Abstract
Small-molecule probes can illuminate biological processes and aid in the assessment of emerging therapeutic targets by perturbing biological systems in a manner distinct from other experimental approaches. Despite the tremendous promise of chemical tools for investigating biology and disease, small-molecule probes were unavailable for most targets and pathways as recently as a decade ago. In 2005, the U.S. National Institutes of Health launched the decade-long Molecular Libraries Program with the intent of innovating in and broadening access to small-molecule science. This Perspective describes how novel small-molecule probes identified through the program are enabling the exploration of biological pathways and therapeutic hypotheses not otherwise testable. These experiences illustrate how small-molecule probes can help bridge the chasm between biological research and the development of medicines, but also highlight the need to innovate the science of therapeutic discovery.
Introduction
Despite remarkable advances in chemistry and biology, small molecule drug discovery remains a slow, costly, and low-yielding activity. In fact, productivity in drug discovery has fallen in recent years. It is estimated that only about 1 in 10 drug candidates entering the clinic are eventually approved – despite increasing investment (Hay et al., 2014). A prime hurdle is our inability to predict the physiologic consequences of modulating candidate drug targets in humans. Currently, lack of sufficient efficacy is the cause of clinical candidates failing in about 50% of Phase-II trials (Arrowsmith, 2011a) and 66% of Phase-III trials (Arrowsmith, 2011b). This high rate of attrition results from an inadequate understanding of how diseases are caused and maintained in human populations.
Expectations regarding the therapeutic relevance of a target are often based on inferences from experiments that alter nucleic acids encoding the putative target (e.g., deleting a DNA sequence; degrading or overexpressing an RNA sequence). Yet most small-molecule drugs alter the functions of proteins, and the correlation between perturbing nucleic acids and their encoded proteins is imperfect. Modulating the functions of candidate drug targets with small molecules would provide insights with far greater relevance to drug discovery. However, the current absence of effective small-molecule probes or ‘tool compounds’ for the vast majority of proteins limits a systematic application of this approach to target validation.
Advances in understanding human biology, particularly ‘experiments of nature’ revealed with increasing frequency by human genetics, are now shining a light on many complex diseases and pointing to targets with a much higher likelihood of providing a therapeutic benefit safely than those derived from model systems (Plenge et al., 2013). And the increasing availability of patient-derived cells and advances in precise genome-editing methods are providing more effective model systems for investigating the functional impact of observed human genetic variation.
But these insights also create a demand for small-molecule probes. The therapeutic implications of these genetic alterations are intriguing, but not certain. Not only do they raise the ‘gene vs. protein’ issue above – they often reflect heritable modulations from birth so the effect of targeting an implicated protein by a therapeutic in an ongoing disease is uncertain. Also, the therapeutic strategies suggested by these studies frequently point to targets that are inaccessible to protein-based drugs and for which small-molecule modulators are currently nonexistent – targets that fall outside traditional drug classes and have been labeled 'undruggable' by some within the pharmaceutical and academic communities. There is an urgent need to innovate chemistry and chemical biology in ways that enable small-molecule modulation of the challenging targets at the heart of disease.
