Abstract
Background
Patient adherence to medications, particularly for conditions requiring prolonged treatment such as tuberculosis (TB), is frequently less than ideal and can result in poor treatment outcomes. Material incentives to reward good behaviour and enablers to remove economic barriers to accessing care are sometimes given in the form of cash, vouchers, or food to improve adherence.
Objectives
To evaluate the effects of material incentives and enablers in patients undergoing diagnostic testing, or receiving prophylactic or curative therapy, for TB.
Search methods
We undertook a comprehensive search of the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; LILACS; Science Citation Index; and reference lists of relevant publications up to 5 June 2015.
Selection criteria
Randomized controlled trials of material incentives in patients being investigated for TB, or on treatment for latent or active TB.
Data collection and analysis
At least two review authors independently screened and selected studies, extracted data, and assessed the risk of bias in the included trials. We compared the effects of interventions using risk ratios (RR), and presented RRs with 95% confidence intervals (CI). The quality of the evidence was assessed using GRADE.
Main results
We identified 12 eligible trials. Ten were conducted in the USA: in adolescents (one trial), in injection drug or cocaine users (four trials), in homeless adults (three trials), and in prisoners (two trials). The remaining two trials, in general adult populations, were conducted in Timor‐Leste and South Africa.
Sustained incentive programmes
Only two trials have assessed whether material incentives and enablers can improve long‐term adherence and completion of treatment for active TB, and neither demonstrated a clear benefit (RR 1.04, 95% CI 0.97 to 1.14; two trials, 4356 participants; low quality evidence). In one trial, the incentive, given as a daily hot meal, was not well received by the population due to the inconvenience of attending the clinic at midday, whilst in the other trial, nurses distributing the vouchers chose to "ration" their distribution among eligible patients, giving only to those whom they felt were most deprived.
Three trials assessed the effects of material incentives and enablers on completion of TB prophylaxis with mixed results (low quality evidence). A large effect was seen with regular cash incentives given to drug users at each clinic visit in a setting with extremely low treatment completion in the control group (treatment completion 52.8% intervention versus 3.6% control; RR 14.53, 95% CI 3.64 to 57.98; one trial, 108 participants), but no effects were seen in one trial assessing a cash incentive for recently released prisoners (373 participants), or another trial assessing material incentives offered by parents to teenagers (388 participants).
Single once‐only incentives
However in specific populations, such as recently released prisoners, drug users, and the homeless, trials show that material incentives probably do improve one‐off clinic re‐attendance for initiation or continuation of anti‐TB prophylaxis (RR 1.58, 95% CI 1.27 to 1.96; three trials, 595 participants; moderate quality evidence), and may increase the return rate for reading of tuberculin skin test results (RR 2.16, 95% CI 1.41 to 3.29; two trials, 1371 participants; low quality evidence).
Comparison of different types of incentives
Single trials in specific sub‐populations suggest that an immediate cash incentive may be more effective than delaying the incentive until completion of treatment (RR 1.11, 95% CI 0.98 to 1.24; one trial, 300 participants; low quality evidence), cash incentives may be more effective than non‐cash incentives (completion of TB prophylaxis: RR 1.26, 95% CI 1.02 to 1.56; one trial, 141 participants; low quality evidence; return for skin test reading: RR 1.13, 95% CI 1.07 to 1.19; one trial, 652 participants; low quality evidence); and higher cash incentives may be more effective than lower cash incentives (RR 1.08, 95% CI 1.01 to 1.16; one trial, 404 participants; low quality evidence).
Authors' conclusions
Material incentives and enablers may have some positive short term effects on clinic attendance, particularly for marginal populations such as drug users, recently released prisoners, and the homeless, but there is currently insufficient evidence to know if they can improve long term adherence to TB treatment.
8 May 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted up to 19 Jul, 2018 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Keywords: Adolescent; Adult; Child; Humans; Male; Young Adult; Motivation; Token Economy; Ill‐Housed Persons; Medication Adherence; Medication Adherence/psychology; Medication Adherence/statistics & numerical data; Patient Compliance; Patient Compliance/psychology; Patient Compliance/statistics & numerical data; Prisoners; Randomized Controlled Trials as Topic; Substance‐Related Disorders; Substance‐Related Disorders/complications; Tuberculin Test; Tuberculin Test/psychology; Tuberculosis, Pulmonary; Tuberculosis, Pulmonary/diagnosis; Tuberculosis, Pulmonary/drug therapy; Tuberculosis, Pulmonary/psychology
Plain language summary
Incentives and enablers for improving patient adherence to tuberculosis diagnosis, prophylaxis, and treatment
Cochrane researchers conducted a review of the effects of material (economic) incentives or enablers on the adherence and outcomes of patients being tested or treated for latent or active tuberculosis (TB). After searching up to 5 June 2015 for relevant trials, they included 12 randomized controlled trials in this Cochrane review.
What are material incentives and enablers and how might they improve patient care?
Material incentives and enablers are economic interventions which may be given to patients to reward healthy behaviour (incentives) or remove economic barriers to accessing healthcare (enablers). Incentives and enablers may be given directly as cash or vouchers, or indirectly in the provision of a service for which the patient might otherwise have to pay (like transport to a health facility).
What the research says
Material incentives and enablers may have little or no effect in improving the outcomes of patients on treatment for active TB (low quality evidence), but further trials of alternative incentives and enablers are needed.
Material incentives and enablers may have some effects on completion of prophylaxis for latent TB in some circumstances but trial results were mixed, with one trial showing a large effect, and two trials showing no effect (low quality evidence).
One‐off material incentives and enablers probably improve rates of return to a single clinic appointment for patients starting or continuing prophylaxis for TB (moderate quality evidence) and may improve the rate of return to the clinic for the reading of diagnostic tests for TB (low quality evidence).
Thus although material incentives and enablers may improve some patients' attendance at the clinic in the short term, more research is needed to determine if they have an important positive effect in patients on long term treatment for TB.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Material incentives compared to routine care for improving patient adherence to TB management | |||||
| Patient or population: Recipients of TB control services Settings: Middle‐income and high‐income country settings (South Africa, Timore‐Leste, USA) Intervention: Material incentives (such as cash, grocery vouchers, or food) Comparison: Routine care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Routine care | Material incentives | ||||
| Sustained incentive programme | |||||
| Cure or completion of treatment for active TB | 721 per 1000 | 750 per 1000 (620 to 807) | RR 1.04 (0.97 to 1.13) | 4356 (2 trials) | ⊕⊕⊝⊝ low1 |
| Completion of TB prophylaxis | — | — | Not pooled | 869 (3 trials) | ⊕⊕⊝⊝ low2,3 |
| Single once‐only incentive | |||||
| Return to clinic to start or continue treatment | 249 per 1000 | 393 per 1000 (316 to 488) | RR 1.58 (1.27 to 1.96) | 595 (3 trials) | ⊕⊕⊕⊝ moderate4 |
| Return for tuberculin skin test reading | 441 per 1000 | 953 per 1000 (622 to 1000) | RR 2.16 (1.41 to 3.29) | 1371 (2 trials) | ⊕⊕⊝⊝ low5,6 |
| The assumed risk is taken from the control groups in the trials. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 2 for indirectness: these two trials evaluated specific interventions amongst specific populations, and problems with the acceptability and implementation of the intervention in both trials limit the generalizability of this finding of no effect. 2Downgraded by 1 for indirectness: these trials were conducted in specific subpopulations from the USA (drug users, recently released prisoners, and adolescents), and the result may not be applicable in other settings. 3Downgraded by 1 for inconsistency: two studies found no suggestion of a benefit with the incentive, and one study found a clinically and statistically significant benefit in drug users in a setting where adherence without incentives was very low. 4Downgraded by 1 for indirectness: these trials were conducted in specific subpopulations from the USA (the homeless or recently released prisoners), and the result may not be applicable in other settings. 5Downgraded by 1 for risk of bias: neither study adequately described the method of randomization. 6Downgraded by 1 for indirectness: these trials were conducted in specific subpopulations from the USA (drug users), and the result may not be applicable in other settings.
Background
Description of the condition
Tuberculosis (TB) is an infectious disease caused by the bacterium Mycobacterium tuberculosis, which spreads from person to person by inhalation of respiratory droplets. Although the global incidence of TB is falling, the burden of disease is still high, especially in low‐ and middle‐income countries (LMICs) where it is associated with poverty (WHO 2013). Impaired immunity, due to co‐infection with the human immuno‐deficiency virus (HIV) or poor nutrition, plays an important role in many infections (WHO 2009a; WHO 2013).
Following the initial infection, most people do not develop symptoms as M. tuberculosis bacteria are completely controlled by the immune system, and lie dormant in a state known as 'latent TB'. Active TB, where the bacterium is no longer controlled by the immune system, can occur at any time following infection and most commonly affects the lungs, causing a chronic cough (which acts to spread the disease), loss of weight, loss of appetite, and general malaise (Harries 2006).
The most widely used method of diagnosing latent TB is the tuberculin test (also known as the Mantoux test), which involves injecting a small amount of a purified M. tuberculosis protein under the skin, usually of the forearm. If the person has previously been exposed to TB, a small swelling occurs due to a localized immune response, and the size of this response is measured 48 to 72 hours later (CDC 2010). Treatment of latent TB, often called TB prophylaxis, aims to prevent the later development of active disease, and reduce transmission.
Effective treatment for both active and latent TB requires regular medication to be taken for six to 12 months, and non‐adherence to this difficult and prolonged schedule is the most common cause of treatment failure (Narayanan 2003; Volmink 2000). Non‐adherence, with prolonged infectiousness, constitutes a health risk to close family and community contacts, and can lead to the development of drug‐resistant organisms which are more difficult and more expensive to treat (Lam 2002).
Adherence is not the sole responsibility of the patient, nor of the health system, but some combination of the two (Garner 2007), and consequently interventions aimed at reducing non‐adherence may need to target both. These interventions may be classified as: technical (making the medications simpler to take, such as reducing doses and personalising packaging); behavioural (establishing a pattern of behaviour through stimuli or positive reinforcement); educational (improving patients' capacity to manage their diseases, often through a cognitive didactic approach); structural (improving the accessibility and acceptability of TB programmes); or complex (a combination of these) (Haynes 2008; Munro 2007; van Dulmen 2007; WHO 2003c). A review of direct observation has been completed indicating little added effect of direct observation (Karumbi 2015), and a review of patient reminders and prompts is also available showing mixed effects (Liu 2014). A further review found that patient education may improve completion of treatment for latent TB (no trials were found investigating the effect of patient education for active TB) (M'Imunya 2012).
Description of the intervention
Incentives and enablers are interventions targeted at the patient which seek to either promote or assist improved adherence (WHO 2003a; WHO 2003b; WHO 2003c). They may be given directly as cash or vouchers for groceries, or indirectly as the provision of a service for which the patient would otherwise have had to pay (for example, transport to and from the clinic).
