Abstract
In western and westernized societies, large portions of the population live in what are considered to be “obesogenic” environments. Among other things, obesogenic environments are characterized by a high prevalence of external cues that are associated with highly palatable, energy-dense foods. One prominent hypothesis suggests that these external cues become such powerful conditioned elicitors of appetitive and eating behavior that they overwhelm the internal, physiological mechanisms that serve to maintain energy balance. The present research investigated a learning mechanism that may underlie this loss of internal relative to external control. In Experiment 1, rats were provided with both auditory cues (external stimuli) and varying levels of food deprivation (internal stimuli) that they could use to solve a simple discrimination task. Despite having access to clearly discriminable external cues, we found that the deprivation cues gained substantial discriminative control over conditioned responding. Experiment 2 found that, compared to standard chow, maintenance on a “western-style” diet high in saturated fat and sugar weakened discriminative control by food deprivation cues, but did not impair learning when external cues were also trained as relevant discriminative signals for sucrose. Thus, eating a western-style diet contributed to a loss of internal control over appetitive behavior relative to external cues. We discuss how this relative loss of control by food deprivation signals may result from interference with hippocampal-dependent learning and memory processes, forming the basis of a vicious-cycle of excessive intake, body weight gain, and progressive cognitive decline that may begin very early in life.
Keywords: Obesity, Cognition, Memory, Saturated fat, Hippocampus, Vicious cycle
Introduction
The control of energy intake and body weight involves the interplay between food-related environmental cues and physiological signals arising from the internal milieu. For example, environmental food cues are often thought to evoke learned appetitive and eating behaviors whereas internal satiety signals suppress the evocation of those responses (Woods, 2004). Within this framework, excess energy intake and body weight gain result when the power of environmental cues to evoke feeding behavior exceeds the ability of internal signals to inhibit feeding.
This shift toward external relative to internal control of intake has been described as a consequence of living in what has been termed an “obesogenic” environment (e.g., Swinburn et al., 2011). Obesogenic environments are characterized by an abundance of low cost, energy-dense, highly palatable foods and beverages. It is thought that external cues associated with these foods and beverages can become strong elicitors of eating. Furthermore, sophisticated marketing and advertising practices maximize exposure to these cues and heighten their salience. It has been hypothesized that these external factors combine to overwhelm the capacity of internal physiological control mechanisms to prevent positive energy balance and avoid body weight gain (e.g., Corsica & Hood, 2011; King, 2013; Zheng, Lenard, Shin, & Berthoud, 2009). The result has been high and/or growing rates of obesity, especially in western or westernized societies where obesogenic environments are most prevalent (e.g., Malik, Willett, & Hu, 2013; Sturm & Hattori, 2013).
Of particular relevance to this Special Issue of Appetite, obesogenic environments have also been linked to excessive weight gain in children and adolescents (Osei-Assibey et al., 2012; Saelens et al., 2012). For example, rates of childhood obesity are elevated in neighborhoods with higher compared to lower numbers of fast food outlets (Carroll-Scott et al., 2013). Obesity rates are also higher for children that attend schools in neighborhoods with relatively high numbers of convenience stores and fast food restaurants (Wasserman et al., 2014; but see Williams et al., 2014). In addition, the results of some studies show that exposure to TV advertising for energy-dense foods (Andreyeva, Kelly, & Harris, 2011; Boyland et al., 2011) and receptivity to this type of advertising are positively correlated with childhood BMI (McClure et al., 2013).
Similar to the population at large, the incidence of obesity has doubled in children ages 6–11 and tripled in adolescents ages 12–19 since 1980 (Ogden, Carroll, Kit, & Flegal, 2012). In addition, like their adult counterparts, children that are overweight or obese exhibit increased risk factors for Type II diabetes, hypertension, and other comorbidities (Daniels et al., 2005). Obese children are also likely to become obese adults - at which time the severity and range of threats to health and quality of life are magnified (Freedman et al., 2005; Guo & Chumlea, 1999).
One of the most pernicious of these threats is cognitive decline. Previous research has identified obesity and increased body adiposity in mid-life with the development of late-life cognitive dementias such as Alzheimer’s disease (Gustafson, 2008; Whitmer, 2007). As several reports in the current issue confirm (e.g., Convit et al., Verdejo-Garcia et al., Nederkoorn et al., Kahn et al., Bruce et al.), evidence is also accumulating that obesity is associated with impaired cognitive functioning in children and adolescents (Kamijo et al., 2012; Liang, Matheson, Kaye, & Boutelle, 2014; Schwartz et al., 2013). These links raise concerns that excessive weight gain and obesity in childhood increase the risk for more serious cognitive disorders that are usually diagnosed much later in life (Elias, Goodell, & Waldstein, 2012; Smith, Hay, Campbell, &Trollor, 2011).
We think that an important first step toward addressing these concerns is to consider why so many people of all ages have such difficulty resisting the temptations of the obesogenic environment. To say that the physiological controls of intake are overwhelmed by an onslaught of environmental cues that goad us to eat provides only a partial answer. A more complete account must identify and explain the mechanisms that initiate and maintain this hypothetical change in the relationship between the physiological and environmental controls of intake.
Previously, we proposed a model that describes how both internal cues corresponding to hunger and satiety and external cues associated with foods and the postingestive consequences of eating participate in the learned control of energy intake and body weight (e.g., Davidson, Sample, & Swithers, 2014; Davidson, Tracy, Schier, & Swithers, 2014). One purpose of the current research is to assess the extent to which these internal cues are able to compete with external cues for the control of conditioned appetitive behavior when both types of cues are valid signals of food reward. A second goal is to examine the hypothesis that dietary factors common to obesogenic environments can weaken the internal relative to external controls of intake. Based on the findings of the present experiments and the results of earlier work, we will also consider how the mechanisms that underlie such a diet-induced shift from internal toward external control of intake may be related to deficits in certain types of cognitive functions.
The rationale for our present studies is based largely on three sets of previous findings. First, research in our laboratory has shown that rats can use the interoceptive stimulus consequences of different levels of food deprivation as discriminative cues for the delivery of either mild shock (e.g., Davidson, 1987) or sucrose pellets (e.g., Davidson et al., 2005). Evidence for this learning has been obtained after as few as three reinforced trials (Davidson, Flynn, & Jarrard, 1992), and discriminative control generalizes from cues produced by food deprivation and satiation to hormonal manipulations that are known to promote or suppress feeding behavior (e.g., exogenous administration of ghrelin (Davidson et al., 2005) or CCK and leptin (Kanoski, Walls, Davidson, 2007), respectively). These latter findings confirm that interoceptive cues arising from hunger and satiety, rather than exteroceptive stimuli produced by features of the deprivation regimen, were the basis of discriminative responding.
Second, other studies have shown that the ability of rats and humans to use their interoceptive energy or hydrational state cues as discriminative stimuli depends on the functional integrity of the hippocampus (e.g., Francis & Stevenson, 2011; Hebben, Corkin, Eichenbaum, & Shedlack, 1985; Hirsh, Leber, & Gillman, 1978). For example, hippocampal lesions have been shown to impair discriminative responding when cues produced either by different levels of food deprivation or by food versus water deprivation serve as discriminative stimuli (Davidson & Jarrard, 1993; Davidson et al., 2010; Kennedy & Dimitropoulos, 2014; Kennedy & Shapiro, 2009). In contrast, simple discrimination performance based on conventional auditory and visual cues is largely unaffected by hippocampal damage (e.g., Jarrard & Davidson, 1991).
Third, studies have shown that rats maintained on a western-style diet high in both saturated fat and sugar exhibit impairments on a variety of learning and memory problems that are known to depend on the hippocampus. These same rats are not impaired in learning simple discriminations and other learning and memory problems that are hippocampal-independent (Davidson et al., 2012; Hargrave et al., in this issue; Kanoski, Zhang, Zheng, & Davidson, 2010; Molteni, Barnard, Ying, Roberts, & Gomez-Pinilla, 2002). Rats maintained on these diets also exhibit signs of brain pathologies such as increased blood-brain barrier permeability, elevated markers of hippocampal inflammation, and reduced levels of brain neurotrophic factors (Grayson et al., 2013; Hsu & Kanoski, 2014; Kanoski, Walls, Davidson, Kanoski, & Walls, 2007; Miller & Spencer, 2014; Molteni et al., 2002; Sobesky et al., 2014). Moreover, some pathological symptoms have been observed most prominently in rats that also showed both heightened sensitivity to the obesity promoting effects of these diets and impaired hippocampal-dependent learning and memory performance (Davidson et al., 2012, 2013). These findings establish a link between the ability of these diets to promote weight gain and their ability to disrupt hippocampal-dependent learning and memory functions.