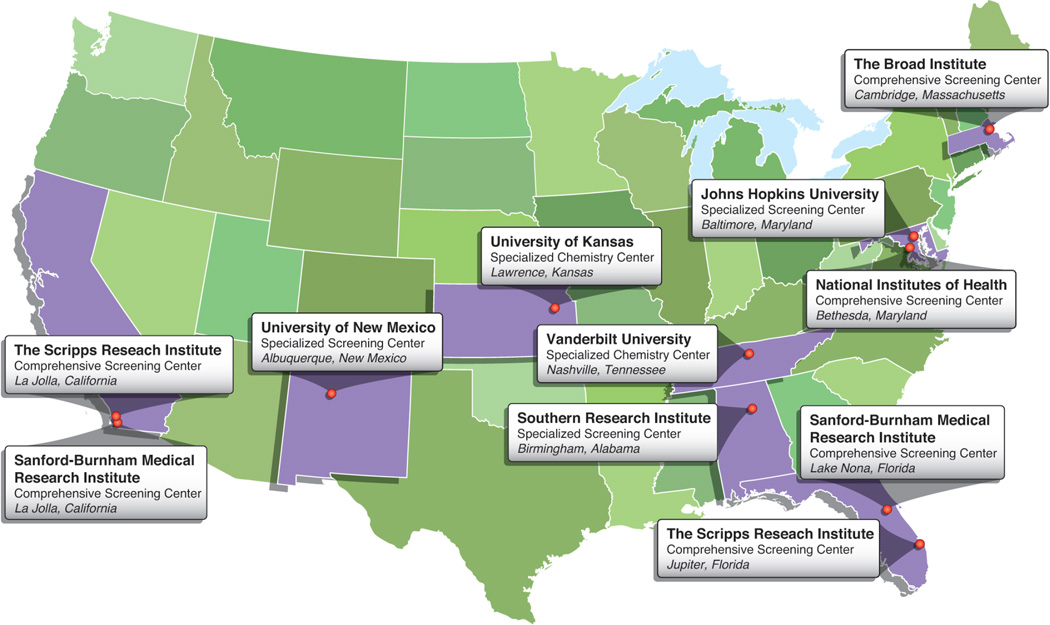
In 2005, in an effort to bridge the chasm between basic biological research and the medicines that should in principle derive from insights into disease, the U.S. National Institutes of Health (NIH) initiated the decade-long Molecular Libraries Program (MLP) (Austin et al., 2004). At that time, small-molecule discovery techniques, including high-throughput screening (HTS) and medicinal chemistry expertise, existed predominantly within pharmaceutical companies. Following a three-year pilot phase, the MLP launched the Molecular Libraries Probe Production Centers Network (MLPCN), which comprised Comprehensive Screening Centers, Specialized Screening Centers and Specialized Chemistry Centers (Fig. 1). These centers were among the earliest efforts to bring systematic small-molecule screening into academic settings.
Fig. 1. Centers comprising the MLPCN Network.
The MLP was designed as an experiment in integrating big science and individual investigator-led science. Individual investigators submitted funding applications to the NIH for assay development or screening projects. For screening projects that were selected through peer review, MLP Centers conducted high-throughput screens using an MLP small-molecule library that grew over the course of the program to a final size of about 390,000 compounds. Novel compounds emerging from the academic synthetic chemistry community, for instance small, chiral compounds having novel skeletons derived from diversity-oriented synthesis (DOS) and other compounds derived from the NIH-funded Chemical Methodologies and Library Development (CMLD) program, made up about 5% of the compound library and yielded small-molecule probes, including some highlighted below, that would not have been discovered otherwise. Following on the screens, the MLP pursued chemical optimization of hits with the aim of identifying small-molecule probes, including ones targeting proteins viewed as challenging, with sufficient potency and selectivity to provide ‘high-quality’ tool compounds (Frye, 2010; Oprea et al., 2009; Workman and Collins, 2010). These probes were envisioned as a first step towards the testing of novel biological and therapeutic hypotheses.
With the conclusion of the program, we can now look back at the progress achieved over the decade of the MLP and forward at how to benefit from the lessons learned. We summarize advances in discovering biologically active small molecules and illustrate how these advances are yielding a rich armamentarium of novel small-molecule probes and, perhaps most notably, experimental paths to therapeutics discovery. The series of 'vignettes' described here provide examples of methodological development and small-molecule probe discovery that is enabling the testing of specific therapeutic hypotheses. These examples highlight the importance of small-molecule probes as critical tools for accelerating translational research, but also emphasize the tremendous hurdles that remain to be cleared if we are to capitalize fully on the rapid pace of biological advances to improve human health. If the lack of productivity of therapeutics discovery is to be reversed, transformative innovation will be needed to push back barriers at every stage of small-molecule therapeutics discovery.