A recent overview of reviews found that material incentives improved adherence and outcomes for a number of health problems, and also increased the utilisation of health services for prevention programmes (Sutherland 2008). Conditional cash transfers, used primarily in Latin America, are essentially material incentives used on a large scale to promote healthy behaviour in poor families and individuals (Lagarde 2007). They have been particularly successful in promoting the use of health services and in improving nutritional and anthropometric outcomes in certain groups (Lagarde 2007).
How the intervention might work
Incentives are based on behavioural theories of reward for 'good' behaviour (van Dulmen 2007), and may be defined as "any financial or material reward that patients and/or providers receive, conditional on their explicitly measured performance or behavior" (Beith 2007). Alternatively, 'enablers' assist patients to adhere by overcoming the financial barriers to treatment. In a recent qualitative review, economic constraints due to absences from work to attend appointments, or the direct and indirect costs of accessing treatment, were commonly cited by patients as important barriers to completing TB treatment (Munro 2007).
As well as potential benefits, the use of material incentives and enablers may also have unintentional negative consequences. Patients who receive incentives to adhere to one health behaviour may be reluctant to adhere to others if they are not also accompanied by incentives (Malotte 1999). This might be especially important where incentives are offered in one of several possible stages in a multi‐stage treatment process such as screening for and treating TB. Further possible negative effects include: resentment in patients who do not receive the incentive (Malotte 2001); fraud and corruption, with patients manipulating the incentive system to gain more; or the creation of 'ghost' patients allowing health staff to steal incentives from the system (White 1998); or the 'perverse incentive' effect, where the incentive induces exactly the opposite behaviour to that intended, ie patients who want to continue receiving the incentive may deliberately not take medications in order to remain ill.
Why it is important to do this review
In light of the increased risk of TB posed by HIV infection (WHO 2013), and the development of epidemics of drug‐resistant forms of TB (Wells 2007; Yang 2011), efforts to help patients complete therapy are of paramount importance. If material incentives and enablers do improve adherence rates amongst patients with TB, they should be used far more widely than they are currently.
Objectives
To evaluate the effects of material incentives and enablers given to patients undergoing diagnostic testing for TB, or receiving drug therapy to prevent or cure TB.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), where the unit of allocation is either an individual or cluster.
Types of participants
People receiving curative treatment for active TB: smear positive cases, smear negative cases, new cases, and re‐treatment cases;
People receiving preventive therapy for latent TB: patients at risk of developing active TB and taking anti‐TB chemoprophylaxis (ie isoniazid preventive therapy);
People suspected of TB undergoing, and collecting results of, diagnostic tests: diagnosis of TB infection (using tuberculin skin tests) and disease (using sputum microscopy and culture) often requires the patient to return to the health facility a few days after the test is performed to receive the results. Incentives have been used to encourage patients to do this.
Types of interventions
Intervention
Interventions included any form of material inducement to return for TB test results, or adhere to or complete anti‐TB preventive or curative treatment. These may have been direct such as cash or vouchers for groceries, or indirect such as the provision of a service for which the patient would otherwise have had to pay (for example, transport to and from the clinic). We did not consider non‐material incentives, such as praise from a health worker, in this Cochrane Review, because their economic value is difficult to quantify and the form of the incentive is difficult to standardize.
In those trials where incentives were combined with other interventions, studies were only eligible for inclusion in a meta‐analysis if disaggregation of the effect of the incentive was possible. Other interventions that could be combined with incentives include health information and education, and increased access to health workers through home visits, or additional appointments.
We only included trials if the standard TB curative or preventive treatment was the same across the control and treatment arms.
Control
Controls were those patients receiving standard TB treatment or preventive treatment, or undergoing testing for suspected TB, who had no incentive or an alternative incentive or intervention.
Types of outcome measures
Primary outcomes
For treatment of active TB: Cure or completion of treatment, or both, using the following World Health Organization (WHO) definitions (WHO 2009b):
Cured: a patient who was initially smear‐positive and who was smear negative in the last month of treatment and on at least one previous occasion;
Completed treatment: a patient who completed the course of treatment as prescribed by the health worker;
Successfully treated: a patient who was cured or who completed treatment (WHO 2009b).
For prophylaxis: cases of active TB; completion of prophylactic treatment.
For diagnostics: number returning to collect test results within the appropriate time frame for that test.
Secondary outcomes
Appointment keeping, presence of urinary markers, and certification by direct observation of treatment.
Adverse events
Adverse events reported in trials, such as expenditure of cash or vouchers on unhealthy items. The latter were defined as commodities that undermine the patient's chance of cure, such as tobacco products or alcohol.
Costs
Cost effectiveness of the intervention; where costs include the direct and indirect costs incurred by patients, and costs to the health system of providing and administering the incentives.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
For this review update we searched the following databases using the search terms and strategy described in Table 2: Cochrane Infectious Diseases Group Specialized Register (5 June 2015); Cochrane Central Register of Controlled Trials (CENTRAL: 5 June 2015); MEDLINE (1966 to 5 June 2015); EMBASE (1974 to 5 June 2015); LILACS (1982 to 5 June 2015); and Science Citation Index (EXPANDED) and Social Sciences Citation index (SSCI) (1956 to 5 June 2015).
1. Detailed search strategies.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | SCI‐EXPANDED and SSC |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis |
| 2 | adherence | PATIENT COMPLIANCE | PATIENT COMPLIANCE | PATIENT‐COMPLIANCE | adherence | adherence |
| 3 | compliance | PATIENT DROPOUTS | PATIENT DROPOUTS | TREATMENT‐REFUSAL | compliance | compliance |
| 4 | Monitor* | MOTIVATION | MOTIVATION | MOTIVATION | Monitor$ | Monitor* |
| 5 | Incentive* | SOCIAL SUPPORT | SOCIAL SUPPORT | SOCIAL SUPPORT | Incentive$ | Incentive* |
| 6 | Reward* | CONTRACTS | CONTRACTS | COMPENSATION | Reward$ | Reward* |
| 7 | Voucher* | Adherence | Adherence | Adherence | Voucher$ | Voucher* |
| 8 | Payment* | Incentive* | Incentive* | Incentive$ | Payment$ | Payment* |
| 9 | Reimbursement* | Reward* | Reward* | Reward$ | Reimbursement$ | Reimbursement* |
| 10 | Concordance | Voucher* | Voucher* | Voucher$ | Concordance | Concordance |
| 11 | Cash transfer* | Payment* | Payment* | Payment$ | Cash transfer$ | Cash transfer* |
| 12 | 2‐11/OR | Reimbursement* | Reimbursement* | Reimbursement$ | 2‐11/OR | 2‐11/OR |
| 13 | 1 AND 12 | Concordance | Concordance | Concordance | 1 AND 12 | 1 AND 12 |
| 14 | — | Cash transfer* | Cash transfer* | Cash transfer$ | — | — |
| 15 | — | 2‐14/OR | 2‐14/OR | 2‐14/OR | — | — |
| 16 | — | 1 AND 15 | 1 AND 15 | 1 AND 15 | — | — |
| 17 | — | — | Limit 16 to Human | Limit 16 to Humans | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Lefebvre 2011); Upper case: MeSH or EMTREE heading; Lower case: free text term.
We also searched the metaRegister of Controlled Trials (mRCT) using 'tuberculosis', 'incentive', 'cash transfer', 'adherence', 'compliance', and 'concordance' as search terms (1998 to 5 June 2015). In addition, we searched the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/search/en/) for ongoing trials (5 June 2015).
This is the same search strategy and the same databases that were used for the original review, Lutge 2012.
Searching other resources
Researchers and organizations
We contacted researchers and other experts in the field of TB and adherence research for unpublished and ongoing trials.
Reference lists
We checked the reference lists of related reviews (Garner 2007; Haynes 2008; Lagarde 2007; Sutherland 2008; Volmink 2000) and all full‐text articles reviewed for inclusion in this review.
Data collection and analysis
Selection of studies
For the original 2012 review, Lutge 2012, Elizabeth Lutge (EL) and Stephen Knight (SK) independently screened all citations and abstracts identified by the search strategy for potentially eligible studies (Lutge 2012). For this review update, EL and Charles Shey Wiysonge (CSW) independently performed screening and study selection. Two review authors independently assessed the full text articles of potentially relevant studies using the pre‐specified trial inclusion criteria. We resolved any disagreements by discussion and consensus. When a disagreement could not be resolved we sought arbitration from a third review author; CSW in the case of the original Cochrane review and Jimmy Volmink (JV) for both the original, Lutge 2012, and this Cochrane review update. We excluded studies that did not meet the inclusion criteria and documented the reasons for exclusion in the 'Characteristics of excluded studies' table.
As EL is the author of a trial included in this Cochrane review update, two review authors who were not involved with this trial (David Sinclair (DS) and CSW) independently applied the inclusion criteria, assessed risk of bias, and performed data extraction of this trial. Two Cochrane Editors provided oversight.
Data extraction and management
In both the original review and its update, EL and CSW independently extracted information from the selected trial reports using a pre‐designed data extraction form on study methods used, participant characteristics, interventions, and outcomes. For all outcomes, we extracted the number of participants randomized and the number analysed. For each study, we extracted the number of participants with an outcome of interest in each group as well as the number of participants randomized to each group, and the number analysed.
We resolved any disagreements through discussion and consensus between EL and CSW initially, and with SK or JV if the disagreement was not resolved.
Assessment of risk of bias in included studies
EL, CSW, and DS independently assessed the risk of bias in each included trial using Cochrane's 'Risk of bias' assessment tool (Higgins 2011), in line with the Cochrane policy on trialists who are also review authors (Kliner 2014). We followed the guidance to assess whether adequate steps were taken to reduce the risk of bias across six specific domains, namely: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessment; incomplete outcome data; selective outcome reporting; and 'other issues'. For each included trial, we independently described what the trial authors reported that they did for each domain and then made a decision relating to the risk of bias for that domain by assigning a judgement of either 'low', 'high', or 'unclear' risk of bias. EL, CSW, and DS compared the results of their independent 'Risk of bias' assessments and resolved any discrepancies by discussion and consensus. A fourth review author (JV) resolved any differences in opinion.
Assessment of reporting biases
If at least 10 trials were included in the meta‐analysis for any outcome, we would have evaluated the likelihood of publication bias and other sources of bias by examining the degree of asymmetry of funnel plots. We chose this number because it has been shown that when there are fewer than 10 studies in a meta‐analysis the power of funnel plot asymmetry tests is too low to distinguish chance from real asymmetry (Higgins 2011).
Data synthesis
We analysed data using Review Manager (RevMan). We analysed trial participants in groups to which they were randomized, regardless of how much of the intended intervention they actually received.
All studies reported only dichotomous data, so we have expressed study results as the risk ratio (RR) with its 95% confidence intervals (CI) for each outcome. We used the fixed‐effect model for the primary analysis. When significant statistical heterogeneity was present and it was appropriate to combine the data, we used the random‐effects model. We stratified analyses according to the type of incentive and control intervention, that is, incentive versus routine care, immediate versus deferred incentive, cash versus non‐cash incentive, and incentive versus any other intervention.
