Abstract
The vertebrate body plan is established through the precise spatiotemporal coordination morphogen signaling pathways that pattern the anteroposterior (AP) and dorsoventral (DV) axes. Patterning along the AP axis is directed by posteriorizing signals Wnt, fibroblast growth factor (FGF), Nodal, and retinoic acid (RA), while patterning along the DV axis is directed by bone morphogenetic proteins (BMP) ventralizing signals. This review addresses the current understanding of how Wnt, FGF, RA and BMP pattern distinct AP and DV cell fates during early development and how their signaling mechanisms are coordinated to concomitantly pattern AP and DV tissues.
Keywords: Morphogens, Gastrulation, BMP, Wnt, FGF, Nodal
1. Introduction
The anteroposterior (AP) and dorsoventral (DV) axes are the foundation for the bilateral body plan. In vertebrates both the AP and DV axes are generated by distinct signaling proteins that act as morphogens. Morphogens specify discrete cell fates over a distance in a concentration-dependent manner and generate activity gradients of high, intermediate, and low to specify distinct cell fates [1]. During axial patterning, these gradients span the entire length of an embryo and the amount of each signal at precise AP and DV locations patterns the entire organism. Accordingly, spatial regulation of morphogen signaling is critical to ensure the correct morphogen levels in particular positions. Moreover, this precise distribution of morphogen signaling across each axis cannot be established instantaneously and entire body axes cannot be patterned all at once. Correct morphogen signaling levels must be maintained throughout the patterning process and cells must also be equipped to know exactly when to respond to morphogen signals to adopt their correct fate [2]. Therefore, temporal regulation of morphogen signaling and target cell competence is also essential to pattern a complete body axis. Furthermore, patterning of all AP and DV tissues spans late blastula, gastrula, and somitogenesis stages, which are distinct and dynamic physical environments. Thus, spatial and temporal regulation of morphogen signaling must also be coordinated to navigate the challenges of early embryo development. Critically, although separate mechanisms exist for patterning the AP and DV axes, both axes are patterned concomitantly; these orthogonal patterning mechanisms must work in harmony across both space and time to properly pattern the organism.
This review will address the current understanding of Xenopus and zebrafish AP and DV axial patterning as separate processes during gastrulation, as well as more recent advances in uncovering the mechanisms that coordinate AP and DV patterning. Finally, this review will discuss current and novel techniques for manipulating spatial and temporal aspects of patterning, including the associated caveats and prospects for future development and application of these techniques.
2. Combinatorial Wnt, FGF, Nodal, and RA morphogenetic signaling patterns the AP axis
Although initial AP polarity in amphibians and fish is determined by the maternally established animal-vegetal axis of the egg, patterning of distinct AP cell fates in all vertebrates is controlled during late blastula and gastrula stages. By the end of gastrulation in Xenopus, zebrafish, chick, and mouse, a clear division of anterior and posterior cell fates has been established [3–11]. AP patterning is mediated by Wnt, fibroblast growth factor (FGF), Nodal, and retinoic acid (RA) signaling. Specifically, Wnt, FGF, Nodal, and RA specify posterior cell fates and the specification of anterior cell fates relies on the graded inhibition of these signals (Fig. 1a – c). During blastula and gastrula stages Wnt, FGF, and Nodal establish the broad regions of the AP body axis (the head, trunk, and tail as most posterior) (Fig. 1a – b). Additionally, Nodal patterns the mesendoderm while Wnt, FGF, and RA specify distinct AP cell fates in the neural plate, dividing it into four distinct regions to establish the central nervous system (CNS). These four rostral (anterior) to caudal (posterior) subdivisions are the forebrain, midbrain, hindbrain, which is further subdivided into rhombomeres (numbered 1 – 7 from rostral to caudal in the zebrafish), and spinal cord (Fig. 1a) [12]. Although the complete specification of CNS fates extends beyond gastrulation, these subdivisions of the CNS can be used as a reliable readout of AP axial patterning. The roles of Wnt, FGF, Nodal, and RA signaling in patterning the AP body axis and/or the CNS are described below.
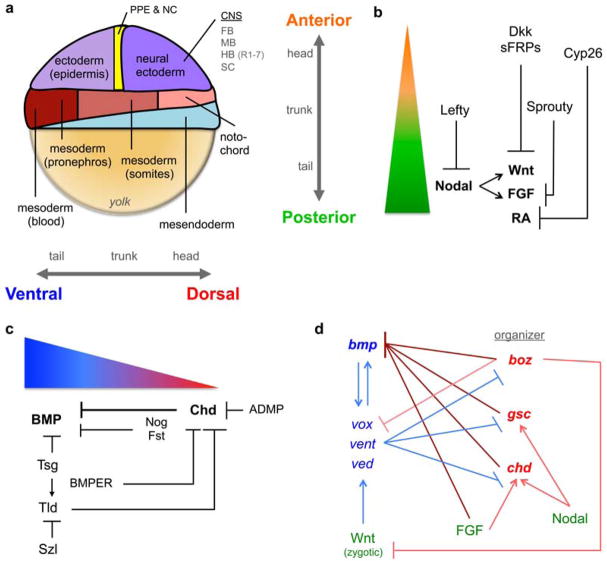
Fig. 1.
The AP and DV axes are patterned by morphogens and their regulators. (a) Fate map of a zebrafish gastrula, adapted from [215], with the orientation of the AP and DV axes. The neural ectoderm can be divided into four regions of the CNS: forebrain (FB), midbrain (MB), hindbrain (HB), which is further subdivided into rhombomeres 1 – 7 in zebrafish, and spinal cord (SC). (b) Wnt, FGF, Nodal, and RA specify posterior fates (green) in a concentration-dependent manner, while their inhibition is required for anterior fate specification (orange). (c) The BMP morphogen gradient specifies ventral cell fates (blue) at high levels and allows dorsal fate specifcation (red) at low levels. BMP signaling is regulated by extracellular factors; the DV localization of their transcriptional domain is depicted. Chd activity is key since it acts as a BMP antagonist and as the substrate for other extracellular modulators. (d) Transcriptional regulation of bmp and dorsal organizer genes. By activating different transcriptional repressors, zygotic Wnt promotes while FGF and Nodal antagonize BMP signaling. Blue lines indicate activity that promotes BMP signaling and red lines indicate activity that limits it (dark shades describe a direct effect, light shades an indirect effect).
2.1. A Wnt gradient specifies posterior cell fates
Wnts are secreted cysteine-rich glycoproteins that bind to the Frizzled (Fz) family of receptors with the assistance of co-receptors such as low-density lipoprotein receptor-related proteins (LRPs) and heparin-sulphate proteoglycans (HSPGs) [13]. During AP patterning, Wnt signaling activates the canonical Wnt/β-catenin pathway and promotes the expression of posterior genes [14,15]. During early and mid-blastula stages in the frog and zebrafish embryo, maternal Wnt signaling is localized dorsally and establishes the dorsal organizer, which establishes DV asymmetry (Section 3) [14]. However, during late blastula and gastrula stages zygotic Wnt signaling is excluded from the dorsal organizer, and localized to the ventrolateral embryo margin; this change in localization during gastrulation coincides with a dramatic change in Wnt function. Zygotic Wnt functions in posterior tissue development (Fig. 1b): increasing zygotic Wnt signaling results in the loss of head structures [16–20], whereas embryos deficient in Wnt signaling exhibit a dramatic loss of the tail and a reciprocally enlarged the head [21–25]. AP patterning by Wnt also depends on Wnt antagonists, including secreted Frizzled-related proteins (sFRPs) and Dickkopf (Dkk), which are localized anteriorly (Fig. 1b) [15,17,26]. sFRPs are secreted proteins that contain domains homologous to the Wnt binding site of Fz receptors and thus bind Wnt and prevent Fz activation, while Dkk proteins are membrane-bound and bind LRP co-receptors to prevent the propagation of Wnt signaling [13].
Wnt signaling is also key to AP neural patterning, specifying caudal CNS cell fates [19,20,23,27–33]. Studies of AP patterning in the CNS beautifully show that Wnt acts as a morphogen to specify caudal cell fates in a concentration-dependent manner [23,27,31–34]. Remarkably, grafting Wnt-expressing cells or beads near forebrain progenitors [33,35] or incubating Xenopus animal cap explants (the most anterior tissue) with Wnt [31] induces caudal cell fates that vary depending on the amount of Wnt expressed. This supports a prominent role for Wnt signaling in establishing the broad subdomains of the AP axis since Wnt can directly convey posterior positional information to specify the proportion and distribution of caudal cell fates in multiple regions of the developing CNS. Importantly, the most rostral cell fates, like the forebrain, require Wnt signal inhibition, which underscores the equal importance of Wnt antagonism in AP patterning (Fig. 1b) [30,36].
Although as a morphogen Wnt must function over a distance, Wnt is post-translationally modified with lipids that make it hydrophobic, insoluble, and poorly mobile, thus limiting its ability to form a signaling gradient by free diffusion [37]. Recent studies of fluorescently tagged Wnt in live zebrafish embryos offer an alternative mechanism for generating a gradient: short, actin-based filopodia can transport Wnt to the contact point between neighboring cells and activate Wnt signaling, increasing its effective signaling range [38].
2.2. An FGF gradient specifies posterior cell fates
FGFs are secreted growth factors that bind and activate FGF receptors (FGFRs). FGFRs are receptor tyrosine kinases (RTKs) and FGF binding results in receptor dimerization and intracellular trans phosphorylation [39]. Activated FGFRs recruit and activate a wide range of effectors, including Grb2 and Ras, which ultimately activate MAPK (mitogen activate protein kinase). Activated MAPK phosphorylates various transcription factors to regulate gene expression [39]. In zebrafish, FGF signaling is first induced during early blastula stages by maternal Wnt signaling [14]. This initial FGF expression localizes to the dorsal margin and contributes to inducing the dorsal organizer in DV axis formation (Section 3). However, similar to Wnt, FGF expression expands throughout the margin during gastrulation. This change in FGF expression coincides with a distinct role for FGF to promote posterior tissues development (Fig. 1b) [40]. Loss of FGF activity results in the loss of trunk and tail [41–43], while gain of FGF activity causes the loss of head tissues [44,45]. FGF signaling is inhibited by Sprouty proteins, which interfere with the activation of the MAPK signaling cascade (Fig. 1b) [46]. Interestingly, since Sprouty can be localized in the cytosol or at the membrane, the mechanism of Sprouty inhibition of MAPK is context dependent and remains to be characterized during AP patterning [46,47].
During CNS development, FGF maintains the midbrain-hindbrain boundary [48,49] and induces caudal cell fates like the hindbrain and spinal cord [50–55]. Unlike Wnt signaling, FGF is not sufficient to ectopically induce caudal cell fates in the forebrain [35] or in animal explants [30]. Although this supports a more prominent role for Wnt signaling in specifying the broad subdivisions of the CNS [12], it is notable that FGF is required to generate a permissive environment for the caudalizing activity of Wnt [30]. The complex relationship between FGF and Wnt during neural patterning remains to be fully characterized, but a recent study suggests that Wnt may regulate Sprouty expression, providing a mechanism to coordinate Wnt and FGF signaling [55].
Evidence indicates that FGF functions as a morphogen, differentially activating posterior genes in a concentration-dependent manner [52,55,56]. Studies of tagged FGF and single molecule fluorescence correlation spectroscopy (FCS) suggest that the FGF gradient is generated by free diffusion of the ligand and receptor-mediated endocytosis [56,57].
2.3. A Nodal gradient specifies mesendoderm and posterior cell fates
Nodal proteins are secreted ligands belonging to the TGFβ superfamily. Nodal signaling is mediated by EGF-CFC (epidermal growth factor-Cripto-1/FRL-1/Cryptic) co-receptors and type I and type II Activin receptors, which are serine/threonine kinases [58,59]. Receptor activation results in the phosphorylation and nuclear accumulation of the Smad2 and Smad3 transcription factors, which then direct the transcriptional activity of target genes [60–62]. The Nodal ligands identified in vertebrates are named as follows: Nodal in mouse; Nodal-related 1 (Ndr1, previously known as Squint), Ndr2 (previously known as Cyclops), and Ndr3 (previously known as Southpaw) in zebrafish; and Xenopus Nodal-related (Xnr) 1, 2,4,5, and 6 [62]. In early zebrafish and Xenopus embryos, Nodals are expressed around the margin and enriched dorsally and have region- and stage-specific functions [22,25,63–67]. Dorsally, Nodal is induced by maternal Wnt signaling during blastula stages [68] and functions in formation of the dorsal organizer (Section 3) [58,63,69–71]. At the margin during gastrulation, Nodal is essential to induce and pattern the mesendoderm [58,63,65–67,70,72–75]. In the mesendoderm, Nodal acts as a morphogen to differentially specify cell fates in a concentration-dependent manner: high levels of Nodal activity specify endoderm while lower levels specify mesoderm [71,73,75–77].
