Abstract
Background
Evidence-based interventions and strategies are needed to improve child survival in countries with a high burden of neonatal and child mortality. An overview of systematic reviews can focus implementation on the most effective ways to increase child survival.
Methods
In this overview we included published Cochrane and other systematic reviews of experimental and observational studies on antenatal, childbirth, postnatal and child health interventions aiming to prevent perinatal/neonatal and child mortality using the WHO list of essential interventions. We assessed the methodological quality of the reviews using the AMSTAR criteria and assessed the quality of the outcomes using the GRADE approach. Based on the findings from GRADE criteria, interventions were summarized as effective, promising or ineffective.
Findings
The overview identified 148 Cochrane and other systematic reviews on 61 reproductive, maternal, newborn and child health interventions. Of these, only 57 reviews reported mortality outcomes. Using the GRADE approach, antenatal corticosteroids for preventing neonatal respiratory distress syndrome in preterm infants; early initiation of breastfeeding; hygienic cord care; kangaroo care for preterm infants; provision and promotion of use of insecticide treated bed nets (ITNs) for children; and vitamin A supplementation for infants from six months of age, were identified as clearly effective interventions for reducing neonatal, infant or child mortality. Antenatal care, tetanus immunization in pregnancy, prophylactic antimalarials during pregnancy, induction of labour for prolonged pregnancy, case management of neonatal sepsis, meningitis and pneumonia, prophylactic and therapeutic use of surfactant, continuous positive airway pressure for neonatal resuscitation, case management of childhood malaria and pneumonia, vitamin A as part of treatment for measles associated pneumonia for children above 6 months, and home visits across the continuum of care, were identified as promising interventions for reducing neonatal, infant, child or perinatal mortality.
Interpretation
Comprehensive adoption of the above six effective and 11 promising interventions can improve neonatal and child survival around the world. Choice of intervention and degree of implementation currently depends on resources available and policies in individual countries and geographical settings.
Funding
This review was part of doctoral thesis which was funded by University of Adelaide, Australia.
Keywords: Stillbirths, Perinatal mortality, Neonatal mortality, Infant mortality, Child mortality, Survival
Highlights
-
•
There are several interventions have showing clear impact on improving neonatal and child survival.
-
•
The most effective include antenatal-corticosteroids, breastfeeding, cord care, kangaroo care, bednets, and vitamin A.
-
•
Countries with high burdens of neonatal and child mortality need to find ways to effectively implement these interventions.
1. Introduction
The global burden of neonatal and child mortality is alarmingly high in low and middle income countries (LMICs). There has been a sharp decline in mortality rates in children under five years of age between 1990 and 2013 (from 90 mortalities per 1000 down to 46 mortalities per 1000 live births between 1990 and 2013). This rate needs to further decrease, to just 30 mortalities per 1000 live births, in order to meet the Millennium Development Goals (MDGs) 2015 target (You et al., 2013).
Despite all the progress made in the last decade, it is very unlikely that the MDG targets will be met in many LMICs, where 99% of global deaths occur (You et al., 2013). In countries with a high burden of neonatal and child mortality, a variety of interventions could substantially reduce deaths and improve maternal and perinatal outcomes. Interventions and care primarily employed during different periods from antenatal to the later childhood period can facilitate reductions in neonatal and later mortality. However, a major obstacle in meeting the proposed reduction is that most neonatal and child health programs do not reach to those who need it the most. Therefore, effective interventions and care-based strategies need to be widely deployed to all and be delivered across the continuum of reproductive, maternal, neonatal and child health (RMNCH) care.
As we approach the deadline for the target of the MDGs and begin the journey towards achieving sustainable development goals (SDGs) we must focus efforts on programs and interventions shown to work. Several systematic reviews have evaluated the role of individual antenatal, natal, postnatal and child health interventions and their potential role at improving morbidity and mortality, however, there has been no overview on these interventions. Such an overview of systematic reviews of interventions to prevent neonatal and child mortality would facilitate the development of a definitive framework for preventing neonatal and child mortality in LMICs.
2. Methodology
In this overview of reviews, we have included all published Cochrane and the most recent (most latest on the given subject) other systematic reviews of randomized, non-randomized controlled trials of interventions and observational studies aiming to prevent perinatal (stillbirths + early neonatal mortality) or neonatal or child mortality (or stillbirths where either of these were not reported). We included interventions considered for improving neonatal and child survival and provided during pre-pregnancy, antenatal, childbirth and postnatal periods to mothers or the infant or child included in a set of 61 RMNCH interventions reported as essential interventions for reproductive, maternal, newborn and child health by the World Health Organization (WHO) (Panel 1) (Pmnch, 2011). We considered reviews that included women of reproductive age, including pregnant women at any stage of gestation, their newborns and children up to five years of age. This overview considered reviews on interventions which were compared against no placebo or treatment or control group (unless otherwise indicated).
Panel 1.
List of interventions reviewed.
| Pre pregnancy interventions |
| Family planning |
| Prevention and management of sexually transmitted infections including HIV |
| Folic acid fortification and/or supplementation |
| Pregnancy interventions |
| Antenatal care |
| Iron and folic acid supplementation during pregnancy |
| Tetanus immunization in pregnancy |
| Prophylactic antimalarial and insecticide treated bednets for preventing malaria in pregnancy |
| Interventions for smoking cessation during pregnancy |
| Screening and treatment of syphilis |
| Prevention and management of HIV and prevention of mother to child transmission in pregnancy |
| Calcium supplementation in pregnancy |
| Low-dose aspirin for the prevention of pre-eclampsia |
| Use of antihypertensive drugs for treating severe hypertension in pregnancy |
| Prevention and treatment of eclampsia |
| Reduce mal presentation at term using external cephalic version (> 36 weeks) |
| Induction of labour for management of premature rupture of membranes at term. |
| Antibiotics for management of preterm rupture of membranes |
| Childbirth interventions |
| Corticosteroids for preventing neonatal respiratory distress syndrome |
| Management of unintended pregnancy |
| Social support during childbirth |
| Prophylactic antibiotic for caesarean-section |
| Prevention of postpartum haemorrhage: prophylactic uterotonic to prevent postpartum haemorrhage |
| Active management of third stage of labour to prevent postpartum haemorrhage |
| Induction of labour for prolonged pregnancy |
| C-section for absolute maternal indication (e.g. obstructed labour and central placenta previa) |
| Management of post-partum haemorrhage e.g. uterine massage |
| Uterotonics |
| Postpartum interventions |
| Advice and provision of family planning |
| Prevent, measure and treat maternal anaemia |
| Detection and management of postpartum sepsis |
| Screening and initiation or continuation of ARV therapy for HIV |
| Neonatal interventions |
| Promotion and provision of thermal care for all newborns to prevent hypothermia |
| Promotion and support for early initiation and exclusive breastfeeding (within the first hour) |
| Promotion and provision of hygienic cord and skin care |
| Neonatal resuscitation with bag and mask for babies who do not breath at birth |
| Newborn immunization |
| Presumptive antibiotic therapy for the newborns at risk of bacterial infection |
| Case management of neonatal sepsis, meningitis and pneumonia |
| Kangaroo mother care for low birth babies |
| Extra support for feeding the small and preterm baby |
| Prophylactic and therapeutic use of surfactant to prevent respiratory distress syndrome in pre-term babies |
| Continuous positive airway pressure (CPAP) to manage pre-term babies with respiratory distress syndrome |
| Management of newborns with jaundice |
| Infant and child health interventions |
| Promotion and support for exclusive breastfeeding for 6 months |
| Continued breastfeeding up to 2 years and beyond |
| Appropriate complementary feeding starting at 6 months |
| Provision and promotion of use of insecticide treated bed nets for children |
| Case management of childhood malaria |
| Comprehensive care of children infected or exposed to HIV infection |
| Promote and provide routine immunization plus H. Influenza, meningococcal, pneumococcal, and rotavirus vaccines |
| Vitamin A supplementation from 6 months of age in Vitamin A deficient populations |
| Management of severe acute malnutrition |
| Case management of childhood pneumonia |
| Vitamin A as part of treatment for measles-associated pneumonia for children above 6 months |
| Vitamin A as part of treatment for non-measles-associated pneumonia for children above 6 months |
| Case management of diarrhoea: Acute watery diarrhoea |
| Dysentery |
| Cross cutting intervention |
| Home visits across the continuum of care women's groups |
All available recent non-Cochrane and updated or most recent Cochrane systematic reviews were identified from the Cochrane Library and PubMed using the search strategy devised for each intervention separately during Nov 2012 to Jan 2013 (Supplementary Table 1). The search terms were limited to title, abstract, or keywords. The methodology for data collection and analysis is based on the Cochrane Handbook of Systematic Reviews of Interventions (Higgins and Green, 2011). The outcomes of interest for this overview of reviews were perinatal mortality, neonatal mortality, infant mortality and under-five mortality reported as primary or secondary outcomes in included reviews.
The protocol for this overview is registered with PROSPERO 2014: CRD42014007091 (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014007091#.U75a1RCLMiw). Two review authors (ZSL and PM) independently assessed the inclusion of all the potential systematic reviews and extracted information using a predefined form (intervention, comparison, mortality outcome, type of studies included — Characteristics of included reviews Supplementary Table 2). Any disagreement was resolved through discussion or, where required, we consulted a third person. We addressed two different quality assessments in this overview: the quality of evidence in the included reviews (Table 1) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al., 2008, Oxman and Group, 2004) and the methodological quality of the systematic reviews using the ‘assessment of multiple systematic reviews’ (AMSTAR) measurement tool (Shea et al., 2007) (Supplementary Table 3). We did not update individual reviews. Where reviews did not prepare and report mortality outcomes using GRADE-pro software (Brozek et al., 2008), we formulated ‘summary of findings’ tables. The following criteria were taken into account to grade the evidence: study limitations (risk of bias for the outcome of interest), consistency of effect, imprecision, indirectness, and publication bias. We summarised the main results of the included reviews into following categories.
-
•What works?
- Effective interventions: indicating that the review found high quality evidence with the effect likely to be similar to research findings.
-
•What might work?
- Promising interventions (more evidence needed): indicating that the review found moderate quality evidence with the effect expected to be similar to research findings, but with a possibility that it will be substantially different in the future.
-
•Insufficient evidence to make judgement
- Ineffective or probably ineffective interventions: indicating that the review found low or very low quality evidence of effectiveness or lack of effectiveness for an intervention.
- For low quality of evidence, it is likely that the effect will be substantially different from research findings, but that these will indicate what might be expected.
- For very low quality of evidence, the anticipated effect is very uncertain and the research does not provide a reliable indication of what might be expected.
