Capsule Summary
Three new BENTA patients sharing the same novel, autosomal dominant gain-of-function missense mutation in CARD11 (C49Y) provide new insight into the progression of this disorder from childhood to adulthood.
Keywords: BENTA, NF-κB, B cell lymphocytosis, primary immunodeficiency, combined immunodeficiency
To the Editor:
The congenital lymphoproliferative disorder known as BENTA (B cell Expansion with NF-κB and T cell Anergy) was recently described in five patients that all presented with the disease shortly after birth (1, 2). Salient clinical features of BENTA include splenomegaly and profound polyclonal B cell lymphocytosis (above 2200/μl, 60-80% CD19+ in peripheral blood), with elevated transitional and mature naïve B cells but few circulating class-switched/memory B cells. Patients experience frequent ear and sinopulmonary infections early in life, with poor antibody responses noted to pneumococcal and meningococcal capsular polysaccharides. Additionally, T cell hyporesponsiveness ex vivo may be linked to a mild T cell immunodeficiency in some patients presenting with molluscum contagiosum or chronic Epstein-Barr virus (EBV) infection.
BENTA disease is genetically linked to germline-encoded, gain-of-function mutations in CARD11, a scaffolding protein largely expressed in lymphocytes and required for antigen receptor (AgR)-induced NF-κB activation (3, 4). Like somatic mutations found in ~10% of diffuse large B cell lymphomas, CARD11 mutations in BENTA patients fall within or immediately adjacent to the coiled-coil (CC) domain (5). CC mutations likely abrogate the requirement for AgR-triggered phosphorylation of the inhibitory linker domain, which supports the “open” conformational change necessary for BCL10-MALT1 recruitment and downstream signal transduction via the IκB kinase (IKK) complex (6).
We recently encountered three new patients with disease symptoms suggesting BENTA, including moderate, polyclonal B cell lymphocytosis with a markedly diminished memory B cell compartment (Table 1). The first (Pt 1) is an 18-year-old Caucasian female initially evaluated at 13 months of age for splenomegaly (10 cm) with splenic hilar adenopathy, leukocytosis (51,000/μL), neutropenia (3%) and lymphocytosis (91%). Secondary to recurrent upper respiratory tract (URT) infections, additional immunologic evaluation was completed. Quantitative immunoglobulin levels documented a serum IgG level of 756 mg/dL (normal 528-2,190), a serum IgM level of 75 mg/dL (normal 48-226), and serum IgA level of 27 mg/dL (normal 44-441). Antibody responses to tetanus (0.44 IU/mL) and Haemophilus influenzae Type b (0.56 mg/L) vaccine were protective; however, antibody responses to a pneumococcal 13-valent conjugate vaccine were suboptimal (<1.3 μg/mL for the 11 serotypes that were assessed). Mitogenic stimulation was normal for phytohemagglutinin (PHA) (SI 17) and pokeweed mitogen (SI 9), but low for concanavalin A (SI 3, laboratory normal SI >3 for all mitogens). Antigen-specific proliferation was low (tetanus toxoid or Varicella zoster (SI 2, normal >3) or even absent (Candida albicans). She remains healthy aside from recurrent URT infections, splenomegaly (22 cm) with associated moderate thrombocytopenia (platelet count = 48,000/μl (normal = 150-450,000)) and persistent B cell lymphocytosis. The second patient (Pt 2) is an 18-year-old Caucasian male initially diagnosed at 2 years of age as a humoral immunodeficiency patient because of recurrent URT infections, especially otitis media, for which the patient had tympanoplasty. In addition, lower respiratory tract infections with bronchiectasis, splenomegaly (12.5 cm at 5 years of age), and peripheral and central lymphadenopathies were reported in childhood. His Ig serum titers at the age of 4 years before onset of Ig replacement therapy were IgG 993 mg/dl (normal 582-1,220), IgM 138 mg/dl (normal 53-162) and IgA 60 mg/dl (normal 46-203), and anti-tetanus toxoid antibodies were present (0.22 IU/mL). He has received Ig substitution from since age 5 and is well under this treatment, with no evidence of thrombocytopenia (platelet count = 262,000/μl). The third patient (Pt 3) is the 51-year-old Caucasian mother of Pt 2, who is clinically asymptomatic. Childhood medical records were unavailable for this patient; however, she reported several periods of sinusitis in the past. Serum Ig levels were normal for IgG (1,113 mg/dl; normal 549-1,278 mg/dl) and IgA (139 mg/dl; normal 41-344), but low for IgM (32 mg/dl; normal 50-209 mg/dl). T cell responses to PHA were close to normal or normal in Pt2 and Pt3 (SI 46 and 82, respectively, laboratory normal SI>50). Antigen-induced proliferation to tuberculin purified protein derivative was normal in both patients (SI of 10 and 63, respectively, normal >10) but responses to Candida albicans and tetanus toxoid were either absent in Pt2 (SI 0, normal>10) or strongly impaired in Pt3 (SI 2).