Nodal also directs AP axial patterning in the zebrafish by specifying posterior cell fates, such as the trunk and tail (Fig. 1b) [60,78,79]. Mis-expressing Nodal and BMP in the most anterior domain of the embryo, the animal pole, ectopically induces trunk and tail tissues [80,81]. The posterior fate of the induced tissue depends on the amount of BMP expressed, suggesting that the relative ratio of BMP to Nodal signaling in the margin directs trunk and tail patterning: an equal BMP/Nodal ratio specifies trunk, while a higher ratio specifies tail [81]. Since specific levels of BMP relative to Nodal are required, it remains unclear whether Nodal is functioning as a morphogen in this context. Furthermore, the ability of Nodal to induce trunk and tail tissues may be indirect: Nodal has been shown to induce Wnt and FGF expression, which promote posterior cell fates (Fig. 1b) [34,82]. Although these studies suggest a role for Nodal in AP patterning, Nodal is also patterning the mesendoderm during the same time period. These two roles of Nodal need further investigation: do they cooperate or inform each other? are they independent and, if so, what mechanisms enable that independence? is Nodal acting as a morphogen in one context but not the other?
In the zebrafish gastrula, the endogenous Nodal signaling gradient has been visualized by fluorescence of Smad2 and Smad3 (intracellular readouts of Nodal activity) and is highest at the margin and decreases anteriorly (animally) [83]. Importantly, the Nodal signaling gradient is shaped by the Nodal antagonist, Lefty, which binds to Nodal and EGF-CFC coreceptors to attenuate Nodal signaling [77,78,84–87]. Furthermore, Nodal and Lefty, as an activator/inhibitor pair, exhibit characteristics described in reaction-diffusion models of pattern formation; mainly that Nodal acts at a short-to midrange distance and induces its own expression and the expression of its inhibitor, Lefty, which acts at a long-range distance [75,88,89]. Recent studies of zebrafish Nodal and Lefty indicate that they have different in vivo diffusion rates (Lefty has a longer range than Nodal), providing strong support to the reaction-diffusion model of pattern formation [86,90].
2.4. An RA gradient specifies posterior CNS
RA, synthesized through the oxidation of retinol (vitamin A), acts as the ligand for nuclear RA receptors (RAR) [91]. Activated RARs dimerize with retinoid X receptors (RXR), then bind specific DNA motifs to regulate gene expression [91,92]. Although embryos lacking RA signaling still develop posterior tissues and, therefore, RA does not play a role in the broad specification of the body plan, RA signaling is essential during gastrulation to correctly pattern the hindbrain [93–96]. Specifically, RA induces genes that specify the identity of more caudal hindbrain segments (rhombomeres 4 – 7 in the zebrafish) [97–99] and thus does not play a role in delineating the broad AP subdivisions of the CNS (Fig. 1a) [12,97,100].
In the context of the hindbrain, RA acts as a morphogen to directly specify distinct posterior cell fates in a concentration-dependent manner (Fig. 1b) [99]. Importantly, discrete levels of RA signaling depend on the active degradation of RA anteriorly by Cyp26 proteins, which degrade RA into its polar metabolites (Fig. 1b) [99,101,102]. cyp26 can be both induced by RA and suppressed by Wnt and FGF signaling, suggesting that Cyp26 integrates the three posterior neural signals to pattern the hindbrain [99,100]. Additionally, cellular retinoic acid-binding proteins (Crabps), which transport RA to Cy26 enzymes for degradation, maintain the robustness of the RA gradient [103]. Recently, a gradient of free, unbound RA has been directly observed in live zebrafish embryos by measuring fluorescence resonance energy transfer (FRET) of novel genetically encoded probes for RA (GEPRAs) [104]. The observed RA gradient is highest in the trunk and then declines in a graded fashion both anteriorly and posteriorly, generating a two-tailed gradient [104].
3. A BMP signaling gradient patterns the DV axis
In Xenopus and zebrafish, the initial DV axis is established by maternal Wnt/β-catenin signaling localized to prospective dorsal cells, which activate dorsal gene expression [14,105–108]. This initial DV polarity depends on the vegetal localization of dorsal determinants in the egg and their asymmetric transport via microtubules to the future dorsal side during cleavage stages. In zebrafish, dorsal activation of maternal Wnt/β-catenin signaling activates the expression of dorsal genes such as bozozok (boz) and fgf that establish and maintain the dorsal organizer after the mid-blastula transition (MBT) [14]. Nodal also contributes to dorsal organizer formation by inducing the expression of other dorsal genes, such as goosecoid (gsc) [63,71]. The organizer protects dorsal cell fates by inducing chordin (chd), noggin (nog), and follistatin (flst) dorsally (Section 3.2) and by repressing the activity of vox/vent/ved, which are transcriptional repressors of dorsal genes (Section 3.3) [109–116]. Given the correct maternal establishment of the dorsal organizer, the patterning of cell fates along the DV axis is achieved by a gradient of BMP signaling, as described below.
3.1. A BMP gradient specifies ventrolateral cell fates
BMPs are secreted growth factors belonging to the TGFβ superfamily that act as a morphogen to pattern DV tissues along the embryonic axis. While there are numerous distinct BMP ligands, the prominent BMPs during DV patterning are BMP2/4 and BMP7 [117]. BMPs are secreted as either covalently linked homodimers or heterodimers and bind a serine/threonine kinase receptor complex composed of two type I (Bmpr1 and/or Acvr1l) and two type II (Bmpr2 and/or Acvr2) receptors [118–122]. In zebrafish, a BMP2/7 heterodimer functions as the obligate ligand, signaling through Bmpr1 and Acvr1l and still unknown type II receptors [118]. The activated type I receptors phosphorylate the C-terminus of the Smad1/5 transcription factor (pSmad1/5)1, resulting in its nuclear accumulation [118,123,124]. In the zebrafish embryo, while initial bmp expression is widespread and present dorsally, it becomes restricted the ventral half of the embryo during DV patterning [130,131]. BMP signaling is essential to specify ventral cell fates: loss of BMP signaling results in complete loss of ventral tissues like epidermis, pronephros, blood and tail with the concurrent expansion of dorsal tissues like neural and anterior somites (Fig. 1a). There is also a dorsally produced BMP, the antidorsalizing morphogenetic protein (ADMP), which synergizes with ventrally produced BMPs to restrict neural tissue to the dorsal side of the embryo [132,133].
BMP acts as a morphogen to directly specify distinct cell fates at discrete DV positions through precise levels of BMP signaling. In the developing zebrafish embryo, high BMP signaling activity specifies ventral cell fates (e.g. epidermis, blood, tail), intermediate BMP signaling levels specify lateral fates (e.g. neural crest), and no BMP signaling results in dorsal fates (e.g. neural, somites) (Fig. 1a and c) [134–139]. The BMP signaling gradient has been visualized by immunofluorescent staining for nuclear pSmad1/5, a direct intracellular readout of BMP signaling. Use of this approach in Xenopus [140–143] and zebrafish [129,139,144,145] reveals a gradient of pSmad1/5 at mid-blastula stages that intensifies during gastrulation, exhibiting high pSmad1/5 intensity ventrally and little to no pSmad1/5 dorsally. However, in zebrafish there are differing reports for nuclear pSmad1/5 in the dorsal organizer: some observe no dorsal pSmad1/5 intensity [129,139,144], while another observes pSmad1/5 in the dorsal organizer [145]. Although all groups use the same antibody and dilution, there are differences in embryo pre-staining processing [145]. An epitope recovery method shows pSmad1/5 in the dorsal organizer, whereas standard methods do not. Further studies are needed to resolve the differences in pSmad1/5 detection in the organizer.
3.2. Extracellular modulators are critical to generate and regulate the BMP signaling gradient
The BMP signaling gradient is generated and further shaped extracellularly. In zebrafish embryos devoid of BMP signaling, BMP heterodimer protein can be injected directly into the extracellular space and completely rescue the pSmad1/5 gradient by the onset of gastrulation [118]. Injection of exogenous heterodimer protein circumvents other potential mechanisms for gradient formation, such as graded bmp expression or transcriptional inputs (BMP transcriptional feedback loops begin after the onset of gastrulation and, therefore, are not contributing to rescue of the BMP gradient prior to the onset of gastrulation; see Section 5). Rescue by the injected heterodimer protein suggests that the BMP gradient is generated largely by extracellular mechanisms. This is consistent with the rescue of severely dorsalized BMP mutants by injecting bmp RNA at the one-cell stage; although bmp RNA is initially present uniformly, the BMP gradient is still able to correctly pattern the DV axis [131,137].
From invertebrates to vertebrates, extracellular modulators are essential to establish and regulate BMP signaling levels [117,133]. Extracellular modulators also enable BMP signaling to act in distinct phases or cellular domains by providing precise spatial and temporal control of signaling, such as in the preplacodal ectoderm (Section 4.5) [146–148]. BMP signaling is required initially to specify these tissues at the onset of gastrulation, but must be completely blocked after gastrulation for their final specification. The primary extracellular modulators of BMP signaling are the BMP antagonists, which are secreted dorsally and bind and sequester BMPs to prevent ligand-receptor binding (Fig. 1c) [149–151]. The BMP antagonists include Noggin (Nog), Follistatin (Flst), and Chordin (Chd) [133,152,153]. Chd, in particular, is vital not only for generating the BMP signaling gradient, but also for continuing to shape the BMP gradient at various stages throughout DV patterning [151,154–159].
Unlike Nog and Flst, Chd itself can be modulated by additional extracellular regulators (Fig. 1c). First, the related metalloproteases Bmp1a and Tolloid (Tld) can cleave and inactivate Chd [160–163], and their inhibitor, Sizzled (Szl), blocks their protease activity, preventing Chd cleavage [163,164]. Twisted-gastrulation (Tsg), which forms a ternary complex with Chd and the BMP ligands, can either promote the cleavage of Chd by Tld or, in the absence of Tld, inhibit BMP signaling [165–170]. Finally, BMP binding endothelial regulator (BMPER, also known as Crossveinless-2) can also antagonize Chd activity and may do so in a complex with Tsg and BMP [146,171–173]. This plethora of Chd regulators suggests that delimiting Chd activity is key to shaping the BMP signaling gradient and DV patterning (Fig. 1c). Furthermore, there is emerging evidence that defining the Chd expression domain is also a crucial parameter for establishing and maintaining a correct BMP signaling gradient [142,145,174,175].
3.3. Transcriptional regulation of BMP by Wnt, FGF, and Nodal signaling
Although Wnt, FGF, and Nodal are clearly required for AP patterning (Section 2), these factors also affect BMP signaling and DV patterning through transcriptional regulatory relationships that persist from their role in establishing early DV polarity. For example fgf, which is initially induced by maternal Wnt/β-catenin, contributes to organizer formation by transcriptionally repressing bmp expression and inducing chd expression, thus antagonizing BMP signaling (Fig. 1d) [176–178]. Similarly, Nodal contributes to organizer formation by inducing gsc [63,71,179] and chd [180], which can inhibit BMP signaling (Fig. 1d). Notably, chd is required for the dorsalizing activity of the organizer [158], but chd expression is not fully dependent on the organizer [180].
On the other hand, the role of Wnt signaling changes when it is zygotically expressed. As opposed to its maternal role in establishing the dorsal organizer, zygotic Wnt signaling promotes vox/vent/ved expression to promote ventral cell fates [181,182]. vox/vent/ved maintain bmp gene expression ventrally and transcriptionally repress boz, chd, and gsc restricting their expression to dorsal regions (Fig. 1d) [114–116,179,183–185]. boz, initially induced by maternal Wnt/β-catenin, promotes dorsal cell fates by acting as a transcriptional repressor of vox/vent/ved, bmp, and zygotic wnt, while also stimulating chd expression (Fig. 1d) [176,186–189]. Interestingly, BMP can also induce vox/vent/ved expression and thus positively regulate its own expression (Fig. 1d) [116,184], though it acquires this ability after the BMP gradient has been established and it has begun patterning the DV axis [130,137,139]. The stage-specific contribution of these transcriptional regulatory relationships remains to be fully characterized and integrated into our understanding of DV patterning.