Bridging the chasm
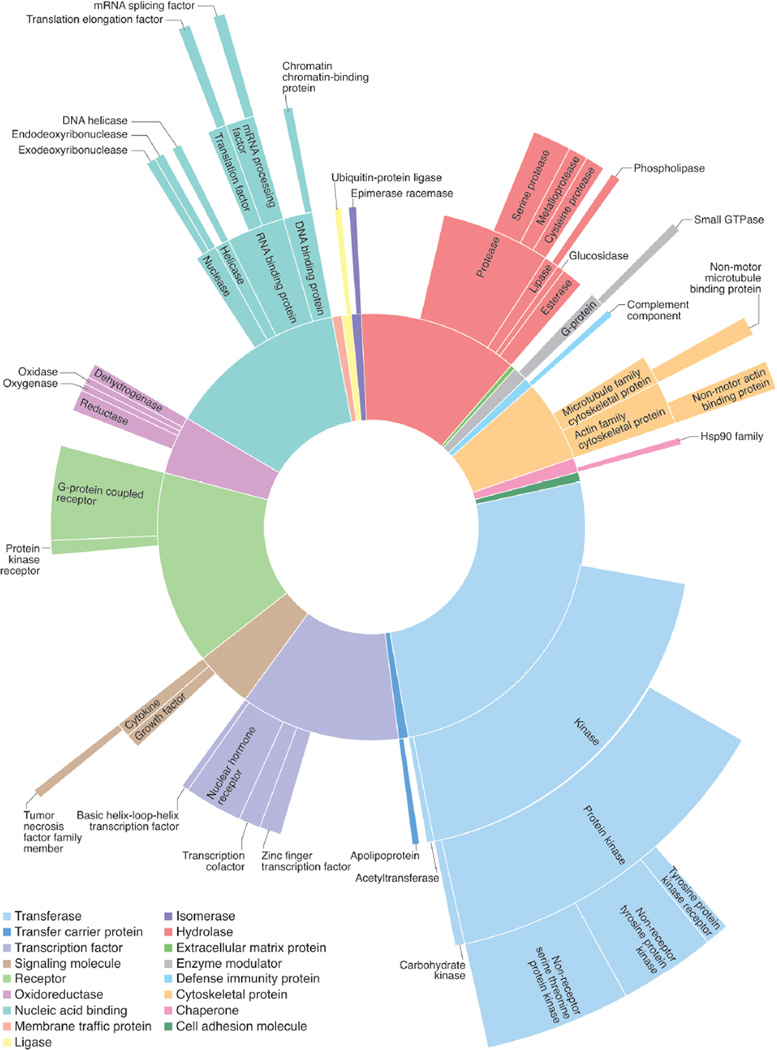
The most visible output of the MLP is a set of 375 small-molecule probes for exploring biology. MLP probes cover a diverse spectrum of target classes, including well-investigated classes such as kinases and G protein-coupled receptors (GPCRs) and less frequently investigated classes such as GTPases, proteases and RNA-binding proteins (Fig. 2). This broad coverage of target space highlights the intentional focus of the MLP Network on relatively under-explored proteins. Many of these compounds were ‘first-in-class’ probes that provided initial evidence for the chemical tractability of a previously recalcitrant target or that modulated a target by a distinct mechanism from previous small molecules (Supplementary Table 1).
Fig. 2. Spectrum of targets modulated by MLP probes.
Target classes modulated by MLP probes are visualized based on HTS data and user-defined thresholds. Arc lengths represent the number of distinct targets within a target class that are modulated by this collection of probes. This graph was generated using the BioAssay Research Database (BARD; bard.nih.gov), which was generated by the MLP to enable users to mine and visualize small-molecule activity data. The graph depicts the bioactivity of about 65% of the total number of MLP probes across 415 unique assays associated with 460 targets, which were categorized using the Panther database into 110 protein categories. The breadth of target classes highlights the diverse biology and target space explored by the MLP.
Perhaps the most exciting outcome of the MLP was the impact of probe-discovery efforts in highlighting paths for therapeutics discovery. Here, we describe a number of vignettes that illustrate the breadth of the translational impact of small-molecule probes identified through MLP research. These vignettes highlight advances in small-molecule science, as well as insights gained through the use of small-molecule probes in diverse disease contexts. Many of these and other MLP projects have directly catalyzed efforts to translate emerging biomedical insights (Table 1).
Table 1.
Translational trajectory for MLP probes.
