Abstract
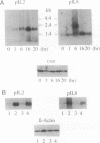
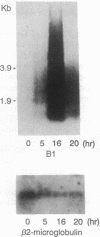
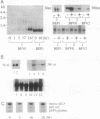
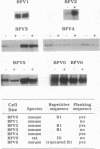
We previously reported that in rat fibroblasts, accumulation of a set of mRNAs ("pIL genes") was modulated as a function of cell growth and transformation, at a posttranscriptional stage, and by a mechanism that depends on a short nucleotide sequence containing an ID repetitive element. In mouse fibroblasts, hybridization with rat pIL probes identified mRNAs with the same pattern of expression, which did not contain ID sequences but contained a different regulatory element, encompassing a repetitive sequence of the B1 family. Expression in mouse cells of a reporter beta-globin gene carrying this element inserted in its 3' noncoding region was growth- and transformation-dependent. The nucleotide sequences of two murine and of three rat pIL cDNAs showed clear similarities in the region immediately adjacent to the ID and B1 repeats. Both the repeat and the flanking sequence were required to confer on beta-globin constructs the pattern of expression characteristic of the pIL genes. The hypothesis is presented that repetitive sequences in the eukaryotic genome might be modular parts of complex regulatory elements ensuring the coordinated expression of various mRNA species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzai K., Kobayashi S., Suehiro Y., Goto S. Conservation of the ID sequence and its expression as small RNA in rodent brains: analysis with cDNA for mouse brain-specific small RNA. Brain Res. 1987 Apr;388(1):43–49. doi: 10.1016/0169-328x(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Carey M. F., Singh K., Botchan M., Cozzarelli N. R. Induction of specific transcription by RNA polymerase III in transformed cells. Mol Cell Biol. 1986 Sep;6(9):3068–3076. doi: 10.1128/mcb.6.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara T. M., Brosius J. Neural BC1 RNA: cDNA clones reveal nonrepetitive sequence content. Proc Natl Acad Sci U S A. 1987 May;84(9):2624–2628. doi: 10.1073/pnas.84.9.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaichenhaus N., Cuzin F. A role for ID repetitive sequences in growth- and transformation-dependent regulation of gene expression in rat fibroblasts. Cell. 1987 Sep 25;50(7):1081–1089. doi: 10.1016/0092-8674(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Glaichenhaus N., Léopold P., Cuzin F. Increased levels of mitochondrial gene expression in rat fibroblast cells immortalized or transformed by viral and cellular oncogenes. EMBO J. 1986 Jun;5(6):1261–1265. doi: 10.1002/j.1460-2075.1986.tb04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A., Lieberburg I., Gardner D., Baxter J. D., Cathala G. G. Transcription of two classes of rat growth hormone gene-associated repetitive DNA: differences in activity and effects of tandem repeat structure. Nucleic Acids Res. 1984 Sep 25;12(18):7153–7173. doi: 10.1093/nar/12.18.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget T., Reich M., Stüber K., Starzinski-Powitz A. Regulated expression of repetitive sequences including the identifier sequence during myotube formation in culture. EMBO J. 1986 Apr;5(4):659–664. doi: 10.1002/j.1460-2075.1986.tb04264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Sakamoto K. Alu interspersed repeats: selfish DNA or a functional gene family? New Biol. 1990 Sep;2(9):759–770. [PubMed] [Google Scholar]
- Kalb V. F., Glasser S., King D., Lingrel J. B. A cluster of repetitive elements within a 700 base pair region in the mouse genome. Nucleic Acids Res. 1983 Apr 11;11(7):2177–2184. doi: 10.1093/nar/11.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert H., Bredow S., Benecke B. J. Expression of a human 7S K RNA gene in vivo requires a novel pol III upstream element. EMBO J. 1990 Mar;9(3):711–718. doi: 10.1002/j.1460-2075.1990.tb08164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lone Y. C., Simon M. P., Kahn A., Marie J. Sequences complementary to the brain-specific "identifier" sequences exist in L-type pyruvate kinase mRNA (a liver-specific messenger) and in transcripts especially abundant in muscle. J Biol Chem. 1986 Feb 5;261(4):1499–1502. [PubMed] [Google Scholar]
- Majello B., La Mantia G., Simeone A., Boncinelli E., Lania L. Activation of major histocompatibility complex class I mRNA containing an Alu-like repeat in polyoma virus-transformed rat cells. Nature. 1985 Apr 4;314(6010):457–459. doi: 10.1038/314457a0. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S. H., Baxter J. D., Gutierrez-Hartmann A. Cell-specific expression of transfected brain identifier repetitive DNAs. Nucleic Acids Res. 1988 May 11;16(9):3963–3976. doi: 10.1093/nar/16.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Rautmann G., Glaichenhaus N., Nahgashfar Z., Breathnach R., Rassoulzadegan M. Complementation of a tsa mutant and replication of a recombinant DNA carrying the viral ori region in mouse cells transformed by polyoma virus. Virology. 1982 Oct 30;122(2):306–317. doi: 10.1016/0042-6822(82)90230-6. [DOI] [PubMed] [Google Scholar]
- Rautmann G., Matthes H. W., Gait M. J., Breathnach R. Synthetic donor and acceptor splice sites function in an RNA polymerase B (II) transcription unit. EMBO J. 1984 Sep;3(9):2021–2028. doi: 10.1002/j.1460-2075.1984.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., St-Jacques B. 'Brain-specific' transcription and evolution of the identifier sequence. 1986 Jan 30-Feb 5Nature. 319(6052):418–420. doi: 10.1038/319418a0. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. Removal of the Alu structural domain from signal recognition particle leaves its protein translocation activity intact. Nature. 1986 Mar 6;320(6057):81–84. doi: 10.1038/320081a0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Gottesfeld J. M., Reynolds W. Control of neuronal gene expression. Science. 1984 Sep 21;225(4668):1308–1315. doi: 10.1126/science.6474179. [DOI] [PubMed] [Google Scholar]