4. Temporally progressive patterning of the AP and DV axes
Tissues along the AP axis are patterned progressively from anterior to posterior, a feature of AP patterning that is well characterized (Fig. 1a) [190]. On the other hand, the temporal patterning of DV tissues has only recently been investigated. While it had been established in Xenopus and zebrafish that BMP signaling patterns most of the DV axis from mid-blastula through gastrula stages, there was only a general understanding of the broad tissue types that were being patterned (Fig. 1a) [148,191,192]. Additionally, the tail is not completely specified within that time window. Although tail progenitors are defined at the onset of gastrulation, their distinct cell fates are not specified until somitogenesis stages [80,159,192–195]. Given this broad time window for DV patterning (mid-blastula to early somitogenesis stages), the dynamic nature of the BMP morphogen gradient, and the progressive patterning of the AP axis that occurs concomitantly, the field was lacking a precise understanding of the temporal control of DV patterning. A pivotal set of experiments tackled this issue and demonstrated in zebrafish that DV patterning, similar to AP patterning, occurs in a temporally progressive manner (Fig. 2) [129,139].
Fig. 2.
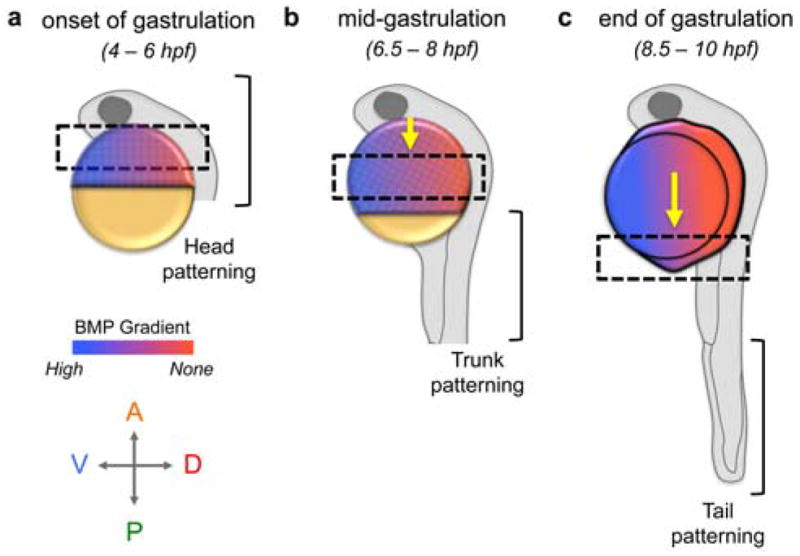
BMP signaling patterns DV cell fates progressively from anterior to posterior. AP and DV coordinates refer to the zebrafish gastrula embryos (a and b), which are depicted with cells atop the yolk. The dashed box indicates the region of active DV patterning with the corresponding portion of the body plan represented by the larval zebrafish (24 hpf). During gastrulation, cells undergo epiboly wherein the multilayered tissue thins and spreads posteriorly to completely envelop the yolk. (a) From late blastula to early gastrula stages (30 – 65% epiboly), the most anterior tissues, i.e. the head, are patterned. (b) As gastrulation proceeds, the region of active patterning progresses posteriorly (yellow arrow). At mid-gastrula stages (65 – 85% epiboly), trunk tissues are patterned. (c) From late gastrula (85 – 100% epiboly) to early somitogenesis, the most posterior tissues, i.e. the tail, are patterned.
4.1. The DV axis is progressively patterned from anterior to posterior
A direct approach to examine when BMP signaling patterns discrete DV tissues is to modulate BMP signaling levels over time. While Section 7.2 details multiple methods to exert temporal control of signaling, our group used heat shock inducible transgene expression in the zebrafish. One transgene used was the wild-type BMP receptor acvr1l (previously called alk8) under the control of the hsp70 promoter in a maternal-zygotic acvr1l mutant (hsp:acvr1l; MZ-acvr1l) [139]. Heat shock induction of acvr1l expression can rescue the severely dorsalized MZ-acvr1l phenotype [196]. Importantly, a normal embryo-wide BMP activity gradient is apparent 30 minutes after heat shock, demonstrating rapid control and robust rescue efficiency via acvr1l transgene induction [139]. Following a series of heat shock inductions at distinct time points, it was found that acvr1l induction as late as the late blastula or onset of gastrulation could fully rescue the gastrula pSmad1/5 gradient and the severe dorsalization of MZ-acvr1l mutant embryos (Fig. 2a) [139]. Surprisingly, although pSmad1/5 is evident during mid-blastula stages, this BMP signaling is not necessary for patterning or to generate a robust pSmad1/5 gradient during gastrulation since acvr1l first expressed at the onset of gastrulation sufficed to pattern the embryo and generate a normal signaling gradient.
However, following heat shock induction, transgene expression persists for up to several hours [159,192]. To determine when BMP signaling is required to pattern tail tissues, the hsp:acvr1l transgene was induced in zygotic (Z) acvr1l mutants, which only display dorsalized tail tissue [196]. By employing a similar developmental series of heat shock rescue experiments detailed above in hsp:acvr1l; Z-acvr1l embryos, acvr1l induction at the one-somite (10.5 hpf) stage fully rescued all Z-acvr1l mutants, whereas induction at later somitogenesis stages only partially rescued the tail dorsalization or did not rescue [139]. This indicates that BMP signaling is sufficient during post-gastrula stages to pattern the tail (Fig. 2c). Therefore, the DV axis is patterned during at least two time windows: BMP initiates patterning of the head and trunk beginning in the late blastula or at the onset of gastrulation and patterns the tail during early somitogenesis stages (Fig. 2a – c).
4.2. BMP signaling progressively patterns the ectoderm and mesoderm from anterior to posterior in a temporal fashion
The studies described above demonstrate that DV axial body patterning initiates during late blastula/early gastrula stages, while patterning of DV tissues of the tail initiates at the end of gastrulation. But these experiments left open when distinct domains along the DV axis are patterned (i.e. are head and trunk DV tissues patterned concomitantly or sequentially). To address this question, a developmental time series of BMP inhibition studies was performed. A transgene was used that expresses the BMP antagonist chd (Section 3.2) under the hsp70 promoter (hsp:chd), which can abolish BMP signaling in a wild-type embryo within 60 minutes of heat-shock induction [129,139]. In these experiments, hsp:chd embryos were heat shocked at distinct 30-minute intervals from blastula through gastrula stages and then phenotyped to determine the extent of dorsalization. If a tissue remains properly patterned (i.e. not dorsalized) after heat-shock induction of Chd at a specific stage, then BMP signaling has already patterned that tissue prior to the stage of heat shock. Since neural tissues are dorsally derived and inhibited by BMP signaling (Fig. 1a and 1c), loss of BMP signaling results in their ventral expansion. Complete dorsalization causes a clear phenotype: neurectodermal markers are radially expanded and encircle the embryo (compare Fig. 3a and 3b). Thus, the ventral expansion of neurectodermal markers was used to gauge the extent of dorsalization.
Fig. 3.
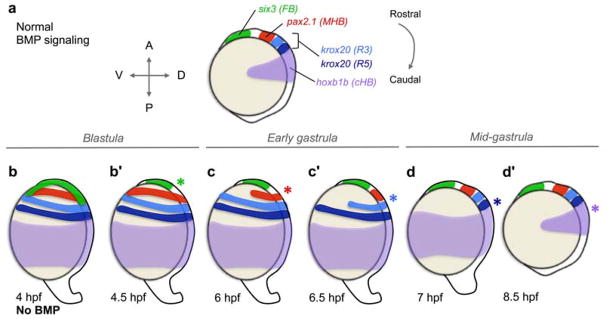
BMP signaling progressively patterns the anterior neurectoderm from rostral to caudal during gastrulation. (a) Wild-type expression pattern of anterior neurectoderm markers [FB, forebrain; MHB, midbrain-hindbrain boundary; R3, rhombomere 3; R5, rhombomere 5; cHB, caudal hindbrain]. (b – d′) Summary of experiments demonstrating that BMP signaling patterns the neurectoderm progressively, from rostral to caudal, in a time-dependent manner. (b) Loss of BMP signaling beginning at a mid-blastula stage (4 hpf) causes severe dorsalization and radial expansion of the dorsally-derived neurectoderm. (b′) Loss of BMP signaling at a late blastula stage (4.5 hpf) causes radial expansion of all markers except six3, which is restricted dorsally (green asterisk). Therefore, BMP patterns six3 between 4 – 4.5 hpf. (c) Loss of BMP signaling at the onset of gastrulation (6 hpf) restricts pax2.1 expression (red asterisk), indicating that BMP patterns the MHB between 4.5 – 6 hpf. (c′) Loss of BMP signaling at 6.5 hpf restricts R3 (light blue asterisk) and (d) loss of BMP signaling at 7 hpf additionally restricts R5 (dark blue asterisk), indicating that BMP patterns R3 and R5 in 30 minute intervals from 6 – 7 hpf. (d′) Loss of BMP signaling at 8.5 hpf restricts hoxb1b expression (purple asterisk), indicating that BMP patterns hoxb1a between 7 – 8.5 hpf.
Heat shock of hsp:chd embryos at a mid-blastula stage (3 hpf) and the subsequent loss of BMP signaling by 4 hpf caused complete dorsalization (Fig. 3b) [129]. Remarkably, loss of BMP signaling at time points at and after 4.5 hpf (resulting from heat shock at and after 3.5 hpf) resulted in the dorsal restriction of neurectodermal markers in a progressive (from rostral to caudal), time-dependent fashion (Fig. 3b – 3d′, asterisks). Loss of BMP signaling at a late blastula stage (4.5 hpf) caused the radial expression of all markers except for the most rostral marker, six3 (forebrain), which was restricted dorsally (Fig. 3b′) [129]. Therefore, BMP signaling acts prior to 4.5 hpf to properly pattern the forebrain, while it functions after 4.5 hpf to pattern more caudal tissues. Loss of BMP signaling at an early gastrula stage (6 hpf) caused the radial expansion of all markers except for six3 and pax2.1 (midbrain-hindbrain boundary, MHB), which were both restricted dorsally (Fig. 3c), demonstrating that BMP signaling patterns the MHB between 4.5 and 6 hpf (Fig. 3c, red asterisk) [139]. Strikingly, hindbrain rhombomeres R3 and R5 (marked by krox20) are patterned in 30-minute intervals (Fig. 3c′ – 3d): R3 requires BMP signaling prior to 6.5 hpf (Fig. 3c′, light blue asterisk) and R5 prior to 7 hpf (Fig. 3d, dark blue asterisk) [139]. Finally, the most caudal hindbrain marker, hoxb1b, requires BMP signaling prior to 8.5 hpf (Fig. 3d′, purple asterisk) [129]. It is important to note that at the later developmental stages BMP signaling is required during specific intervals, as opposed to being required for a longer duration [129].
Notably, BMP signaling also patterns the mesoderm in a progressive, temporal manner. In the hsp:chd experiments discussed above, the expression of mesodermal markers were also characterized in parallel to those of the neurectoderm [139]. Like the neurectoderm, the DV fates of the mesoderm are progressively patterned from anterior to posterior: the anterior pronephros requires BMP signaling prior to 6 hpf, the posterior pronephros prior to 6.5 hpf, and blood precursors prior to 7 hpf (Fig. 1a) [139].
Overall, the studies summarized here and in the previous section demonstrate that BMP signaling specifies the entire DV axis in a time-dependent, progressive fashion.
4.3. An identical patterning clock coordinates DV and AP progressive patterning
The discovery that DV axial patterning progresses along the AP axis analogous to AP patterning (Sections 4.1 – 4.2) and the fact that both axes are patterned during gastrulation prompt the question of whether DV and AP patterning are coordinated in time and/or space or are regulated by independent temporal mechanisms. Recently, this question was addressed by simultaneously manipulating DV and AP patterning, which demonstrated that the patterning of both axes is temporally coordinated [129]. These experiments relied on markers with expression domains dually specified by BMP and either FGF, Wnt, or RA, respectively. For example, otx2 is a marker of anterior neurectoderm (forebrain and midbrain) [197] that is restricted anteriorly by FGF and Wnt signaling and dorsally by BMP signaling, which together define its expression domain (Fig. 4a, left panel). In contrast, hoxb1b is a marker of caudal hindbrain that requires posterior FGF, Wnt, or RA signaling in conjunction with dorsal restriction by BMP signaling, to define its posterior-dorsal expression domain (Fig. 4c, left panel).
Fig. 4.