| Targets | MLP probes | Post-MLP trajectory |
|---|---|---|
| Serine hydrolases | ML081, ML174, ML211, ML225, ML226, ML256, ML257, ML294, ML295, ML296 | Screening and assay platforms, serine hydrolase-directed small-molecule libraries and serine hydrolase inhibitors, resulting in part from MLP projects, have been licensed to Abide Therapeutics, which is pursuing the further identification, preclinical, and clinical development of serine hydrolase inhibitors for a variety of indications, in particular neurological, immunological and metabolic diseases. |
| P. falciparum, T. cruzi | ML238, ML341 | The identification of the MLP malaria probe led to a Gates Foundation-funded Chemical Diversity Initiative that has explored the use of the DOS collections more broadly in infectious disease and has yielded promising compounds that target the etiological agents of malaria tuberculosis via novel mechanisms. In work supported by the Global Health Innovation Technology (GHIT) fund and in collaboration with Eisai, the MLP anti-trypanosomal probe is being optimized to enable in vivo proof of concept studies and a DOS-based antimalarial agent is being optimized towards IND-enabling studies. |
| S1P1 agonists | ML007 | Receptos licensed intellectual property surrounding S1P1 agonists including the racemate of its clinical candidate RPC1063, which is currently in two Phase- III studies in relapsing multiple sclerosis and about to enter Phase-III testing in ulcerative colitis and Phase-II testing in Crohn’s disease. |
| M4 mAChR | ML108, ML253 | AstraZeneca has licensed intellectual property associated with the M4 PAMs and the company is pursuing preclinical development of the compounds as a potential treatment for the neuropsychiatric symptoms associated with AD and schizophrenia. |
| GCase | ML198 | This probe and related analogs have been licensed to Lysosomal Therapeutics Inc., which is pursuing preclinical development of these non-inhibitory GCase chaperones. |
| Integrin αIIbβ3 receptor antagonist | ML165 | Rockefeller Medical School and NCATS are pursuing an IND based on a derivative of ML165 indicated for pre-hospital therapy of patients with ST segment elevated myocardial infarction (STEMI). |
| P97 AAA ATPase inhibitor | ML240 | Cleave BioSciences licensed intellectual property surrounding ML240. A derivative of this probe, CB-5083, is currently in two Phase-I studies, one in relapsed and refractory multiple myeloma and the other in solid tumors refractory to the standard-of-care. |
Targeting poorly characterized enzymes
Small-molecule probes can aid efforts to annotate the function of poorly characterized enzymes, including those that human genetic studies have implicated in disease. However, it is difficult to establish a typical biochemical high-throughput screening assay to identify modulators of an enzyme if the substrate is not known. Activity-based protein profiling (ABPP) makes use of reactive small-molecule ligands to label covalently the active sites of members of large enzyme families, including those that are uncharacterized (Nomura et al., 2010). In this approach, broadly reactive ABPP probes can be used in a competition experiment to identify small molecules that selectively reduce labeling of a desired enzyme target. Despite the potential, this substrate-free screening approach has not been widely used because the current competitive ABPP methods relies on SDS-PAGE or mass spectrometry assays and can only be used to evaluate, at most, a few hundred compounds.
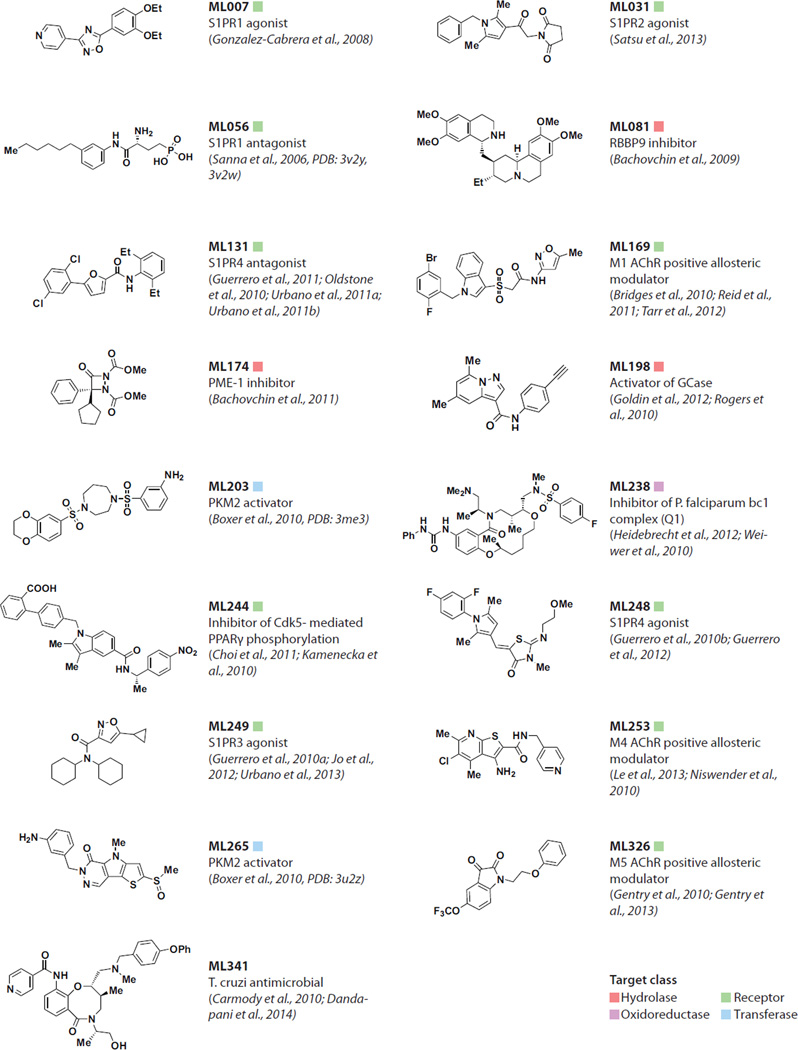
The MLP undertook a project to develop a high-throughput fluorescence polarization technology for ABPP-based screening (fluopol-ABPP) (Bachovchin et al., 2009). A small fluorophore in solution rotates rapidly and emits a low fluorescence polarization signal, whereas a fluorophore bound to a protein rotates more slowly and emits a higher signal. Based on this known property, an ABPP assay was developed in which fluorescent ABPP probe labeling of an enzyme target generates a signal that is then reduced when a small molecule ‘hit’ effectively competes for binding. This approach proved generally applicable to high-throughput screening in multi-well format. For instance, screening 20,000 small molecules in 384-well plates led to the identification of multiple small molecules that blocked serine fluorophosphonate (FP)-rhodamine labeling of the serine hydrolase retinoblastoma-binding protein-9 (RBBP9), a cancer-associated enzyme. Secondary chemoproteomic assays in mouse brain fractions and human cell lysates subsequently identified the natural product emetine (ML081; Fig. 3) as the first RBBP9 inhibitor that is selective for this serine hydrolase family member (Bachovchin et al., 2009).