Considered together, these three sets of findings indicate that (a) interoceptive stimuli arising from different levels of food deprivation can gain associative control over behavior; (b) the hippocampus is a neural substrate for this type of associative control, but for not simple associative learning about external stimuli; and (c) consuming a high-saturated fat, high-sugar diet characteristic of obesogenic environments is associated not only with obesity but also with the development of hippocampal pathologies and impairments in hippocampal-dependent learning and memory processes. Against this backdrop, this paper evaluates the possibility that diets high in saturated fat and sugar interfere with hippocampal functioning and thereby reduce the control of energy intake by internal relative to external cues.
Experiment 1
Our theoretical framework suggests that appetitive behavior is normally under the joint control of external food-related cues and interoceptive cues related to hunger and satiety. Experiment 1 assessed this hypothesis by training rats with both food deprivation cues and external cues as compound discriminative cues for sucrose pellets. After asymptotic discrimination performance was achieved, the external cues were removed to assess discriminative control by deprivation cues alone. Then, following retraining with the deprivation cue/external cue compound, rats were tested with the discriminative contingencies reversed for the external cues, while the deprivation cue contingency remained the same. Thus, the training contingencies of the deprivation cues were now in opposition to the training contingencies of the external cues (i.e., the non-reinforced external cue was presented in the compound with the reinforced deprivation cue and vice versa). This procedure allowed us to determine which type of stimuli – the internal deprivation cues versus the external cues – had primary control over conditioned behavior. If the rats failed to show significant discriminative responding when tested with the deprivation cues alone and when the deprivation cues were placed in direct competition with the external cues, this would indicate that deprivation state signals have little or no control over appetitive behavior when relevant external cues that predict food reward are also present. However, the extent to which deprivation cue-based discriminative control is maintained under these conditions would suggest that deprivation states fare well in competition with external cues for the control of appetitive responding.
Methods
Subjects
Subjects were 32 male Sprague-Dawley rats (Harlan, Indianapolis) weighing 328–365 g at the start of testing. Animals were housed individually in tub cages with pellet laboratory chow (Lab Diets 5001) and water available ad libitum except as described below. The colony room was maintained on a 12:12 h light-dark cycle with lights on at 0700. Temperature in the colony room was maintained at 21–23 °C. The Purdue Animal Care and Use Committee approved all procedures for the care and treatment of animals in this experiment.
Apparatus
All training and testing sessions were conducted in 8 identical conditioning chambers constructed of aluminum end walls and Plexiglas sidewalls, measuring 59.7 × 34.3 × 26.35 cm (Lafayette Instruments, Lafayette, IN). The floors of the chamber consisted of stainless steel metal rods measuring .48 cm in diameter and 1.07 cm apart. A recessed food magazine was in the center of one end wall of each chamber. Entries into the food magazine were recorded by a computer-operated infrared monitoring system. One infrared photo transmitter and one receiver were located on each side wall immediately in front of the recessed food magazine, such that rats would have to break the beam to gain entry to the food magazine. Reinforcers were 45 mg sucrose pellets (Research Diets Inc., Lancaster, NH).
Procedures
Training
The design of Experiment 1 is shown in Table 1. Prior to the beginning of training, rats were assigned to two groups (n = 16 each) that were matched on body weight (M ± SD = 346 ± 10 g). For all rats, food deprivation levels alternated each day between 0 h and 24 h food deprivation. Operationally defined, on 0 h food deprivation days, all rats had free access to food for approximately 24 h before the beginning of a training session. On 24 h food deprivation days, rats had no access to food for approximately 24 h prior to the beginning of the training session.
Table 1.
Design Experiment 1.
| Group | n | Compound cue training |
Deprivation cue test |
Compound cue retraining |
Cue competition test |
|---|---|---|---|---|---|
| 0+ | 16 | 0A+, 24B− | 0+, 24− | 0A+, 24B− | 0B−. 24A− |
| 24+ | 16 | 24A+, 0B− | 24+, 0− | 24A+, 0B− | 24B−, 0A− |
Note: 0+ = rewarded under 0 h but not 24 h deprivation; 24+ = rewarded under 24 h but not 0 h deprivation; A and B = external tone or white noise cues counterbalanced across groups.
Training trials consisted of a 4 min presentation of a discrete auditory cue, which was either a 3000 Hz tone (T) or a 3 Hz white noise (WN). On reinforced trials, the auditory cue terminated in the delivery of 5 sucrose pellets. During non-reinforced trials, the feeders operated at the end of 4 min but no pellets were delivered. Rats in Group 0+ were reinforced at the end of each training session that took place under 0 h food deprivation and received no pellets during a training sessions that took place under 24 h food deprivation. Group 24+ received the opposite contingency between food deprivation level and presentation of sucrose pellets. The auditory cues co-varied with the rats’ non-reinforced levels of food deprivation, such that the rats could solve the discrimination either by paying attention to the auditory cues (e.g., WN+, T−) or to their interoceptive cues (e.g., 0 h+, 24 h−). The auditory cues were counterbalanced such that half of the animals in each group were trained with the WN as the reinforced stimulus and the T as the non-reinforced stimulus and vice versa. On both reinforced and non-reinforced trials, the rats were removed from the conditioning chambers and returned to their home cages approximately 2 min after feeder operation.
All of the rats were trained and tested in four squads of 8 animals, with each rat in a squad assigned to a different conditioning chamber. Training sessions were always held at the same time of day (1700 h) but did not occur every day in order to prevent the reward from being delivered according to a single-alternating schedule (approximately 5 training sessions per week). The schedule was designed so that the number of transitions from 0 h to 24 h and from 24 h to 0 h food deprivation during training was equated. The number of 0–0 h and 24–24 h transitions was also equated. Initial training consisted of 52 sessions (26 sessions under 0 h- and the remaining 26 sessions under 24 h-food deprivation).
Throughout the experiment, the 4 min period that ended with feeder activation was further subdivided into twenty-four 10 s intervals. The percent of these intervals during which the photo beam was interrupted was calculated over the 4 min of each session prior to feeder activation. This percentage was used as the index of discriminative responding.
Testing
After asymptotic performance was achieved, a series of tests were conducted to determine the extent to which the rats had relied on their internal deprivation cues as opposed to the external auditory cues to solve the discrimination problem. Testing took place across 6 days, during which time the rats were maintained on their alternating schedule of food deprivation.
Deprivation cue test
One day after the rats’ last training trial, the rats were tested on two consecutive days under 0 h and then 24 h food deprivation without presentation of the auditory cues. During these trials, the rats were placed in the conditioning chamber for 4 min as in original training except that neither the T or WN stimuli were turned on. No pellets were delivered during these test sessions.
Compound cue retraining
Beginning on the day following the deprivation cue test, the rat were given two sessions that were identical to the non-reinforced and reinforced sessions that took place during original training (i.e., the reinforced auditory cue was presented under the rats’ reinforced level of food deprivation, and the non-reinforced auditory cue was presented under the rats’ non-reinforced level of food deprivation). The purpose of these sessions was to determine if discrimination performance based on the compound cue was reinstated following testing with only deprivation stimuli.
Cue-competition test
Beginning on the day following the compound cue retraining, the rats were tested with the auditory cues switched across the two levels of food deprivation. Thus, on one of these sessions, the rats were presented with their reinforced auditory cue when they were tested under their non-reinforced level of food deprivation, and on the other session they were presented with their non-reinforced auditory cue when tested under their reinforced level of food deprivation (see Table 1). Thus, each trial contained both a reinforced and non-reinforced cue, with the interoceptive cue signaling a different outcome than the auditory cue. This test allowed us to determine the extent to which the deprivation cues would control appetitive responding even in the face of direct competition from external auditory cues that signaled the opposite contingency. No sucrose pellets were delivered during this test phase.