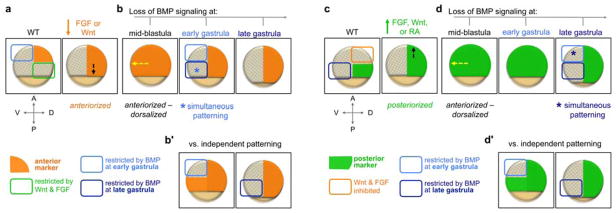
Summary of experiments demonstrating that AP and DV patterning are temporally coordinated throughout gastrulation. Marker expression is depicted in embryos at mid- or late gastrula stage. (a) BMP restricts anterior marker expression (orange) at early gastrula stages. Embryo anteriorized by inhibiting FGF or Wnt (dashed black arrow: posterior expansion of anterior marker). (b) Inhibiting BMP signaling in an anteriorized embryo at a mid-blastula stage causes a compound anteriorized-dorsalized phenotype (dashed yellow arrow: ventral expansion of the anterior marker). Since AP and DV patterning are temporally coordinated, inhibiting BMP at an early gastrula stage (when the anterior marker is normally patterned) causes complete dorsal restriction of the anterior marker. (b′) If AP and DV patterning were temporally independent, inhibiting BMP at an early gastrula stage would restrict the normal domain and the posteriorly expanded region would be restricted at a later gastrula stage. (c) BMP restricts posterior marker expression (green) at late gastrula stages. Embryo posteriorized by overexpressing FGF, Wnt, or RA (dashed black arrow: anterior expansion of posterior marker). (d) Inhibiting BMP signaling in a posteriorized embryo at a mid-blastula stage causes a compound posteriorized-dorsalized phenotype (dashed yellow arrow: ventral expansion of the posterior marker). Since AP and DV patterning are temporally coordinated, inhibiting BMP signaling only at a late gastrula stage (when the posterior marker is normally patterned) causes complete dorsal restriction of the posterior marker. (d′) If AP and DV patterning were temporally independent, inhibiting BMP at an early gastrula stage would still restrict the anteriorly expanded domain, but not the normal domain of the posterior marker.
If AP and DV patterning are temporally coordinated, then alterations in AP patterning would similarly alter the temporal patterning of DV tissues. To evaluate this, embryos were either anteriorized by inhibiting FGF or Wnt, or posteriorized by overexpressing FGF, Wnt, or RA (Fig. 4a and 4c, right panels). These AP alterations were performed in hsp70:chd embryos (described in Section 4.2) to enable concurrent temporal manipulation of BMP signaling and DV patterning. Since BMP is required at late blastula stages (4.5 – 5 hpf), heat shock at 4 hpf in an already anteriorized or posteriorized embryo resulted in the ventral expansion of the anterior or posterior marker, respectively (Fig. 4b and 4d, left panels). These compound phenotypes can be described as anteriorized-dorsalized or posteriorized-dorsalized.
The key question was whether the anteriorized or posteriorized regions in the compound phenotypes would be patterned by BMP signaling simultaneously at the time point when BMP normally patterns the marker (Fig. 4b and 4d), or independently at the time point when BMP normally patterns each position along the AP axis (Fig. 4b′ and 4d′). If patterning of the anteriorized or posteriorized regions occurs based on the normal timing of the marker, then DV and AP patterning are coordinated (Fig. 4b and 4d); conversely, if DV and AP patterning are not coordinated, then BMP would pattern the anteriorized or posteriorized regions of the marker at independent time points (Fig. 4b′ and 4d′). Strikingly, the compound phenotype was always patterned simultaneously at the time point when BMP normally patterns the marker, showing that AP and DV patterning are temporally coordinated throughout gastrulation along these orthogonal axes [129]. This intimate coordinated patterning enables cells to adopt both an AP and DV identity simultaneously, integrating the positional information of two orthogonal axes to progressively pattern the embryo from head to tail (Fig. 1a – c).
4.4. The Smad1/5 linker region coordinates AP and DV patterning
Several in vitro and in vivo studies offer a potential mechanism to coordinate AP and DV patterning: the phosphorylation state of Smad1. Although C-terminally phosphorylated Smad1/5 (referred to as Ct-pSmad1/5 in this section) is the primary downstream nuclear effector of BMP signaling (Section 3.1), FGF and Wnt signaling components can also alter the phosphorylation state of Smad1/5, specifically in the linker region between its N- and C-terminal domains (Fig. 5). MAPK, activated by FGF signaling, can phosphorylate four conserved sites in the Smad1/5 linker (pSmad1/5-LMAPK) [198–200], while Wnt signaling inhibits GSK3 phosphorylation of the Smad1/5 linker (pSmad1/5-LGSK3) (Fig. 5) [201]. There is a model of sequential Smad1/5 phosphorylation: first by BMPR on the C-terminus, second by MAPK in the linker, and third by GSK3 in the linker (Fig. 5a, blue arrows) [200,201]. Studies in Xenopus and MEFs demonstrate that pSmad1/5-LMAPK represses Smad1/5 activity and that pSmad1/5-LGSK3 enhances this inhibition [198–200]. Specifically, pSmad1/5-LMAPK and dual pSmad1/5-LMAPK+GSK3 promote Smad1/5 polyubiquitinylation by the Smurf1 E3 ligase, which results in Smad1/5 proteosomal degradation outside the nucleus (Fig. 5a) [200–202]. Thus, the Smad1/5 response to BMP signaling, which directs DV patterning, also depends on FGF and Wnt activity, which direct AP patterning.
Fig. 5.
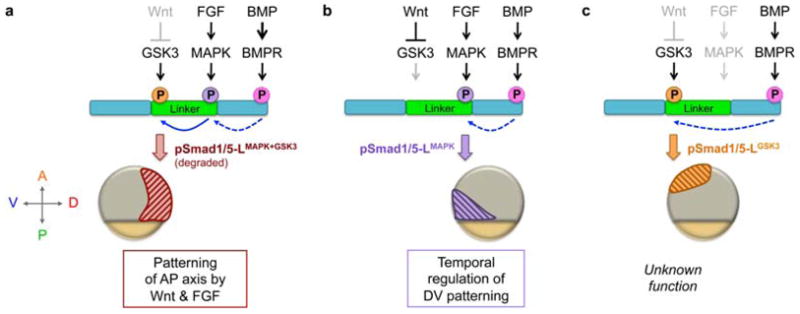
Model of spatially restricted functions of distinct phosphorylated Smad1/5 linker forms, depicted in mid-gastrula stage embryos. The N- and C-termini of Smad1/5 are shown in teal and the linker region is in green. C-terminal phosphorylation of Smad1/5 (Ct-pSmad1/5) a prerequisite to linker phosphorylation (dashed blue arrow). (a) In dorsal regions, MAPK and GSK3 sequentially phosphorylate the Smad1/5 linker (solid blue arrow), leading to pSmad1/5-LMAPK+GSK3 degradation and blockage of BMP signaling. This mechanism may be most important during blastula stages when BMP signaling is more widespread and present dorsally (Section 3.1). (b) In ventral-posterior regions both FGF and Wnt are present, activating MAPK and inhibiting GSK3, respectively, to generate pSmad1/5-LMAPK. pSmad1/5-LMAPK may have reduced activity, which regulates timing of DV patterning. During epiboly, pSmad1/5-LMAPK localizes progressively more posteriorly with the margin, patterning anterior DV tissues in its wake (not shown). (c) In animal regions GSK3 is uninhibited by Wnt, resulting in pSmad1/5-LGSK3, though its significance remains unknown. Since pSmad1/5-LGSK3 does not overlap with pSmad1/5-LMAPK, it is possible that in the zebrafish embryo (versus in vitro studies) either GSK3 does not require MAPK to prime the linker or those residues may be rapidly de-phosphorylated.
Since the signaling molecules that direct AP and DV patterning converge on Smad1/5 phosphorylation at distinct sites (with either inhibitory or permissive effects, respectively), differential Smad1/5 phosphorylation could coordinate the timing of DV and AP patterning (Fig. 5) [203]. Antibody staining of mid- to late gastrula stage embryos for the three distinct Smad1/5 phosphorylation states reveals that each is spatially restricted: Ct-pSmad1/5 is only observed ventrally, pSmad1/5-LMAPK is localized ventral-vegetally, and pSmad1/5-LGSK3 is predominantly restricted to ventral-animal regions (Fig. 5) [129]. This spatial restriction is wholly dependent on the Smad1/5 phosphorylation state since Smad1/5 (phosphorylated and un-phosphorylated) is uniformly present across the embryo [129]. Furthermore, the more vegetal localization of pSmad1/5-LMAPK overlaps with the region of active BMP patterning at the margin (Fig. 2), while the animal localization of pSmad1/5-LGSK3 does not; therefore only FGF/MAPK activity is favorably positioned to temporally regulate BMP signaling.
Strikingly, experiments with mRNA encoding a human Smad1 resistant to MAPK phosphorylation (hSmad1-MM) disrupt coordinated DV and AP patterning. In zebrafish embryos deficient for endogenous Smad5, mis-expressed wild-type hSmad1 fully rescues the embryo, whereas hSmad1-MM results in anterior and posterior tissue markers being patterned by BMP 30 minutes earlier than normal [129]. This indicates that pSmad1/5-LMAPK regulates the timing of DV patterning; presumably, pSmad1/5-LMAPK slows or inhibits the cellular response to BMP signaling by 30-minutes to ensure that AP and DV patterning occur simultaneously (Fig. 5b).
Exclusive MAPK phosphorylation of the Smad1/5 linker in the ventral-vegetal region is consistent with known FGF and Wnt activity at the margin. While FGF induces pSmad1/5-LMAPK, Wnt inhibits additional pSmad1/5-LGSK3 possibly preventing pSmad1/5 degradation (Fig. 5b). Conversely, at the animal pole, the absence of both FGF and Wnt signaling results in pSmad1/5-LGSK3 localized animally (Fig. 5c). Thus, the localization of each phosphorylated Smad1/5 linker state is an amalgamation of the spatial distributions of BMP, FGF, and Wnt activity, providing a mechanism to coordinate AP and DV. This parallels regulatory mechanisms used by other TGFβ family members, which maximize different Smad2 or Smad3 phosphorylation states for distinct functions [204,205]. However, other mechanisms likely also modulate the temporal function of BMP signaling since DV tissues continue to be patterned progressively with hSmad1-MM despite being precocious by 30 minutes. For example, temporal regulation of chromatin state could also contribute to the progressive patterning of DV tissues.
4.5. BMP patterning of anterior neural tissues offers an additional setting to study spatiotemporal mechanisms that regulate BMP signaling
Although BMP signaling must be inhibited for neural tissue to be induced (Fig 1a and 1c) [206], recent studies have revealed additional spatial and temporal complexities in the role of BMP signaling in patterning distinct neural fates. First, BMP actively patterns the anterior neural ectoderm (i.e. inhibiting eye specification to protect forebrain telencephalon fates). Based on small molecule BMP receptor inhibitor studies, BMP patterns the forebrain during late blastula to early gastrula stages (though see a discussion of timing delays in Section 7.2), thus preceding the function of Wnt antagonists in forebrain specification [207]. Since BMP also restricts forebrain markers dorsally during the same time interval (Fig. 3b – b′), studies to address the finer spatial domain of BMP activity in conjunction with the role of distinct activity levels in forebrain patterning are needed.
Second, the boundary of neural and nonneural ectoderm presents additional spatial and temporal challenges for BMP signaling regulation. Distinct levels of BMP signaling may pattern the neural crest (NC) and preplacodal ectoderm (PPE), which gives rise to sensory organs like the inner ear and olfactory epithelium (Fig. 1a) [137,148,208]. BMP signaling would need to be tightly regulated to generate such distinct domains in this very narrow region and BMPER (Section 3.2) may play a key role in this process [146]. Furthermore, PPE patterning requires two contrary phases of BMP signaling: at late blastula stages, BMP signaling specifies PPE precursors, while at late gastrula stages BMP antagonists must block BMP signaling for further PPE development [147]. Thus, patterning of the forebrain, PPE, and NC present unique environments to study spatial and temporal mechanisms (e.g. expression of competency factors) that could regulate BMP signaling and BMP antagonist activity to pattern the most anterior regions of the embryo.
5. Do the morphogenetic movements of gastrulation impact cell fate specification?
The previous section discusses recent progress in understanding basic spatiotemporal features of AP and DV patterning: both AP and DV cell fates are 1) progressively patterned along the AP axis, 2) patterned in a coordinated manner by an identical patterning clock, which is 3) mediated in part by FGF phosphorylation of the Smad1/5 linker in ventral regions of the embryo (Section 4). However, this coordinated AP and DV patterning takes place during the dynamic and rapid process of gastrulation. Gastrulation shapes the germ layers of the embryo through the conserved morphogenetic movements of cell internalization, epiboly, convergence, and extension, all of which result in dramatic cell movements and rearrangements of cellular contacts [209]. The relationship between these morphogenetic movements and concurrent AP and DV cell fate specification is key to fully understand these processes, yet this relationship is complex and requires further investigation.