Fig. 3. MLP probes highlighted in vignettes.
Small molecule inhibitors of protein phosphatase methylesterase-1 PME-1, a regulator of protein phosphatase 2A (PP2A) that has been linked to cancer and neurodegeneration, had not previously been identified, in part due to the challenges of designing an in vitro biochemical assay suitable for monitoring the carboxymethylation state of PP2A. A fluopol-ABPP screen yielded initial hits, from which four novel aza-β-lactams were shown in follow-up assays to selectively inhibit PME-1 in human cells. The most potent inhibitor, ML174 (ABL127; Fig. 3), inhibited PME-1 with a low nanomolar IC50 and had no activity against more than 50 other serine hydrolases. In mice, ML174 selectively inactivated PME-1 and increased the levels of PP2A carboxymethylation in the brain (Bachovchin et al., 2011). Strikingly, the aza-β-lactam hits are examples of innovations resulting from diversity synthesis performed by an academic chemistry laboratory – such compounds are distinct from conventional screening compounds available from vendors. The aza-β-lactam scaffold has subsequently proven to be a versatile chemotype for developing selective serine hydrolase inhibitors (Zuhl et al., 2012).
The MLP has also developed a modified ABPP method that can be used to read out cellular and in vivo target engagement, an often-missing link in the preclinical development of drug leads (Simon et al., 2013). Using fluopol-ABPP, the first selective inhibitors of the serine hydrolases lysophospholipase 1 (LYPLA1) and lysophospholipase 2 (LYPLA2) were identified and found to act reversibly. For reversible inhibitors that lack known biomarkers such as endogenous substrates or products, demonstrating target engagement is challenging. To overcome this limitation, new ABPP probes were designed that reacted with serine hydrolases over an observable time window. In human cells and in mice, these moderately reactive ABPP probes could be used to accurately measure the target engagement and selectivity of the LYPLA1 and LYPA2 inhibitors. For instance, dosing mice with the inhibitors blocked more than 90% of LYPLA1 and LYPA2 activity in lung, heart and kidney tissue, whereas the activity of the enzymes was only reduced by about 50% in the brain and no detectable enzyme inhibition was seen in the liver (Adibekian et al., 2012).
More generally, fluopol-ABPP has proven useful for enzyme classes beyond serine hydrolases and has been used for screening glutathione S-transferases (Tsuboi et al., 2011), arginine methyltransferases (Dillon et al., 2012) and protein arginine deiminases (Knuckley et al., 2010).
Toward novel mechanisms of action compounds through multiplexed bioactivity data
Identifying small-molecule probes that modulate challenging targets often requires accessing new chemical starting points. Previous efforts to address this gap have typically focused on increasing diversity from a chemical structure perspective, a metric known to predict imperfectly the identity of compounds with new bioactivities. The MLP worked to develop high-throughput and inexpensive methods that would enable multiplexed cellular bioactivity measurements to guide directly the building of compound collections whose members have novel mechanisms of action.