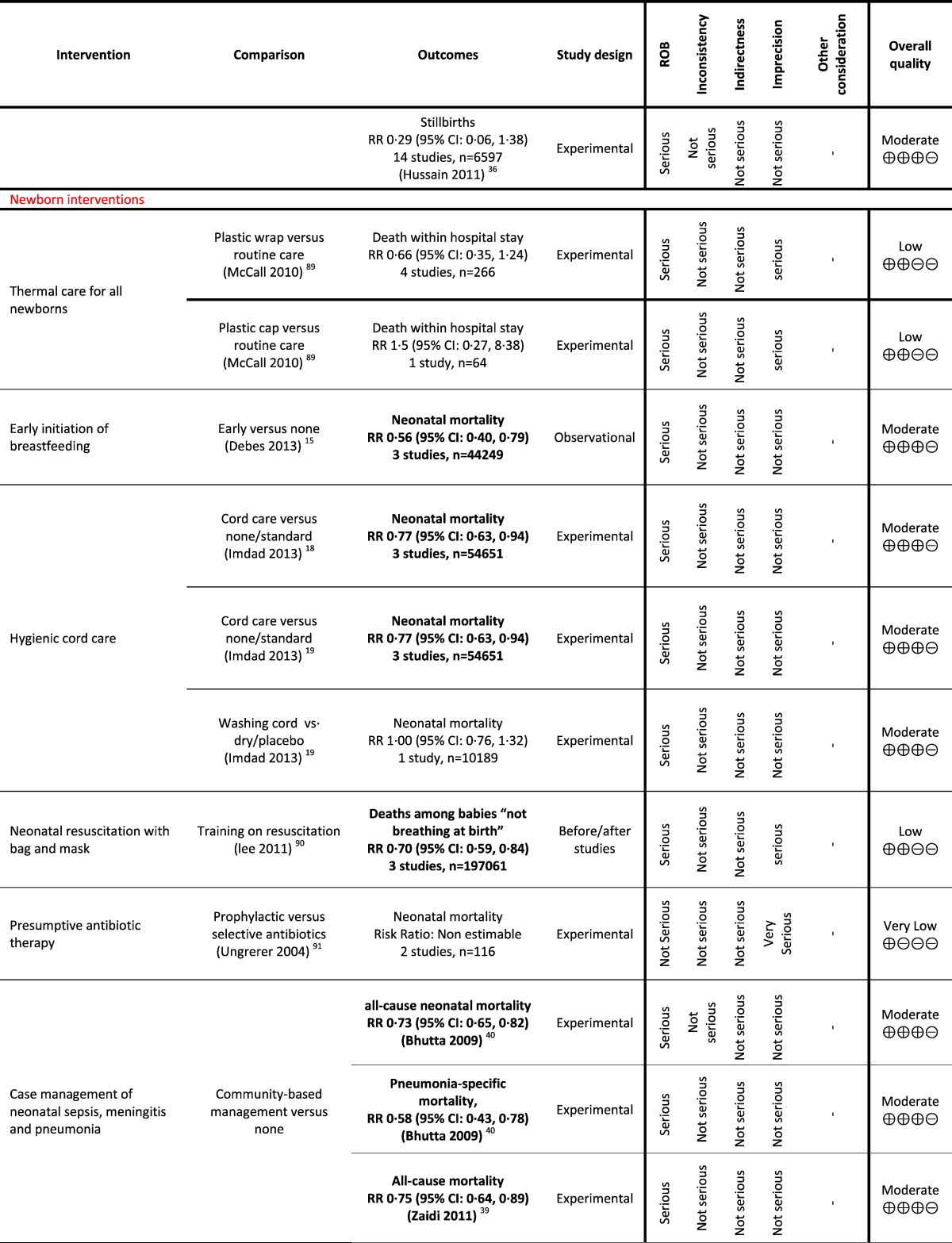
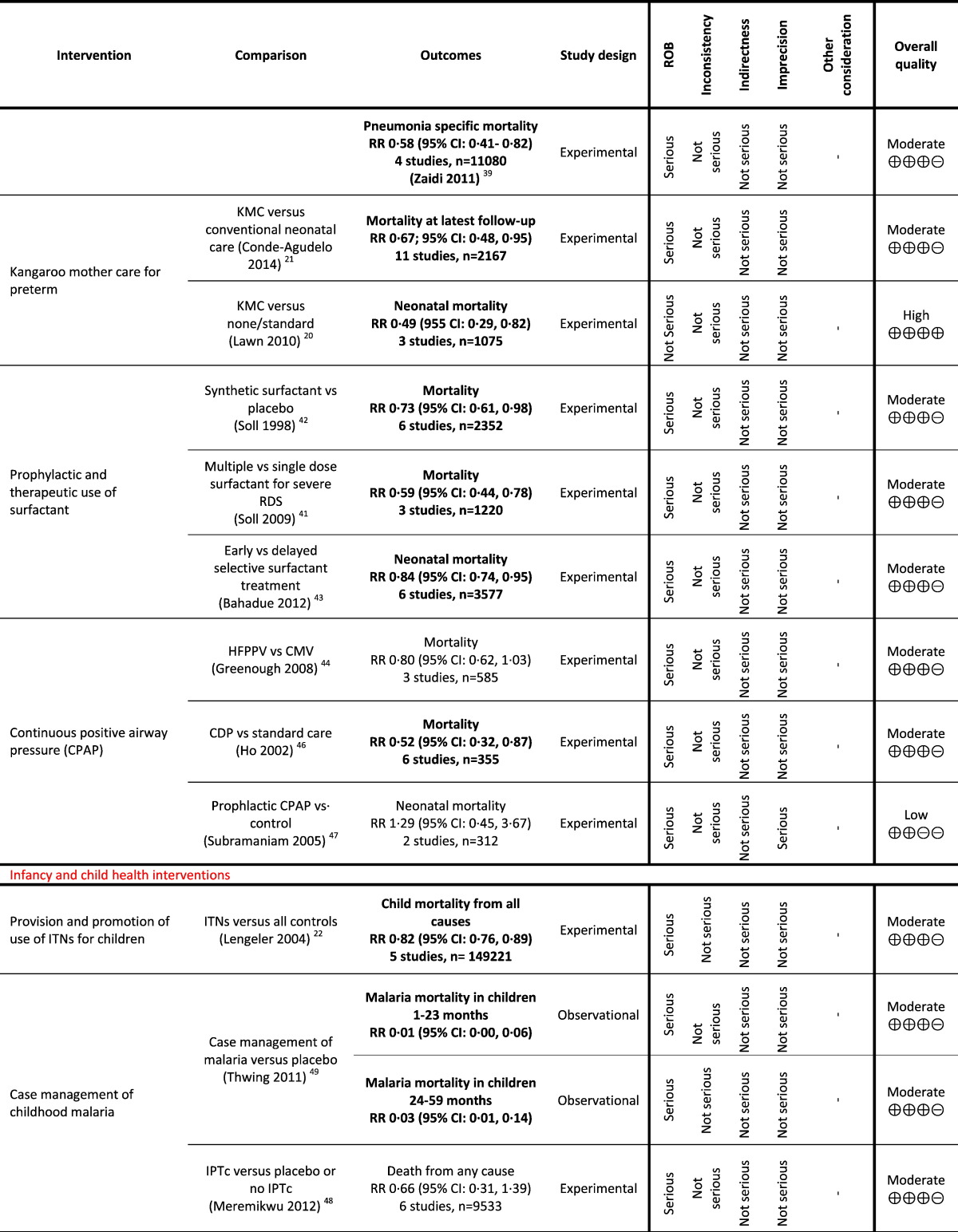
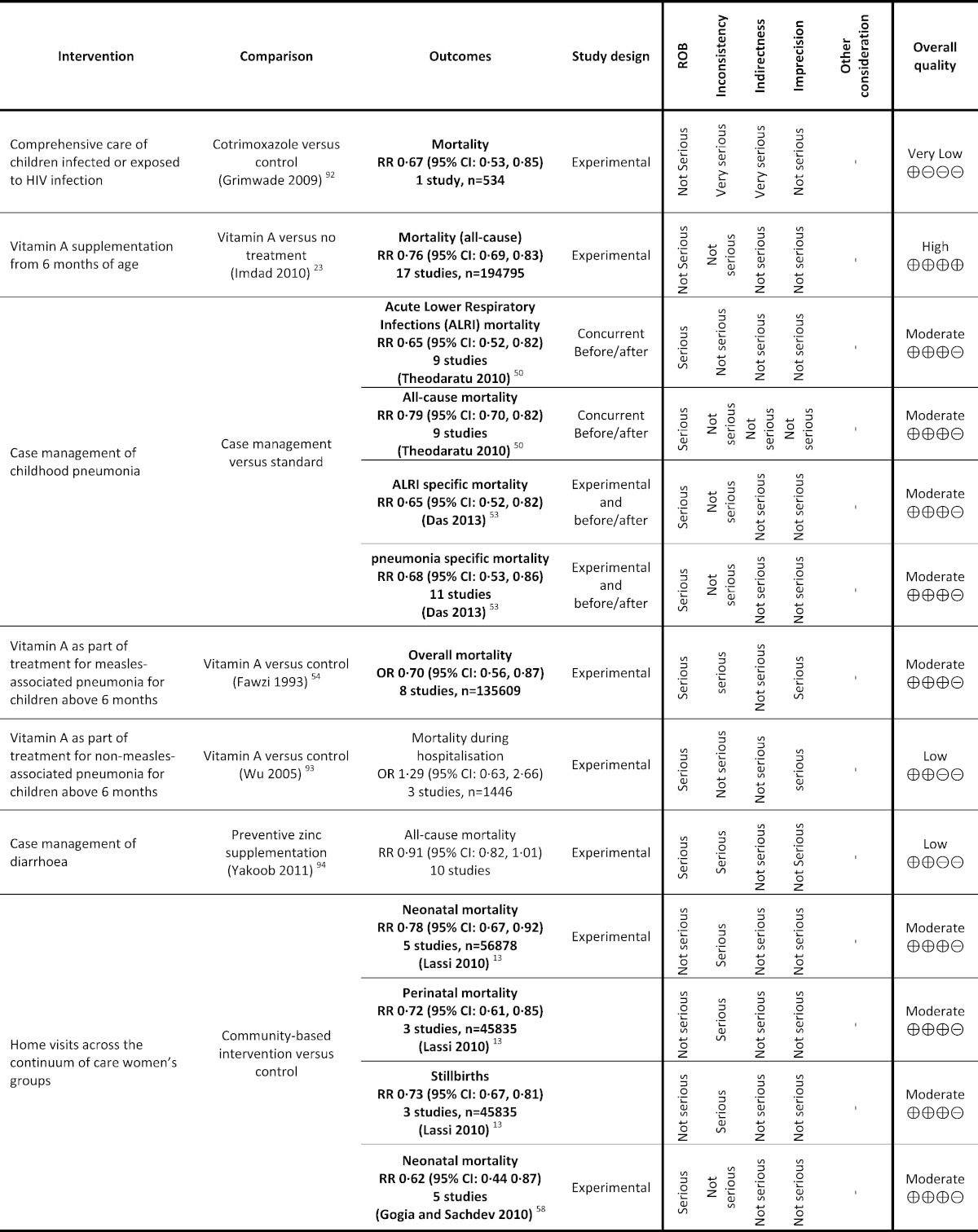
Table 1.
Grading analysis of mortality outcomes from included reviews.