5.1. Morphogenetic movements and AP and DV signaling and patterning
Evidence suggests that Wnt, Nodal, FGF, and BMP signaling can direct morphogenetic cell movements independently of their roles in cell specification [210]. For example, the BMP signaling gradient, in addition to DV fate specification, also directs domains of distinct convergent extension movements [211], possibly through the regulation of cell-cell adhesion [212]. However, since cell fate specification is difficult to truly uncouple from cell behavior experimentally, differential cell movements may still be a result of DV cell specification. Alternatively, the DV positional information supplied by the BMP gradient may independently inform cell movements [209]. Further studies are needed to distinguish between these two possibilities. There are similar studies and open questions concerning AP patterning and gastrulation associated with Nodal signaling [209].
Some cells dramatically change their position during dorsal convergence and extension and are exposed to different levels of morphogen signaling. It remains mostly unknown whether these cells are already specified, bring their fate with them, and are refractory to the new signaling environment they move through. Interestingly, BMP signaling patterns prospective head DV tissues prior to the major cell movements of dorsal convergence. Thus these rostral cells are expected to sense the same BMP signaling level during the first half of gastrulation and are specified at the time they converge dorsally (Section 4.2 and Fig. 3) [139]. In other contexts cells may be specified by morphogen signals during discrete time windows, responding to gradient thresholds, or may measure signal over a window of time as they move through a gradient. Lastly, cells may require exposure to multiple morphogen signaling levels for their specification. Further studies are required to decipher precisely the relationships between cell movements, morphogen gradients, and cell specification and the mechanisms that intertwine these processes.
5.2. How do changes in DV signaling pole proximity and gastrulation cell movements affect gradient formation?
Gastrulation from fish to mammals entails dramatic rearrangements in cellular contacts. Though the types of cell movements during gastrulation are diverse, each type consistently results in a change in cell-cell contacts, which may impact the functionality of morphogen gradients [209]. A clear example arises when considering the BMP gradient during gastrulation in the zebrafish (Fig. 2 and 6, yellow arrows). Gastrulation begins at the vegetal margin of the zebrafish embryo (50% epiboly) where the ventral-most cells, which have the highest levels of BMP signaling, are the farthest possible distance (~675 μm, embryo diameter) from the dorsal-most cells, which have no BMP signaling (Fig. 6a, compare white and black asterisks). As gastrulation and epiboly proceed, the margin progresses vegetally (posteriorly) and the ventral- and dorsal-most cells continuously move closer to each other until they eventually meet (100% epiboly) (Fig. 6b – d). The distance between these cells of opposing signals drastically decreases from ~675 μm at the onset of gastrulation to their direct apposition in the tailbud at the end of gastrulation. Thus, the cells presumed to have the highest and lowest levels of BMP signaling progressively converge until meeting in the tailbud. This dramatic increase in the proximity of cells with opposing signal may have profound effects on the shape of the BMP morphogen gradient during gastrulation [159,213].
Fig. 6.
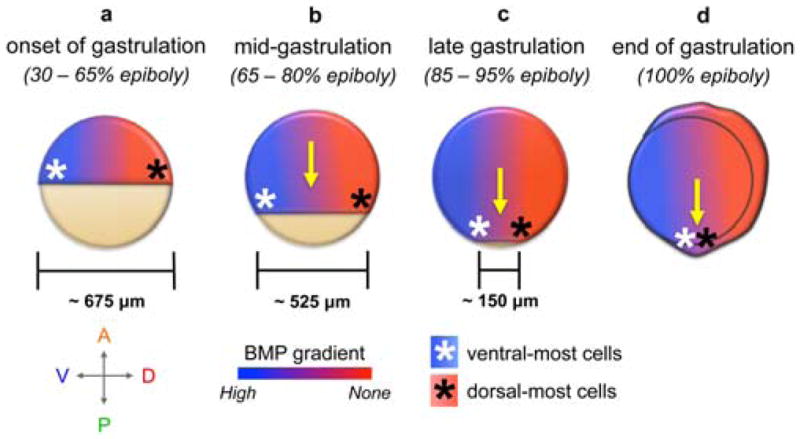
During gastrulation, there is a dramatic decrease in distance between the ventral- and dorsal-most cells. (a) From late blastula to early gastrula stages (4 – 6 hpf), the ventral-most cells (white asterisk), which have the highest levels of BMP signaling, are farthest (approximately 675 μm) from the dorsal-most cells (black asterisk), which have the lowest levels of BMP signaling. (b – d) As gastrulation proceeds (6 – 10hpf), epiboly movements advance the margin posteriorly (yellow arrow) and the distance between the ventral- and dorsal-most cells decreases rapidly until, by the end of epiboly, they are in direct contact.
Epiboly likely plays a key role in regulating the temporal patterning of DV tissues through FGF signaling. As epiboly proceeds, pSmad1/5-LMAPK is localized to progressively more posterior (vegetal) regions (Fig. 5b), which would enable both the temporal progressivity and coordination of DV and AP tissue patterning [129]. Future studies are needed to address whether these changing signaling contexts (i.e. the proximity of cells with opposing signals or the progressively posterior restriction of pSmad1/5-LMAPK) are a principal spatial mechanism to direct the shape and timing of morphogen gradients throughout gastrulation.
5.3. Morphogenetic movements reorganize the DV axis established by the onset of gastrulation
It is worth noting that, due to the massive cell movements during gastrulation, the dorsal-ventral axis defined in late blastula/early gastrula stages (Fig. 1a) is distinct from the dorsoventral axis of the post-gastrula embryo, which has a body plan that resembles the mature organism. That is, while the broad territories of the embryo can be mapped by the onset of gastrulation (Fig. 1a), these tissues are dramatically reorganized during gastrulation and neurulation [214,215]. In particular, dorsal convergence combined with extension along the AP axis results in many tissues being oriented along the AP axis after gastrulation and during somitogenesis. However, this does not mean that there is only one axis [216–219]. Prior to the onset of gastrulation, these tissues are oriented along a coordinate orthogonal to the AP axis, i.e. the DV axis in Fig. 1a (gray arrows). For example, by the onset of gastrulation the somites are oriented along the DV axis of the early gastrula (Fig. 1a) and this orientation informs the organization of the somites along the AP axis: dorsal somitic mesoderm develops into more anterior somites, while ventral somitic mesoderm develops into more posterior somites. Moreover, the epidermis is specified by BMP signaling ventrally during gastrulation, but later is present throughout all regions of the embryo; the neural crest is specified by BMP signaling in lateral regions of the gastrula embryo, but comes to lie dorsally in the neural tube following neurulation. Thus, the DV axis of the early gastrula is an independent coordinate system to that of the post-gastrula and neurula embryo.
Furthermore, visualization of morphogen gradients (Section 7.1) demonstrates that there are indeed two orthogonal axes of the embryo at the onset of gastrulation. Gradients of Nodal [83,220] and Wnt [20,221] signaling are observed along the AP axis and a gradient of BMP signaling is observed orthogonally, revealing two distinct axial coordinate systems (Fig. 1a and 1c) [129,139]. Moreover, AP patterning continues in the absence of DV patterning (e.g. in BMP loss-of-function contexts), making evident the independent patterning of these axes (Fig. 3 and 4). Thus, the DV and AP axes are essential coordinates for cell fate specification and patterning of the body plan during gastrulation. Organization of tissues along the AP axis at the end of gastrulation results from integrating orthogonal morphogen gradients with dorsal convergence and extension morphogenetic movements. Further studies are needed to understand how these distinct axes are integrated to coordinate progressive patterning of the embryo (Section 4) and how they account for cell movements.
6. What is the role of autoregulatory feedback loops in regulating and/or coordinating AP and DV patterning?
Another aspect of AP and DV patterning that remains to be fully characterized is the role of, and crosstalk between, the autoregulatory transcriptional feedback mechanisms that are activated by AP and DV signaling. Across zebrafish, Xenopus, and mouse, there are known feedback mechanisms that regulate FGF, Nodal, and BMP signaling. FGF and Nodal signaling transcriptionally activate their respective inhibitors. FGF signaling induces the expression of sprouty (spry) and Spry proteins comprise a major class of FGF/RTK inhibitors [46,222]. Nodal signaling induces the expression of Antivin/Lefty proteins, which antagonize Nodal signaling [84,85,223,224].
BMP signaling is regulated by multiple feedback mechanisms. High levels of BMP signaling ventrally promote bmp, tld, tsg, and cv-2 expression, all BMP-promoting factors [130,170,171,213]. However, high BMP signaling also induces szl, which antagonizes BMP signaling by inhibiting Tld/Bmp1a cleavage of Chd [225,226]. High levels of BMP signaling also repress chd expression, restricting it to dorsal regions [227]. Additionally, there is a recent report in zebrafish that factors in extraembryonic tissues can initiate a positive feedback loop on BMP signaling [228]. BMP signaling also induces the expression of bambi, which encodes a transmembrane protein implicated in attenuating BMP signaling [146,229–231], although loss-of-function studies have yet to determine a role for bambi in DV patterning [232]. Importantly, since all of these feedback loops are active after the onset of gastrulation [127,128,130,132,137] they regulate and maintain the BMP signaling gradient rather than establish it.
Transcriptional feedback may be integral to regulate and/or shape FGF, Nodal and BMP signaling gradients. Indeed, studies applying mathematical models support a key role for both activating and inhibitory feedback loops in stabilizing and refining morphogen gradients for pattern formation [90,145,174,233–236]. However, the requirement for these feedback loops and whether they primarily confer robustness to the morphogen gradient or serve to refine the AP and DV pattern remain unknown. It would also be interesting to consider the changing environment of the gastrula embryo when modeling the role of feedback regulation. Furthermore, the coordination of feedback mechanisms either within or between signaling pathways remains unknown and could represent an additional mechanism to coordinate AP and DV patterning.
7. Current and emergent methods of spatial and temporal manipulation of AP and DV patterning
In this section we focus primarily on in vivo genetic and fluorescent visualization approaches, the majority of which have been developed in zebrafish.
7.1. Morphogen visualization and use of reporters
A major difficulty in studying the morphogens that pattern the AP and DV axes is that they are secreted and difficult to visualize by immunostaining at endogenous levels. Most studies of ligand expression and dynamics rely on overexpression of fluorescently labeled constructs [38,57,90,142] or the use of antibodies that recognize the immature ligand, as opposed to its fully processed form [144,145]. While the endogenous RA gradient has recently been visualized and quantified [104], that approach relies on FRET from a direct ligand-receptor interaction, which is less applicable for the Wnt, FGF, and BMP gradients since these ligands signal through more complex mechanisms (i.e. ligand bound to receptor may not be indicative of active signaling depending on the presence of co-receptors or complex stoichiometry). However, the advent of CRISPR/Cas9 genome editing offers a new approach to tag these ligands at their endogenous loci and even employ signal amplification techniques to visualize the endogenous morphogen gradient in fixed or live samples [237–239]
As secreted factors, the mRNA expression domains of these morphogens may inform gradient formation, yet this role remains uncharacterized. In situ transcript detection methods are qualitative and describe a transcript domain spatially, but do not provide quantitative data of transcript levels. However, recent advances in quantitative, single-molecule RNA transcript detection offer new possibilities [240–242]. For example, there are reports that bmp ligand expression during gastrulation is graded across the ventral half embryo, with highest expression ventrally and lowest expression laterally [144,178], while others do not report a difference in bmp expression [127,128,130,137,243]. Quantitative transcript detection can directly determine if graded bmp ligand expression could contribute to the BMP morphogen gradient.
A complementary approach to ligand visualization is the visualization of downstream readouts of the morphogen. For example, the intracellular transducer of BMP signaling is nuclear pSmad1/5, which can be directly visualized by immunofluorescence [129,139,244,245]. There are also various transgenic reporters in zebrafish for Wnt [221,246,247], FGF [248], Nodal [83], RA [99,249], and BMP signaling [144,250–252]. These reporter transgenes utilize sequences from a promoter that responds to the morphogen signal to drive reporter expression e.g. of luciferase or GFP. But, how rapidly the reporter is expressed after signal induction and how long the reporter signal persists after signal repression must be carefully characterized to determine the responsiveness of each reporter. To visualize morphogen signaling, which can change in a relatively short time period, it is likely best to us rapidly folding (e.g. Venus) or destabilized fluorescent proteins [83,221,246,248,252].