Statistical analysis
The dependent measure for training and testing was the percentage of 10 s periods in which the photo beam was interrupted throughout the 4 minutes preceding sucrose delivery. Training data were analyzed using a repeated measures ANOVA with Session, and +/− trial type (“+” = rewarded; “-” = nonrewarded) as within-subjects factors, and Group (0 + and 24+) as a between-subjects factor. A similar analysis was used to evaluate the data during testing except that Phase (End of Training Baseline, Dep Cue alone test, Retraining with compound cues, and Cue Competition test) replaced Sessions and a between-subjects factor. Significant interactions and main effects were further analyzed with post-hoc tests with Bonferonni correction. For all statistical comparisons α-level was set at p < 0.05.
Results
Training
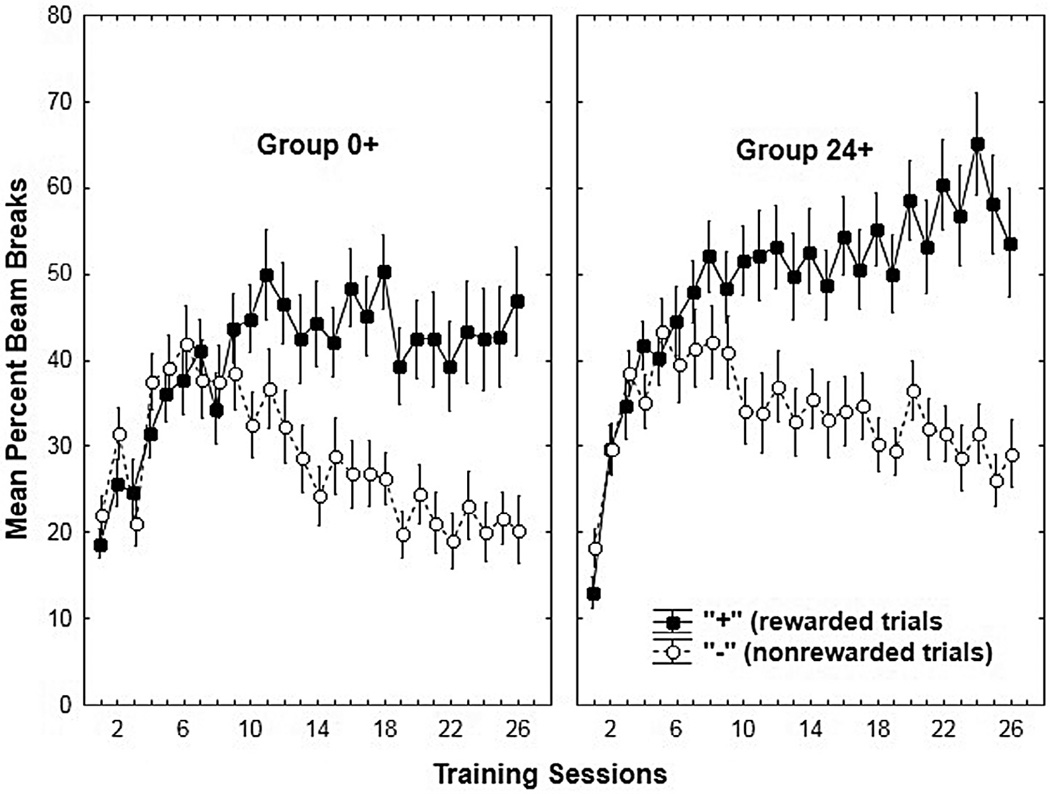
The rats in Groups 0+ and 24+ each showed that they solved the food deprivation intensity discrimination by learning to respond more on their rewarded (+) compared to their norewarded (−) sessions (see Fig. 1). Repeated measures analysis of variance (ANOVA) yielded a significant (F (1, 28) = 50.24, p < 0.01) main effect of +/− as well as a significant +/−× Session interaction (F(25, 700) = 12.62, p < 0.01). In addition, there was a main effect of Group (F(1, 28) = 4.51, p < 0.05) indicating that Group 24+ exhibited significantly more responding overall than Group 0+. However, neither the Group ×+/− interaction nor the Group ×+/−× Session interaction was significant, indicating that the groups did not differ in their ability to solve the discrimination task. A separate one-way ANOVA compared difference scores for each group (obtained by subtracting percent beam breaks on nonrewarded (−) trials from percent beam breaks on rewarded (+) trials) over the last two-session block of training. This analysis also failed to yield evidence of a significant difference in discrimination performance between the two groups (F(1, 30) < 1). Post-hoc tests were used to evaluate the +/−× Session interaction collapsed across the two groups (i.e., 0 + and 24+). This analysis revealed that responding was significantly greater on the rewarded versus nonrewarded sessions from sessions 10–26 of training.
Fig. 1.
Mean ± S.E.M. percent magazine entries during 4-minute period preceding sucrose delivery on reinforced (+) and nonreinforced (−) deprivation sessions for Group 0+ (left panel) and Group 24+ (right panel) during Experiment 1 acquisition.
Testing
As in training, when Group (0+ and 24+) was included as a factor and analyzed across the phases of testing, a significant main effect was obtained (F(1, 30) = 4.53, p < .05). This indicated that overall level of responding on both + and - trials was higher for Group 24 + compared to Group 0+. However, neither the Group ×+/− (F(1, 30) = 1.105, p> .30), Group × Phase (F(2, 60) = 2.52; p > .08), nor the Group ×+/−× Phase (F (2, 60) = 1.76, p > .18) interactions achieved significance. Based on the failure of Group to interact with +/− in any way, the results provided no evidence that discrimination performance for the 0 + and 24+ groups differed during testing, and therefore the remaining analyses will make no further mention of the Group variable.
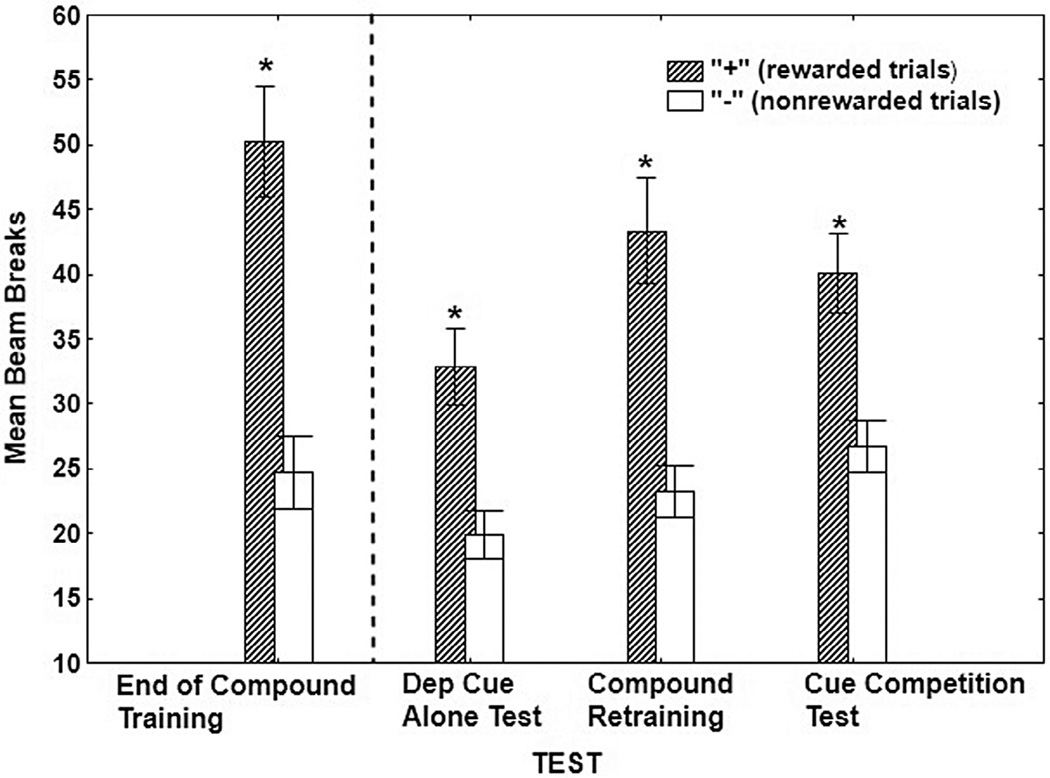
Figure 2 shows responding during rewarded and nonrewarded sessions on the last two sessions of training when both food deprivation and external cues were presented as compound discriminative stimuli; on the deprivation cue test when food deprivation cues were presented without external cues; during compound cue retraining when the originally trained deprivation cue/auditory cue compound discriminative contingencies were reinstated; and on the cue-competition test when the auditory cues were tested in compound with deprivation cues that signaled the opposite discriminative contingency. These results show that relative to the end of training and retraining with the original deprivation cue/auditory cue compound, the overall level of appetitive responding was reduced on the Deprivation Cue test when the auditory cues were removed. However, despite this drop, the deprivation cues nevertheless maintained significant discriminative control over responding. In fact, the deprivation cues maintained strong discriminative control even when those cues were placed in direct competition with the external auditory cues.