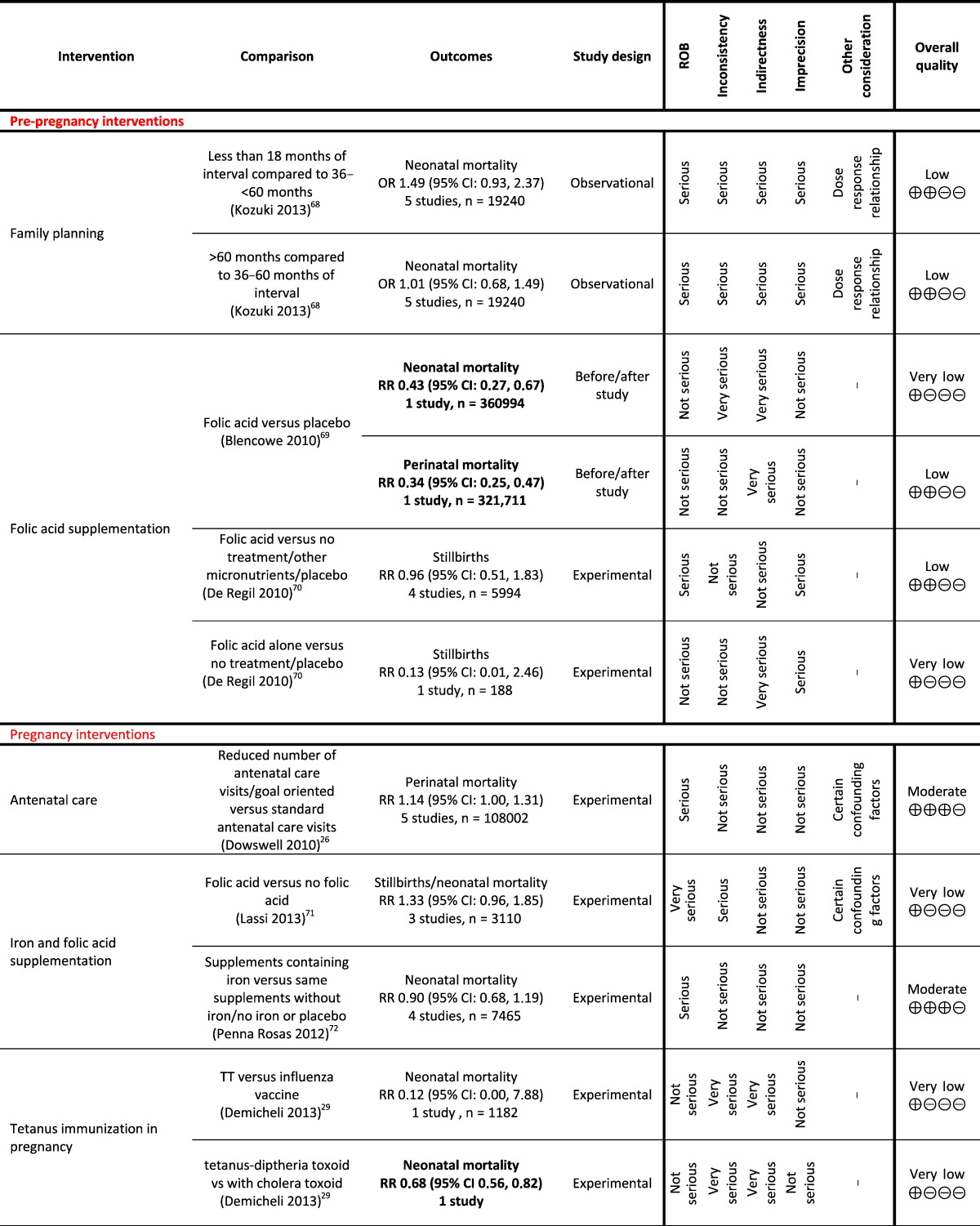
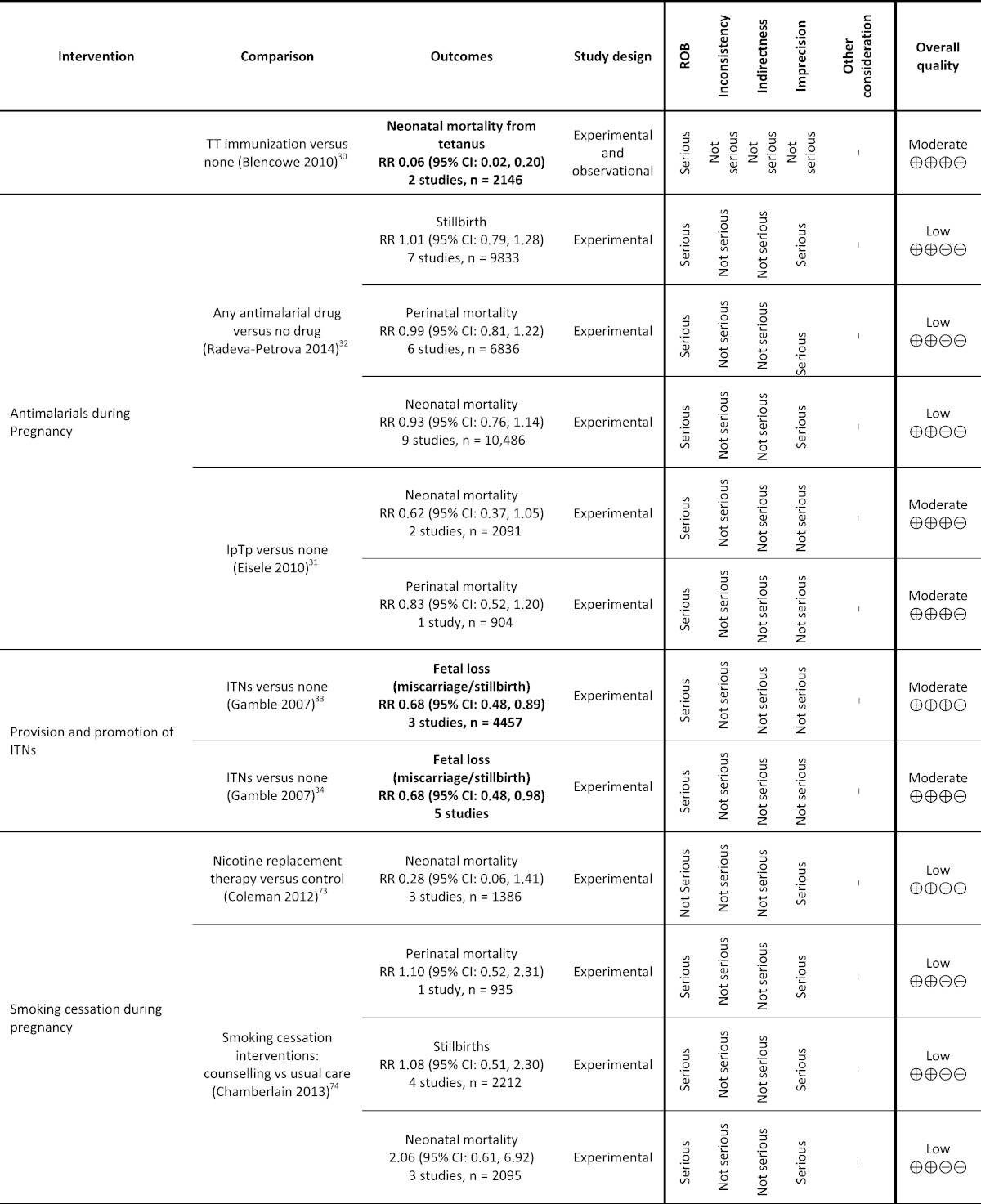
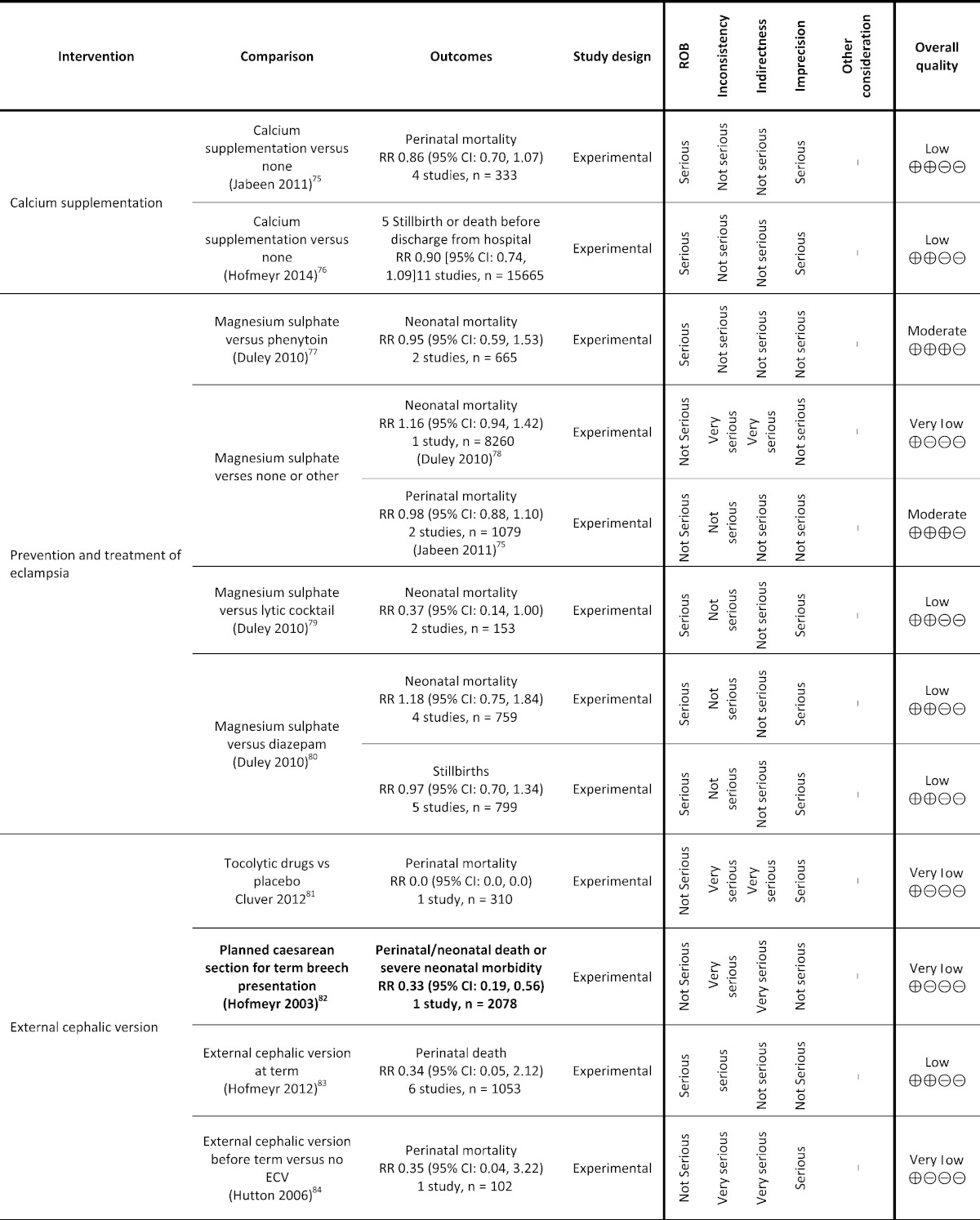
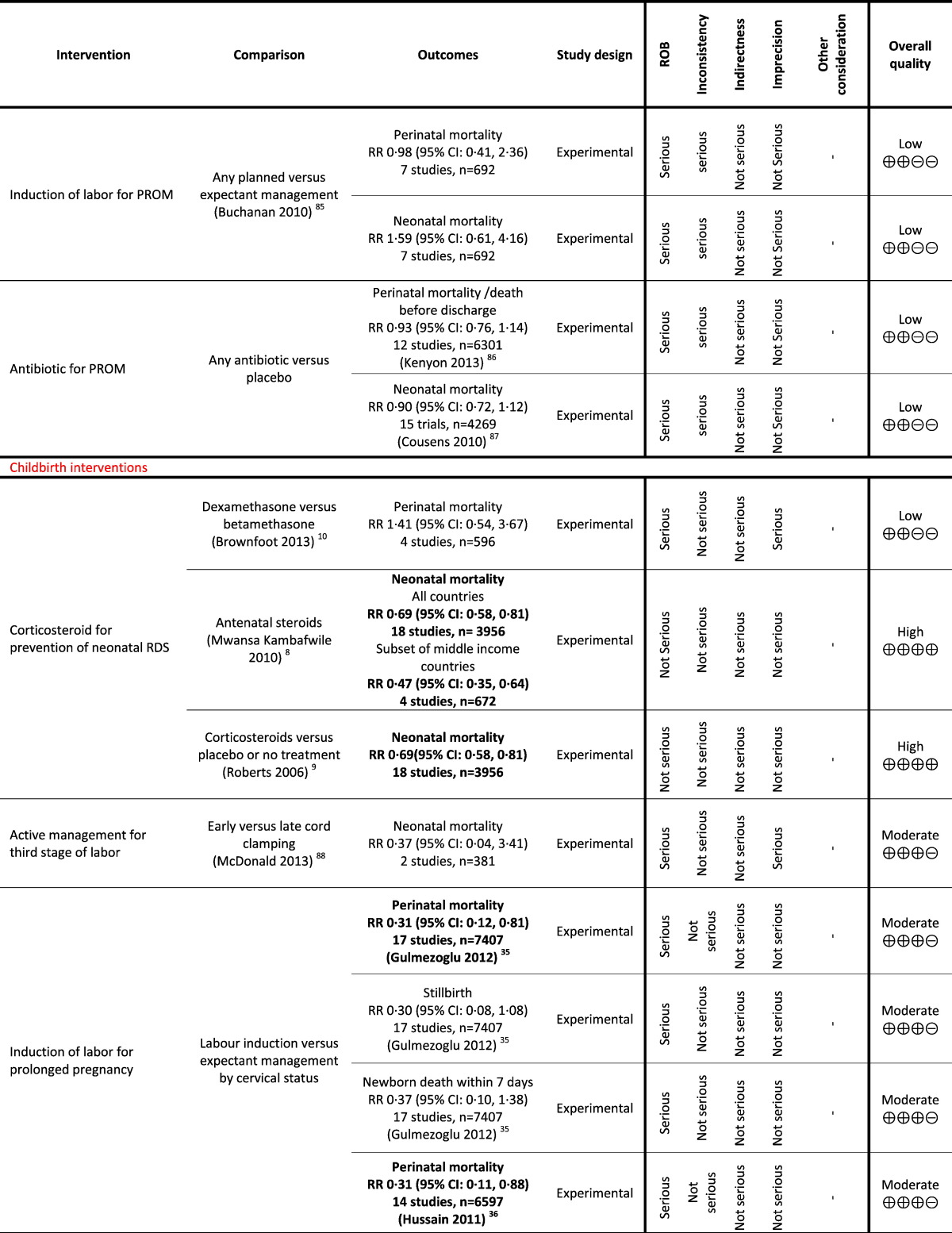
3. Funders and Their Role
This review was part of doctoral thesis which was funded by University of Adelaide, Australia. The funders had no role in the study design, study conduct, data analysis, data interpretation, or writing of the report. All authors take responsibility for the integrity and the accuracy of the data. The corresponding author had final responsibility to submit the report for publication.
4. Results
The overview included 61 reproductive (n = 3), maternal (pregnancy: n = 15; childbirth: n = 11; postpartum: n = 4), newborn (n = 12) and child (n = 16) health interventions to assess their impact on neonatal and child survival (Panel 1). A total of 148 systematic reviews were identified for these 61 RMNCH interventions, of which 92 were Cochrane reviews, 55 were non-Cochrane reviews and one was a WHO guideline on management of unintended pregnancy. Of these 148 reviews, only 57 reviews reported mortality outcomes (Panel 2).
Panel 2.
GRADE interventions according to outcomes.
| What works | What might work | Insufficient evidence |
|---|---|---|
| Mortality (neonatal or infant or child) | ||
| Corticosteroid for prevention of neonatal respiratory distress syndrome | Tetanus immunization in pregnancy (tetanus toxoid vs. placebo) | Family planning |
| Early initiation of breastfeeding | Prophylactic antimalarial during pregnancy | Periconceptional folic acid supplementation |
| Hygienic cord care | Induction of labour for prolonged pregnancy | Folic acid supplementation during pregnancy⁎ |
| Kangaroo mother care for low birth weight babies | Case management of neonatal sepsis, meningitis and pneumonia | Iron supplementation during pregnancy |
| Provision and promotion of use of insecticide treated bed nets for children | Prophylactic and therapeutic use of surfactant | Tetanus immunization in pregnancy (TT vs. diphtheria and influenza) |
| Vitamin A supplementation from 6 months of age | Continuous positive airway pressure (CPAP) | Smoking cessation during pregnancy |
| Case management of childhood malaria | Prevention and treatment of eclampsia | |
| Case management of childhood pneumonia | Active management for third stage of labour | |
| Vitamin A as part of treatment for measles associated pneumonia for children above 6 months | Induction of labour for PROM | |
| Home visits across the continuum of care women's groups | Antibiotic for PROM | |
| Thermal care for all newborns | ||
| Neonatal resuscitation with bag and mask | ||
| Presumptive antibiotic therapy for newborns | ||
| Case management of childhood malaria (monthly sulfadoxine pyrimethamine (SP) compared to standard 2-dose SP) | ||
| Comprehensive care of children infected or exposed to HIV infection | ||
| Vitamin A as part of treatment for non-measles-associated pneumonia for children above 6 months | ||
| Case management of diarrhoea | ||
| Perinatal mortality | ||
| Antenatal care | Periconceptional folic acid supplementation vs. placebo | |
| Prophylactic antimalarial during pregnancy | Smoking cessation during pregnancy | |
| Induction of labour for prolonged pregnancy | Calcium supplementation | |
| Home visits across the continuum of care women's groups | Prevention and treatment of eclampsia (MgSO4 vs. none or other) |
|
| External cephalic version | ||
| Induction of labour for PROM | ||
| Antibiotic for PROM⁎⁎ | ||
| Corticosteroid for prevention of neonatal RDS (dexamethasone versus betamethasone) | ||
| Stillbirths | ||
| Provision and promotion of ITNs⁎⁎⁎ | Periconceptional folic acid supplementation vs. no treatment/placebo | |
| Prophylactic antimalarial during pregnancy | Folic acid supplementation during pregnancy⁎ | |
| Induction of labour for prolonged pregnancy | Smoking cessation during pregnancy | |
| Home visits across the continuum of care women's groups | ||
Interventions in bold indicate that the outcomes estimates were statistically significant.
Stillbirths + neonatal mortality.
Perinatal mortality or death before discharge.
Foetal loss (miscarriage and stillbirths).