7.2. Methods for temporal manipulation of signaling
Our knowledge of when Wnt, FGF, RA, Nodal and BMP signals are required for AP and DV patterning comes from experiments that activate or inhibit these signals at specific developmental time points. A particularly expedient approach in Xenopus and zebrafish is to incubate embryos in media containing various chemical inhibitors or activators. These pharmacological treatments include SU5402 (inhibits FGFR), LiCl (inhibits the Wnt inhibitor GSK3), DEAB (inhibits RA processing), Dorsomorphin DMH1 (inhibit BMP type I receptors), and RA itself [129]. Although these chemicals have well-characterized direct effects, studies of temporal function must determine the delay between drug application and complete inhibition or activation of signaling. This delay is infrequently defined; instead it is assumed that inhibition or activation ensues immediately after drug application, which may not be the case. Surprisingly, for example, the small molecule BMP signaling inhibitor, DMH1, takes 3 hours to fully inhibit pSmad1/5 during gastrulation [129]. Determining the delay between drug removal and reversal of its impact on signaling activity is also important to define a temporal window or duration of signaling. Furthermore, one must account for the multiple functions of a signaling pathway during development. For example, Wnt establishes the dorsal organizer during mid- to late blastula stages, which is unrelated to its role directing AP patterning during gastrula stages (Sections 2.1 and 3). Therefore, any experiment that aims to understand the posteriorizing role of Wnt signaling must manipulate Wnt signaling after blastula stages.
Another method of temporal manipulation is to generate transgenic lines that can be induced to either inhibit or activate signaling. Previously we discussed hsp70 promoter driven genes that inhibit or activate BMP signaling (Sections 4.1 – 4.3) [129,139,159,192,253]). When using heat shock-inducible transgenes, it is important to determine the appropriate duration of heat shock to activate or inhibit signaling, which can vary from only 10 minutes [159] to as long as an hour [139,192]. Here, too, it is important to factor in the time it takes for signaling to be effectively induced or fully repressed when defining the time of signaling function. Finally, inducing heat shock results in embryo-wide expression of the transgene. To achieve spatially restricted gene control, one may induce local heat shock by sublethal laser irradiation [254] or a microheater [255]. Alternatively, one may transplant cells from the transgenic line into a background without a heat shock transgene and heat shock the entire embryo after transplantation (cell transplantations are described below).
7.3. Methods for spatial manipulation of signaling
Morphogen signaling relies on the restricted localization and differential mobility of the morphogens themselves and their regulators. An elegant, direct, and versatile method to investigate spatial mechanisms is by generating chimeric organisms by grafting or transplanting donor cells into a host embryo [256]. First, cell transplantation or grafting assays can determine the importance of localization by altering the spatial expression of the protein of interest. For example, to determine where tolloid must be expressed to inhibit Chordin function, tld-expressing cells were transplanted into tld mutant embryos. Only cells transplanted into the ventral vegetal region rescued the tld mutant phenotype, thus revealing the region where Tld cleaves Chd to promote BMP signaling [159]. Second, cell transplantation assays can be used to further evaluate whether a signaling factor functions directly at a distance. Such studies clearly established that the zebrafish Nodal signal, Squint, functions directly on its gene targets at a distance and therefore is a morphogen [76].
Additionally, the cell transplantation approach may be extended to address questions not only of space but also of time. As noted in Section 7.2, cells from heat shock-inducible transgenic lines may be used as transplant donors to incorporate temporal and spatial control of gene expression [192,253]. Furthermore, transplantation of various regions of the zebrafish blastula-gastrula margin has revealed there are distinct cell fate organizing centers in the margin, and that these organizing centers are fully active by the onset of gastrulation, including the one that specifies tail tissue [80,81].
7.4. Conclusion
Establishing the vertebrate body plan requires the coordination and integration of AP and DV axial patterning across the entire length of the embryo and over multiple developmental stages. The spatiotemporal regulation of this process is complex, but it can reveal the essential and conserved mechanisms used to generate and maintain morphogen signaling gradients. With the advent of quantitative measurement and visualization techniques, we are closer to understanding the mechanisms that drive patterning and body plan formation.
Acknowledgments
We would like to thank Allison Jamieson-Lucy and Benjamin Tajer for comments and careful reading of the manuscript. We would also like to acknowledge grants from the National Institutes of Health R01-GM56326-16 (MCM), T32-GM007229 (FBT), and F31-GM113362-01 (FBT).
Footnotes
In Xenopus, Smad1 is the primary transducer of BMP signaling during gastrulation, while in zebrafish smad5 functions predominantly over smad1 [125–128]. Since Smad1 and Smad5 have equivalent phosphorylation sites and ventralizing activity [129], they will be referred to as Smad1/5 in this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolpert L. Positional information and the spatial pattern of cellular differentiation. Journal of Theoretical Biology. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 2.Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, et al. Gene Regulatory Logic for Reading the Sonic Hedgehog Signaling Gradient in the Vertebrate Neural Tube. Cell. 2012;148:273–84. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamse J, Sive H. Vertebrate anteroposterior patterning: the Xenopus neurectoderm as a paradigm. Bioessays. 2000;22:976–86. doi: 10.1002/1521-1878(200011)22:11<976::AID-BIES4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 5.Gawantka V, Pollet N, Delius H, Vingron M, Pfister R, Nitsch R, et al. Gene expression screening in Xenopus identifies molecular pathways, predicts gene function and provides a global view of embryonic patterning. Mechanisms of Development. 1998;77:95–141. doi: 10.1016/s0925-4773(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 6.Grinblat Y, Gamse J, Patel M, Sive H. Determination of the zebrafish forebrain: induction and patterning. Development. 1998;125:4403–16. doi: 10.1242/dev.125.22.4403. [DOI] [PubMed] [Google Scholar]
- 7.Darnell DK, Stark MR, Schoenwolf GC. Timing and cell interactions underlying neural induction in the chick embryo. Development. 1999;126:2505–14. doi: 10.1242/dev.126.11.2505. [DOI] [PubMed] [Google Scholar]
- 8.Knoetgen H, Viebahn C, Kessel M. Head induction in the chick by primitive endoderm of mammalian, but not avian origin. Development. 1999;126:815–25. doi: 10.1242/dev.126.4.815. [DOI] [PubMed] [Google Scholar]
- 9.Rowan AM, Stern CD, Storey KG. Axial mesendoderm refines rostrocaudal pattern in the chick nervous system. Development. 1999;126:2921–34. doi: 10.1242/dev.126.13.2921. [DOI] [PubMed] [Google Scholar]
- 10.Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Current Biology. 1996;6:1487–96. doi: 10.1016/s0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- 11.Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D’Apice MR, Nigro V, et al. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. The EMBO Journal. 1993;12:2735–47. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green D, Whitener AE, Mohanty S, Lekven AC. Vertebrate nervous system posteriorization: Grading the function of Wnt signaling. Dev Dyn. 2014;244:507–12. doi: 10.1002/dvdy.24230. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–24. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- 14.Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–77. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- 15.Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harbor Perspectives in Biology. 2013;5:a007955. doi: 10.1101/cshperspect.a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes & Development. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Glinka A, Wu W, Delius H, Blumenstock C, Niehrs C, Monaghan AP. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 18.Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-Dependent Phosphorylation during Vertebrate Axis Specification. Developmental Cell. 2010;19:521–32. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C-H, Oda T, Itoh M, Di Jiang, Artinger KB, Chandrasekharappa SC, et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–6. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsky RI, Itoh M, Moon RT, Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–47. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- 21.Heasman J, Kofron M, Wylie C. β-Catenin Signaling Activity Dissected in the Early Xenopus Embryo: A Novel Antisense Approach. Dev Biol. 2000;222:124–34. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu T, Bae Y-K, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–41. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Developmental Cell. 2001;1:103–14. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Shinagawa A. Marked Alteration at Midblastula Transition in the Effect of Lithium on Formation of the Larval Body Pattern of Xenopus laevis. Dev Growth Differ. 1989;31:531–41. doi: 10.1111/j.1440-169X.1989.00531.x. [DOI] [PubMed] [Google Scholar]
- 25.Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, et al. Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–309. doi: 10.1242/dev.02295. [DOI] [PubMed] [Google Scholar]
- 26.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–56. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh K, Sokol SY. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mechanisms of Development. 1997;61:113–25. doi: 10.1016/s0925-4773(96)00627-2. [DOI] [PubMed] [Google Scholar]
- 28.Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the Lateral Neural Plate Is Dependent on a Wnt-Mediated Signal from Posterior Nonaxial Mesoderm. Dev Biol. 1999;212:366–80. doi: 10.1006/dbio.1999.9319. [DOI] [PubMed] [Google Scholar]
- 29.McGrew LL, Lai C-J, Moon RT. Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin. Dev Biol. 1995;172:337–42. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- 30.McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mechanisms of Development. 1997;69:105–14. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 31.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 32.Rhinn M, Lun K, Luz M, Werner M, Brand M. Positioning of the midbrain-hindbrain boundary organizer through global posteriorization of the neuroectoderm mediated by Wnt8 signaling. Development. 2005;132:1261–72. doi: 10.1242/dev.01685. [DOI] [PubMed] [Google Scholar]
- 33.Nordström U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–32. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- 34.Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–83. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- 35.Woo K, Fraser SE. Specification of the zebrafish nervous system by nonaxial signals. Science. 1997;277:254–7. doi: 10.1126/science.277.5323.254. [DOI] [PubMed] [Google Scholar]
- 36.Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–65. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- 37.Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11:1265–71. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 38.Stanganello E, Hagemann AIH, Mattes B, Sinner C, Meyen D, Weber S, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- 39.Pownall ME, Isaacs HV. FGF Signalling in Vertebrate Development. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 40.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–42. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–70. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 42.Draper BW, Stock DW, Kimmel CB. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–54. doi: 10.1242/dev.00671. [DOI] [PubMed] [Google Scholar]
- 43.Griffin KJP, Kimelman D. Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development. Dev Biol. 2003;264:456–66. doi: 10.1016/j.ydbio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Christen B, Slack JMW. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev Biol. 1997;192:455–66. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- 45.Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. The EMBO Journal. 1994;13:4469. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason JM, Morrison DJ, Albert Basson M, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 48.Reifers F, Böhli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–95. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- 49.Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–36. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- 50.Cox WG, Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995;121:4349–58. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- 51.Doniach T. Basic FGF as an inducer of anteroposterior neural pattern. Cell. 1995;83:1067–70. doi: 10.1016/0092-8674(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 52.Kengaku M, Okamoto H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development. 1995;121:3121–30. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- 53.Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–92. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labalette C, Bouchoucha YX, Wassef MA, Gongal PA, Le Men J, Becker T, et al. Hindbrain patterning requires fine-tuning of early krox20 transcription by Sprouty 4. Development. 2011;138:317–26. doi: 10.1242/dev.057299. [DOI] [PubMed] [Google Scholar]
- 55.Dyer C, Blanc E, Hanisch A, Roehl H, Otto GW, Yu T, et al. A bi-modal function of Wnt signalling directs an FGF activity gradient to spatially regulate neuronal differentiation in the midbrain. Development. 2014;141:63–72. doi: 10.1242/dev.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scholpp S, Brand M. Endocytosis controls spreading and effective signaling range of Fgf8 protein. Current Biology. 2004;14:1834–41. doi: 10.1016/j.cub.2004.09.084. [DOI] [PubMed] [Google Scholar]
- 57.Yu SR, Burkhardt M, Nowak M, Ries J, Petrášek Z, Scholpp S, et al. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–6. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]
- 58.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC Protein One-Eyed Pinhead Is Essential for Nodal Signaling. Cell. 1999;97:121–32. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 59.Schier AF. Axis formation and patterning in zebrafish. Curr Opin Genet Dev. 2001;11:393–404. doi: 10.1016/s0959-437x(00)00209-4. [DOI] [PubMed] [Google Scholar]
- 60.Schier AF, Shen MM. Nodal signaling in vertebrate development. Nature. 2000;403:385–9. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 61.Schier AF, Talbot WS. Molecular Genetics of Axis Formation in Zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 62.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 63.Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–5. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 64.Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci USa. 1998;95:9932–7. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- 66.Smith WC, McKendry R, Ribisi S, Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 67.Joseph EM, Melton DA. Xnr4: a Xenopus nodal-related gene expressed in the Spemann organizer. Dev Biol. 1997;184:367–72. doi: 10.1006/dbio.1997.8510. [DOI] [PubMed] [Google Scholar]