Fig. 2.
Mean ± S.E.M. percent magazine entries during the last two rewarded (+) and nonrewarded (−) sessions of Compound Training (i.e., food deprivation cues and auditory cues presented as compound discriminative stimuli), the Deprivation Cue Test (i.e., deprivation cues only), Compound Retraining with the deprivation/auditory cue compound, and the Cue Competition Test (external cue contingencies reversed while deprivation contingencies remained the same).
An ANOVA comparing discrimination performance across all of these phases obtained a significant main effect of +/− (F(1, 28) = 58.54, p< 0.001) and a significant +/−× Test interaction (F(3, 84) = 3.67, p < 0.05). Despite this significant interaction, Bonferroni tests showed that responding was significantly greater on “+” compared to “-” sessions in each phase shown in Fig. 2 regardless of whether or not the external cues were presented or whether discriminative control by the deprivation cues was opposed by external cues that had been trained with the opposite discriminative contingencies (all p values < .01).
Discussion
The results of Experiment 1 show that rats can learn to use interoceptive cues arising from 0 and 24 h food deprivation as discriminative stimuli even when those cues are trained in compound with highly relevant external cues. This was shown by findings that, following training with deprivation and auditory cues as compound discriminative stimuli, discriminative responding to deprivation cues was observed (a) when external cues were removed the discriminative contingencies were reversed for the auditory while being held constant for the deprivation cues.
These findings are important for two reasons. First, they show that food deprivation cues can compete with external cues for the control of appetitive responding. Indeed, had the deprivation cues not been able to compete successfully with the external cues, this would reduce the plausibility of the idea that reduced interoceptive control might be partially responsible for the excess intake and body weight gain observed in obesogenic environments. In fact, rather than being overshadowed by the external cues, the results of the cue-competition test suggest the possibility that the deprivation cues had more influence over appetitive behavior than the external cues.
Second, the results of Experiment 1 suggest that associative control by internal deprivation stimuli and exteroceptive cues may depend on different mechanisms. Food deprivation cues were present before, during, and after each training and test session in Experiment 1, whereas the external cues were present during only the 4 min period immediately prior to the delivery of the sucrose pellets or prior to the time when delivery of those pellets was withheld. Based on both temporal contiguity and correlation with the delivery of the sucrose pellets, one might expect that external cues would have had much stronger associative control over appetitive behavior compared to the deprivation cues (Murphy & Baker, 2004; Rescorla & Wagner, 1972). We have previously suggested that interoceptive energy state cues function like contextual stimuli that are established pre-experimentally as signals for when food-related environmental events will be followed by rewarding postingestive consequences (e.g., Davidson, Tracy, et al., 2014). Such “occasion setting” stimuli are not thought to be directly associated with food or other types of unconditioned stimuli (USs). Rather they influence behavior based on their ability to modulate the capacity of cues that are directly associated with food or other USs to excite the memories of those events (Swartzentruber, 1995). This analysis suggests that the internal deprivation cues modulated the ability of the food-related external cues to evoke appetitive responding for sucrose, without being embedded in a direct association with sucrose pellets themselves. From this perspective, for the rats in Experiment 1, learning about deprivation cues did not involve competition with external cues for a direct association with the sucrose pellet US, and thus the deprivation cues could acquire discriminative control over behavior even when highly valid external cues were also available.
Experiment 2
Experiment 1 showed that rats are able to learn about interoceptive food deprivation stimuli even when salient external cues also served as valid predictors of food reward. The purpose of Experiment 2 was to assess whether stimulus control by food deprivation cues would be diminished by chronic exposure to a diet high in saturated fat and processed sugars. This diet was selected because it is similar in macronutrient content to the human “western diet” so named because of its widespread consumption in western and westernized societies (e.g., Hintze, Benninghoff, & Ward, 2012). If intake of a western-style diet (WD) impairs the ability of interoceptive state cues to control appetitive responding, and the function of those state cues is to modulate the ability of external food cues to evoke eating and appetitive behavior, then this impairment may be one mechanism by which consuming a WD makes it difficult to resist responding to environmental food cues in an obesogenic environment.
In Experiment 2, two groups of rats were trained using the same deprivation discrimination paradigm that was used in Experiment 1 (see Table 2), with the exception that rats were trained only with the deprivation cues (i.e., no external cues present during training). When performance reached asymptote for both groups, training was suspended and dietary manipulations were introduced. Half of the animals were given WD, while the remaining animals continued to receive standard chow. After 42 days of diet exposure, animals were tested on their ability to use deprivation states as discriminative cues for sucrose. To assess whether or not any WD-induced impairment was specific to the interoceptive cues, external cues (i.e., a tone or light) were then introduced as additional predictors of sucrose. Following 10 sessions of compound training with both internal and external cues present, the external cues were removed to reassess the degree of discriminative control by internal cues alone.
Table 2.
Pre-diet training and post-diet testing (Experiment 2).
| Group | n | Deprivation cue training |
Ad libitum diet (42 days) |
Deprivation cue test |
Compound internal/ external cue test |
Final deprivation cue test |
|---|---|---|---|---|---|---|
| 0+ | 8 | 0+, 24− | WD | 0+, 24− | 0A+, 24B− | 0+, 24− |
| 0+ | 8 | 0+, 24− | Chow | 0+, 24− | 0A+, 24B− | 0+ , 24− |
| 24+ | 8 | 0−, 24+ | WD | 0−, 24+ | 0A−, 24B+ | 0−, 24+ |
| 24+ | 8 | 0−, 24+ | Chow | 0−, 24+ | 0A−, 24B+ | 0−, 24+ |
Note: 0+ = rewarded under 0 h but not 24 h deprivation; 24+ = rewarded under 24 h but not 0 h deprivation; WD = western diet; A and B = external tone or light cues counterbalanced across groups.
Methods
Subjects
Subjects were 32 naïve male Sprague-Dawley rats, weighing approximately 250–300 g upon arrival from Harlan. Rats were individually housed in plastic tubs and maintained on a 14:10 h light:dark cycle with lights on at 0900 h. Temperature in the colony room was maintained at 21–23 °C. All procedures for the care and treatment of the rats in this experiment were approved by the American University Institutional Animal Care and Use Committee.
Diets
The WD was a lard-based diet high in saturated fat and dextrose-based sugars (Harlan Teklad, TD.10768). The WD had a caloric density of approximately 4.4 kcal/g (approximately 42% kcal from fat, 37% kcal from carbohydrates) and contained the following (g/kg): 270 g casein, 220.5 g dextrose, 120 g maltodextrin, 170 g lard, 15 g safflower oil, 15 g soybean oil, 80 g corn starch, and 50 g cellulose. The control diet used was standard laboratory rodent chow (LabDiet, Formula 5001). The caloric density of the chow diet was 3.0 kcal/g (approximately 13% kcal from fat, 56% kcal from carbohydrates). Both diets were provided in pelleted form in the home cage. All animals received ad libitum water throughout the experiment.
Apparatus
All training and testing sessions took place in 8 identical conditioning chambers that were of the same description as those used for Experiment 1, with the following exceptions: the auditory stimulus was a 1500 Hz, 74–76 db tone (Sonalert, Lafayette Instruments). The 6 W jeweled light measuring 2.4 cm in diameter located 5 cm to the left of and 6 cm above the recessed food magazine served as a visual conditioned stimulus. The infrared photo beam transmitters and receivers used to record food magazine approaches were located just inside the recessed food magazine.
Procedures
Training
Prior to the start of training, rats were assigned to two groups (n = 16 each) matched on body weight (means = 349.12 g and 350.44 g; SEM = 3.17). Rats were trained to discriminate between 0 h and 24 h food deprivation as described in Experiment 1; however, no external cues were provided. Training sessions took place at approximately 0930 h every day. Training consisted of 32 sessions, 16 sessions under 0 h food deprivation and 16 sessions under 24 h food deprivation. As in Experiment 1, the percent of 10 s intervals in which the infrared photo beam inside the food magazine was interrupted during the 4 min period that preceded feeder activation served as the index of appetitive behavior.