In addition, we used the GRADE approach to summarise the quality of the evidence on the effects of material incentives on each outcome (Guyatt 2008). In the GRADE system, randomized trials without important limitations constitute high quality evidence. However, the system considers five factors that can lower the quality of the evidence: study limitations; inconsistent results across studies; indirectness of the evidence; imprecision; and publication bias. Overall, the GRADE system classifies research evidence into four categories (high, moderate, low, or very low quality). High quality evidence implies that we "are very confident that the true effect lies close to that of the estimate of the effect", while very low quality evidence implies that the "true effect is likely to be substantially different from the estimate of effect" found in the review (Balshem 2011).
Subgroup analysis and investigation of heterogeneity
We determined the presence of statistical heterogeneity across trials by visually inspecting the forest plots to check for overlapping CIs and by means of the Chi² test for heterogeneity with a P value of < 0.10 indicating statistical significance. Furthermore, we use the I² statistic to quantify the amount of heterogeneity as either low (I² statistic value ≤ 25%), moderate (I² statistic value between 25% and 75%), or high (I² statistic value of ≥ 75%). If we had at least 10 studies in any meta‐analysis that showed significant statistical heterogeneity, we would have explored the possible sources of heterogeneity by performing subgroup analyses; with subgroups defined by age, gender, socioeconomic status, and risk of bias (low versus high/unclear).
Results
Description of studies
For Lutge 2012, we obtained 733 titles and abstracts from the electronic search of databases, and no additional articles from contacting researchers or screening reference lists. After removal of duplicates, 225 records remained. Following discussion and consensus, we obtained 21 potentially eligible articles. Eleven RCTs met our inclusion criteria (Chaisson 2001; Malotte 1998; Malotte 1999; Malotte 2001; Martins 2009; Morisky 2001; Pilote 1996; Tulsky 2000; Tulsky 2004; White 1998; White 2002). The final article, Kominski 2007, was a cost‐effectiveness analysis of an included study (Morisky 2001).
In the 2014 update to the review, we obtained five records from the searches. We screened the abstracts of theses publications and deemed three irrelevant (one was a report of an education intervention and two were reports of text messaging interventions for improving TB treatment adherence). Two full articles were retrieved (Gärden 2013; Lutge 2013). We excluded Gärden 2013 because it was not a RCT; it used an historical control group as comparator (Gärden 2013). We included the second potentially eligible study, Lutge 2013, in this review. The search conducted in 2015 yielded 11 records, seven published articles, and four ongoing studies. Of the seven published articles, five were clearly irrelevant to this review and were excluded. One of the remaining two articles reported a qualitative process evaluation of an included study (Lutge 2013). The other article reported data from an observational cohort study (Chua 2015), and excluded because it was not a RCT. All four ongoing studies were found to be irrelevant to the review and discarded.
The search and selection of studies for the original review, Lutge 2012, and its update are shown in Figure 1.
1.
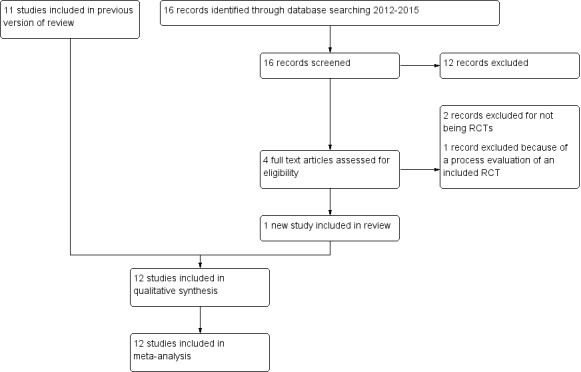
PRISMA diagram showing the search and selection of studies
Included studies
The trials identified and included in this review all randomized individual participants and reported only dichotomous outcomes.
Ten of the 12 included trials were conducted in the USA, and only two are from low‐ and middle‐income countries (LMICs) (Lutge 2013: South Africa; Martins 2009: Timor‐Leste).
Studies varied in size from 79 to 4091 participants, with a mean of 735 participants, and most studies focused on very specific patient subgroups. Four studies were conducted among injection drug or cocaine users (Malotte 1998; Malotte 1999; Chaisson 2001; Malotte 2001), three among homeless or marginally housed adults (Pilote 1996; Tulsky 2000; Tulsky 2004), two studies in prisoners (White 1998; White 2002), and one assessed incentives given to adolescents aged 11 to 19 years (Morisky 2001). Only two studies involved members of the general adult population with TB (Lutge 2013; Martins 2009).
The studies assessed adherence to different stages of TB management. Some investigated the use of incentives in improving return for reading of tuberculin skin test results (Malotte 1998; Malotte 1999) while others focused on improving attendance at the clinic for initiation of treatment (Pilote 1996), adherence to preventive TB treatment (Chaisson 2001; Malotte 2001; Morisky 2001; White 1998; White 2002; Tulsky 2000; Tulsky 2004) and two looked at adherence to treatment for active TB (Lutge 2013; Martins 2009).
The trials investigated various types of incentives, and several trials had multiple study arms receiving different forms of both material and non‐material incentives. Eight studies included a study arm given cash in values of USD 5 or USD 10 (Chaisson 2001; Malotte 1998; Malotte 1999; Malotte 2001; Pilote 1996; Tulsky 2000; Tulsky 2004; White 1998). Four studies gave vouchers that could be redeemed for groceries, food, transport, meals at fast food outlets or phone calls (Lutge 2013; Malotte 1999; Tulsky 2004; White 2002), and one study gave food as a hot daily meal (Martins 2009). In one trial, adolescent patients negotiated the incentive they received from their parents (Morisky 2001). Common choices included special meals at home, going to a movie, or renting a video.
These material incentives were compared with routine care, and in multi‐arm trials also with motivational education (Malotte 1998; Malotte 1999), peer counselling (Morisky 2001; Pilote 1996; Tulsky 2000), and standardized education sessions (White 1998; White 2002). In addition, one study compared different levels of incentive (Malotte 1998), one study compared an immediate incentive, given monthly throughout treatment, with a lump sum given on completion (Chaisson 2001), and two studies compared different forms of incentive (Malotte 1999; Tulsky 2004).
Excluded studies
We excluded trials for the following reasons: patients in both trial arms were given the same incentive (Nyamathi 2006); quasi‐randomized with randomization done either by day of the week (Cheng 1997) or by the last digits in the participants' clinic record numbers (Morisky 1990); cross sectional studies where one group was given the incentive and the other was not (Cantalice Filho 2009; FitzGerald 1999; Yao 2008); or the main intervention was community health‐worker delivered TB treatment combined with food supplements (Jahnavi 2010). In this review update, we excluded one new potentially eligible study because the control was an historical group of patients on treatment for TB (Gärden 2013), and another study because the intervention was not randomized (Chua 2015).
Risk of bias in included studies
We have summarized our 'Risk of bias' judgements for each included trial in Figure 2 and Figure 3.
2.
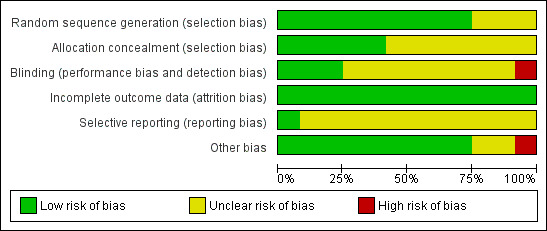
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
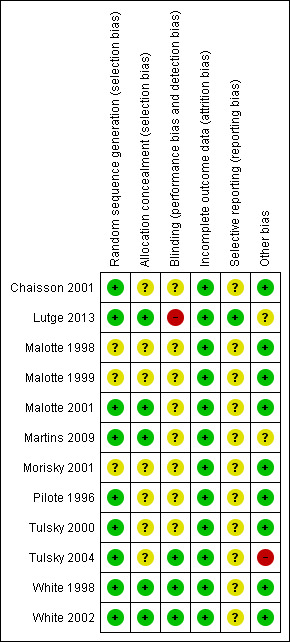
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered the generation of the randomization sequence adequate in nine trials (Chaisson 2001; Lutge 2013; Malotte 2001; Martins 2009; Pilote 1996; Tulsky 2000; Tulsky 2004; White 1998; White 2002) and unclear risk in the remainder (Malotte 1998; Malotte 1999; Morisky 2001). Allocation concealment was judged to be adequate in five trials (Lutge 2013; Malotte 2001; Martins 2009; White 1998; White 2002) and unclear risk in the rest.
Blinding
Three trials had adequate blinding of outcome assessors (White 1998; White 2002; Tulsky 2004), Lutge 2013 had no blinding of outcome assessors, and the remaining trials were unclear.
Incomplete outcome data
All the included trials addressed incomplete outcome data adequately.
Selective reporting
It was unclear to us whether or not 11 of the 12 included RCTs were free of selective outcome reporting since the study protocols were unavailable and there was no earlier methods paper listing the pre‐specified outcomes for any of the trials. The one trial which was prospectively registered prior to commencement (Current Controlled Trials ISRCTN50689131) was free of selective reporting (Lutge 2013).
Other potential sources of bias
Tulsky 2004 compared the effects of cash and non‐cash incentives among homeless adults on adherence to treatment for latent TB infection as well as the length of time needed to look for participants who missed their dose of medications. Although the participants were described as homeless, the study groups were not the same with respect to their primary housing in the year prior to the study. In the cash incentive arm, 23% had lived in a shelter or on the street, whilst 41% of the non‐cash incentive arm had done so (Tulsky 2004). This baseline difference had the potential to introduce systematic differences in study outcomes.
The conduct of the two trials amongst patients on treatment for active TB were affected by contextual issues that had important implications for their results (Lutge 2013; Martins 2009). In Martin's trial, two important factors affected the conduct of the trial and undermined the potential effectiveness of the intervention. The first was civil conflict that arose during the last few months of the trial, resulting in the displacement of approximately 70% of the local population, and dramatically increasing the rate of default from treatment (Martins 2009). The second factor was the timing of the provision of meals to patients in the intervention group. Many patients found the provision of meals, together with treatment, at midday inconvenient, and this may have become a barrier to adherence to treatment (Martins 2009). In Lutge 2013, fidelity to the intervention was poor, with only 31.5% of patients receiving their vouchers for most of their treatment. More than a third (36.2%) of eligible patients did not receive a voucher at all and the remainder received vouchers for between one and three months of treatment. This low fidelity was attributed largely to nurses' rationing of the vouchers to those whom they felt were more deprived and therefore more deserving (Lutge 2013). It is important that such contextual issues be considered in further trials of this nature. In the very environments where extensive poverty or conflicts prevail and TB is likely to be more prevalent, contextual issues may profoundly affect the conduct and the results of the trials.