Using the GRADE approach, we identified six interventions to be clearly effective in reducing neonatal, infant or child mortality (corticosteroids for preventing neonatal respiratory distress syndrome in preterm infants; early initiation of breastfeeding; hygienic cord care; kangaroo care for preterm infants; provision and promotion of use of insecticide treated bed nets (ITNs) for children; and vitamin A supplementation for infants from six months of age).
We identified 11 promising interventions for reducing neonatal, infant, child or perinatal mortality (antenatal care; tetanus immunization in pregnancy; prophylactic antimalarial during pregnancy; induction of labour for prolonged pregnancy; case management of neonatal sepsis, meningitis and pneumonia; prophylactic and therapeutic use of surfactant; continuous positive airway pressure; case management of childhood malaria; case management of childhood pneumonia; vitamin A as part of treatment for measles associated pneumonia for children above 6 months; and home visits across the continuum of care) and a further four interventions were rated as promising for reducing stillbirths (prophylactic antimalarial during pregnancy; provision and promotion of ITNs during pregnancy; induction of labour for prolonged pregnancy; and home visits across the continuum of care). Eighteen interventions showed insufficient evidence of benefit in one or more of the mortality categories (Table 1).
4.1. Effective Interventions
4.1.1. Corticosteroids for Preventing Neonatal Respiratory Distress Syndrome (RDS)
This overview identified three reviews (Mwansa-Kambafwile et al., 2010, Roberts and Dalziel, 2006, Brownfoot et al., 2013), of which two (Mwansa-Kambafwile et al., 2010, Roberts and Dalziel, 2006) reviewed the impact of antenatal corticosteroids on the mother before anticipated preterm birth (with additional analysis for women in LMICs) (Mwansa-Kambafwile et al., 2010). Brownfoot and colleagues (Brownfoot et al., 2013) assessed different corticosteroid regimens. Two reviews reported the impact of corticosteroids on neonatal mortality (Mwansa-Kambafwile et al., 2010, Roberts and Dalziel, 2006). Roberts and Dalziel pooled 18 trials on 3956 women at risk of preterm birth and found a 31% (Risk Ratio (RR) 0.69; 95% Confidence Interval (CI): 0.58, 0.81) reduction in neonatal mortality (high GRADE rating) in women who were given antenatal corticosteroids compared to women who were not given any corticosteroids or given placebo (Roberts and Dalziel, 2006). Mwansa-Kambafwile and colleagues (Mwansa-Kambafwile et al., 2010) reported a 31% (RR 0.69; 95% CI: 0.58, 0.81) reduction (high GRADE rating) in preterm-specific mortality on pooling 18 trials on 3956 women mostly from high-income countries and 53% (RR 0.47; 95% CI: 0.35, 0.64) reduction in preterm-specific mortality on pooling a subset of four trials on 672 women from middle-income countries who were given antenatal corticosteroids.
4.1.2. Early Initiation of Breastfeeding
The overview identified six reviews (Dyson et al., 2005, Lewin et al., 2010, Lassi et al., 2010, Imdad et al., 2011a, Debes et al., 2013, Lumbiganon et al., 2012) that reported the impact of different interventions on improving early initiation of breastfeeding. Lewin and colleagues (Lewin et al., 2010) and Lassi and colleagues (Lassi et al., 2010) assessed the impact of interventions delivered through lay health workers and in the form of packages, respectively, on improving breastfeeding rates. These reviews reported reductions in mortality; however, reduction in deaths may have been achieved by other parts of the intervention package and therefore the reduction does not necessarily reflect the impact of a breastfeeding intervention alone. Dyson and colleagues (Dyson et al., 2005), Imdad and colleagues (Imdad et al., 2011a), and Lumbiganon and colleagues (Lumbiganon et al., 2012) did not report outcomes on mortality. The review by Debes and colleagues (Debes et al., 2013) identified 18 studies, of which three prospective cohort studies (including 44,249 newborns) with moderate GRADE quality showed neonatal mortality was reduced by 44% (RR 0.56; 95% CI: 0.40, 0.79) with early initiation of breastfeeding (within less than 24 h of birth).