Dietary treatment
When performance reached asymptote, training was suspended, and Groups 0+ and 24+ were each divided into WD and Chow control subgroups. Half of the rats in Group 0+ (n = 8) and half of the rats in Group 24+ (n = 8) were assigned to receive ad libitum WD for 42 days, while the remaining animals were maintained on standard chow. When training and testing resumed after 42 days, the rats remained on their WD or chow diet on the alternating 0- and 24-hr deprivation schedule. These two groups were matched on discrimination performance in the last two training sessions under each deprivation level.
Deprivation cue test 1
Six weeks after the introduction of the diets, rats were retrained on the deprivation discrimination for 14 sessions, with 7 sessions under each deprivation condition. Including days on which sessions were not conducted, this first phase of deprivation cue testing lasted for 20 days.
Training with external cues
Following the post-diet retraining with deprivation cues, external light and tone cues were introduced and presented with food deprivation cues as compound discriminative signals for sucrose pellets. For half of the rats in each deprivation and diet condition (e.g., WD 24+), a tone served as the additional signal for sucrose delivery, while a light predicted no sucrose. The remaining rats received the reverse contingency (i.e., a light signaled sucrose delivery while a tone signaled no sucrose delivery). These compound internal/external cues were available for 20 sessions, 10 sessions under each deprivation level. Including days in which the rats were not run, this phase lasted 28 days.
Final deprivation cue test
After training the animals with the compound stimuli, we reassessed their ability to solve the discrimination using deprivation cues alone. External light and tone cues were removed, and rats were tested for four sessions, two under each deprivation level, with the external cues removed.
Statistical analyses
For training, the primary dependent measure was the percentage of 10 s periods in which the photo beam was interrupted throughout the 4 minutes preceding sucrose delivery. Training data were analyzed using ANOVA with Deprivation level (0 h and 24 h deprivation), Session, and +/− (“+” = rewarded; “-” = nonrewarded) as within-subjects factors, and Group (0+ and 24+) as a between-subjects factor. Data from testing were converted to difference scores (calculated as mean percentage beam breaks on rewarded (+) trials minus mean percentage beam breaks on nonrewarded (−) trials). These difference scores were evaluated using ANOVA with Diet (WD and chow) and Group (0+ and 24+) as between-subjects factors and with Phase (Training, Sessions 1 and 16; Testing, Deprivation Cue Test Sessions 1 and 7; External Cue Test Sessions 1 and 10; and Final Deprivation Cue Test Session) as a within-subjects factor. Single factor ANOVA for body weight employed Diet as a between-subjects factor. Significant interactions and main effects were further analyzed with post-hoc Fisher’s Least Significant Difference (LSD) tests. For all statistical comparisons α-level was set at p < 0.05.
Results
Deprivation intensity discrimination training
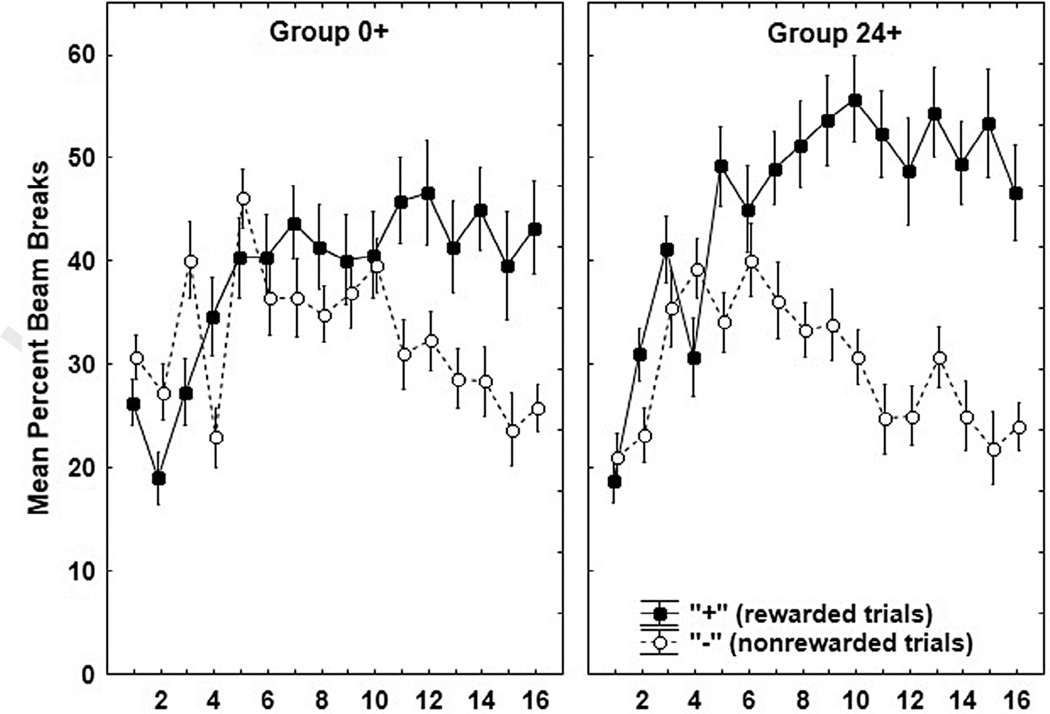
Both Groups 0+ and 24+ learned to solve the discrimination. As seen in Fig. 3, by the end of training both Groups 0+ and 24+ exhibited more responding on rewarded compared to nonrewarded sessions although the magnitude of this difference was somewhat smaller for Group 0+. An overall ANOVA yielded a significant Group ×+/−× Session (F (15, 450) = 3.10, p < .01) interaction. To further evaluate this interaction, the data for Groups 0+ and 24+ were analyzed separately. For Group 0+, ANOVA yielded significant main effects of +/− (F(1, 15) = 15.70, p < .01), Session (F(1, 225) = 3.95, p < .01) and a significant +/−× Session interaction (F(15, 225) = 5.26, p< .01). Post-hoc tests revealed that the rats in Group 0+ responded significantly more on “+” compared to “–” trials on sessions 11–16. The main effects of +/− (F(1, 15) = 48.88, p < .01), and Session (F(15, 225) = 7.27, p < .01) were also significant for Group 24+, as was the +/−× Session interaction (F(15, 225) = 7.69, p < .01). Post-hoc tests showed that responding on reinforced trials was significantly greater than on nonreinforced trials for Group 24+ on sessions 5 and 7–16.
Fig. 3.
Mean ± S.E.M. percent magazine entries during the 4-minute period preceding sucrose delivery for rewarded (+) and nonrewarded (−) sessions during Experiment 2 acquisition. The left panel shows data for Group 0+, while the right panel shows data for Group 24+.