Effects of interventions
See: Table 1
Incentives versus routine care
Completion of treatment for active TB
Two trials examined the effect of material incentives in patients with active TB. One trial evaluated incentives given as food (in the form of hot meals at the clinic during the intensive phase of treatment followed by monthly food parcels) compared to usual care with nutritional advice (Martins 2009), and the second trial compared vouchers, redeemable for food and household goods, with usual care alone (Lutge 2013). Neither trial found statistically significant differences in treatment completion rates between participants given incentives and those receiving usual care (RR 1.04, 95% CI 0.97 to 1.14; two trials, 4356 participants; Analysis 1.1).
1.1. Analysis.
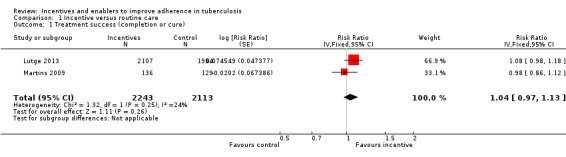
Comparison 1 Incentive versus routine care, Outcome 1 Treatment success (completion or cure).
Completion of TB prophylaxis
Three studies from the USA examined the effect of incentives on completion of TB prophylaxis. Malotte 2001 gave a USD 5 cash incentive to drug users on attendance for twice weekly directly observed treatment, Morisky 2001 established an incentive agreement between adolescents aged between 11 to 19 years and their parents, where parents provided cash or treats at various stages in the treatment process, and White 2002 gave recently released prisoners transportation vouchers worth USD 25 upon first presentation at a TB clinic.
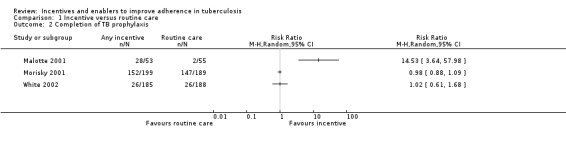
Malotte 2001 showed large effects with incentives, with over half the 53 patients completing treatment with an incentive, contrasted with very low completion in the control (2/55). In Morisky 2001, completion of treatment was better in the control group (77.8%), and was similar with the incentive (76.4%), while in White 2002 completion remained low in both groups despite the intervention (13.8% control versus 14.1% intervention).
Return to clinic for initiation or continuation of TB prophylaxis
Three studies from the USA compared material incentives with routine care (Pilote 1996; White 1998; White 2002). Pilote 1996 gave USD 5 to homeless people on return to a clinic after a positive tuberculin skin test, White 1998 gave USD 5 when recently released prisoners attended a community clinic for continuation of TB prophylaxis, and White 2002 gave recently released prisoners food or transportation vouchers worth USD 25 upon presentation at a TB clinic.
Incentives improved clinic attendance for initiation or continuation of treatment for latent TB infection (RR 1.58, 95% CI 1.27 to 1.96; three trials, 595 participants; Analysis 1.3). Completion rates in the control groups varied, and this was also the case in the intervention group, so that despite the apparent benefit in terms of relative risk, in two trials in prisoners, attendance at clinics remained lower than 25% even in the intervention groups.
1.3. Analysis.
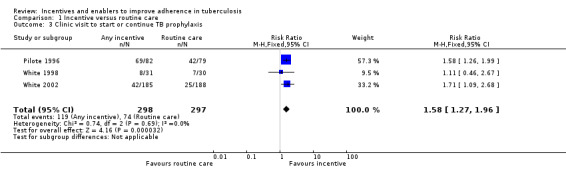
Comparison 1 Incentive versus routine care, Outcome 3 Clinic visit to start or continue TB prophylaxis.
Return to clinic for tuberculin skin test reading
Two studies in drug users from the USA compared material incentives (USD 5 to USD 10) with routine care alone (Malotte 1998; Malotte 1999).
Material incentives increased the proportion of people who returned for reading of the tuberculin skin test (RR 2.16, 95% CI 1.41 to 3.29; two trials, 1371 participants; Analysis 1.4), both with quantitatively important large effects.
1.4. Analysis.
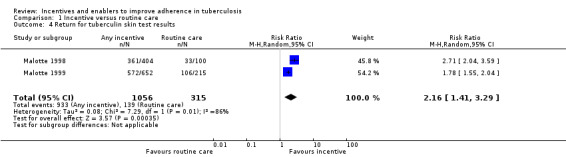
Comparison 1 Incentive versus routine care, Outcome 4 Return for tuberculin skin test results.
Immediate versus deferred incentive
Completion of TB prophylaxis
One study among drug users (Chaisson 2001), compared the effects of an immediate incentive (USD 10 for each monthly appointment attended) with the promise of a deferred lump sum (USD 10 for each appointment attended) on completion of TB prophylaxis.
The participants who received the immediate incentives completed treatment more often than those whose incentives were deferred (83% versus 75%), but the difference was not statistically significant (RR 1.11, 95% CI 0.98 to 1.24; one trial, 300 participants; Analysis 2.1).
2.1. Analysis.
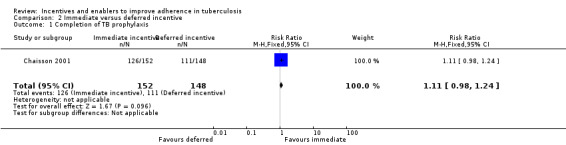
Comparison 2 Immediate versus deferred incentive, Outcome 1 Completion of TB prophylaxis.
Cash versus non‐cash incentives
Completion of TB prophylaxis
One study among homeless and marginally housed adults with latent TB infection (Tulsky 2004) compared a cash incentive (USD 5), with non‐cash incentives (patients could choose between fast food or grocery store coupons, phone cards or bus tokens equivalent to USD 5). The cash incentive was more effective than the non‐cash incentives (RR 1.26, 95% CI 1.02 to 1.56; one trial, 141 participants; Analysis 3.1).
3.1. Analysis.
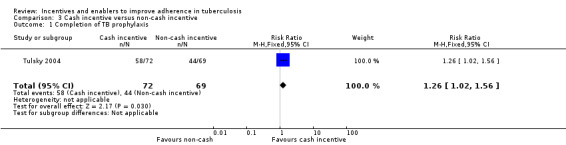
Comparison 3 Cash incentive versus non‐cash incentive, Outcome 1 Completion of TB prophylaxis.
Return to clinic for tuberculin skin test reading
One study amongst injection drug and crack cocaine users (Malotte 1999), directly compared a cash incentive (USD 10) with non‐cash incentives (grocery store coupons, bus tokens and fast food coupons equivalent in value to USD 10).
The cash incentive was significantly more effective at increasing return for reading of tuberculin skin tests than any of the non‐cash incentives (RR 1.13, 95% CI 1.07 to 1.19; one trial, 652 participants; Analysis 3.2).
3.2. Analysis.
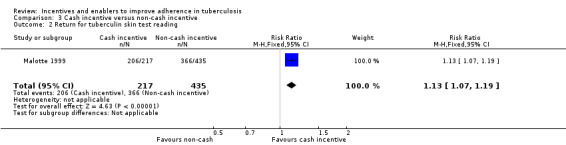
Comparison 3 Cash incentive versus non‐cash incentive, Outcome 2 Return for tuberculin skin test reading.
Different values of cash incentive
Return to clinic for tuberculin skin test reading
One trial, Malotte 1998, also compared different values of cash incentive (USD 10 versus USD 5).
The USD 10 incentive significantly increased the proportion of patients returning to clinic to collect their TB test result compared to the USD 5 incentive (RR 1.08, 95% CI 1.01 to 1.16; one trial, 404 participants; Analysis 5.1).
5.1. Analysis.
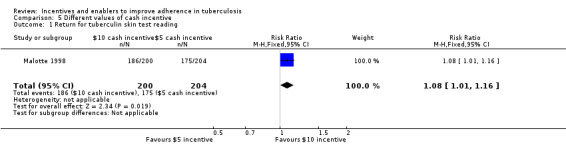
Comparison 5 Different values of cash incentive, Outcome 1 Return for tuberculin skin test reading.
Incentives versus any other intervention
Completion of TB prophylaxis
Three trials also used peer counselling or education sessions to promote completion of TB prophylaxis: one among jail inmates (White 2002), one amongst homeless adults (Tulsky 2000), and one among adolescents (Morisky 2001). There was no significant difference between material incentives and education or counselling (RR 1.04, 95% CI 0.59 to 1.83; 3 trials, 837 participants; Analysis 4.1).
4.1. Analysis.
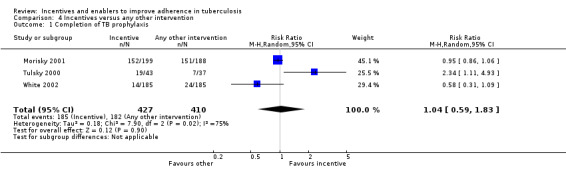
Comparison 4 Incentives versus any other intervention, Outcome 1 Completion of TB prophylaxis.
Return to clinic for initiation or continuation of TB prophylaxis
Two trials assessing return to clinic for TB prophylaxis compared material incentives with education or counselling. Both are from the USA; Pilote 1996 used peer counsellors to encourage homeless men and women to attend clinic after a positive test result, and White 2002 gave education sessions every two weeks to jail inmates to encourage attendance at a community clinic upon release.There was no significant difference between material incentives and education or peer counselling (RR 1.10, 95% CI 0.92 to 1.31; two trials, 535 participants; Analysis 4.2).
4.2. Analysis.
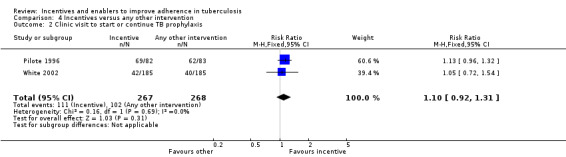
Comparison 4 Incentives versus any other intervention, Outcome 2 Clinic visit to start or continue TB prophylaxis.
Return to clinic for tuberculin skin test reading
The two trials among drug users in the USA (Malotte 1998; Malotte 1999), also had a treatment arm which received 5 to 10 minutes of motivational education .
The material incentives (USD 5 to USD 10) significantly increased the rate of return for tuberculin skin test reading compared to motivational education alone (RR 2.16, 95% CI 1.56 to 3.00; two trials, 1366 participants; Analysis 4.3).
4.3. Analysis.
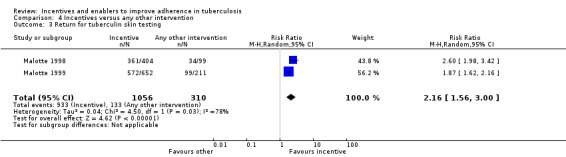
Comparison 4 Incentives versus any other intervention, Outcome 3 Return for tuberculin skin testing.
Potential effect modifiers
The most commonly reported potential effect modifier was educational status. Six trials assessed this and no effect on outcomes was noted (Malotte 1998; Malotte 1999; Malotte 2001; Pilote 1996; Tulsky 2004; White 2002). In one trial, it was noted that the following groups had significantly lower treatment success rates: patients who were unemployed (compared to those who were employed); children older than 13 years (compared to children younger than 13 years); men (compared to women); and patients with smear negative TB (compared to patients with smear positive TB) (Lutge 2013).