4.1.3. Hygienic Cord Care
The overview identified two reviews, of which Zupan and colleagues assessed topical cord care (Zupan et al., 2004) and the other two by Imdad and colleagues assessed chlorhexidine application alone and other application for cord care and included almost similar studies (Imdad et al., 2013a, Imdad et al., 2013b). The latter two reported neonatal mortality (Imdad et al., 2013a, Imdad et al., 2013b). Pooled analysis of three studies (n = 54,561) found a moderate GRADE quality and significant 23% (RR 0.77; 955 CI: 0.63, 0.94) reduction in neonatal mortality with the application of chlorhexidine when compared with no application to the umbilical cord (dry cord care) (Imdad et al., 2013a, Imdad et al., 2013b). However the Cochrane review by Imdad and colleagues also compared washing the cord with dry care, reporting no difference in all-cause mortality (RR 1.00; 95% CI: 0.76, 1.32, moderate GRADE quality) (Imdad et al., 2013b).
4.1.4. Kangaroo Mother Care for Preterm Infants
The overview identified two reviews (Lawn et al., 2010, Conde-Agudelo and Díaz-Rossello, 2014) that assessed the impact of kangaroo mother care (KMC) on preterm and low birth weight infants (< 2000 g) and reported mortality outcome. Pooled analysis of 11 studies from 2167 infants reported a significant 33% reduction in mortality (moderate GRADE quality) at the latest follow up (RR 0.67; 95% CI: 0.48, 0.95) (Conde-Agudelo and Díaz-Rossello, 2014). The meta-analysis of three randomized controlled trials (RCTs) (n = 1075) — a subset of those pooled in the latest Cochrane review (Conde-Agudelo and Díaz-Rossello, 2014) — that provided KMC to infants in the first week of life showed a significant 51% reduction in neonatal mortality (RR 0.49; 95% CI: 0.29, 0.82 — high GRADE quality) when compared to standard care (Lawn et al., 2010). This review also pooled three observational studies and found a similar beneficial impact on neonatal mortality (RR 0.68; 95% CI: 0.58, 0.79) (Lawn et al., 2010).
4.1.5. Provision and Promotion of use of ITNs for Children
The overview identified one review that pooled five studies on 149,221 children and compared ITNs with control and found a significant 18% reduction in child mortality (RR 0.82; 95% CI: 0.76, 0.89 — moderate GRADE quality) (Lengeler, 2004).
4.1.6. Vitamin A Supplementation From 6 Completed Months of age
The overview identified three reviews from the same review authors who assessed the impact of vitamin A supplementation from six months of age, and reported neonatal mortality (Imdad et al., 2010, Imdad et al., 2011b, Mayo-Wilson et al., 2011). In the latest of these, pooling of 17 trials including 194,795 children found that vitamin A supplementation is effective in reducing all-cause mortality by 24% (RR 0.76; 95% CI: 0.69, 0.83) when compared with no treatment or placebo (Imdad et al., 2010). The quality was high on GRADE analysis.
4.2. Promising Interventions
4.2.1. Antenatal Care
The overview identified two reviews (Dowswell et al., 2010, Carroli et al., 2001) assessing the impact of fewer than usual antenatal care visits. This review of five trials including 108,002 pregnant women identified that reduced number of antenatal care visits (ranged 4–9) was associated with 14% higher risk of perinatal mortality (RR 1.14; 95% CI: 1.00, 1.31) when compared with standard antenatal care visits (ranged 12–14 +) (Dowswell et al., 2010), indicating that fewer antenatal visits than the standard number may be harmful. Another review, comparing group with standard antenatal care did not detect significant differences in perinatal mortality (RR 0.59; 95% CI: 0.22, 1.52; 2 trials, n = 1315) (Homer et al., 2012).
4.2.2. Tetanus Immunization in Pregnancy
The overview identified two reviews on tetanus toxoid (TT) vaccination versus placebo: Demicheli and colleagues (Demicheli et al., 2013) compared TT vaccination with influenza and cholera vaccination, whereas Blencowe and colleagues (Blencowe et al., 2010a) compared TT immunization with no immunization. The comparison of TT with influenza and cholera was judged as low quality and therefore included in “insufficient evidence interventions” section. The meta-analyses from Blencowe and colleagues (Blencowe et al., 2010a) displayed a significant impact of TT immunization on reducing neonatal mortality when compared with no immunization (RR 0.06; 95% CI: 0.02, 0.20; two studies, n = 2146). This review pooled two studies, of which one was an experimental trial and the other was an observational study.
4.2.3. Prophylactic Antimalarials During Pregnancy
The overview identified four reviews on prophylactic antimalarial and intermittent preventive treatment (IPT) in pregnancy (Eisele et al., 2010, Radeva-Petrova et al., 2014). Two reviews reported outcomes on neonatal mortality and perinatal mortality (Eisele et al., 2010, Radeva-Petrova et al., 2014), whereas one reported stillbirths (Radeva-Petrova et al., 2014).
4.2.3.1. Neonatal Mortality
Radeva-Petrova and colleagues assessed antimalarial drug prophylaxis (e.g. chloroquine given weekly) or IPT (typically sulfadoxine-pyrimethamine given two to three times during pregnancy) with no regular or routine antimalarial or comparator IPT and found a non-significant 7% reduction in neonatal and infant mortality (RR 0.93; 95% CI: 0.76, 1.14 — low GRADE quality) on pooling nine trials including 10,486 women in their first or second pregnancy (Radeva-Petrova et al., 2014). The review by Eisele and colleagues compared IPT with control and found a non-significant 17% reduction in neonatal mortality (RR 0.83; 95% CI: 0.52, 1.20 — moderate GRADE quality) (Eisele et al., 2010).
4.2.3.2. Perinatal Mortality
Radeva-Petrova and colleagues pooled six trials on 6836 women in their first or second pregnancy and found a non-significant 1% reduction in perinatal mortality (RR 0.99; 95% CI: 0.81, 1.22 — low GRADE quality) (Radeva-Petrova et al., 2014). Eisele and colleagues found a non-significant 17% reduction in perinatal mortality (RR 0.83; 95% CI: 0.52, 1.20 — moderate GRADE quality) (Eisele et al., 2010).
4.2.3.3. Stillbirths
Radeva-Petrova and colleagues pooled seven trials on 9833 women in their first or second pregnancy and reported a non-significant increase in stillbirths (RR 1.01; 95% CI: 0.79, 1.28 — low GRADE quality) (Radeva-Petrova et al., 2014).
4.2.4. Provision and Promotion of ITNs During Pregnancy
The overview identified two reviews by Gamble and colleagues (Gamble et al., 2006, Gamble et al., 2007) that studied the effect of ITN on pregnant women and reported moderate GRADE quality foetal loss. Pooled analysis of five trials reported a significant 32% reduction in foetal loss (miscarriage or stillbirths) (RR 0.68; 95% CI: 0.48, 0.98) (Gamble et al., 2006). A subset of those trials pooled in the Cochrane review reported a significant 32% reduction in foetal loss (miscarriage or stillbirths) (RR 0.68; 95% CI: 0.48, 0.89; three studies, n = 4557) (Gamble et al., 2007).
4.2.5. Induction of Labour for Prolonged Pregnancy
The overview identified two reviews that evaluated the benefits and harms of a policy of labour induction at term or post-term compared with awaiting spontaneous labour or later induction of labour (Gulmezoglu et al., 2012, Hussain et al., 2011). Both of the reviews included almost the same set of studies and reported outcomes on perinatal mortality and stillbirths (Gulmezoglu et al., 2012, Hussain et al., 2011), while only Gulmezoglu and colleagues reported neonatal mortality (Gulmezoglu et al., 2012).
4.2.5.1. Neonatal Mortality
The meta-analysis by Gulmezoglu and colleagues found a moderate GRADE quality non-significant 63% (RR 0.37; 95% CI: 0.10, 1.38; 17 studies, n.7407) reduction in neonatal deaths within seven days when compared with labour induction at term or post-term with awaiting spontaneous labour or later induction of labour (Gulmezoglu et al., 2012).
4.2.5.2. Perinatal Mortality
The meta-analysis of 17 studies on 7407 women by Gulmezoglu and colleagues found a significant 69% (RR 0.31; 95% CI: 0.12, 0.81 — moderate GRADE quality) (Gulmezoglu et al., 2012) and meta-analysis of 14 studies on 6597 women by Hussain and colleagues found a significant 69% (RR 0.31; 95% CI: 0.11, 0.88 — moderate GRADE) (Hussain et al., 2011) reduction in perinatal mortality with induced labour at term or post-term.
4.2.5.3. Stillbirths
The meta-analysis of 17 studies on 7407 women by Gulmezoglu and colleagues found a non-significant 70% (RR 0.30; 95% CI: 0.08, 1.08 — moderate GRADE quality) (Gulmezoglu et al., 2012) and meta-analysis of 14 studies on 6597 women by Hussain and colleagues found a 71% (RR 0.29; 95% CI: 0.06, 1.38 — moderate GRADE quality) (Hussain et al., 2011) reduction in stillbirths.
4.2.6. Case Management of Neonatal Sepsis, Meningitis and Pneumonia
The overview identified four reviews that assessed the impact of case management of diagnosed sepsis, meningitis and pneumonia among neonates (Gordon and Jeffery, 2005, Sazawal and Black, 2003a, Zaidi et al., 2011, Bhutta et al., 2009a). Among these, two reviews reported an impact on mortality which was moderate on GRADE quality (Zaidi et al., 2011, Bhutta et al., 2009a). Case management of neonatal infectious diseases reported 27% (RR 0.73; 95% CI: 0.65, 0.82) (Bhutta et al., 2009a) and 25% (RR 0.75, 95% CI: 0.64, 0.89; 4 studies) (Zaidi et al., 2011) reduction in all-cause mortality. Similarly, the reviews also reported reduction in pneumonia specific mortality by 42% (RR 0.58; 95% CI: 0.43, 0.78) (Bhutta et al., 2009a) and (RR 0.58; 95% CI: 0.41, 0.82; 3 studies) (Zaidi et al., 2011).
4.2.7. Prophylactic and Therapeutic use of Surfactant
The overview identified three reviews on the impact of prophylactic and therapeutic use of surfactant and reported moderate quality GRADE outcomes on neonatal mortality (Soll and Ozek, 2009, Soll, 1998, Bahadue and Soll, 2012). Soll pooled six studies on 2352 newborns that compared synthetic surfactant with placebo and found a significant 27% reduction in neonatal mortality (RR 0.73; 95% CI: 0.61, 0.88) (Soll, 1998). Soll and Ozek assessed the impact of multiple doses of surfactant with single dose from three trials on 1220 newborns with severe RDS and found a significant 41% reduction in neonatal mortality (RR 0.59; 95% CI: 0.44, 0.78) (Soll and Ozek, 2009). Bahadue and Soll compared early versus delayed selective surfactant treatment for RDS from six studies (n = 3577) and found a significant 16% reduction in neonatal mortality (RR 0.84; 95% C: 0.74, 0.95) (Bahadue and Soll, 2012).