Gene-expression studies have demonstrated the impact of unbiased, high-dimensional profiling data in defining disease states and also in inferring compound mechanism of action (Hughes et al., 2000; Lamb et al., 2006; Weinstein et al., 1997). High-content imaging could provide a complementary measurement for capturing complex disease phenotypes in unbiased, high-throughput formats. However, current high-content screening assays have either focused on a specific cellular process of interest or a limited set of cellular readouts, often based on labeled proteins or qPCR of target transcripts, or have been limited in throughput (Loo et al., 2007; Weinstein et al., 1997; Young et al., 2008). The MLP therefore focused on a ‘cell-painting assay’ that enables a more unbiased approach for characterizing alterations induced by disease states or small-molecule perturbagens (Gustafsdottir et al., 2013). Using six dyes measured across five channels, 824 distinct cellular morphological features could be quantitatively imaged. In a pilot assay, small molecules known to have similar bioactivities were accurately clustered together based on similarities between image-based profiles. As cell morphology is impacted by many factors ranging from genetic and epigenetic to metabolic and environmental, we anticipate that the low cost, but high-dimensional imaging assay will facilitate identification of unexpected cellular alterations associated with small-molecule perturbations and disease.
While methods such as gene-expression profiling and the new image-based profiling assay contain rich information describing biological responses to small molecules, few approaches are available to use these data to understand structure–activity relationships (SAR). To enable more effective multi-parameter optimization of probes and therapeutic leads, the MLP developed a computational method to generate SAR information automatically from high-dimensional small-molecule profiling data (Wawer et al., 2014a). \Gene expression profiling data and cell-painting data were collected for about 20,000 DOS molecules and just over 2000 bioactive compounds. Association-rule mining was then used to define ‘rules’ of varying strengths that connected chemical attributes (e.g., contiguous substructures within compounds, shared synthetic histories) to biological effect patterns in the gene expression and imaging data. In one application of the methodology, identification of a DOS-based molecule that co-clustered with structurally unrelated microtubule destabilizers provided an opportunity for ‘scaffold hopping’ based on the signatures derived from this high-content information.
Rooted in these methodological advances, the question became whether multiplexed cellular small-molecule activity data could be used to create a screening library with maximal bioactivity (performance) diversity. There is general consensus that a small-molecule library that perturbs as many biological targets and induces as many distinct cellular phenotypes as possible would be most effective for cell-based screening. However, the data needed to create a priori such a screening collection were not previously available. As a consequence, assembling diverse screening collections has thus far focused primarily on the computed diversity of chemical structures. In one notable recent paper, a retrospective analysis of high-throughput screening data was used to assemble a library on the basis of biological activity (Petrone et al., 2012); however, this method, which is based on analysis of historical data, is not extensible to new candidate library members. The MLP measured gene-expression and image-based profiles for over 12,000 bioactive compounds and over 17,000 diversity-oriented synthesis (DOS)-based molecules for which HTS results from up to 178 cell-based screens were available (Wawer et al., 2014b). We asked if the profiling data could select for compounds with diverse bioactivities, as judged by comparing patterns of activity in cells or against target proteins. Compounds selected based on varied activity in the imaging and gene expression assay profiles significantly outperformed compounds selected randomly or based on chemical diversity when their performance diversity in historical HTS assays was compared. In addition, we discovered thousands of compounds, many of which formed scaffold-based clusters, that induced cell morphology and gene expression patterns not seen within the 12,000 bioactives, likely hinting at novel mechanisms of action. These methods and others that yield multiplexed data reporting complementary measures of compound activity offer a powerful route for prioritizing the synthesis and testing of small molecules more likely to modulate novel therapeutic targets.
New antiinfectives through chemical diversity
Many neglected infectious diseases are endemic to developing nations and the socioeconomic impact of these diseases is devastating. The impact of drug resistance and the lack of effective drugs are well exemplified by malaria and Chagas disease, respectively. In the case of malaria, there are several drugs available for treatment, but rapid emergence of resistant parasites poses a serious problem (Fidock, 2013). The currently available treatment options for Chagas disease are very limited and are efficacious only during the acute phase of the disease, not the chronic phase. Moreover, the severe adverse effects of the current anti-Chagas drugs lead to poor patient compliance (Bern, 2011). The development of safe new drugs for treating malaria and Chagas and other infectious diseases is urgently needed.
Identifying antimalarials and anti-trypanosomal agents with novel chemical structures and mechanisms of action may facilitate the development of therapies that are effective against parasites resistant to current drugs. DOS pathways have proven highly effective at rapidly generating novel, stereochemically rich compounds, including ones containing 8- to 14-membered rings (Gerard et al., 2011; Lowe et al., 2012; Marcaurelle et al., 2010) and the MLP screening library contained more than 5,000 DOS compounds in this vein. These compounds, in conjunction with certain MLP probe-discovery projects that used a larger collection of 100,000 DOS compounds, have enabled the identification of small-molecule probes that act against these infectious agents through new mechanisms.