Deprivation intensity discrimination testing
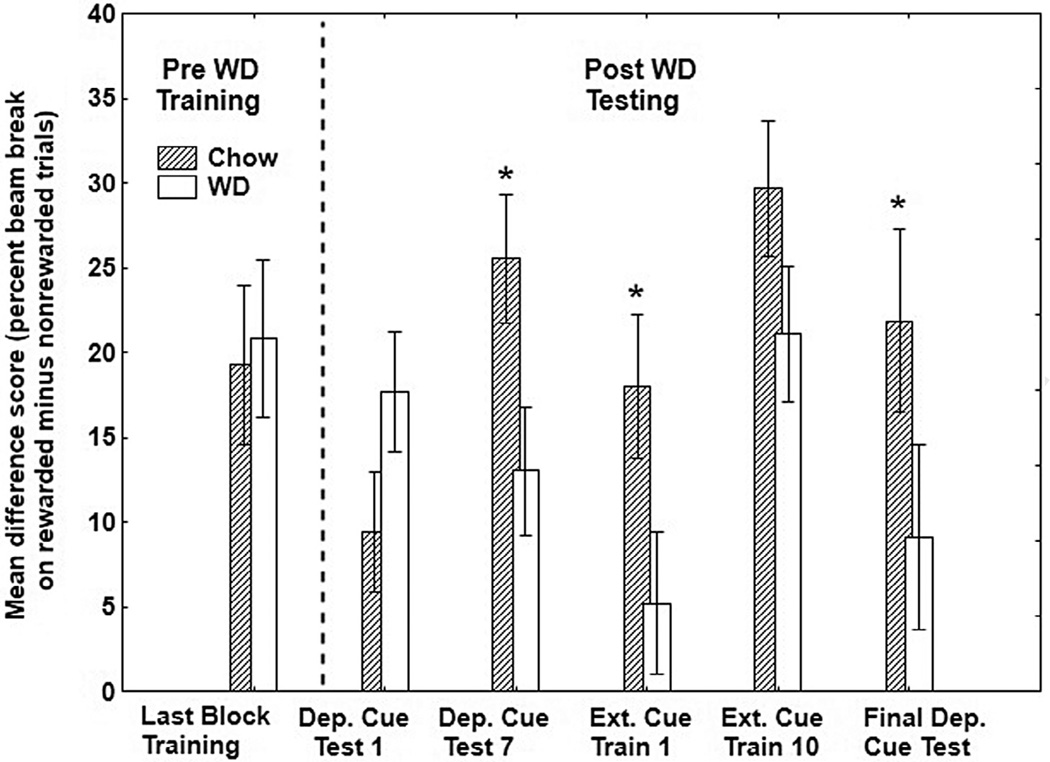
As seen in Fig. 4, the difference in percent beam breaks recorded on the last rewarded and the last nonrewarded session of training prior to WD exposure (Last Block of Training) differed little between rats that were subsequently given WD and chow. Although performance did not differ between the two diet groups in initial training, differences between the two diet groups began to emerge after prolonged exposure to the diets. Specifically, difference scores showed that the magnitude of the discrimination was significantly greater for the group fed chow compared to the group fed WD at the conclusion of the first of seven “+” and seven “-” sessions of deprivation cue testing (Dep. Cue Test 7). This pattern continued on the first trial after external cues were introduced and presented in the compound with the food deprivation stimuli (Ext. Test 1). In contrast, the difference scores for the two diet conditions did not vary significantly at the end of 10 sessions with external cues (Ext. Test 10), based mostly on improved performance for the WD-fed rats. However, when the external cues were removed (Final Dep. Cue Test), discrimination performance for the WD-fed rats fell once again to levels that were significantly lower compared to the rats fed chow. Thus, the results indicated that with the exception of the first deprivation cue test, discrimination performance based on food deprivation stimuli was worse for rats fed WD compared to chow, whereas discrimination performance when external cues were present did not differ between the two groups.
Fig. 4.
Mean ± S.E.M. difference scores (i.e., nonreinforced subtracted from reinforced responding) of percent magazine entries during the 4-minute period preceding sucrose delivery for WD-fed and chow-fed rats during first and last rewarded and nonrewarded sessions (i.e., +/− session) of each phase including: the last block of training prior to the introduction of the WD (Last Block of Training), the first +/− session following six weeks on the diets (Dep. Cue Test 1), the seventh +/− session with the deprivation cues only (Dep. Cue Test 7), the first +/− session in which the external light and tone cues were introduced (Ext. Cue Test 1), the tenth and last session with the external light and tone cues in combination with the deprivation cues (Ext. Cue Test 10), and the last +/− session in which external light and tone cues were removed for the Final Deprivation Cue Test.
Analysis of the results obtained on the Last Block of Training (i.e., prior to the diet manipulations) revealed no significant main effects of Group (0+ and 24+) or of the to be assigned Diet (WD and chow), nor was the interaction between these variables significant. This was supported by ANOVA, which yielded significant main effects of Diet (F(1, 28) = 5.54, p < .05) and Test Session (F(4,112) = 3.74, p< .01), along with a significant Diet × Test Session interaction (F(4, 112) = 2.61, p< .05). Group did not interact significantly with either Diet or Test Session. Post hoc tests confirmed that difference scores were significantly higher for rats fed chow compared to rats fed WD on Dep. Cue Test 7, on Ext. Cue Test 1, and on the Final Dep. Cue Test after the external cues had been removed (all ps < .05). Difference scores for the WD and chow diet conditions were not significantly different on Dep. Cue Test 1 or on the final test with external cues (i.e., Ext. Cue Test 10).
To compare learning about external cues and food deprivation stimuli, analyses of simple main effects were used to compare the size of the difference scores for each group on (a) the first day of testing with external cues and deprivation cues combined, (b) on the last day of testing with both of those cues, and (c) on the final test session when the external cues were removed. For rats fed WD, the main effect of Test Session was significant (F(2,30) = 7.67, p < .01). Post-hoc tests found that difference scores for Group WD were significantly higher on the last test session with external cues compared to both the first session with external cues and the final test session when external cues were removed (both p values < .01). For the chow-fed rats, the main effect of test was not significant (F(2, 30) = 1.44, p > .25), indicating that difference scores did not differ on any of those test sessions.
We also compared discrimination performance on each test with baseline performance on the Last Block of Training prior to the diet manipulation. For rats maintained on chow, there were no significant differences in discrimination performance on any test session compared to the last block of food deprivation training. In contrast, for WD-fed rats, discrimination performance was significantly below this baseline when external cues were introduced on Ext. Cue Test 1 and after the external cues were removed on the Final Dep. Cue Test (ps< .05).
Body weight
Prior to the beginning of food deprivation discrimination training, the mean weights of the rats that were later assigned to receive ad libitum WD (380.13 g; SEM = 5.97) and chow (382.06 g; SEM = 4.37) prior to testing did not differ significantly (F(1, 30) < 1). At the end of training, half of the rats in Group 0+ and half in Group 24+ were matched on body weight and were placed on ad libitum WD while the remaining rats in each group were placed on ad libitum chow. Mean body weight immediately prior to this diet manipulation was 383.25 g (SEM = 5.47) for the rats placed on WD and 382.56 g (SEM = 4.37) for the rats maintained on chow. This difference also failed to achieve significance. Following 42 days of ad libitum maintenance on these diets, mean weight for rats fed WD rose to 468.50 g (SEM = 6.88), while mean weight for rats fed chow was 449.5 g (SEM = 5.06). An ANOVA comparing weight gain for these two groups (85.25 g for WD; 67.44 g for chow; SEM = 3.10) yielded a significant main effect of Diet (F(1, 30) = 16.48, p< .01). The rats remained on their respective WD and chow diets until the end of testing. However, following the 6-week period of ad libitum exposure to the diets, the rats were returned to the alternating 0- and 24-h schedule of food deprivation that was used during original deprivation discrimination training. At the end of this 50-day period of testing, the rats fed WD weighed a mean of 485.31 g (SEM = 7.95), whereas the rats fed chow weighed a mean of 456.19 g (SEM = 5.92). The difference in weight gain from the first day compared to the last day of testing was 16.81 g for the WD-fed rats and 6.69 g for the chow-fed rats. One-way ANOVAs confirmed that these differences in mean weight (F(1, 30) = 8.63, p < .05 ) and mean weight gain were significant (F(1, 30) = 5.94, p < .05).
Discussion
The results of Experiment 2 showed that, compared to rats maintained on standard chow, rats maintained on WD were impaired in using deprivation cues as discriminative signals for the delivery of sucrose pellets. With the exception of the first test, rats fed WD showed weaker discrimination performance based on deprivation cues than did the rats fed chow. In contrast, discriminative responding for both diet conditions was comparable at the end of training after external cues had been added to deprivation cues as relevant signals for the delivery of sucrose pellets. However, WD-fed rats’ discrimination performance once again fell significantly below that of the chow-fed-rats during the final test in which the external cues were removed, leaving only deprivation cues as relevant stimuli. Thus, discrimination performance by WD-fed rats appeared to be based on their ability to use external cues, rather than their food deprivation cues as discriminative stimuli.
It is unlikely that the impaired discrimination performance based on food deprivation cues was the result of WD reducing the reinforcing power or incentive value of sucrose. If that were the case, the discrimination performance of the WD-fed rats should have been impaired relative to rats fed chow with both external and food deprivation cues because both were associated with same sucrose reward. That WD had different effects on discriminative control by each type of cue argues against this hypothesis.
A more plausible possibility is that intake of WD reduced discriminability of deprivation cues relative to external cues. For example, if rats fed WD were less hungry when food deprived compared to rats fed chow, then the difference between the internal cues produced by 0 h and 24 h food deprivation may have become too small to support the same level of discriminative responding exhibited by the chow-fed rats. Although intake was not measured in this study, the rats fed WD gained more weight during the initial 6-week period of ad libitum feeding and continued to gain more weight compared to chow controls during testing under alternating 24 h periods of food deprivation and free access to food. This difference in weight gain suggests that when food was available, rats fed WD were consuming a greater amount of calories relative to their energy needs compared to rats fed chow. This could have made the WD-fed rats less deprived (“hungry”) than chow-fed rats following 24 h without food and thus less able to discriminate between the interoceptive stimulus consequences of 0- and 24-h food deprivation.