4.2.8. Continuous Positive Airway Pressure (CPAP)
The overview identified three reviews (Greenough et al., 2008, Lemyre et al., 2002, Ho et al., 2002, Subramaniam et al., 2005), of which two reported mortality as an outcome (Greenough et al., 2008, Ho et al., 2002, Subramaniam et al., 2005). Greenough 2008 compared high frequency positive pressure ventilation (HFPPV) with conventional ventilation (CMV) and reported a non-significant 20% reduction in neonatal mortality (RR 0.80; 95% CI: 0.62, 1.03; three studies, n = 585 — moderate GRADE) (Greenough et al., 2008). Ho and colleagues compared continuous distending pressure (CDP) with standard care and found a significant 48% reduction in neonatal mortality (RR 0.52; 95% CI: 0.32, 0.87; six studies, n = 355 — moderate GRADE) (Ho et al., 2002). Subramaniam and colleagues, compared prophylactic CPAP with control and reported an increase in neonatak deaths with prophylactic use (RR 1.29; 95% CI: 0.45, 3.67 — low GRADE) (Subramaniam et al., 2005).
4.2.9. Case Management of Childhood Malaria
The overview identified four reviews (Eisele et al., 2010, Meremikwu et al., 2012, Thwing et al., 2011), of which Thwing and colleagues reported a reduction in malaria mortality in children 1 to 23 months (RR 0.01; 95% CI: 0.00, 0.06) and in children 24 to 59 months of age (RR 0.03; 95% CI: 0.01, 0.14 — moderate GRADE quality) (Thwing et al., 2011). Meremikwu and colleagues compared IPT versus placebo or no IPT and reported a non-significant reduction in child mortality (RR 0.66; 95% CI: 0.31, 1.39; six studies, n = 9533 — moderate GRADE quality) (Meremikwu et al., 2012).
4.2.10. Case Management of Childhood Pneumonia
The overview identified four reviews (Theodoratou et al., 2010, Sazawal and Black, 2003b, Lamberti et al., 2013, Das et al., 2013), of which two reviews reported mortality as an outcome. Both of these reviews reported a significant reduction in acute lower respiratory tract infections (ALRI) specific mortality (RR 0.65; 95% CI: 0.52, 0.82; nine studies) (Theodoratou et al., 2010); (RR 0.65; 95% CI: 0.52, 0.82) (Das et al., 2013) and all-cause mortality (RR 0.79; 95% CI: 0.70, 0.82; nine studies);Theodoratou et al., 2010 (RR 0.68; 95% CI: 0.53, 0.86) (Das et al., 2013) with case management of pneumonia when compared to standard or no care. The evidence was moderate quality on GRADE analysis.
4.2.11. Vitamin A as Part of Treatment for Measles-Associated Pneumonia for Children Above 6 Months
The overview identified two reviews (Fawzi et al., 1993, Sudfeld et al., 2010), of which one reported mortality (Fawzi et al., 1993). This review pooled eight studies on 135,609 children and compared vitamin A supplementation with none for measles associated pneumonia and reported a significant 30% reduction in child mortality (RR 0.70; 95 CI: 0.56, 0.87 — moderate GRADE quality) (Fawzi et al., 1993).
4.2.12. Home Visits Across the Continuum of Care women's Groups
The overview identified four reviews (Lassi et al., 2010, Kidney et al., 2009, Bhutta et al., 2009b, Gogia and Sachdev, 2010). Only two reviews (Lassi et al., 2010, Gogia and Sachdev, 2010) assessed home visitation as part of delivery strategy. Both of these reviews reported outcome on neonatal mortality (Lassi et al., 2010, Gogia and Sachdev, 2010), whereas only one reported outcomes on perinatal mortality and stillbirths (Lassi et al., 2010).
4.2.12.1. Neonatal Mortality
The review by Lassi and colleagues reported a 22% reduction in neonatal mortality (RR 0.78; 95% CI: 0.67, 0.92 — moderate GRADE quality) on pooling five studies on 56,878 participants (Lassi et al., 2010). On the other hand, Gogia 2010 pooled five studies and reported a 38% reduction in neonatal mortality (RR 0.62; 95% CI: 0.44, 0.87 — moderate GRADE quality) (Gogia and Sachdev, 2010).
4.2.12.2. Perinatal Mortality
The review by Lassi and colleagues pooled three studies on 45,835 participants and reported a 28% reduction in perinatal mortality (RR 0.72; 95% CI: 0.61, 0.85 — moderate GRADE quality) (Lassi et al., 2010).
4.2.12.3. Stillbirths
The review by Lassi and colleagues pooled three studies on 45,835 participants and reported a 27% reduction in stillbirths (RR 0.73; 95% CI: 0.67, 0.81 — moderate GRADE quality) (Lassi et al., 2010).
4.3. Ineffective or probably ineffective interventions
Panel 2 reports the list of interventions which were low or very low on GRADE quality and thus were categorized as interventions with insufficient evidence. Some of those interventions reported their impact on stillbirths, perinatal or neonatal mortality and those includes family planning (Kozuki et al., 2013), periconceptional folic acid supplementation (Blencowe et al., 2010b, De-Regil et al., 2010), folic acid supplementation during pregnancy (Lassi et al., 2013b, Pena-Rosas et al., 2012), smoking cessation during pregnancy (Coleman et al., 2012, Chamberlain et al., 2013), calcium supplementation during pregnancy (Imdad et al., 2011c, Hofmeyr et al., 2014), magnesium sulphate compared to phenytoin for prevention and management of pre-eclampsia (Duley et al., 2010a, Duley et al., 2010b, Duley et al., 2010c, Duley et al., 2010d), external cephalic version (Cluver et al., 2012, Hofmeyr et al., 2003, Hofmeyr and Kulier, 2012, Hutton and Hofmeyr, 2006), induction of labor for PROM (Buchanan et al., 2010), antibiotics for PROM (Kenyon et al., 2013, Cousens et al., 2010), active management for third stage of labor (McDonald et al., 2013,), thermal care (McCall et al., 2010), neonatal resuscitation with bad and mask (Lee et al., 2011), presumptive antibiotic therapy for newborn (Ungerer et al., 2004,), Comprehensive care of children infected or exposed to HIV infection (Grimwade and Swingler, 2006), Vitamin A as part of treatment for non-measles-associated pneumonia for children above 6 months (Wu et al., 2005), and case management of diarrhea (Yakoob et al., 2011).
5. Discussion
There have been many great successes in reducing neonatal mortality as part of the MDGs, however, the current rates are still too high since each year 2.9 million newborns do not live to their first month of life (Berkley et al., 2014). In order to accelerate the progress towards reaching the targets set for 2015, this overview aimed to identify key interventions for neonatal and later survival. Review of all the recent Cochrane and other reviews on pre-pregnancy, pregnancy, neonatal and child health interventions which have reported perinatal or neonatal and child mortality identified six highly effective and 11 promising interventions which are likely to improve health and survival among babies. During the past decade, notable advances have been made in reviewing the evidence base for newborn interventions (Bhutta et al., 2013, Bhutta et al., 2014), especially in the context of essential interventions, packages of care and their interconnections (Lassi et al., 2013a).
The key effective interventions for improving the survival identified in this overview include antenatal corticosteroids for preventing neonatal RDS in preterm infants; early initiation of breastfeeding; hygienic cord care; KMC for preterm infants; provision and promotion of use of ITNs for children; and vitamin A supplementation for infants from six months of age. Among these, four are particularly effective for neonates, while two had clear implications for improving the survival among infants and children. Most of the interventions identified are very effective for premature infants, as deaths from preterm births complications are the leading cause for neonatal deaths (Bhutta et al., 2013). Every year, an estimated 15 million babies are born preterm. Of these over one million die. The common cause of neonatal mortality is RDS which is related to prematurity. The incidence of mortality due to prematurity is highest in LMIC (Blencowe et al., 2012) where even moderately preterm babies strive for survival. Preventing deaths from preterm births, is therefore of the utmost importance. Administration of antenatal corticosteroids to women at risk of preterm birth can prevent deaths among babies related to RDS. This overview further suggests that the risk of deaths among those who are born too soon can be halved (50%) by encouraging KMC which not only ensures skin-to-skin contact, but promotes breastfeeding and early recognition of danger signs and illnesses in newborns. Similarly, the benefits of breastfeeding have been well documented; with studies suggesting much greater benefits of early vs. late feeding (Debes et al., 2013). Early initiation of breastfeeding can reduce neonatal deaths by 44%. At the same time hygienic cord care can further reduces mortality by 23%. For children under the age of five years, infections accounts for a large number of deaths. Prevention of malaria particularly in malaria endemic countries can ensure 18% reduction in mortality. Provision of vitamin A for children above 6 months of age, which decreases the susceptibility towards infection, can also improve survival and health.
Despite the clear evidence of these interventions, coverage is still low and therefore their impact to reduce mortality among newborns and children is very poor. The recent Lancet every newborn series (Bhutta et al., 2013, Bhutta et al., 2014) has clearly highlighted that approximately three-quarters of deaths under five years can be averted if countries implement interventions at a coverage of 70–90% by 2025 (Bhutta et al., 2013). Considering the example of TT immunization, it is quite evident that 60% increase in coverage in last 25 years has led to 90% reduction in tetanus mortality in babies (Blencowe et al., 2010a). However, the coverage for insecticide treated bed nets in 2011 is still low 35.3% (5.2%–75.5%) and countries should prioritize mechanisms to increase coverage (Hill et al., 2014). Moreover, effective interventions such as hygienic cord care, which includes chlorhexidine cord cleansing, and adopting antenatal corticosteroids for preventing neonatal respiratory distress syndrome in preterm infants have very low coverage according to surveys with less than a third of women and neonates in need receiving them (Mason et al., 2014). Therefore, integrating these interventions into existing neonatal and childhood programs whereby mothers may also receive interventions such TT immunization, ITNs and corticosteroids when at risk at the same time may be an effective way to increase coverage.
High coverage of available interventions by 2025 can prevent almost three-quarters of neonatal deaths, and can save around 2 million lives per year (Bhutta et al., 2014). Interventions delivered in packages, especially for the care of small and ill neonates have the potential to save 1.9 million newborn infants (Bhutta et al., 2014). Estimate suggests that available interventions can reduce neonatal deaths related to prematurity by 58%, intrapartum by 79% and infections by 84% among neonates (Bhutta et al., 2014). Therefore, the implementation of the interventions identified in this overview will be of paramount importance for improving neonatal and child survival especially in the countries with the highest burden of mortality. It is vital to understand that these interventions are central for LMIC where neonatal and child health indicators are still not up to a high standards and many lives are either lost or their quality compromised due to a dearth of simple and effective actions (Bhutta et al., 2005). These interventions need to be deployed to all and promoted from the very outset, including the preconception period, which is vital to ensuring that women of child bearing age understand the importance of these interventions for their babies' health and survival.