A cell-based malaria viability screen using an 8,000 DOS compounds led to the identification of a highly active hit with four stereogenic elements. Of the 15 other possible stereoisomers of the molecule that were present in the screening collection, only one had any detectable activity. Subsequent optimization, guided by these stereochemical insights, led to the identification of ML238 (Fig. 3), which is potent against both the chloroquine-resistant Dd2 strain (IC50 = 0.4 nM) and the wild-type 3d7 strain (IC50 = 0.6 nM) of Plasmodium falciparum (Heidebrecht et al., 2012; Weiwer et al., 2010). In addition, ML238 has good water solubility and does not lyse red blood cells (EC50 > 40,000 nM), which is important because it suggests a lack of broad cytotoxicity. To determine the compound’s mechanism of action, MLP generated ML238-resistant parasites and conducted whole-genome sequencing. These studies revealed that ML238 acts at the quinone reductase site of the bc1 complex (Q1) (Lukens et al., 2014), which is on the opposite side of the cell membrane from the quinol oxidase site targeted by the antimalarial drug atovaquone. ML238 and atovaquone lacked cross-resistance and were synergistic in combination, highlighting the quinone reductase site as a antimalarial therapeutic target that may overcome atovaquone resistance (Lukens et al., 2014).
Similarly, a cell-based screen for molecules with selective lethality against Trypanosoma cruzi, the etiological agent of Chagas disease, identified ML341 (Fig. 3), a DOS-derived smallmolecule probe (Carmody et al., 2010; Dandapani et al., 2014). ML341 is potent against several strains of T. cruzi including the clinical cardiotropic isolate CA-1 strain, clone 72 (CA-I/72) (IC50 = 1 nM). Next, ML341 was evaluated for in vitro cidal activity against a CA-I/72 strain in bovine skeletal muscle (BESM) cells. Emergence of the trypomastigote form of T. cruzi was monitored daily in infected BESM cells treated with ML341 or benznidazole, the current first line drug. In this stringent assay, ML341 was cidal at 40 nM, whereas benznidazole was cidal only at 6.6 uM.
In addition to this potency, ML341 was stable to mouse liver microsomes, which will facilitate the use of this probe in in vivo studies. Out of the 8 possible stereoisomers of ML341, only 2 stereoisomers were stable to mouse liver microsomes, a finding that helped guide further medicinal chemistry optimization. As seen in these two examples, having a comprehensive set of stereoisomers in the screening collection was crucial for evaluating rapidly stereochemistry-based SAR for potency and ancillary pharmacology.
Sphingosine-1-phosphate receptor modulators
Characterizing the mechanisms of action behind the anti-inflammatory properties of FTY720, a derivative of the fungal metabolite-derived serine palmityoltransferase inhibitor myriocin (Chen et al., 1999), led to the discovery that FTY720 modulates sphingosine-1-phosphate (S1P) receptors to regulate lymphocyte tracking (Mandala et al., 2002). This finding then paved the way for the subsequent clinical development of FTY720 (fingolimod), a sphingosine analog that modulates four of the five human S1P receptors, for the treatment of multiple sclerosis (Rosen et al., 2008). S1P receptors are expressed predominantly in immune, neural, endothelial and smooth muscles cells, and are believed to play roles that include regulating immune cell trafficking, endothelial barrier integrity and blood pressure, suggesting the potential to modulate specific receptor subtypes to treat a variety of diseases (Rosen et al., 2013).
To better validate individual S1P receptors as therapeutic targets, the MLP set out to identify small-molecule probes that selectively agonized or antagonized each receptor. A selective S1P1 antagonist (ML056, Fig. 3) with in vivo activity was developed through rational synthesis and used to explore the impact of perturbing this receptor on lymphocyte movement and function, providing evidence that the therapeutic effects of FTY720 are primarily mediated through this receptor (Sanna et al., 2006). The concomitant reduction in lymphocytes by the antagonist, resulting in endothelial leakage and severe pulmonary edema, showed that agonist therapy was the only tolerable choice for autoimmune disease (Rosen et al., 2013).
The poor water solubility of ML056 imparted by a hydrophobic strong zwitterion could be overcome both in vitro and in vivo by formulation as the reversible carbonate adduct. This derivatization was essential for the maintenance of complete receptor occupancy of stable S1PR1, which enabled the first successful crystal structure of a lipid-sensing GPCR (Hanson et al., 2012). When combined with biochemical studies, the S1P1 structure also provided insights into a new agonist binding mode used by compounds that include the second-generation S1P1 agonist ML007 (Fig. 3). This series, identified in an allosteric agonist primary screen, was determined in a secondary assay to be subject to non-competitive inhibition by ML056, an orthosteric agonist, occupying the same site as the receptor's endogenous ligand. These studies defined a binding pocket distinct from S1P that affords greater receptor selectivity and potentially a better pharmacokinetic profile and therapeutic index (Gonzalez-Cabrera et al., 2008).