None of the studies reported their results subgrouped by HIV status. In three studies it was noted that HIV positive patients were included (Malotte 1998; Malotte 1999; Malotte 2001), and in one study it was noted that the population from which the study sample was drawn had a generally low HIV prevalence (Martins 2009). In one trial, missing data on HIV status precluded its analysis (Lutge 2013) and in a further three trials HIV positive patients were actively excluded (Tulsky 2000; Tulsky 2004; White 2002).
Adverse events
Although adverse events due to the anti‐TB drugs administered (such as isoniazid) were noted in some trials, adverse effects of the incentives and enablers themselves were only investigated in one study (Lutge 2013). Lutge 2013 recorded very few incidents of theft of vouchers, and no obvious perverse incentive effect. Patients were not inclined to stop taking their TB treatment, in order to remain ill and therefore continue receiving their vouchers.
Cost effectiveness
We found one paper reporting a cost analysis, Kominski 2007, which related to an included trial (Morisky 2001). This trial involved the administration of an incentive to adolescents with latent TB (in the form of a "contingency contract" with their parents). In a second trial, the costs of providing the intervention were described as effectively doubling the cost of treatment per patient (Martins 2009). However, since neither of these trials demonstrated any clinical benefit with the use of these interventions, any further appraisal of the cost components is inappropriate.
Discussion
Summary of main results
Only two trials have assessed whether material incentives and enablers can improve long‐term adherence and completion of treatment for active TB, and neither demonstrated a clear benefit (low quality evidence).
Three trials assessed the effects of material incentives on completion of TB prophylaxis with mixed results (low quality evidence). A large effect was seen with regular cash incentives given to drug users at each clinic visit in a setting with extremely low treatment completion in the control group. A second trial in prisoners found no effect with a cash incentive at the start of treatment, and a third trial from a setting with reasonably high treatment completion among teenagers found that material incentives offered by parents did not improve treatment completion compared to parental supervision alone.
However in specific populations, such as recently released prisoners, drug users, and the homeless, trials show that material incentives probably do improve one off clinic re‐attendance for initiation or continuation of anti‐TB prophylaxis (moderate quality evidence), and may increase the return rate for reading of tuberculin skin test results (low quality evidence).
Overall completeness and applicability of evidence
Only two studies have evaluated the long term effects of material incentives on TB treatment outcomes. Although both were conducted in highly endemic settings, their findings are not easily generalized to wider populations because of specific contextual factors affecting each trial. In one trial, the incentive, given as a hot daily meal, was not well received by the population due to the inconvenience of attending the clinic at midday. In addition, conflict in the area affected clinic attendance and therefore participation in the trial. In the second trial, the nurses distributing the vouchers chose to "ration" their distribution among eligible patients and consequently decreased the power of this trial to detect a statistically significant result. These factors served to reduce the quality of the evidence presented by these trials and therefore, although neither trial showed a benefit, other trials not affected by such factors may do so. These trials demonstrate the importance of context in the conduct of research around economic interventions to improve TB treatment outcomes. Although relevant in all research, the context in which trials of social interventions take place may profoundly affect the trials. Trials of economic interventions tested in different contexts may have different results, depending on the levels of poverty, social stability, and social cohesion prevailing at the time. Such factors, whilst having an important effect on the incidence and outcomes of TB, may also have an impact on the effectiveness of economic interventions designed to reduce incidence and improve outcomes. Therefore the context of such trials, and the effect of the context on the generalizability of trial results, should be carefully considered in trial design and reporting.
The remaining studies were all conducted in traditionally hard to reach or marginal populations; injecting drug users, homeless people, prison inmates, and adolescents. It is possible that these subpopulations have different relationships with material incentives, than the general population. Economic incentives and enablers may be perceived and used differently by different groups, and although these marginalized groups are vulnerable to TB, and therefore trials in these groups remain important, the effects of economic interventions may be different in these groups compared to the broader adult population. In addition, the trials that tested economic incentives and enablers in marginalized groups took place in the USA, where the context in terms of the health services, poverty levels, social stability and social cohesion may be different from that of LMICs, where the burden of TB is highest. Thus the results of these trials may be applicable only in the setting of the USA, and only to the groups in which the trials occurred.
One important consideration in extrapolating these results to other populations is HIV. It is possible that HIV co‐infection may affect adherence to anti‐TB medications, either positively (for example, through adherence education received in the HIV programme), or negatively (for example, because illness prevents patients from attending the clinic, or because patients are already taking a number of medications for HIV). However, HIV was not considered in most of these studies. Since the risk of developing TB among patients with HIV is far higher than in those who are HIV negative (WHO 2009a), future studies on economic incentives and enablers for TB should specifically investigate the effect of HIV status on outcomes.
In some settings, health workers and managers may be concerned about giving cash to patients. Indeed, this was the rationale for the inclusion of non‐cash incentives in one trial included in this review (Malotte 1999). The reason for this concern was not described in the trial, but could be related to the expenditure of cash on unhealthy purchases. Vouchers for specified goods cannot be spent on such items and in fact were demonstrated by this trial to have a beneficial effect on return for tuberculin skin testing.
A further objection to the use of incentives may be to the rationale of 'paying the patient' to behave in a healthy way (when it is considered the patients' responsibility to do so). However in poor settings, it may be difficult, if not impossible, for the patient to access the clinic or pay for medicines (McIntyre 2006). This acknowledgement underlies the Opportunidades Programme (now Progressa Programme) in Mexico, where patients are assisted financially in return for behaviours that will promote the health of families (Lagarde 2007). This programme has been shown to have benefits in a poor population (Lagarde 2007), as well as in groups of vulnerable patients in wealthier settings (such as homeless people in the USA) (Pilote 1996). Indeed, in the process evaluation of one of the included trials, nurses responsible for administering the vouchers perceived them to be helping needy patients, rather than paying them to enable a certain behaviour (Lutge 2013).
Quality of the evidence
We assessed the quality of evidence in this review using GRADE methods, and presented it in five 'Summary of findings' tables (Table 1; Appendix 1; Appendix 2; Appendix 3; Appendix 4). The evidence is generally considered to be of low or very low quality, which indicates that further research is very likely to change these estimates of effect.
The main reason for downgrading quality was the indirectness of the evidence, with only two trials from the general adult population of LMICs.
Potential biases in the review process
We minimised potential biases in the review process by adhering to Cochrane guidelines (Higgins 2011). We conducted comprehensive searches of both peer‐reviewed and grey literature, without limiting the searches to a specific language. Two review authors independently assessed study eligibility, extracted data, and assessed the risk of bias in each included study.
Agreements and disagreements with other studies or reviews
Consistent with the findings of relevant previous reviews (Haynes 2008; Lagarde 2007; Sutherland 2008), we found that material incentives and enablers may promote the uptake of health services in certain settings. However, to the best of our knowledge, our review is the most comprehensive synthesis of existing evidence on the effects of material incentives in patients undergoing diagnostic testing for TB or receiving drug therapy to prevent or cure TB.
Authors' conclusions
Implications for practice.
Material incentives may have some positive effects on adherence in the short term, particularly for marginal populations such as drug users, recently released prisoners, and the homeless, but there is currently insufficient evidence to know if they can improve the long term adherence to TB treatment.
Implications for research.
Further high‐quality studies will help explore and delineate the effects and costs of incentives and enablers to improve adherence to the long‐term treatment of active TB.
Future studies should specifically investigate the role of HIV and socioeconomic status in modifying the effects of incentives for TB treatment. The possible adverse effects of incentives, such as misuse of incentives, fraudulent practices, the effect of incentives on non‐recipients, and the perverse incentive effect, should also be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2015 | Amended | Typo corrected in abstract and in main text. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 1, 2012
| Date | Event | Description |
|---|---|---|
| 19 August 2015 | New search has been performed | We performed a search update and identified two potentially eligible studies. We included one new study and excluded the other. Although we had originally intended to include only studies focusing on adults aged 16 years and over, we dropped this age limitation as a few trials were found that included children or adolescents. |
| 19 August 2015 | New citation required but conclusions have not changed | Review updated June 2015 |
Acknowledgements
CSW's work is funded by Stellenbosch University; the National Research Foundation of South Africa; and the Effective Health Care Research Consortium (www.evidence4health.org), which is funded by the UK Department for International Development (DFID). We thank Paul Garner, Xiaolin Wei, Sally Jackson, Sarah Donegan, Anne‐Marie Stephani, and Simon Lewin for support and advice.
The academic editor for this Cochrane Review was Professor Paul Garner. The editorial base for the Cochrane Infectious Disease Group is funded by the DFID, UK, for the benefit of LMICs.
Appendices
Appendix 1. Summary of findings table 2
| Immediate versus deferred incentive for improving patient adherence to TB management | |||||
| Patient or population: people at high risk of developing TB Settings: high‐ and low‐income settings Intervention: immediate incentive (received on a regular basis during treatment) Comparison: deferred incentive (received only at end of treatment). | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Deferred incentive | Immediate | ||||
| Completion of TB prophylaxis | 750 per 1000 | 832 per 1000 (735 to 930) | RR 1.11 (0.98 to 1.24) | 300 (1 trial) | ⊕⊕⊝⊝ low1,2 |
| The assumed risk is taken from the control group in the trial. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 1 for indirectness: this trial was conducted in specific subpopulations from the USA and the result may not be applicable to other settings. 2Downgraded by 1 for imprecision: the 95% CI of the estimate of effect includes both clinically important benefit and no effect.
Appendix 2. Summary of findings table 3
| Cash versus non‐cash incentive for improving patient adherence to TB management | |||||
| Patient or population: people at high risk of developing TB Settings: high‐ and low‐income settings Intervention: cash incentive Comparison: non‐cash incentive | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Non‐cash incentive | Cash | ||||
| Return for tuberculin skin test reading | 841 per 1000 | 950 per 1000 (900 to 992) | RR 1.13 (1.07 to 1.18) | 652 (1 trial) | ⊕⊕⊝⊝ low1 |
| Completion of TB prophylaxis | 638 per 1000 | 804 per 1000 (651 to 995) | RR 1.26 (1.02 to 1.56) | 141 (1 trial) | ⊕⊕⊝⊝ low1 |
| The assumed risk is taken from the control group in the trial. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 2 for indirectness: these trials were conducted in specific subpopulations from the USA and the results may not be applicable to other settings.
Appendix 3. Summary of findings table 4
| Comparison of different values of cash incentives for improving patient adherence to TB management | |||||
| Patient or population: people at high risk of developing TB Settings: high‐ and low‐income settings Intervention: higher cash value (USD 10) Comparison: lower cash value (USD 5) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| lower cash value | higher cash value | ||||
| Return for tuberculin skin test reading | 858 per 1000 | 927 per 1000 (867 to 995) | RR 1.08 (1.01 to 1.16) | 404 (1 trial) | ⊕⊕⊝⊝ low1 |
| The assumed risk is taken from the control group in the trial. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 2 for indirectness: one trial conducted in a specific subpopulation from the USA and the result may not be applicable to other settings.