A step forward to seeing improvements in annual reductions in neonatal mortality rates would be to pay more attention to the target group for the interventions; funding and resources may need to be reallocated to include stillbirth prevention which has received very little attention so far (Frøen et al., 2011). High fertility rates may also be adding to the problem. Care and resources in LMICs may be inadequate to cover already existing newborns; and increasing numbers of neonates will lead to strains on existing health care systems. Improved access to family planning, contraceptive methods, awareness and education will decrease the disparity and help efforts to achieve decreased neonatal mortality rates (Bhutta et al., 2014).
Community-based delivery strategies to increase access to needed care must be foremost to bringing about a positive change in the LMICs because appropriate education and awareness needs to precede interventions. Empowerment of women, removing barriers to accessibility to health care services, increased education and awareness in communities, and shifting the focus to evidence based interventions may help in adopting healthy practices among mothers and improve child survival rates (Bhutta et al., 2014). Appropriate, culturally sensitive education and awareness provided to the communities, followed by timely implementation of discussed interventions which can be integrated with existing healthcare practices, will definitely bring the required improvement in child health and survival.
Several limitations do however need to be recognised. First, it is important to consider that many of the interventions assessed in this review demonstrated important reductions in morbidity but may have been underpowered to show differences in neonatal and later survival. Second, it is also important to be aware that some clearly effective interventions, such TT immunization during pregnancy for reducing tetanus related mortality in neonates do not rate highly on GRADE, due to the study designs required to address this issue. Third, it is not possible to account for all the biases involved in the individual primary studies during the conduct of an overview of systematic reviews, where only systematic reviews and not individual primary studies are included. In addition, the high level synthesis of an overview may not always capture important contextual factors, such as educational attainment, socio-economic status, and access to care.
6. Conclusion
The implementation of these interventions will help in achieving the targets set for MDGs 4 and 5. Adoption of effective interventions promises a much needed improvement in neonatal and child outcomes around the world, especially if selected depending on the clinical indications and keeping in mind the need for cost-effectiveness in view of the limited resources in LMICs.
Research in Context
The synthesis of findings from 148 reviews on interventions for mothers and babies showed that steroids for pregnant mothers at risk of delivering babies early, breastfeeding, cord care, kangaroo care for babies born early, treated bednets for children, and vitamin A for babies from six months of age, are effective interventions for improving survival among babies and children. Antenatal care, tetanus injection during pregnancy, drugs to prevent malaria during pregnancy, inducing labour during prolonged pregnancy, use of surfactant and resuscitation to improve breathing among babies, management of infections among babies and children, and home visits during pregnancy and postnatal period, are the promising interventions for their survival.
Author's Contribution
ZSL conceptualised the review in consultation with PM, CC, and ZAB and wrote the first draft of the paper with substantial inputs from PM. ZSL, PM contributed to the scientific literature search, screening, collection, and analysis of data for all the included interventions with close inputs from CC and ZAB. All authors saw successive drafts of the paper and provided input. ZSL, PM, CC and ZAB finalized the paper and ZSL is the overall guarantor.
Conflict of Interest
None.
Contributor Information
Zohra S. Lassi, Email: zohra.lassi@adelaide.edu.au.
Philippa F. Middleton, Email: philippa.middleton@adelaide.edu.au.
Caroline Crowther, Email: caroline.crowther@adelaide.edu.au, c.crowthe@auckland.ac.nz.
Zulfiqar A. Bhutta, Email: Zulfiqar.bhutta@sickkids.ca, Zulfiqar.bhutta@aku.edu.
References
- Bahadue F.L., Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 2012;(11) doi: 10.1002/14651858.CD001456.pub2. (Art. No.: CD001456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley S., Dybul M., Godal T., Lake A. Integration and innovation to advance newborn survival. Lancet. 2014;384(9938):e22-3. doi: 10.1016/S0140-6736(14)60691-7. (Published, Online May 20, 2014) [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Darmstadt G.L., Hasan B.S., Haws R.A. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(Supplement 2):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Zaidi A.K.M., Thaver D., Humayun Q., Ali S., Darmstadt G.L. Management of newborn infections in primary care settings: a review of the evidence and implications for policy? Pediatr. Infect. Dis. J. 2009;28(1):S22–S30. doi: 10.1097/INF.0b013e31819588ac. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Darmstadt G.L., Haws R.A., Yakoob M.Y., Lawn J.E. Delivering interventions to reduce the global burden of stillbirths: improving service supply and community demand. BMC Pregnancy Childbirth. 2009;9(Suppl. 1):S7. doi: 10.1186/1471-2393-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta Z.A., Das J.K., Rizvi A. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Das J.K., Bahl R. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- Blencowe H., Lawn J., Vandelaer J., Roper M., Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int. J. Epidemiol. 2010;39(Suppl. 1):i102–i109. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Modell B., Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010;39(Supplement 1):i110. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M. National, regional and worldwide estimates of preterm birth. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Brownfoot F.C., Gagliardi D.I., Bain E., Middleton P., Crowther C.A. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2013;(8) doi: 10.1002/14651858.CD006764.pub3. (Art. No.: CD006764) [DOI] [PubMed] [Google Scholar]
- Brozek Jan, Oxman Andrew, Schünemann Holger. 2008. GRADEpro. [Computer program]. Version 3.2 for Windows. [Google Scholar]
- Buchanan S.L., Crowther C.A., Levett K.M., Middleton P., Morris J. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 2010;3(3) doi: 10.1002/14651858.CD004735.pub3. (Art. No.: CD004735) [DOI] [PubMed] [Google Scholar]
- Carroli G., Villar J., Piaggio G. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357(9268):1565–1570. doi: 10.1016/S0140-6736(00)04723-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain C., O'Mara-Eves A., Oliver S. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst. Rev. 2013;10(10) doi: 10.1002/14651858.CD001055.pub4. (Art. No.: CD001055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluver C., Hofmeyr G.J., Gyte G.M., Sinclair M. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database Syst. Rev. 2012;1(1) doi: 10.1002/14651858.CD000184.pub3. (Art. No.: CD000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T., Chamberlain C., Davey M.-A., Cooper S.E., Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2012;9(9) doi: 10.1002/14651858.CD010078. (Art. No.: CD010078) [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A., Díaz-Rossello J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2014;(4) doi: 10.1002/14651858.CD002771.pub3. (Art. No.: CD002771) [DOI] [PubMed] [Google Scholar]
- Cousens S., Blencowe H., Gravett M., Lawn J.E. Antibiotics for pre-term pre-labour rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. Int. J. Epidemiol. 2010;39(Suppl. 1):i134–i143. doi: 10.1093/ije/dyq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J.K., Lassi Z.S., Salam R.A., Bhutta Z.A. Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health. 2013;13(Suppl. 3):S29. doi: 10.1186/1471-2458-13-S3-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes A.K., Kohli A., Walker N., Edmond K., Mullany L.C. Time to initiation of breastfeeding and neonatal mortality and morbidity: a systematic review. BMC Public Health. 2013;13(3):1–14. doi: 10.1186/1471-2458-13-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli V., Barale A., Rivetti A. Vaccines for women to prevent neonatal tetanus. Cochrane Database Syst. Rev. 2013;5(5) doi: 10.1002/14651858.CD002959.pub3. (Art. No.: CD002959) [DOI] [PubMed] [Google Scholar]
- De-Regil L.M., Fernández-Gaxiola A.C., Dowswell T., Peña-Rosas J.P. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2010;10(10) doi: 10.1002/14651858.CD007950.pub2. (Art. No.: CD007950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowswell T., Carroli G., Duley L. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst. Rev. 2010;10(10) doi: 10.1002/14651858.CD000934.pub2. (Art. No.: CD000934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L., Henderson-Smart D.J., Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst. Rev. 2010;10(10) doi: 10.1002/14651858.CD000128.pub2. (Art. No.: CD000128) [DOI] [PubMed] [Google Scholar]
- Duley L., Gulmezoglu A.M., Henderson-Smart D.J., Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst. Rev. 2010;11(11) doi: 10.1002/14651858.CD000025.pub2. (Art. No.: CD000025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L., Gulmezoglu A.M., Chou D. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database Syst. Rev. 2010;9(9) doi: 10.1002/14651858.CD002960.pub2. (Art. No.: CD002960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L., Henderson-Smart D.J., Walker G.J., Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst. Rev. 2010;12(12) doi: 10.1002/14651858.CD000127.pub2. (Art. No.: CD000127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson L., McCormick F., Renfrew M.J. Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst. Rev. 2005;2(2) doi: 10.1002/14651858.CD001688.pub2. (Art. No.: CD001688) [DOI] [PubMed] [Google Scholar]
- Eisele T.P., Larsen D., Steketee R.W. Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas. Int. J. Epidemiol. 2010;39(Suppl. 1):i88–i101. doi: 10.1093/ije/dyq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzi W.W., Chalmers T.C., Herrera M.G., Mosteller F. Vitamin A supplementation and child mortality. JAMA. 1993;269(7):898–903. [PubMed] [Google Scholar]
- Frøen J.F., Cacciatore J., McClure E.M., for The Lancet's Stillbirths Series steering committee Stillbirths: why they matter. Lancet. 2011;377(9774):1353–1366. doi: 10.1016/S0140-6736(10)62232-5. [DOI] [PubMed] [Google Scholar]
- Gamble C., Ekwaru J.P., ter Kuile F.O. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst. Rev. 2006;2(2) doi: 10.1002/14651858.CD003755.pub2. (Art. No.: CD003755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble C., Ekwaru P.J., Garner P., ter Kuile F.O. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4(3):e107. doi: 10.1371/journal.pmed.0040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogia S., Sachdev H.S. Home visits by community health workers to prevent neonatal deaths in developing countries: a systematic review. Bull. World Health Organ. 2010;88(9):658–666. doi: 10.2471/BLT.09.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A., Jeffery H.E. Antibiotic regimens for suspected late onset sepsis in newborn infants. Cochrane Database Syst. Rev. 2005;3(3) doi: 10.1002/14651858.CD004501.pub2. (Art. No.: CD004501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A., Dimitriou G., Prendergast M., Milner A.D. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst. Rev. 2008;1(1) doi: 10.1002/14651858.CD000456.pub3. (Art. No.: CD000456) [DOI] [PubMed] [Google Scholar]