A related idea is that instead of reducing the discriminability of interoceptive food deprivation stimuli, WD reduced the ability of rats to interpret or use the information provided by those stimuli. As noted in the Introduction, rats and people with hippocampal damage are also impaired in using their deprivation state cues (food, water) to solve certain types of spatial (Hirsh et al., 1978; Kennedy & Shapiro, 2004) and nonspatial (Davidson et al., 2010) discrimination problems. This impairment exemplifies a broader deficit in the ability of hippocampal-lesioned rats to use both interoceptive and exteroceptive contextual stimuli (e.g., Good & Honey, 1991; Maren, Phan, & Liberzon, 2013; Smith & Bulkin, 2014). Because consuming WD is associated with signs of hippocampal pathology and hippocampal-dependent memory deficits, it is possible that WD intake impaired food deprivation discrimination performance in the present study by interfering with the ability of rats to use the contextual information provided by their interoceptive food deprivation stimuli.
From this perspective, it is noteworthy that while deficits in hippocampal-dependent learning and memory performance can occur following 3–10 days of WD exposure (Kanoski & Davidson, 2010; Murray et al., 2009), other studies have found that these early deficits soon disappear and are not apparent again until after more than 60 days on the diet (Davidson et al., 2013; Hargrave et al., in this issue). This time course may account for our present finding that discrimination performance based on food deprivation cues was unimpaired for rats fed WD on Dep. Cue Test 1, which took place after 42 days of ad libitum diet exposure, but was impaired for WD-fed relative to chow-fed rats on both Dep. Cue Test 7, which took place 62 days after the beginning of WD, and on the Final Dep. Cue Test that occurred 90 days after WD was introduced. It should be noted that unlike our previous studies, which involved continuous ad libitum feeding of WD and chow diets, the present study exposed the rats to alternating 24 h periods with free access and no access to food for all but the first 42 days of diet exposure. Nonetheless, rats fed WD continued to gain weight and gain more weight compared to rats fed chow, even during the period when they were exposed to this regimen of free-feeding and food restriction.
General discussion
The results of Experiment 1 showed that rats can use interoceptive stimuli arising from food deprivation and satiation as discriminative stimuli to predict the delivery of sucrose, even when those cues are trained in the compound with external cues that are equally valid, if not better, predictors of sucrose. Thus, interoceptive food deprivation cues can compete with exteroceptive cues for control of appetitive behavior when both were trained as valid signals for a sucrose US. Experiment 2 provided evidence that consumption of WD, relative to standard chow, reduced the ability of interoceptive food deprivation cues to exert discriminative control over conditioned appetitive behavior. However, when exteroceptive cues became available as valid predictors of sucrose pellets, rats fed WD exhibited a level of discrimination performance comparable to that shown by chow-fed rats. This outcome suggests that WD intake reduced the salience of interoceptive food deprivation cues relative to that of exteroceptive stimuli in the learned control of appetitive behavior. This pattern of results is consistent with the idea that WD promotes excess weight gain by reducing regulatory control over energy intake by internal physiological hunger and satiety signals in the face of environmental stimuli that strongly elicit conditioned appetitive and eating behaviors. This relative shift from internal toward external control may be central to the emergence of obesity in environments where external food-related cues are thought to overwhelm the physiological mechanisms that regulate intake.
While our findings provide evidence that WD intake reduces the influence of internal food deprivation cues relative to external cues over energy intake and body weight regulation, the specific mechanisms that underlie this effect remain to be determined. We propose that this question can be addressed on associative grounds. It is clear that the meaning of an event and its associated consequences often varies depending on the context (Bouton, 1993). Contextual cues similarly modulate our memories and responses to foods and food-related stimuli. For example, encountering a favorite food may elicit quite different thoughts or expectations about the consequences of consuming it when one is hungry compared to food sated. In this case, interoceptive stimuli corresponding to hunger and satiety can be said to provide a context that facilitates retrieval of the likely future consequences of the appetitive and eating behavior. Our findings suggest that consuming a western-style diet that is high is saturated fat and sugar impairs the ability of rats to utilize the contextual information that is normally provided by their interoceptive food deprivation cues. In contrast, WD intake has much less or even no effect on responding evoked by more punctate exteroceptive food-related stimuli. Thus, a WD-induced reduction in the ability to use contextual information provided by interoceptive energy state signals without a similar reduction in the ability of exteroceptive stimuli to evoke eating and appetitive behavior would result in excess eating and weight gain.
This result is especially likely if one accepts the view that the adaptive regulation of energy intake depends much less on eating in response to physiological hunger than on the ability of physiological satiety or meal termination signals to suppress eating in response to environmental food cues (Woods, 2004). Under these conditions, a reduction in the ability to detect or utilize interoceptive signals produced by food deprivation and satiation would increase pressure to overeat, because the role for satiety cues in the inhibition of intake is more important than the role of hunger signals in the excitement of appetitive and eating behaviors.
The hippocampus has long been regarded as a brain substrate for the utilization of contextual information (Maren & Holt, 2000; Wiltgen et al., 2010), including that provided by interoceptive signals related to energy state (e.g., Hirsh et al., 1978). Our present findings are consistent with the idea that WD intake reduces the discriminability of energy state cues before they are processed by the hippocampus. Alternatively, our current findings, as well as much other evidence, are also consistent with the hypothesis that WD degrades the information processing capabilities of the hippocampus. Rats that consume diets high in saturated fat and sugar exhibit impairments in a variety of hippocampal-dependent learning and memory tasks compared to rats fed standard chow, including tasks in which the exteroceptive cues function like deprivation cues by signaling when other external cues or responses will or will not be followed by rewarding appetitive outcomes (Davidson, Tracy, et al., 2014). Several reports have also shown that such deficits in hippocampal-dependent learning and memory functions are accompanied by signs of pathology in the hippocampus such as reductions in brain-derived neurotrophic factor (BDNF), reduced neurogenesis, increased levels of pro-inflammatory cytokines, and increased BBB permeability, which seems to have negative effects that are preferential with respect to the hippocampus (for reviews see Hsu & Kanoski, 2014; Kanoski & Davidson, 2011; Shefer, Marcus, & Stern, 2013). Thus, consuming WD may promote energy dysregulation and weight gain not only by reducing discriminability of interoceptive energy state signals but also by impairing the function of the brain substrate responsible for processing the information provided by these as well as other types of contextual stimuli.
A number of recent reports indicate that diet-induced hippocampal pathology and impairments in hippocampal learning and memory functions can occur at a young age in both rodents and humans. For example, Boitard et al. (2012) found that juvenile rats maintained on a high-fat diet exhibited both impaired hippocampal-dependent spatial memory performance in the radial maze and reduced levels of hippocampal neurogenesis compared to chow-fed controls. The same group also reported that young rats exhibited both deficits in spatial memory performance in the water maze and increased markers of hippocampal inflammation following maintenance on a high-fat diet (Boitard et al., 2014). Impairments in hippocampal-dependent spatial memory along with changes in hippocampal morphology and expression of neurotrophic factors have also been reported for rats exposed to diets high in saturated fats beginning either prenatally or immediately after weaning (e.g., Page, Jones, & Anday, 2014). Furthermore, in humans, Baym et al. (2014) found that consuming high amounts of saturated fat is negatively correlated with performance on hippocampal-dependent relational memory in children as young as 7 years of age. Body adiposity is also negatively correlated with relational memory performance for children in the same age group (Khan et al., 2014).
One implication of the present findings is that if WD intake progressively weakens the ability of interoceptive satiety cues to counter the evocation of conditioned appetitive and eating behaviors by environmental cues, this could set the stage for a “vicious cycle” of obesity and cognitive decline that may begin early in life (Davidson, Kanoski, Schier, Clegg, & Benoit, 2007; Davidson, Sample, et al., 2014). With this scenario, eating WD could produce impairments in either the detection of satiety cues or in the utilization of the contextual information which those cues provide. Either of these deficits could lead to overeating based on the reduced ability of satiety signals to curb response evocation by environmental cues associated with food and the rewarding postingestive consequences of intake. As exposure to WD increases, so do the harmful effects of that diet on hippocampal function, including those involved with using contextual information provided energy state cues. The result is increasing intake and body weight gain accompanied by progressively deteriorating hippocampal-dependent learning and memory capacity. Within this framework, diet-induced deficits in energy regulation and cognitive ability during childhood and adolescence could be precursors for both obesity and serious cognitive dysfunction much later in life. Other papers in this special issue focus directly on the impact of diet and obesity on cognitive function early in human development.