Appendix 4. Summary of findings table 5
| Incentives versus educational or motivational interventions for improving patient adherence to anti‐TB treatment | |||||
| Patient or population: patients at high risk of developing TB Settings: high‐ and low‐income settings Intervention: an incentive Comparison: any educational or motivational intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| any other intervention | material incentives | ||||
| Return for tuberculin skin test reading | 429 per 1000 | 927 per 1000 (669 to 1000) | RR 2.16 (1.56 to 3.00) | 1366 (2 trials) | ⊕⊕⊝⊝ low1,2 |
| Return to clinic to start or continue treatment | 381 per 1000 | 419 per 1000 (351 to 499) | RR 1.10 (0.92 to 1.31) | 535 (2 trials) | ⊕⊕⊝⊝ low2,3 |
| Completion of prophylaxis for latent TB | 444 per 1000 | 462 per 1000 (262 to 813) | RR 1.04 (0.59 to 1.83) | 837 (3 trials) | ⊕⊕⊝⊝ low2,4 |
| The assumed risk is taken from the control group in the trial. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 1 for risk of bias: neither study adequately described the method of randomization. 2Downgraded by 1 for indirectness: these trials were conducted in specific subpopulations from the USA and the result may not be applicable to other settings. 3Downgraded by 1 for imprecision: the 95% CI includes what may be clinically important benefits and no effect. 4Downgraded by 1 for inconsistency: two studies found no suggestion of a benefit with the incentive, and just one study found a clinically and statistically significant benefit in drug users.
Data and analyses
Comparison 1. Incentive versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment success (completion or cure) | 2 | 4356 | Risk Ratio (Fixed, 95% CI) | 1.04 [0.97, 1.13] |
| 2 Completion of TB prophylaxis | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clinic visit to start or continue TB prophylaxis | 3 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.27, 1.96] |
| 4 Return for tuberculin skin test results | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.41, 3.29] |
1.2. Analysis.
Comparison 1 Incentive versus routine care, Outcome 2 Completion of TB prophylaxis.
Comparison 2. Immediate versus deferred incentive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Completion of TB prophylaxis | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.98, 1.24] |
Comparison 3. Cash incentive versus non‐cash incentive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Completion of TB prophylaxis | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.02, 1.56] |
| 2 Return for tuberculin skin test reading | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.07, 1.19] |
Comparison 4. Incentives versus any other intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Completion of TB prophylaxis | 3 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.83] |
| 2 Clinic visit to start or continue TB prophylaxis | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 3 Return for tuberculin skin testing | 2 | 1366 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.56, 3.00] |
Comparison 5. Different values of cash incentive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return for tuberculin skin test reading | 1 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.01, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chaisson 2001.
| Methods | Individually RCT, factorial design Duration of enrolment: June 1995 to August 1997 |
|
| Participants | Number enrolled: 300 Inclusion criteria: injection drug users over 18 years old, with tuberculin skin test reading of more than 5 mm induration if HIV positive or more than 10 mm if HIV negative, on preventive treatment for TB Exclusion criteria: evidence of active TB, history of serious adverse reaction to INH (isoniazid) treatment, previous INH treatment for ≥ 6 months, serum ALT elevated > 5 times normal levels, or HIV disease with CD4 count < 200/mm³. (Isoniazid is a standard TB medication used for both prophylaxis and treatment of active TB) |
|
| Interventions | All participants were randomly assigned to receive either:
The immediate payment was given at the end of each month when the patient had completed a routine assessment for adherence and drug toxicity. The deferred payment was credited each month a patient in this group completed assessment for adherence and toxicity, but payment was made when treatment was completed or when the patient withdrew from the study. Each arm was on prophylaxis for TB. |
|
| Outcomes | Completion of 6 months of INH preventive treatment (reporting for each of 6 monthly visits and taking at least 80% of medication). | |
| Notes | Independent of the material incentive, all patients were randomly assigned to directly observed preventive therapy (i.e. outreach meeting with a nurse twice a week; peer support counselling (i.e. monthly support group meetings); or routine care (i.e. monthly clinic visits). Trial location: Baltimore, USA Setting: community‐based TB clinic Source of funding: National Institute on Drug Abuse (DA 08992) and the National Institute of Allergy and Infectious Diseases (AI 01637) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation performed by computer algorithm. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not known if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers presented for whole group and each arm, intention‐to‐treat (ITT) analysis. Withdrawals included "failure to return (37 patients), voluntary withdrawal (4)...and other reasons (13)". These do not seem to be related to the material incentives. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Lutge 2013.
| Methods | Cluster RCT using primary health care clinics as clusters Duration: July 2009 to March 2010 |
|
| Participants | Number enrolled: 20 clinics randomized, enrolling 4091 TB patients Inclusion criteria: all patients diagnosed with pulmonary, drug sensitive TB, and starting TB treatment during the study period. Includes both adults and children Exclusion criteria: none stated |
|
| Interventions | Primary health care clinics were randomized to provide:
|
|
| Outcomes | Primary outcome: TB treatment success defined as cure or treatment completion Secondary outcomes:
|
|
| Notes | Trial location: KwaZulu‐Natal, South Africa Setting: urban and rural primary healthcare clinics Source of funding: Research Programme of the National Department of Health (South Africa), the Tuberculosis Control Assistance Program (TP CAP, the Netherlands), the Wellcome Trust (UK) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 20 study clinics were randomly selected from the 26 eligible clinics stratified by district. Within the two districts, the study clinics were randomly assigned in a 1:1 ratio, using a randomisation list generated by the study statistician." |
| Allocation concealment (selection bias) | Low risk | "Clinics were allocated to intervention or control groups by the study statistician and no changes were made to this allocation". Personal communication with the trial author: The study statistician had no knowledge of the clinic sites being randomized and was based Cape town. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Because of the nature of the intervention, no blinding was possible." "Data extractors were not blinded as it was considered neither practical nor feasible to conceal from them the intervention status of the clinic." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss of clusters. Outcome data were unavailable on 0.2% of patients in intervention clinics and 0.7% of patients in control clinics. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting identified. |
| Other bias | Unclear risk | Fidelity to the intervention was poor, with only 31.5% of patients receiving their vouchers for most of their treatment. More than a third (36.2%) of eligible patients did not receive a voucher at all and the remainder received vouchers for between 1 and 3 months of treatment. This low fidelity was attributed largely to nurses' rationing of the vouchers to those whom they felt were more deprived and therefore more deserving. |
Malotte 1998.
| Methods | Individually RCT Duration: April 1994 to August 1995 |
|
| Participants | Number enrolled: 1004 Inclusion criteria: injection drug and crack cocaine users, who had tuberculin skin tests and were required to return for the reading Exclusion criteria: none stated |
|
| Interventions | Participants were divided into 6 arms, which received the following interventions:
|
|
| Outcomes | Return for tuberculin skin test reading within 96 hours | |
| Notes | Trial location: Long Beach, California, USA Setting: urban research clinic Source of funding: National Institute on Drug Abuse (grant RO1‐DA08799) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No omissions from final analysis. 1004 enrolled, ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Malotte 1999.
| Methods | Individually RCT Duration: September 1995 to September 1997 |
|
| Participants | Number enrolled: 1078 Inclusion criteria: injection drug and crack cocaine users who had tuberculin skin tests and were required to return for the reading (age restrictions not specifically stated but all participants were over age of 18 years) Exclusion criteria: participation in group's previous studies |
|
| Interventions |
|
|
| Outcomes | Return for TB skin test reading within 96 hours | |
| Notes | Study was a follow‐up to Malotte 1998 ‐ trial authors wanted to test effectiveness of non‐cash incentives, as they felt health departments might object to giving cash out to patients as this was considered controversial. Trial location: Long Beach, California, USA Setting: urban research clinic Source of funding: National Institute on Drug Abuse (grant RO1‐DA08799) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No omissions from final analysis. 1078 randomized, ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Malotte 2001.
| Methods | Individually RCT Duration: April 1994 to September 1997 (recruitment period) |
|
| Participants | Number enrolled: 169 Inclusion criteria: injection drug or crack cocaine users, needing INH treatment for TB prophylaxis Exclusion criteria: active TB or medical contraindications to the use of isoniazid |
|
| Interventions |
Participants in both arms received INH prophylaxis. |
|
| Outcomes | Completion of course of INH (6 months if patient HIV negative, 12 months if patient HIV positive). Also percentage of medications taken on time (all doses in all arms were directly observed). | |
| Notes | Trial location: Long beach, California, USA Setting: urban research clinic Source of funding: National Institute on Drug Abuse (grant RO1‐DA08799) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization in blocks of 18, assumed to have been done by computer. |
| Allocation concealment (selection bias) | Low risk | Allocation was kept in "numbered, opaque, sealed envelopes" and "staff ...were unaware of block size". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 169 patients randomized. Six excluded from analysis for medical reasons which were unlikely to have been related to the study outcome. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Martins 2009.
| Methods | Individually RCT conducted at three sites in Dili, Timor Leste Duration: enrolment 16 March to 9 November 2005; follow‐up continued until July 2006 |
|
| Participants | Patients with newly diagnosed pulmonary TB, both positive and negative results on sputum tests Eligible: 833 (563 excluded) Randomized: 270 (133 control, 137 intervention group) Most participants were poor, malnourished men living close to the clinics |
|
| Interventions |
Both groups received standard TB treatment |
|
| Outcomes | Primary outcomes: completion of treatment, including cure Secondary outcomes: adherence to treatment, weight gain, and clearance of sputum smears |
|
| Notes | Outbreak of civil conflict in the country three months before completion of study disrupted service delivery and access of patients to health care (70% of the population were displaced). However, it is likely that this affected intervention and control groups similarly. Most participants were poor and malnourished men who lived close to the clinics and this may limit the external generalizability of the study. Substantial missing data for intermediate outcomes implies that participants did not attend clinics regularly. Also, intervention was not well received by many participants as it was inconvenient to attend the clinics at midday for the meal (this was also the reason for a high number of patients' refusal to participate in the trial). 70% of participants had negative smear results, which means that cure could not be objectively verified. Adherence was not objectively assessed. Adverse events: None necessitated stopping treatment. Itch with or without rash was more than twice as likely to occur in the intervention group (RR 2.27, 95% CI 1.20 to 4.26). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random allocation sequence with randomly varying block sizes (done by independent statistician using STATA). Allocation was stratified by community health clinic and by diagnosis of TB (positive or negative smear). |
| Allocation concealment (selection bias) | Low risk | Concealed from all investigators with sequentially numbered opaque sealed envelopes prepared distant from study site. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of participants and treatment providers not done, but independent observer who determined the primary outcome was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants received allocated intervention and loss to follow‐up (transfer to another clinic during treatment) was very small (1% in intervention group and 4% in control group). ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Unclear risk | Two important factors affected the conduct of the trial and undermined the potential effectiveness of the intervention. The first was civil conflict that arose during the last few months of the trial, resulting in the displacement of approximately 70% of the local population, and dramatically increasing the rate of default from treatment. The second factor was the timing of the provision of meals to patients in the intervention group. Many patients found the provision of meals, together with treatment, at midday inconvenient, and this may have become a barrier to adherence to treatment. |
Morisky 2001.
