(3):642–8. [DOI] [PubMed] [Google Scholar]
- 14.Elter T, Gercheva-Kyuchukova L, Pylylpenko H, Robak T, Jaksic B, Rekhtman G, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. Lancet Oncol. 2011;12(13):1204–13. [DOI] [PubMed] [Google Scholar]
- 15.Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(19):1756–65. [DOI] [PubMed] [Google Scholar]