Table 1. Phenotype analysis of new BENTA patients.
Ranges for BENTA (based on previous pediatric patients identified (1, 2)) versus normal adults (values from the Department of Laboratory Medicine, NIH) are listed at right for comparison.
| Pt 1 | Pt 2 | Pt 3 | BENTA | Normal
adult range |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marker | % | Abs #/μl |
% | Abs #/μl |
% | Abs #/μl |
% | Abs # | % | Abs # |
| CD19+ B | 82 | 2801 | 50 | 1900 | 48 | 912 | 63.2- 87.4 |
2253- 48262 |
3-19 | 59-329 |
|
Immature/
Transitional B * |
3 | 84 | 2 | 38 | 3 | 27 | 27-35 | 3150- 19570 |
2-11 | 1-36 |
| Memory B | 0 | 0 | 0.1 | 5 | 0.1 | 3 | 0.4-1 | 16-552 | 0.4- 2.3 |
5-46 |
| CD3+ T | 24 | 999 | 44 | 1672 | 42 | 798 | 10- 31.5 |
1682- 5522 |
60-84 | 714-2266 |
| CD4+ T | 14 | 592 | 29 | 1102 | 27 | 513 | 4.8- 17.9 |
622-2651 | 32-62 | 359-1565 |
| CD8+ T | 8 | 342 | 11 | 418 | 8 | 152 | 3.4- 17.9 |
408-1877 | 11-35 | 178-853 |
|
NK
(CD16+CD56+) |
7 | 283 | 6 | 228 | 10 | 190 | 2.3-5.6 | 226-1270 | 6-35 | 126-729 |
| WBC # | 5900 | 7900 | 5600 | 5750- 60810 |
4500- 10000 |
|||||
| Lympho # | 4340 | 3800 | 1900 | 4040- 55220 |
800-4800 | |||||
| Gender | Female | Male | Female | 1 Male, 3 Female | ||||||
|
Age at
analysis |
16 years | 18 years | 51 years | 8-12 years old | ||||||
Immature/transitional B cells = CD19+CD10+ (Pt 1) or CD19+ / CD21+CD24++ according to institutional flow cytometry guidelines. Memory B cells = CD19+CD27+.