Footnotes
Acknowledgement: This research was supported by Grant R01HD028792 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. We thank Ms. Jennie Mak for assistance with data collection for Experiment 2.
References
- Andreyeva T, Kelly IR, Harris JL. Exposure to food advertising on television. Associations with children’s fast food and soft drink consumption and obesity. Economics & Human Biology. 2011;9:221–233. doi: 10.1016/j.ehb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, et al. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. American Journal of Clinical Nutrition. 2014;99:1026–1033. doi: 10.3945/ajcn.113.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain, Behavior, and Immunity. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Boyland EJ, Harrold JA, Kirkham TC, Corker C, Cuddy J, Evans D, et al. Food commercials increase preference for energy-dense foods, particularly in children who watch more television. Pediatrics. 2011;128:E93–E100. doi: 10.1542/peds.2010-1859. [DOI] [PubMed] [Google Scholar]
- Carroll-Scott A, Gilstad-Hayden K, Rosenthal L, Peters SM, McCaslin C, Joyce R, et al. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity. The role of built, socioeconomic, and social environments. Social Science &Medicine. 2013;95:106–114. doi: 10.1016/j.socscimed.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsica JA, Hood MM. Eating disorders in an obesogenic environment. Journal of the American Dietetic Association. 2011;111:996–1000. doi: 10.1016/j.jada.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents. Pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101:198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Flynn FW, Jarrard LE. Potency of food deprivation intensity cues as discriminative stimuli. Journal of Experimental Psychology. Animal Behavior Processes. 1992;18:174–181. doi: 10.1037//0097-7403.18.2.174. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, et al. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253C:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behavioral & Neural Biology. 1993;59:167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behavioral Neuroscience. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Current Opinion in Pharmacology. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology and Behavior. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiology of Learning and Memory. 2014;108:172–184. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Tracy AL, Schier LA, Swithers SE. A view of obesity as a learning and memory disorder. Journal of Experimental Psychology. Animal Behavior Processes. 2014;40:261–279. doi: 10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Goodell AL, Waldstein SR. Obesity, cognitive functioning and dementia. Back to the future. Journal of Alzheimers Disease. 2012;30:S113–S125. doi: 10.3233/JAD-2011-111175. [DOI] [PubMed] [Google Scholar]
- Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behavioral Neuroscience. 2011;125:943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity. The Bogalusa Heart Study. Pediatrics. 2005;115:22–27. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behavioral Neuroscience. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, et al. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. International Journal of Obesity (2005) 2013 doi: 10.1038/ijo.2013.100. [DOI] [PubMed] [Google Scholar]
- Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. The American Journal of Clinical Nutrition. 1999;70:145S–148S. doi: 10.1093/ajcn/70.1.145s. [DOI] [PubMed] [Google Scholar]
- Gustafson D. A life course of adiposity and dementia. European Journal of Pharmacology. 2008;585:163–175. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection. Case HM. Behavioral Neuroscience. 1985;99:1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Hintze KJ, Benninghoff AD, Ward RE. Formulation of the total Western diet (TWD) as a basal diet for rodent cancer studies. Journal of Agricultural and Food Chemistry. 2012;60:6736–6742. doi: 10.1021/jf204509a. [DOI] [PubMed] [Google Scholar]
- Hirsh R, Leber B, Gillman K. Fornix fibers and motivational states as controllers of behavior. A study stimulated by the contextual retrieval theory. Behavioral Biology. 1978;22:463–478. doi: 10.1016/s0091-6773(78)92583-x. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Kanoski SE. Blood-brain barrier disruption. Mechanistic links between Western diet consumption and dementia. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Davidson TL. On the hippocampus and learned conditional responding. Effects of aspiration versus ibotenate lesions. Hippocampus. 1991;1:107–117. doi: 10.1002/hipo.450010110. [DOI] [PubMed] [Google Scholar]
- Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, et al. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20:2406–2411. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology. Animal Behavior Processes. 2010;36:313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment. Links to hippocampal dysfunction and obesity. Physiology and Behavior. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Walls EK, Davidson TL, Kanoski SE, Walls EK. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28:988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. Journal of Alzheimer’s Disease. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J, Dimitropoulos A. Influence of feeding state on neurofunctional differences between individuals who are obese and normal weight. A meta-analysis of neuroimaging studies. Appetite. 2014;75:103–109. doi: 10.1016/j.appet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Baym CL, Monti JM, Raine LB, Drollette ES, Scudder MR, et al. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. Journal of Pediatrics. 2014;166(2):302–308. doi: 10.1016/j.jpeds.2014.10.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The modern obesity epidemic, ancestral hunter-gatherers, and the sensory/reward control of food intake. The American Psychologist. 2013;68:88–96. doi: 10.1037/a0030684. [DOI] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity. 2014;38:494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity. Trends, risk factors and policy implications. Nature Reviews Endocrinology. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural Brain Research. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain. Implications for fear conditioning, extinction and psychopathology. Nature Reviews. Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure AC, Tanski SE, Gilbert-Diamond D, Adachi-Mejia AM, Li ZG, Li ZZ, et al. Receptivity to television fast-food restaurant marketing and obesity among U.S. youth. American Journal of Preventive Medicine. 2013;45:560–568. doi: 10.1016/j.amepre.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation. A pathway to cognitive impairment. Brain, Behavior, and Immunity. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Baker AG. A role for CS-US contingency in Pavlovian conditioning. Journal of Experimental Psychology. Animal Behavior Processes. 2004;30:229–239. doi: 10.1037/0097-7403.30.3.229. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. The FASEB Journal. 2009;23:4353–4360. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass Index among US children and adolescents, 1999–2010. Jama-Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Assibey G, Dick S, Macdiarmid J, Semple S, Reilly JJ, Ellaway A, et al. The influence of the food environment on overweight and obesity in young children. A systematic review. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KC, Jones EK, Anday EK. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 2014;306:R527–R537. doi: 10.1152/ajpregu.00319.2013. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning. Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Saelens BE, Sallis JF, Frank LD, Couch SC, Zhou C, Colburn T, et al. Obesogenic neighborhood environments, child and parent obesity. The neighborhood impact on kids study. American Journal of Preventive Medicine. 2012;42:E57–E64. doi: 10.1016/j.amepre.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DH, Leonard G, Perron M, Richer L, Syme C, Veillette S, et al. Visceral fat is associated with lower executive functioning in adolescents. International Journal of Obesity. 2013;37:1336–1343. doi: 10.1038/ijo.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neuroscience and Biobehavioral Reviews. 2013;37:2489–2503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Smith DM, Bulkin DA. The form and function of hippocampal context representations. Neuroscience and Biobehavioral Reviews. 2014;40:52–61. doi: 10.1016/j.neubiorev.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan. Implications for novel approaches to prevention and treatment. Obesity Reviews. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1 beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain, Behavior, and Immunity. 2014;42:22–32. doi: 10.1016/j.bbi.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. International Journal of Obesity. 2013;37:889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Animal Learning&Behavior. 1995;23:123–143. [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. Obesity 1. The global obesity pandemic. Shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Wasserman JA, Suminski R, Xi J, Mayfield C, Glaros A, Magie R. A multi-level analysis showing associations between school neighborhood and child body mass index. International Journal of Obesity. 2014;38:912–918. doi: 10.1038/ijo.2014.64. [DOI] [PubMed] [Google Scholar]
- Whitmer RA. The epidemiology of adiposity and dementia. Current Alzheimer Research. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- Williams J, Scarborough P, Matthews A, Cowburn G, Foster C, Roberts N, et al. A systematic review of the influence of the retail food environment around schools on obesity-related outcomes. Obesity Reviews. 2014;15:359–374. doi: 10.1111/obr.12142. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Current Biology. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world. Reward-driven brain overrides repletion signals. International Journal of Obesity. 2009;33(Suppl. 2):S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited references
- Berthoud HR. The neurobiology of food intake in an obesogenic environment. The Proceedings of the Nutrition Society. 2012;71:478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]