- Grimwade K., Swingler G.H. Cotrimoxazole prophylaxis for opportunistic infections in children with HIV infection. Cochrane Database Syst. Rev. 2006;1(1) doi: 10.1002/14651858.CD003508.pub2. (Art. No.: CD003508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulmezoglu A.M., Crowther C.A., Middleton P., Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst. Rev. 2012;6(6) doi: 10.1002/14651858.CD004945.pub3. (Art.No.:CD004945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G.H., Oxman A.D., Vist G.E. Rating quality of evidence and strength of recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. (Available from www.cochranehandbook.org) [Google Scholar]
- Hill J., Hoyt J., van Eijk A.M., ter Kuile F.O., Webster J., Steketee R.W. Prioritizing pregnant women for long-lasting insecticide treated nets through antenatal care clinics. PLoS Med. 2014;11(9):e1001717. doi: 10.1371/journal.pmed.1001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Subramaniam P., Henderson-Smart D., Ho JJ Davis P., Subramaniam P., Henderson-Smart D.J., Davis P.G. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Database Syst. Rev. 2002;2(2) doi: 10.1002/14651858.CD002271. (Art. No.: CD002271) [DOI] [PubMed] [Google Scholar]
- Hofmeyr G.J., Kulier R. External cephalic version for breech presentation at term. Cochrane Database Syst. Rev. 2012;10(10) doi: 10.1002/14651858.CD000083.pub2. (Art. No.: CD000083) [DOI] [PubMed] [Google Scholar]
- Hofmeyr G.J., Hannah M.E., Lawrie T.A. Planned caesarean section for term breech delivery. Cochrane Database Syst. Rev. 2003;2(2) doi: 10.1002/14651858.CD000166.pub2. (Art. No.: CD000166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr G.J., Lawrie T.A., Atallah Á.N., Duley L., Torloni M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2014;(6) doi: 10.1002/14651858.CD001059.pub4. (Art. No.: CD001059) [DOI] [PubMed] [Google Scholar]
- Homer C.S.E., Ryan C., Leap N., Foureur M., Teate A., Catling-Paull C.J. Group versus conventional antenatal care for women. Cochrane Database Syst. Rev. 2012;11(11) doi: 10.1002/14651858.CD007622.pub2. (Art. No.: CD007622) [DOI] [PubMed] [Google Scholar]
- Hussain A.A., Yakoob M.Y., Imdad A., Bhutta Z.A. Elective induction for pregnancies at or beyond 41 weeks of gestation and its impact on stillbirths: a systematic review with meta-analysis. BMC Public Health. 2011;11(Suppl. 3):S5. doi: 10.1186/1471-2458-11-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton E.K., Hofmeyr G.J. External cephalic version for breech presentation before term. Cochrane Database Syst. Rev. 2006;1(1) doi: 10.1002/14651858.CD000084.pub2. (Art. No.: CD000084) [DOI] [PubMed] [Google Scholar]
- Imdad A., Herzer K., Mayo-Wilson E., Yakoob M.Y., Bhutta Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst. Rev. 2010;12(12) doi: 10.1002/14651858.CD008524.pub2. (Art. No.: CD008524) [DOI] [PubMed] [Google Scholar]
- Imdad A., Yakoob M.Y., Bhutta Z.A. Effect of breastfeeding promotion interventions on breastfeeding rates, with special focus on developing countries. BMC Public Health. 2011;11(Suppl. 3):S24. doi: 10.1186/1471-2458-11-S3-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A., Yakoob M.Y., Sudfeld C., Haider B., Black R., Bhutta Z. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11(Suppl. 3):S20. doi: 10.1186/1471-2458-11-S3-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A., Jabeen A., Bhutta Z.A. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: a meta-analysis of studies from developing countries. BMC Public Health. 2011;11(Suppl. 3):S18. doi: 10.1186/1471-2458-11-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A., Mullany L.C., Baqui A.H. The effect of umbilical cord cleansing with chlorhexidine on omphalitis and neonatal mortality in community settings in developing countries: a meta-analysis. BMC Public Health. 2013;13(Suppl. 3):S15. doi: 10.1186/1471-2458-13-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A., Bautista R.M.M., Senen K.A.A., Uy M.E.V., Mantaring J.B., III, Bhutta Z.A. Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database Syst. Rev. 2013;(5) doi: 10.1002/14651858.CD008635.pub2. (Art. No.: CD008635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S., Boulvain M., Neilson J.P. Antibiotics for preterm rupture of membranes. Cochrane Database Syst. Rev. 2013;(12) doi: 10.1002/14651858.CD001058. (Art. No.: CD001058) [DOI] [PubMed] [Google Scholar]
- Kidney E., Winter H.R., Khan K.S. Systematic review of effect of community-level interventions to reduce maternal mortality. BMC Pregnancy Childbirth. 2009;9(1):2. doi: 10.1186/1471-2393-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuki N., Lee A.C.C., Silveira M.F. The associations of birth intervals with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13(Suppl. 3):S3. doi: 10.1186/1471-2458-13-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti L.M., Zakarija-Grković I., Fischer Walker C.L. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13(Suppl. 3):S18. doi: 10.1186/1471-2458-13-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi Z.S., Haider B.A., Bhutta Z.A. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database Syst. Rev. 2010;11(11) doi: 10.1002/14651858.CD007754.pub2. (Art. No.: CD007754) [DOI] [PubMed] [Google Scholar]
- Lassi Z.S., Majeed A., Rashid S., Yakoob M.Y., Bhutta Z.A. The interconnections between maternal and newborn health-evidence and implications for policy. J. Matern. Fetal Neonatal Med. 2013;26(Suppl. 1):3–53. doi: 10.3109/14767058.2013.784737. [DOI] [PubMed] [Google Scholar]
- Lassi Z., Salam R., Haider B., Bhutta Z. Folic acid supplementation during pregnancy formaternal health and pregnancyoutcomes. Cochrane Database Syst. Rev. 2013;3(3) doi: 10.1002/14651858.CD006896.pub2. (Art. No.: CD006896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn J.E., Mwansa-Kambafwile J., Horta B.L., Barros F.C., Cousens S. Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. Int. J. Epidemiol. 2010;39(Suppl. 1):i144–i154. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Cousens S., Wall A. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11(Suppl. 3):S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemyre B., Davis P.G., De Paoli A.G. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database Syst. Rev. 2002;1(1) doi: 10.1002/14651858.CD002272. (Art. No.: CD002272) [DOI] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2004;2(2) doi: 10.1002/14651858.CD000363.pub2. (Art. No.: CD000363) [DOI] [PubMed] [Google Scholar]
- Lewin S., Munabi-Babigumira S., Glenton C. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst. Rev. 2010;3(3) doi: 10.1002/14651858.CD004015.pub3. (Art. No.: CD004015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbiganon P., Martis R., Laopaiboon M., Festin M.R., Ho J.J., Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database Syst. Rev. 2012;(9) doi: 10.1002/14651858.CD006425.pub3. (Art. No.: CD006425) [DOI] [PubMed] [Google Scholar]
- Mason E., McDougall L., Lawn J.E., for The Lancet Every Newborn Study Group†on behalf of the Every Newborn Steering Committee From evidence to action to deliver a healthy start for the next generation. Lancet. 2014;384(9941):455–467. doi: 10.1016/S0140-6736(14)60750-9. [DOI] [PubMed] [Google Scholar]
- Mayo-Wilson E., Imdad A., Herzer K., Yakoob M.Y., Bhutta Z.A. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343 doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall E.M., Alderdice F., Halliday H.L., Jenkins J.G., Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst. Rev. 2010;3(3) doi: 10.1002/14651858.CD004210.pub4. (Art. No.: CD004210) [DOI] [PubMed] [Google Scholar]
- McDonald S.J., Middleton P., Dowswell T., Morris P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst. Rev. 2013;7(7) doi: 10.1002/14651858.CD004074.pub3. (Art. No.: CD004074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meremikwu M.M., Donegan S., Sinclair D., Esu E., Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst. Rev. 2012;2(2) doi: 10.1002/14651858.CD003756.pub4. (Art. No.: CD003756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwansa-Kambafwile J., Cousens S., Hansen T., Lawn J.E. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int. J. Epidemiol. 2010;39(Suppl. 1):i122–i133. doi: 10.1093/ije/dyq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman A.D., Group G.W. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(19):1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Rosas J., De-Regil L., Dowswell T., Viteri F. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2012;12(12) doi: 10.1002/14651858.CD004736.pub4. (Art. No.: CD004736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pmnch W. World Health Organization; Geneva: 2011. Essential interventions, commodities and guidelines for reproductive maternal, newborn, and child health. [Google Scholar]
- Radeva-Petrova D., Kayentao K., ter Kuile F.O., Sinclair D., Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst. Rev. 2014;(10) doi: 10.1002/14651858.CD000169.pub3. (Art. No.:CD000169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2006;3(3) doi: 10.1002/14651858.CD004454.pub2. (Art. No.: CD004454) [DOI] [PubMed] [Google Scholar]
- Sazawal S., Black R.E. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect. Dis. 2003;3(9):547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- Sazawal S., Black R.E. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect. Dis. 2003;3(9):547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- Shea B.J., Grimshaw J.M., Wells G.A. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll R. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 1998;3(3) doi: 10.1002/14651858.CD001149. (Art. No.: CD001149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll R., Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 2009;1(1) doi: 10.1002/14651858.CD000141.pub2. (Art. No.: CD000141) [DOI] [PubMed] [Google Scholar]
- Subramaniam P., Henderson-Smart D.J., Davis P.G. Prophylactic nasal continuous positive airways pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst. Rev. 2005;(3) doi: 10.1002/14651858.CD001243.pub2. (Art. No.: CD001243) [DOI] [PubMed] [Google Scholar]
- Sudfeld C.R., Navar A.M., Halsey N.A. Effectiveness of measles vaccination and vitamin A treatment. Int. J. Epidemiol. 2010;39(Suppl. 1):i48–i55. doi: 10.1093/ije/dyq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E., Al-Jilaihawi S., Woodward F. The effect of case management on childhood pneumonia mortality in developing countries. Int. J. Epidemiol. 2010;39(Suppl. 1):i155–i171. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwing J., Eisele T.P., Steketee R.W. Protective efficacy of malaria case management and intermittent preventive treatment for preventing malaria mortality in children: a systematic review for the Lives Saved Tool. BMC Public Health. 2011;11(3):1–9. doi: 10.1186/1471-2458-11-S3-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer R.L.S., Lincetto O., McGuire W., Saloojee H., Gulmezoglu A.M. Prophylactic versus selective antibiotics for term newborn infants of mothers with risk factors for neonatal infection. Cochrane Database Syst. Rev. 2004;4(4) doi: 10.1002/14651858.CD003957.pub2. (Art. No.:CD003957) [DOI] [PubMed] [Google Scholar]
- Wu T., Ni J., Wei J. Vitamin A for non-measles pneumonia in children. Cochrane Database Syst. Rev. 2005;3(3) doi: 10.1002/14651858.CD003700.pub2. (Art. No.: CD003700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoob M.Y., Theodoratou E., Jabeen A. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011;11(Suppl. 3):S23. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You D., New J.R., Wardlaw T. World Bank; Washington DC: 2013. Levels and trends in child mortality: estimates developed by the UN Inter-agency Group for child Mortality Estimation (IGME) - report 2013. [Google Scholar]
- Zaidi A.K.M., Ganatra H.A., Syed S. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health. 2011;11(Suppl. 3):S13. doi: 10.1186/1471-2458-11-S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan J., Garner P., Omari A.A. Topical umbilical cord care at birth. Cochrane Database Syst. Rev. 2004;3(3) doi: 10.1002/14651858.CD001057.pub2. (Art. No.: CD001057) [DOI] [PMC free article] [PubMed] [Google Scholar]