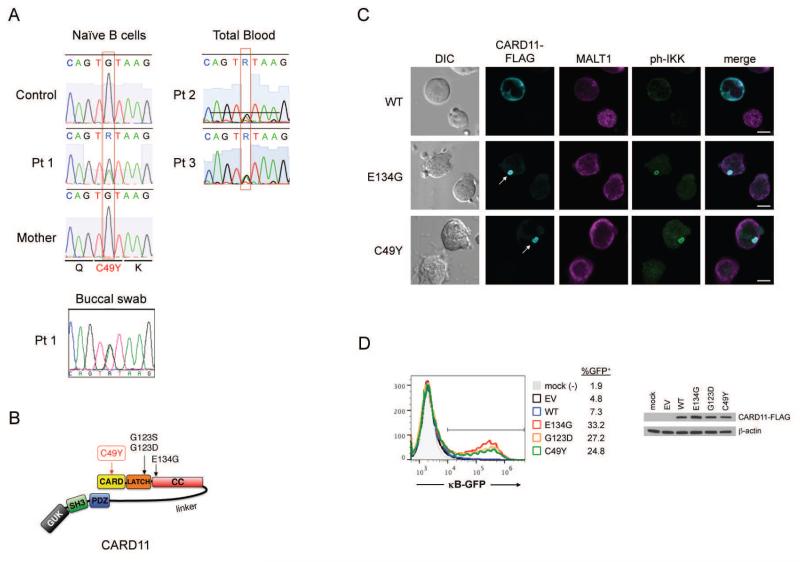
RNA-Seq (Pt 1) and whole exome DNA sequencing (Pt 2, Pt 3) analyses revealed that all three patients shared the same heterozygous, missense mutation in exon 3 of CARD11 (chr7: 2987283 C>T; C49Y), confirmed by traditional Sanger sequencing of DNA from purified B cells and buccal swabs for Pt 1 and from total blood for Pt 2 and Pt 3 (Figure 1A). Unlike other BENTA-associated CARD11 mutations described to date, C49Y falls within the N-terminal caspase recruitment domain (CARD) (Figure 1B). The CARD domain is critical for BCL10 interaction and downstream TCR signaling, as well as regulatory T cell development (7). This mutation was previously reported in one case of DLBCL (8), and identified in an unbiased screen for gain-of-function CARD11 mutants capable of activating NF-κB and promoting human DLBCL tumor growth in vitro (6). Indeed, we confirmed that transfection of the C49Y CARD11 mutant into the CARD11-deficient Jurkat T cell line JPM50.6 resulted in spontaneous protein aggregation, colocalization with MALT1 and active IKK (9), and constitutive NF-κB activation in the absence of AgR stimulation, comparable to other BENTA mutants described (Figure 1C-D). Similar results were obtained in the BJAB B cell line (Suppl. Fig 1). Collectively these results are consistent with biochemical data suggesting that the CARD domain also physically associates with the inhibitory linker domain to keep CARD11 in a closed, inactive conformation (6). We can therefore conclude that C49Y is the first germline-encoded, autosomal dominant gain-of-function mutation reported outside of the LATCH-CC region of the CARD11 protein, manifesting as BENTA disease in 3 patients.
Figure 1. Gain-of-function missense mutation in CARD11 (C49Y) in three BENTA patients.
(A) Sanger gDNA sequencing of C49Y BENTA patients. (B) Schematic diagram of CARD11 with BENTA-associated mutations. (C) Fluorescent microscopy of CARD11-deficient JPM50.6 T cells transfected with FLAG-CARD11 constructs. White arrows indicate CARD11 aggregates. Scale bars = 5 μm. (D) Flow cytometric quantitation of κB-driven GFP reporter and immunblotting of CARD11-FLAG in transfected JPM50.6 cells. Mock = untransfected cells, EV=empty vector, WT=wild type.
Interestingly, all three cases sharing the C49Y mutation presented with milder disease when compared with previous cases. Much of this may be attributed to a marked decrease in B cell lymphocytosis noted over time in most pediatric BENTA patients as they age (2). More specifically, reduced output of new transitional B cells from the bone marrow may explain this phenomenon (10). Consistent with this idea, relative proportions of circulating immature B cells were within normal range in all three C49Y patients (Table 1). On the other hand, two known BENTA patients maintained high lymphocyte counts well into adulthood (~1-4×104 cells/μl), including ~70-90% CD19+ B cells (2) (I. Morison, personal communication). However, these two patients were also splenectomized in childhood. Indeed, splenectomy appears to be associated with significantly higher peripheral blood B cell counts in patients with BENTA, and may be a counterproductive clinical measure that predisposes patients to certain infections (e.g. encapsulated bacteria) and/or greater risk of B cell malignancy (1, 2). We can only speculate that specific CARD11 mutations may be intrinsically linked to mild (C49Y) or severe (G123D) B cell lymphocytosis, although comparable gain-of-function activity was measured in various in vitro systems (1, 6). However, the presence of additional genomic modifications as well as environmental factors and infection history may also influence disease severity in individual patients. Additional basic research focusing on mutant CARD11 signal transduction, combined with careful longitudinal analysis of confirmed BENTA patients, will help clarify those determinants that influence BENTA pathogenesis as new patients continue to be identified.