| Methods | Individually RCT. Duration: not stated |
|
| Participants | Number enrolled: 794 Inclusion criteria: adolescents aged 11 to 19 years who needed treatment for latent TB infection. Exclusion criteria: not stated |
|
| Interventions |
Participants in intervention and control arms received INH prophylaxis. |
|
| Outcomes | Completion of 6 months of INH prophylaxis; measured using the discharge summary recorded in the patient's medical chart. | |
| Notes | Trial location: Los Angeles County, USA Setting: urban community based clinics Source of funding: National Heart, Lung and Blood Institute (ROI‐55770). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors state that ITT model was used (pg 570). 794 adolescents enrolled and analysed. No omissions from final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | Over and above the interventions described above, patients were interviewed three times during the study and at each interview received USD 15. The additional interest in the participants, plus the cash which may have acted as a further incentive to adhere, may be regarded as interventions in themselves. However, this applied to all participants and would not have introduced bias. |
Pilote 1996.
| Methods | Individually RCT Duration: June 1992 to April 1994 |
|
| Participants | Number enrolled: 244 Inclusion criteria: Homeless "men and women", age not specified (but all over 18 years in results), who had a tuberculin skin test and were required to attend a clinic to initiate treatment for latent or active TB Exclusion criteria: Recent investigation for TB |
|
| Interventions |
|
|
| Outcomes | Attendance at clinic appointment within three weeks of positive reading of tuberculin skin test. | |
| Notes | Second phase of this study reported in Tulsky et al 2000. Trial location: San Fransisco, California, USA Setting: urban community based TB clinic (attached to San Francisco General Hospital) Source of funding: Kaiser Family Foundation, Acquired Immunodeficiency Syndrome Clinical Research Center, San Francisco, California; Universitywide Acquired Immunodeficiency Syndrome Research Program, University of California; and by grant R01‐DA04363‐07 from the National Institute on Drug Abuse, Bethesda, MD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "treatment group was assigned by sampling without replacement from blocks of nine". Assumed to be done by computer. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 244 patients randomized, 244 analysed. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Tulsky 2000.
| Methods | Individually RCT Duration: June 1992 to May 1995 (recruitment period June 1992 to December 1994, plus 6 months of patient follow‐up time). |
|
| Participants | Number enrolled: 118 Inclusion criteria: homeless adults, with positive tuberculin skin test or credible history of prior positive tuberculin skin test but no follow‐up for this in the 6 months prior to the study Exlusion criteria: receiving treatment or prophylaxis for TB at the time of the study, or HIV positive |
|
| Interventions |
Participants in intervention and control arms received INH prophylaxis. |
|
| Outcomes | Completion of 6 months of INH preventive treatment as documented in patients' clinic charts; number of months of INH dispensed. | |
| Notes | Trial location: San Francisco, California, USA Setting: community‐based TB clinic Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization used, therefore allocation sequence assumed to have been generated by computer. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 330 patients randomized, 195 found to require further evaluation and 37 needed further diagnostic tests (sputum cultures and liver function tests). Of 121 who were prescribed INH, 118 were analysed ‐ 3 were excluded from study because of "toxic effects of INH". These reasons unlikely to be related to final outcome. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Tulsky 2004.
| Methods | Individually RCT. Duration: May 1996 to May 1998 (based on recruitment period of May 1996 to December 1997, plus 6 months for patient follow‐up). |
|
| Participants | Number enrolled: 119 (85% male; median age 41 years, range 21 to 79) Inclusion criteria: homeless adults who were eligible for preventive TB treatment. Adults who were "truly homeless" (living in street and shelter dwellings) and those who were "marginally housed" (living in residential hotels) were recruited into the study Exclusion criteria: active TB or HIV positive |
|
| Interventions |
Participants in intervention and control arms received INH prophylaxis. |
|
| Outcomes |
|
|
| Notes | As the cash incentive arm did so much better than the non incentive arm in the study performed by this group previously (Tulsky 2000), the trial authors felt it would be unethical to continue to randomize one group to no incentive. Trial location: San Francisco, California, USA Setting: urban, community‐based TB clinic Source of funding: National Heart, Lung, and Blood Institute (grant HL55729) and the National Institute of Mental Health (grant MH54907). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated "...from a list of randomly generated numbers". |
| Allocation concealment (selection bias) | Unclear risk | "...numbers previously sealed into individual envelopes and selected in consecutive order". Unclear if these envelopes were opaque. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "TB clinic physicians were blinded with respect to the results of the randomisation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 141 patients randomized but 16 not prescribed INH (4 in cash incentive arm, 12 in non‐cash incentive arm). Reasons for exclusion clinical and unlikely to be related to allocation. 6 patients censored (5 for clinical reasons, 1 because died in hotel fire). Again, reasons for exclusion unlikely to be related to allocation or outcome. 119 patients analysed. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | High risk | The study groups were not the same with respect to their primary housing in the year prior to the study. In the cash incentive arm, 23% had lived in a shelter or on the street, whilst 41% of the non‐cash incentive arm had done so. |
White 1998.
| Methods | Individually RCT Duration: one year (1996) |
|
| Participants | Number enrolled: 79 (98% male, mean age 32.0 years) Inclusion criteria: jail inmates eligible for INH prophylaxis for latent TB infection Exclusion criteria: unable to speak English or Spanish, or sequestration from jail population due to violence or mental illness |
|
| Interventions |
Participants in intervention and control arms received INH prophylaxis. |
|
| Outcomes | Attendance at first visit to community TB clinic to continue INH prophylaxis after release from jail | |
| Notes | Trial location: San Francisco, California, USA Setting: Prison Source of funding: Academic Senate of the University of California, San Francisco. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done using table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Previously sealed, ordered, opaque envelopes used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Research assistants collecting clinic data (as to whether participant attended first appointment or not) were blinded as to participants' assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 79 inmates enrolled in the study, 18 remained in prison for the full duration of their INH treatment (and so were never required to present at a community TB clinic). 61 were analysable, and there were no differences between treatment allocations in this group. "Data were rechecked for internal validity and there were no differences by study group in any of the variables collected for this analytic sample of 61 persons" (pg 508). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
White 2002.
| Methods | Individually RCT Duration of enrolment: 1 March 1998 to 31 May 1999 |
|
| Participants | Number enrolled: 558 (82% male; median age 28.5 years in incentive arm, 29.7 years in routine care arm, and 29.5 years in education arm) Inclusion criteria: Jail inmates with latent TB infection, eligible for and agreeable to INH prophylaxis Exclusion criteria: HIV positive, not able to speak English or Spanish, assessed by Sherriff's personnel to be violent, or by mental health staff to have a serious psychiatric illness |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | HIV positive patients on INH prophylaxis receive very different programme of treatment, including incentives. Trial location: San Francisco, California, USA Setting: prison Source of funding: National Institute of Nursing Research, National Institutes of Health, Bethesda, Md. (grant R01 NR04456). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done using table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Ordered, opaque, sealed envelopes used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Research assistants collecting clinic data (as to whether participant attended first appointment or not) were blinded as to participants' assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 558 inmates enrolled, 48 discontinued INH treatment whilst in jail, and 185 completed INH treatment whilst in jail. Thus 325 were eligible for analysis. There were no differences between study group in either the 325 analysable patients or 558 initially enrolled patients. Reasons for exclusion from analysis not likely to be related to final outcome. ITT analysis for those released while taking INH. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and there is no earlier methods paper listing the pre‐specified outcomes. |
| Other bias | Low risk | The study appears to be free of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cantalice Filho 2009 | Not a RCT; essentially two cross‐sectional studies where first group was not given incentive and second group was. |
| Cheng 1997 | Not a RCT, as allocation to treatment interventions was done by day of the week. |
| Chua 2015 | Not a RCT; allocation to receive the grocery voucher intervention was not randomized. |
| FitzGerald 1999 | Not a RCT; essentially two cross‐sectional studies where first group was not given incentive and second group was. |
| Gärden 2013 | Not a RCT; comparison group was an historical control. |
| Jahnavi 2010 | A trial of community health worker delivered TB treatment combined with food supplements; and not a trial of food incentives per se. |
| Morisky 1990 | Not a RCT, as allocation to treatment interventions was done by the last digits of the participants' clinic numbers. |
| Nyamathi 2006 | Both the intervention and control arms received a USD 5 cash incentive for each dose of INH prophylaxis taken. It was therefore not possible to assess the effect of the incentive in this study. The main intervention was a nurse case management programme. |
| Yao 2008 | Controlled before‐and‐after study, with no evidence of randomization to control or intervention groups. Incentives were provided to health care workers as well as patients, and the effect of patients' incentives only was not disaggregated. |
Differences between protocol and review
.
Contributions of authors
All five review authors contributed to the review, with EL taking primary responsibility. EL, SK, DS, and CSW participated in study selection, data extraction and analysis, and writing of the report. JV resolved any disputes arising during the selection of trials to include in the review, and assisted with data analysis and report writing.
Sources of support
Internal sources
Centre for Evidence‐based Health Care, Stellenbosch University (CSW), South Africa.
External sources
-
Effective Health Care Research Consortium, Stellenbosch University's Centre for Evidence Based Health Care, and South African Cochrane Centre (EL), South Africa.
EL received a travel grant from the Effective Health Care Research Consortium, through the Centre for Evidence‐based Health Care (Stellenbosch University), to spend one week at the South African Cochrane Centre (South African Medical Research Council) and work with CSW in updating the review.
Declarations of interest
EL was the principal investigator in the new study included in the current review update (Lutge 2013). However, two review authors who were not involved with this trial (DS and CSW) independently extracted and verified data. Two Cochrane Editors provided oversight.