- 68.Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES. Maternally controlled beta-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- 69.Toyama R, O’Connell ML, Wright CV, Kuehn MR, Dawid IB. Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development. 1995;121:383–91. doi: 10.1242/dev.121.2.383. [DOI] [PubMed] [Google Scholar]
- 70.Erter CE, Solnica-Krezel L, Wright CV. Zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev Biol. 1998;204:361–72. doi: 10.1006/dbio.1998.9097. [DOI] [PubMed] [Google Scholar]
- 71.Gritsman K, Talbot WS, Schier AF. Nodal signaling patterns the organizer. Development. 2000;127:921–32. doi: 10.1242/dev.127.5.921. [DOI] [PubMed] [Google Scholar]
- 72.Kimelman D, Griffin KJ. Vertebrate mesendoderm induction and patterning. Curr Opin Genet Dev. 2000;10:350–6. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- 73.Green JB, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–9. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- 74.Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–83. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schier AF. Nodal Morphogens. Cold Spring Harbor Perspectives in Biology. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Schier AF. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–10. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- 77.Feldman B, Concha ML, Saúde L, Parsons MJ, Adams RJ, Wilson SW, et al. Lefty antagonism of Squint is essential for normal gastrulation. Curr Biol. 2002;12:2129–35. doi: 10.1016/s0960-9822(02)01361-1. [DOI] [PubMed] [Google Scholar]
- 78.Thisse B, Wright CV, Thisse C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature. 2000;403:425–8. doi: 10.1038/35000200. [DOI] [PubMed] [Google Scholar]
- 79.Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signaling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–9. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- 80.Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–52. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- 81.Fauny J-D, Thisse B, Thisse C. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of Nodal to BMP activity. Development. 2009;136:3811–9. doi: 10.1242/dev.039693. [DOI] [PubMed] [Google Scholar]
- 82.Mathieu J, Griffin K, Herbomel P, Dickmeis T, Strähle U, Kimelman D, et al. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development. 2004;131:629–41. doi: 10.1242/dev.00964. [DOI] [PubMed] [Google Scholar]
- 83.Harvey SA, Smith JC. Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol. 2009;7:e1000101. doi: 10.1371/journal.pbio.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–40. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- 85.Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development. 1999;126:3253–62. doi: 10.1242/dev.126.14.3253. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y, Schier AF. Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol. 2002;12:2124–8. doi: 10.1016/s0960-9822(02)01362-3. [DOI] [PubMed] [Google Scholar]
- 87.Chen C, Shen MM. Two modes by which Lefty proteins inhibit nodal signaling. Curr Biol. 2004;14:618–24. doi: 10.1016/j.cub.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 88.Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1952;237:37–72. [Google Scholar]
- 89.Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. Bioessays. 2000;22:753–60. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 90.Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, et al. Differential Diffusivity of Nodal and Lefty Underlies a Reaction-Diffusion Patterning System. Science. 2012;336:721–4. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rhinn M, Dollé P. Retinoic acid signaling during development. Development. 2012;139:843–58. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 92.Linville A, Radtke K, Waxman JS, Yelon D, Schilling TF. Combinatorial roles for zebrafish retinoic acid receptors in the hindbrain, limbs and pharyngeal arches. Dev Biol. 2009;325:60–70. doi: 10.1016/j.ydbio.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, et al. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–4. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 94.Grandel H, Lun K, Rauch G-J, Rhinn M, Piotrowski T, Houart C, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–65. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- 95.Begemann G, Marx M, Mebus K, Meyer A, Bastmeyer M. Beyond the neckless phenotype: influence of reduced retinoic acid signaling on motor neuron development in the zebrafish hindbrain. Dev Biol. 2004;271:119–29. doi: 10.1016/j.ydbio.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 96.Schilling TF. Anterior-posterior patterning and segmentation of the vertebrate head. Integr Comp Biol. 2008;48:658–67. doi: 10.1093/icb/icn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maves L, Kimmel CB. Dynamic and sequential patterning of the zebrafish posterior hindbrain by retinoic acid. Dev Biol. 2005;285:593–605. doi: 10.1016/j.ydbio.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 98.Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–22. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White RJ, Nie Q, Lander AD, Schilling TF. Complex Regulation of cyp26a1 Creates a Robust Retinoic Acid Gradient in the Zebrafish Embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–46. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- 101.Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–87. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White RJ, Schilling TF. How degrading: Cyp26s in hindbrain development. Dev Dyn. 2008;237:2775–90. doi: 10.1002/dvdy.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai AQ, Radtke K, Linville A, Lander AD, Nie Q, Schilling TF. Cellular retinoic acid-binding proteins are essential for hindbrain patterning and signal robustness in zebrafish. Development. 2012;139:2150–5. doi: 10.1242/dev.077065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimozono S, Iimura T, Kitaguchi T, Higashijima S-I, Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013:1–5. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- 105.Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and β-catenin. Current Biology. 1998;8:591–4. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 106.Schroeder KE, Yost HJ. Xenopus poly (A) binding protein maternal RNA is localized during oogenesis and associated with large complexes in blastula. Dev Genet. 1996;19:268–76. doi: 10.1002/(SICI)1520-6408(1996)19:3<268::AID-DVG10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 107.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. Maternal Wnt11 Activates the Canonical Wnt Signaling Pathway Required for Axis Formation in Xenopus Embryos. Cell. 2005;120:857–71. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 108.Moon RT, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays. 1998;20:536–45. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 109.Onichtchouk D, Glinka A, Niehrs C. Requirement for Xvent-1 and Xvent-2 gene function in dorsoventral patterning of Xenopus mesoderm. Development. 1998;125:1447–56. doi: 10.1242/dev.125.8.1447. [DOI] [PubMed] [Google Scholar]
- 110.Melby AE, Clements WK, Kimelman D. Regulation of dorsal gene expression in Xenopus by the ventralizing homeodomain gene Vox. Dev Biol. 1999;211:293–305. doi: 10.1006/dbio.1999.9296. [DOI] [PubMed] [Google Scholar]
- 111.Trindade M, Tada M, Smith JC. DNA-binding specificity and embryological function of Xom (Xvent-2) Dev Biol. 1999;216:442–56. doi: 10.1006/dbio.1999.9507. [DOI] [PubMed] [Google Scholar]
- 112.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flores MVC, Lam EYN, Crosier KE, Crosier PS. Osteogenic transcription factor Runx2 is a maternal determinant of dorsoventral patterning in zebrafish. Nat Cell Biol. 2008;10:346–52. doi: 10.1038/ncb1697. [DOI] [PubMed] [Google Scholar]
- 114.Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB. Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci USa. 2000;97:12121–6. doi: 10.1073/pnas.97.22.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu T, Yamanaka Y, Nojima H, Yabe T, Hibi M, Hirano T. A novel repressor-type homeobox gene, ved, is involved in dharma/bozozok-mediated dorsal organizer formation in zebrafish. Mechanisms of Development. 2002;118:125–38. doi: 10.1016/s0925-4773(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 116.Melby AE, Beach C, Mullins M, Kimelman D. Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol. 2000;224:275–85. doi: 10.1006/dbio.2000.9780. [DOI] [PubMed] [Google Scholar]
- 117.Dutko JA, Mullins MC. SnapShot: BMP signaling in development. Cell. 2011;145:636–636. e1–2. doi: 10.1016/j.cell.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–43. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 120.Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–79. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 121.Nikaido M, Tada M, Takeda H, Kuroiwa A, Ueno N. In vivo analysis using variants of zebrafish BMPR-IA: range of action and involvement of BMP in ectoderm patterning. Development. 1999;126:181–90. doi: 10.1242/dev.126.1.181. [DOI] [PubMed] [Google Scholar]
- 122.Armes NA, Smith JC. The ALK-2 and ALK-4 activin receptors transduce distinct mesoderm-inducing signals during early Xenopus development but do not co-operate to establish thresholds. Development. 1997;124:3797–804. doi: 10.1242/dev.124.19.3797. [DOI] [PubMed] [Google Scholar]
- 123.Feng X-H, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 124.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 125.Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–84. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- 126.Dick A, Meier A, Hammerschmidt M. Smad1 and Smad5 have distinct roles during dorsoventral patterning of the zebrafish embryo. Developmental Dynamics. 1999;216:285–98. doi: 10.1002/(SICI)1097-0177(199911)216:3<285::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 127.Hild M, Dick A, Rauch GJ, Meier A, Bouwmeester T, Haffter P, et al. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–59. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- 128.Kramer C, Mayr T, Nowak M, Schumacher J, Runke G, Bauer H, et al. Maternally Supplied Smad5 Is Required for Ventral Specification in Zebrafish Embryos Prior to Zygotic Bmp Signaling. Dev Biol. 2002;250:263–79. [PubMed] [Google Scholar]
- 129.Hashiguchi M, Mullins MC. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development. 2013;140:1970–80. doi: 10.1242/dev.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmid B, Fürthauer M, Connors SA, Trout J, Thisse B, Thisse C, et al. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–67. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- 131.Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, et al. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–54. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- 132.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–60. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today. 2006;78:224–42. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- 134.Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–85. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- 135.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 136.Knecht AK, Harland RM. Mechanisms of dorsal-ventral patterning in noggin-induced neural tissue. Development. 1997;124:2477–88. doi: 10.1242/dev.124.12.2477. [DOI] [PubMed] [Google Scholar]
- 137.Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- 138.Schumacher JA, Hashiguchi M, Nguyen VH, Mullins MC. An Intermediate Level of BMP Signaling Directly Specifies Cranial Neural Crest Progenitor Cells in Zebrafish. PLoS ONE. 2011;6:e27403. doi: 10.1371/journal.pone.0027403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Developmental Cell. 2008;14:108–19. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faure S, Lee MA, Keller T, Dijke Ten P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917–31. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- 141.Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- 142.Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proceedings of the National Academy of Sciences. 2013;110:20372–9. doi: 10.1073/pnas.1319745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cho G-S, Choi S-C, Han J-K. BMP signal attenuates FGF pathway in anteroposterior neural patterning. Biochem Biophys Res Commun. 2013;434:509–15. doi: 10.1016/j.bbrc.2013.03.105. [DOI] [PubMed] [Google Scholar]
- 144.Ramel M-C, Hill CS. The ventral to dorsal BMP activity gradient in the early zebrafish embryo is determined by graded expression of BMP ligands. Dev Biol. 2013;378:170–82. doi: 10.1016/j.ydbio.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xue Y, Zheng X, Huang L, Xu P, Ma Y, Min Z, et al. Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat Commun. 2014;5:3766. doi: 10.1038/ncomms4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reichert S, Randall RA, Hill CS. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development. 2013;140:4435–44. doi: 10.1242/dev.098707. [DOI] [PubMed] [Google Scholar]
- 147.Kwon H-J, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wawersik S, Evola C, Whitman M. Conditional BMP inhibition in Xenopus reveals stage-specific roles for BMPs in neural and neural crest induction. Dev Biol. 2005;277:425–42. doi: 10.1016/j.ydbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 149.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann Organizer Signal noggin Binds and Inactivates Bone Morphogenetic Protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 150.Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, et al. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mechanisms of Development. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- 151.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–98. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dal-Pra S, Fürthauer M, Van-Celst J, Thisse B, Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol. 2006;298:514–26. doi: 10.1016/j.ydbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Developmental Cell. 2005;8:401–11. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 154.Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, et al. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- 155.Fisher S, Amacher SL, Halpern ME. Loss of cerebum function ventralizes the zebrafish embryo. Development. 1997;124:1301–11. doi: 10.1242/dev.124.7.1301. [DOI] [PubMed] [Google Scholar]
- 156.Fisher S, Halpern ME. Patterning the zebrafish axial skeleton requires early chordin function. Nat Genet. 1999;23:442–6. doi: 10.1038/70557. [DOI] [PubMed] [Google Scholar]
- 157.Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes & Development. 1996;10:2452–61. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- 158.Schulte-Merker SS, Lee KJK, McMahon APA, Hammerschmidt MM. The zebrafish organizer requires chordino. Nature. 1997;387:862–3. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 159.Connors SA, Tucker JA, Mullins MC. Temporal and spatial action of Tolloid (Mini fin) and Chordin to pattern tail tissues. Dev Biol. 2006;293:191–202. doi: 10.1016/j.ydbio.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 160.Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–16. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blader P, Rastegar S, Fischer N, Strähle U. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science. 1997;278:1937–40. doi: 10.1126/science.278.5345.1937. [DOI] [PubMed] [Google Scholar]