Supplementary Material
Acknowledgements
We thank the patients and their families for participating in our research studies. Patient blood samples were obtained after provision of informed consent. We also thank Angela Wang for arranging blood draws and Julie Niemela for conducting CLIA confirmation of the CARD11 mutation in Pt. 1, Marie-Céline Deau, Monique Forveille, Stéphanie Ndaga, and Aminata Diabate for excellent technical support and Pr Capucine Picard and Pr Marina Cavazzana for scientific advice.
Funding: J.R.S. and A.L.S. were supported by grants from the Concern Foundation and Uniformed Services University. H.S. and Y.Z. are supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases. L.H., A.D., A.F. and S.K. were supported by the “Institut National de la Santé et de la Recherche Médicale, the European Union’s 7th RTD Framework Programme (ERC advanced grant PID-IMMUNE contract 249816), the Fondation pour la Recherche Médicale (grant number: ING20130526624), la Ligue Contre le Cancer (Comité de Paris) and a government grant managed by the French Agence Nationale de la Recherche as part of the “Investments for the Future” program (ANR-10-IAHU-01). S.K is a Centre National de la Recherche Scientifique (CNRS) researcher.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brohl AS, Stinson JR, Su HC, Badgett T, Jennings CD, Sukumar G, et al. Germline CARD11 Mutation in a Patient with Severe Congenital B Cell Lymphocytosis. Journal of clinical immunology. 2014 doi: 10.1007/s10875-014-0106-4. Epub 2014/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow AL, Xiao W, Stinson JR, Lu W, Chaigne-Delalande B, Zheng L, et al. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med. 2012;209(12):2247–61. doi: 10.1084/jem.20120831. Epub 2012/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell research. 2011;21(1):55–70. doi: 10.1038/cr.2010.182. Epub 2010/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turvey SE, Durandy A, Fischer A, Fung SY, Geha RS, Gewies A, et al. The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency. The Journal of allergy and clinical immunology. 2014;134(2):276–84. doi: 10.1016/j.jaci.2014.06.015. Epub 2014/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–9. doi: 10.1126/science.1153629. Epub 2008/03/08. [DOI] [PubMed] [Google Scholar]
- 6.Chan W, Schaffer TB, Pomerantz JL. A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival. Mol Cell Biol. 2013;33(2):429–43. doi: 10.1128/MCB.00850-12. Epub 2012/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salisbury EM, Wang L, Choi O, Rutschmann S, Ashton-Rickardt PG. N-Ethyl-N-nitrosourea mutagenesis in the mouse provides strong genetic and in vivo evidence for the role of the Caspase Recruitment Domain (CARD) of CARD-MAGUK1 in T regulatory cell development. Immunology. 2014;141(3):446–56. doi: 10.1111/imm.12207. Epub 2014/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459(7247):717–21. doi: 10.1038/nature07968. Epub 2009/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinohara H, Behar M, Inoue K, Hiroshima M, Yasuda T, Nagashima T, et al. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-kappaB activation. Science. 2014;344(6185):760–4. doi: 10.1126/science.1250020. Epub 2014/05/17. [DOI] [PubMed] [Google Scholar]
- 10.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clinical and experimental immunology. 2010;162(2):271–9. doi: 10.1111/j.1365-2249.2010.04206.x. Epub 2010/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.