Unchanged
References
References to studies included in this review
Chaisson 2001 {published data only}
- Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, et al. A randomised, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. American Journal of Medicine 2001;110(8):610‐15. [DOI] [PubMed] [Google Scholar]
Lutge 2013 {published data only}
- Lutge E, Lewin S, Volmink J. Economic support to improve tuberculosis treatment outcomes in South Africa: a qualitative process evaluation of a cluster randomized controlled trial. Trials 2014;15:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutge E, Lewin S, Volmink J, Friedman I, Lombard C. Economic support to improve tuberculosis treatment outcomes in South Africa: a pragmatic cluster randomised controlled trial. Trials 2013;14:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malotte 1998 {published data only}
- Malotte CK, Rhodes F, Mais KE. Tuberculosis screening and compliance with return for skin test reading among active drug users. American Journal of Public Health 1998;88(5):792‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malotte 1999 {published data only}
- Malotte CK, Hollingshead JR, Rhodes F. Monetary versus nonmonetary incentives for TB skin test reading among drug users. American Journal of Preventive Medicine 1999;16(3):182‐8. [DOI] [PubMed] [Google Scholar]
Malotte 2001 {published data only}
- Malotte CK, Hollingshead JR, Larro M. Incentives vs outreach workers for latent tuberculosis treatment in drug users. American Journal of Preventive Medicine 2001;20(2):103‐7. [DOI] [PubMed] [Google Scholar]
Martins 2009 {published data only}
- Martins N, Morris P, Kelly PM. Food incentives to improve completion of tuberculosis treatment: randomised controlled trial in Dili, Timor Leste. BMJ 2009;339:b4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morisky 2001 {published data only}
- Kominski GF, Varon SF, Morisky DE, Malotte CK, Ebin VJ, Coly A, et al. Costs and cost‐effectiveness of adolescent compliance with treatment for latent tuberculosis infection: results from a randomised trial. Journal of Adolescent Health 2007;40(1):61‐8. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Malotte KC, Ebin V, Davidson P, Cabrera D, Trout PT, et al. Behavioural interventions for the control of tuberculosis among adolescents. Public Health Reports 2001;116(6):568‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilote 1996 {published data only}
- Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless: a trial to improve adherence to referral. Archives of Internal Medicine 1996;156(2):161‐5. [PubMed] [Google Scholar]
Tulsky 2000 {published data only}
- Tulsky JP, Pilote L, Hahn JA, Zolopa AJ, Burke M, Chesney M, et al. Adherence to isoniazid prophylaxis in the homeless: a randomised controlled trial. Archives of Internal Medicine 2000;160(5):697‐702. [DOI] [PubMed] [Google Scholar]
Tulsky 2004 {published data only}
- Tulsky JP, Hahn JA, Long HL, Chambers DB, Robertson MJ, Chesney MA, et al. Can the poor adhere? Incentives for adherence to TB prevention in the homeless. International Journal of Tuberculosis and Lung Disease 2004;8(1):83‐91. [PubMed] [Google Scholar]
White 1998 {published data only}
- White MC, Tulsky JP, Reilly P, McIntosh HW, Hoynes TM, Goldenson J. A clinical trial of a financial incentive to go to the tuberculosis clinic for isoniazid after release from jail. International Journal of Tuberculosis and Lung Disease 1998;2(6):506‐12. [PubMed] [Google Scholar]
White 2002 {published data only}
- White MC, Tulsky JP, Goldenson J, Portillo CJ, Kawamura M, Menendez E. Randomised controlled trial of interventions to improve follow‐up for latent tuberculosis infection after release from jail. Archives of Internal Medicine 2002;162(9):1044‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cantalice Filho 2009 {published data only}
- Cantalice Filho JP. Food baskets given to tuberculosis patients at a primary health care clinic in the city of Duque de Caxias, Brazil: effect on treatment outcomes. Jornal Brasileiro de Pneumologia 2009;35(10):992‐7. [DOI] [PubMed] [Google Scholar]
Cheng 1997 {published data only}
- Cheng TL, Ottolini MC, Baumhaft K, Brasseux C, Wolf MD, Scheidt PC. Strategies to increase adherence with tuberculosis test reading in a high‐risk population. Pediatrics 1997;100(2 Pt 1):210‐3. [DOI] [PubMed] [Google Scholar]
Chua 2015 {published data only}
- Chua AP, Lim LK, Ng H, Chee CB, Wang YT. Outcome of a grocery voucher incentive scheme for low‐income tuberculosis patients on directly observed therapy in Singapore. Singapore Medical Journal 2015;56(5):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
FitzGerald 1999 {published data only}
- FitzGerald JM, Patrick DM, Strathdee S, Rekart M, Elwood RK, Schecter MT, et al. Use of incentives to increase compliance for TB screening in a population of intravenous drug users. International Journal of Tuberculosis and Lung Diseases 1999;3(2):153‐5. [PubMed] [Google Scholar]
Gärden 2013 {published data only}
- Gärden B, Samarina A, Stavchanskaya I, Alsterlund R, Övregaard A, Taganova O, et al. Food incentives improve adherence to tuberculosis drug treatment among homeless patients in Russia. Scandinavian Journal of Caring Sciences 2013;27(1):117‐22. [DOI] [PubMed] [Google Scholar]
Jahnavi 2010 {published data only}
- Jahnavi G, Sudha CH. Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting. Singapore Medical Journal 2010;51(12):957‐62. [PubMed] [Google Scholar]
Morisky 1990 {published data only}
- Morisky DE, Malotte KC, Choi P, Davidson P, Rigler S, Sugland B, et al. A patient education program to improve adherence rates with antituberculosis drug regimens. Health Education Quarterly 1990;17(3):253‐67. [DOI] [PubMed] [Google Scholar]
Nyamathi 2006 {published data only}
- Nyamathi A, Nahid P, Berg J, Burrage J, Christiani A, Aqtash S, et al. Efficacy of nurse case‐managed intervention for latent tuberculosis among homeless subsamples. Nursing Research 2008;57(1):33‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamathi A, Stein J, Schumann A, Tyler D. Latent variable assessment of outcomes in a nurse‐managed intervention to increase latent tuberculosis treatment completion in homeless adults. Health Psychology 2007;26(1):68‐76. [DOI] [PubMed] [Google Scholar]
- Nyamathi AM, Christiani A, Nahid P, Gregerson P, Leake B. A randomised controlled trial of two treatment programs for homeless adults with latent tuberculosis infection. International Journal of Tuberculosis and Lung Disease 2006;10(7):775‐82. [PubMed] [Google Scholar]
Yao 2008 {published data only}
- Yao H, Wei X, Liu J, Zhao J, Hu D, Walley JD. Evaluating the effects of providing financial incentives to tuberculosis patients and health care providers in China. International Journal of Tuberculosis and Lung Disease 2008;12(10):1166‐72. [PubMed] [Google Scholar]
Additional references
Balshem 2011
- Balshem B, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Broze J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Beith 2007
- Beith A, Eichler R, Weil D. Performance‐Based Incentives for Health: A Way to Improve Tuberculosis Detection and Treatment Completion?. Center for Global Development Working Paper 122 2007.
CDC 2010
- Centers for Disease Control and Prevention. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. Atlanta, Georgia: CDC, 2010. [Google Scholar]
Garner 2007
- Garner P, Smith H, Munro S, Volmink J. Promoting adherence to tuberculosis treatment. Bulletin of the World Health Organization 2007;85(5):404‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harries 2006
- Harries AD, Dye C. Tuberculosis. Annals of Tropical Medicine and Parasitology 2006;100(5‐6):415‐31. [DOI] [PubMed] [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karumbi 2015
- Karumbi J, Garner P. Directly observed therapy for treating tuberculosis.. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD003343.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kliner 2014
- Kliner M, Garner P. When trial authors write Cochrane Reviews: competing interests need to be better managed [editorial]. Cochrane Database of Systematic Reviews 2014;9:10.1002/14651858.ED000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kominski 2007
- Kominski GF, Varon SF, Morisky DE, Malotte CK, Ebin VJ, Coly A, et al. Costs and cost‐effectiveness of adolescent compliance with treatment for latent tuberculosis infection: results from a randomised trial. Journal of Adolescent Health 2007;40(1):61‐8. [DOI] [PubMed] [Google Scholar]
Lagarde 2007
- Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health interventions in low‐ and middle‐income countries: a systematic review. Journal of the Americal Medical Association 2007;298(16):1900‐10. [DOI] [PubMed] [Google Scholar]
Lam 2002
- Lam TH, Hedley AJ. Respiratory disease. In: Detels R, McEwen J, Beaglehole R, Tanaka H editor(s). Oxford Textbook of Public Health. 4th Edition. Vol. 3, Oxford: Oxford University Press, 2002:1227‐54. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins J, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
Liu 2014
- Liu Q, Abba K, Alejandria MM, Sinclair D, Balanag VM, Lansang MAD. Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD006594.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
M'Imunya 2012
- M'Imunya MJ, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006591.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2006
- McIntyre D, Thiede M, Dahlgren G, Whitehead M. What are the economic costs for households of illness and of paying for health care in low‐ and middle‐income country contexts?. Social Science and Medicine 2006;62(4):858‐65. [DOI] [PubMed] [Google Scholar]
Munro 2007
- Munro S, Lewin S, Smith H, Engel M, Fretheim A, Volmink J. Adherence to tuberculosis treatment: a qualitative systematic review of stakeholder perceptions. PLoS Medicine 2007;4(7):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanan 2003
- Narayanan PR, Garg R, Santha T, Kumaran PP. Shifting the focus of tuberculosis research in India. Tuberculosis 2003;83(1‐3):135‐42. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sutherland 2008
- Sutherland K, Leatherman S, Christianson J. Paying the patient: does it work? A review of patient‐targeted incentives. http://www.health.org.uk/sites/default/files/PayingThePatientDoesItWork.pdf (accessed 24 July 2011).
van Dulmen 2007
- Dulmen S, Sluijs E, Dijk L, Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Services Research 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volmink 2000
- Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003343.pub2] [DOI] [PubMed] [Google Scholar]
Wells 2007
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug‐resistant tuberculosis: the perfect storm. Journal of Infectious Diseases 2007;196(Suppl 1):S86‐107. [DOI] [PubMed] [Google Scholar]
WHO 2003a
- World Health Organization. Defining Adherence. Adherence to long‐term therapies: evidence for action. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2003b
- World Health Organization. The magnitude of the problem of poor adherence. Adherence to long‐term therapies: evidence for action. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2003c
- World Health Organization. Lessons Learned. Adherence to long‐term therapies: evidence for action. Geneva: World Health Organization, 2003:19‐24. [Google Scholar]
WHO 2009a
- World Health Organization. TB incidence, prevalence and mortality. Global Tuberculosis Control 2009: Epidemiology, strategy, financing. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2009b
- World Health Organization. Global Tuberculosis Control 2009: Epidemiology, strategy, financing. Annex 2: Methods. Geneva: WHO, 2009:173‐85. [Google Scholar]
WHO 2013
- World Health Organization. Global Tuberculosis Report 2013. Global Tuberculosis Report. Geneva: World Health Organization, 2013. [Google Scholar]
Yang 2011
- Yang Y, Li X, Zhou F, Jin Q, Gao L. Prevalence of drug‐resistant tuberculosis in mainland China: systematic review and meta‐analysis. PLoS One 2011;6(6):e20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lutge 2012
- Lutge EE, Wiysonge CS, Knight SE, Volmink J. Material incentives and enablers in the management of tuberculosis. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD007952.pub2] [DOI] [PubMed] [Google Scholar]