- 162.Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–91. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- 163.Muraoka O, Shimizu T, Yabe T, Nojima H, Bae Y-K, Hashimoto H, et al. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–38. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- 164.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–59. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oelgeschlager M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–63. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–83. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 167.Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–8. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- 168.Larraín J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–47. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oelgeschlager M. The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development. 2003;130:4047–56. doi: 10.1242/dev.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Little SC, Mullins MC. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development. 2004;131:5825–35. doi: 10.1242/dev.01464. [DOI] [PubMed] [Google Scholar]
- 171.Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–11. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- 172.Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Developmental Cell. 2008;15:248–60. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, et al. Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev Biol. 2010;337:405–14. doi: 10.1016/j.ydbio.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 174.Inomata H, Shibata T, Haraguchi T, Sasai Y. Scaling of Dorsal-Ventral Patterningby Embryo Size-Dependent Degradation of Spemann’s Organizer Signals. Cell. 2013;153:1296–311. doi: 10.1016/j.cell.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 175.Genikhovich G, Fried P, Prünster MM, Schinko JB, Gilles AF, Fredman D, et al. Axis Patterning by BMPs: Cnidarian Network Reveals Evolutionary Constraints. Cell Rep. 2015;10:1646–54. doi: 10.1016/j.celrep.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maegawa S, Varga M, Weinberg ES. FGF signaling is required for beta-catenin-mediated induction of the zebrafish organizer. Development. 2006;133:3265–76. doi: 10.1242/dev.02483. [DOI] [PubMed] [Google Scholar]
- 177.Fürthauer M, Thisse C, Thisse B. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development. 1997;124:4253–64. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- 178.Fürthauer M, Van-Celst J, Thisse C, Thisse B. Fgf signaling controls the dorsoventral patterning of the zebrafish embryo. Development. 2004;131:2853–64. doi: 10.1242/dev.01156. [DOI] [PubMed] [Google Scholar]
- 179.Fox MD, Bruce AEE. Short- and long-range functions of Goosecoid in zebrafish axis formation are independent of Chordin, Noggin 1 and Follistatin-like 1b. Development. 2009;136:1675–85. doi: 10.1242/dev.031161. [DOI] [PubMed] [Google Scholar]
- 180.Sirotkin HI, Dougan ST, Schier AF, Talbot WS. bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development. 2000;127:2583–92. doi: 10.1242/dev.127.12.2583. [DOI] [PubMed] [Google Scholar]
- 181.Ramel M-C, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- 182.Ramel M-C, Buckles GR, Baker KD, Lekven AC. WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol. 2005;287:237–48. doi: 10.1016/j.ydbio.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 183.Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development. 2001;128:2407–20. doi: 10.1242/dev.128.12.2407. [DOI] [PubMed] [Google Scholar]
- 184.Gilardelli CN, Pozzoli O, Sordino P, Matassi G, Cotelli F. Functional and hierarchical interactions among zebrafish vox/vent homeobox genes. Dev Dyn. 2004;230:494–508. doi: 10.1002/dvdy.20073. [DOI] [PubMed] [Google Scholar]
- 185.Gonzalez EM, Fekany-Lee K, Carmany-Rampey A, Erter C, Topczewski J, Wright CVE, et al. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes & Development. 2000;14:3087–92. doi: 10.1101/gad.852400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leung T, Bischof J, Söll I, Niessing D, Zhang D, Ma J, et al. bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development. 2003;130:3639–49. doi: 10.1242/dev.00558. [DOI] [PubMed] [Google Scholar]
- 187.Solnica-Krezel L, Driever W. The role of the homeodomain protein Bozozok in zebrafish axis formation. Int J Dev Biol. 2001;45:299–310. [PubMed] [Google Scholar]
- 188.bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. 2000;127:2583–92. doi: 10.1242/dev.127.12.2583. [DOI] [PubMed] [Google Scholar]
- 189.Fekany-Lee K, Gonzalez E, Miller-Bertoglio V, Solnica-Krezel L. The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development. 2000;127:2333–45. doi: 10.1242/dev.127.11.2333. [DOI] [PubMed] [Google Scholar]
- 190.Stern CD, Charité J, Deschamps J, Duboule D, Durston AJ, Kmita M, et al. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- 191.Marom K, Levy V, Pillemer G, Fainsod A. Temporal analysis of the early BMP functions identifies distinct anti-organizer and mesoderm patterning phases. Dev Biol. 2005;282:442–54. doi: 10.1016/j.ydbio.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 192.Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–43. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- 193.Tucker AS, Slack JMW. Tail bud determination in the vertebrate embryo. Current Biology. 1995;5:807–13. doi: 10.1016/s0960-9822(95)00158-8. [DOI] [PubMed] [Google Scholar]
- 194.Tucker AS, Slack JMW. The Xenopus laevis tail-forming region. Development. 1995;121:249–62. [Google Scholar]
- 195.Beck CW, Whitman M, Slack JM. The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev Biol. 2001;238:303–14. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- 196.Mintzer KA, Lee MA, Runke G, Trout J, Whitman M, Mullins MC. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–69. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 197.Li Y, Allende ML, Finkelstein R, Weinberg ES. Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mechanisms of Development. 1994;48:229–44. doi: 10.1016/0925-4773(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 198.Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes & Development. 2003;17:3023–8. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes & Development. 2005;19:1022–7. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sapkota G, Alarcón C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell. 2007;25:441–54. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 201.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–93. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–93. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 203.Eivers E, Fuentealba LC, De Robertis EM. Integrating positional information at the level of Smad1/5/8. Curr Opin Genet Dev. 2008;18:304–10. doi: 10.1016/j.gde.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Matsuzaki K. Smad phospho-isoforms direct context-dependent TGF-β signaling. Cytokine Growth Factor Rev. 2013;24:385–99. doi: 10.1016/j.cytogfr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 205.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, et al. Transforming growth factor-β signalling: Role and consequences of Smad linker region phosphorylation. Cellular Signalling. 2013;25:2017–24. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 206.Marchal L, Luxardi G, Thomé V, Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci USa. 2009;106:17437–42. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bielen H, Houart C. BMP Signaling Protects Telencephalic Fate by Repressing Eye Identity and Its Cxcr4-Dependent Morphogenesis. Developmental Cell. 2012;23:812–22. doi: 10.1016/j.devcel.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–20. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- 209.Solnica-Krezel L. Conserved Patterns of Cell Movements during Vertebrate Gastrulation. Current Biology. 2005;15:R213–28. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 210.Heisenberg C-P, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr Opin Genet Dev. 2008;18:311–6. doi: 10.1016/j.gde.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- 212.Hardt von der S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg C-P, et al. The Bmp Gradient of the Zebrafish Gastrula Guides Migrating Lateral Cells by Regulating Cell-Cell Adhesion. Current Biology. 2007;17:475–87. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 213.Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–30. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- 214.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–94. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 215.Kimelman D, Martin BL. Anterior–posterior patterning in early development: three strategies. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1:253–66. doi: 10.1002/wdev.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lane MC, Sheets MD. Designation of the anterior/posterior axis in pregastrula Xenopus laevis. Dev Biol. 2000;225:37–58. doi: 10.1006/dbio.2000.9803. [DOI] [PubMed] [Google Scholar]
- 217.Lane MC, Smith WC. The origins of primitive blood in Xenopus: implications for axial patterning. Development. 1999;126:423–34. doi: 10.1242/dev.126.3.423. [DOI] [PubMed] [Google Scholar]
- 218.Lane MC, Sheets MD. Rethinking axial patterning in amphibians. Developmental Dynamics. 2002;225:434–47. doi: 10.1002/dvdy.10182. [DOI] [PubMed] [Google Scholar]
- 219.Kumano G, Smith WC. Revisions to the Xenopus gastrula fate map: Implications for mesoderm induction and patterning. Developmental Dynamics. 2002;225:409–21. doi: 10.1002/dvdy.10177. [DOI] [PubMed] [Google Scholar]
- 220.Dubrulle J, Jordan BM, Akhmetova L, Farrell JA, Kim S-H, Solnica-Krezel L, et al. Response to Nodal morphogen gradient is determined by the kinetics of target gene induction. Elife. 2015:4. doi: 10.7554/eLife.05042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shimizu N, Kawakami K, Ishitani T. Visualization and exploration of Tcf/Lef function using a highly responsive Wnt/β-catenin signaling-reporter transgenic zebrafish. Dev Biol. 2012;370:71–85. doi: 10.1016/j.ydbio.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 222.Fürthauer M, Reifers F, Brand M, Thisse B, Thisse C. sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. Development. 2001;128:2175–86. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- 223.Cheng AM, Thisse B, Thisse C, Wright CV. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in xenopus. Development. 2000;127:1049–61. doi: 10.1242/dev.127.5.1049. [DOI] [PubMed] [Google Scholar]
- 224.Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–98. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- 225.Yabe T, Shimizu T, Muraoka O, Bae Y-K, Hirata T, Nojima H, et al. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–16. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- 226.Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–16. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- 227.Miller-Bertoglio VE, Fisher S, Sánchez A, Mullins MC, Halpern ME. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol. 1997;192:537–50. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- 228.Sun Y, Tseng W-C, Fan X, Ball R, Dougan ST. Extraembryonic signals under the control of MGA, Max, and Smad4 are required for dorsoventral patterning. Developmental Cell. 2014;28:322–34. doi: 10.1016/j.devcel.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 229.Grotewold L, Plum M, Dildrop R, Peters T, Rüther U. Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mechanisms of Development. 2001;100:327–30. doi: 10.1016/s0925-4773(00)00524-4. [DOI] [PubMed] [Google Scholar]
- 230.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massagué J, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–5. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 231.Tsang M, Kim R, de Caestecker MP, Kudoh T, Roberts AB, Dawid IB. Zebrafish nma is involved in TGFbeta family signaling. Genesis. 2000;28:47–57. doi: 10.1002/1526-968x(200010)28:2<47::aid-gene20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 232.O’Connor MN, Salles II, Cvejic A, Watkins NA, Walker A, Garner SF, et al. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood. 2009;113:4754–62. doi: 10.1182/blood-2008-06-162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 234.Meinhardt H. Models for the generation and interpretation of gradients. Cold Spring Harbor Perspectives in Biology. 2009;1:a001362. doi: 10.1101/cshperspect.a001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Barkai N, Shilo B-Z. Robust generation and decoding of morphogen gradients. Cold Spring Harbor Perspectives in Biology. 2009;1:a001990. doi: 10.1101/cshperspect.a001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee HX, Mendes FA, Plouhinec J-LJ, De Robertis EM. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes & Development. 2009;23:2551–62. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Le Cong, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Meth. 2013;10:957–63. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–9. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gaspar I, Ephrussi A. Strength in numbers: quantitative single-molecule RNA detection assays. Wiley Interdisciplinary Reviews: Developmental Biology. 2015;4:135–50. doi: 10.1002/wdev.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Campbell PD, Chao JA, Singer RH, Marlow FL. Dynamic visualization of transcription and RNA subcellular localization in zebrafish. Development. 2015;142:1368–74. doi: 10.1242/dev.118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gross-Thebing T, Paksa A, Raz E. Simultaneous high-resolution detection of multiple transcripts combined with localization of proteins in whole-mount embryos. BMC Biology. 2014;12:55. doi: 10.1186/s12915-014-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hemmati Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: Expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17:78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- 244.Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engström U, et al. The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434:83–7. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- 245.Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917–31. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- 246.Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–37. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- 247.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 248.Molina GA, Watkins SC, Tsang M. Generation of FGF reporter transgenic zebrafish and their utility in chemical screens. BMC Dev Biol. 2007;7:62. doi: 10.1186/1471-213X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- 250.Korchynskyi O, Dijke ten P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–91. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 251.Monteiro RM, de Sousa Lopes SMC, Korchynskyi O, Dijke ten P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. Journal of Cell Science. 2004;117:4653–63. doi: 10.1242/jcs.01337. [DOI] [PubMed] [Google Scholar]
- 252.Collery RF, Link BA. Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish. Dev Dyn. 2011;240:712–22. doi: 10.1002/dvdy.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Row RH, Kimelman D. Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Dev Biol. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shoji W, Sato-Maeda M. Application of heat shock promoter in transgenic zebrafish. Dev Growth Differ. 2008;50:401–6. doi: 10.1111/j.1440-169X.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 255.Placinta M, Shen M-C, Achermann M, Karlstrom RO. A laser pointer driven microheater for precise local heating and conditional gene regulation in vivo. Microheater driven gene regulation in zebrafish. BMC Dev Biol. 2009;9:73. doi: 10.1186/1471-213X-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kemp HA, Carmany-Rampey A, Moens C. Generating Chimeric Zebrafish Embryos by Transplantation. JoVE. 2009:e1394–4. doi: 10.3791/1394. [DOI] [PMC free article] [PubMed] [Google Scholar]