Agonizing the S1P1 receptor to modulate immune cell trafficking may provide a therapeutic approach for diseases beyond multiple sclerosis. Clinical outcomes following influenza infection are determined by properties instrinsic to the virus strain and also differences in the host immune response. In particular, high cytokine levels and immune cell recruitment correlate with a poor outcome. In mice infected with a 2009 H1N1 pandemic influenza viral isolate, CYM-5442, an optimized derivative of ML007 with in vivo activity, inhibited the secretion of cytokines, reduced immune cell infiltration into the lung, and improved survival – while having no impact on viral titers (Teijaro et al., 2011; Walsh et al., 2011).
The MLP has also identified agonists of the S1P2 (ML031; Fig. 3) (Satsu et al., 2013), S1P3 (ML249; Fig. 3) (Guerrero et al., 2010a; Jo et al., 2012; Urbano et al., 2013) and S1P4 (ML248; Fig. 3) (Guerrero et al., 2010b; Guerrero et al., 2012) receptors, as well as an S1P4 receptor antagonist (ML131; Fig. 3) (Guerrero et al., 2011; Oldstone et al., 2010; Urbano et al., 2011a; Urbano et al., 2011b), providing multiple first-in-class small-molecule probes for further eludidating the functions of this class of GPCRs. ML249, an allosteric agonist for the S1P receptor has been used to define orthosteric, allosteric and biopic space within the S1P3 receptor binding pocket (Jo et al. 2012).
Antagonists of PPARγ phosphorylation
The thiazolidinedione (TZD) class of antidiabetic drugs comprises PPARγ agonists that increase expression of the insulin-sensitizing hormone adiponectin and decrease expression of insulin resistance-inducing hormones – in part contributing to their potent insulin-sensitizing efficacy (Yu et al., 2002). Although drugs in this class are marketed to treat type-2 diabetes, their clinical utility has been limited by serious side effects seen in a minority of patients, including fluid retention (Staels, 2005), congestive heart failure (Nesto et al., 2003), and decreased bone density (Aubert et al., 2010).
The antidiabetic efficacy of these drugs was long thought to result from receptor agonism. Strikingly though, as additional PPARγ modulators were developed, drug efficacy correlated more tightly with ligand binding affinity than with agonist activity, prompting a closer examination of the mechanism through which PPARγ ligands improve insulin sensitization. PPARγ ligands that were potent insulin sensitizers were found to be highly effective at reducing obesity-induced phosphorylation of PPARγ at S273 in mice, despite varying levels of receptor agonism. Also, the therapeutic efficacy of the TZD rosiglitazone correlated with how effectively the drug reduced PPARγ phosphorylation at S273 in individual patients. Expression profiling in adipocytes revealed that phosphorylation of PPARγ at S273 leads to repression of a subset of target genes associated with obesity and insulin sensitivity (Choi et al., 2010). Recent studies have further shown that docking of the transcriptional repressor Thrap3 to phospho-S273 on PPARγ regulates the expression of diabetes-associated genes (Choi et al., 2014). Collectively these results suggest that blocking phosphorylation of PPARγ at S273, by normalizing expression of insulin sensitizing genes, is the dominant therapeutic mechanism of this drug class. However, it was not clear if small molecules could be identified that sufficiently separated the two activities of TZDs and, if found, what the efficacy and safety profile of such compounds would be.
Starting from known potent partial agonists of PPARγ, the MLP synthesized and tested analogs to look for molecules that bind the receptor tightly and inhibit phosphorylation of S273, but are devoid of receptor agonist activity. Identifying small molecules that block a post-translational modification of a receptor is a challenging objective that was aided by a toolbox of methods developed by the MLP for characterizing nuclear receptor structure-function (Marciano et al., 2014a). These efforts led to the non-agonist (antagonist) probe ML244 (SR1664; Fig. 3) (Choi et al., 2011; Kamenecka et al., 2010). Hydrogen-deuterium exchange (HDX), which has proven particularly effective for characterizing ligand-induced conformational dynamics of nuclear receptors (Marciano et al., 2014b), revealed that ML244 directly binds PPARγ, does not stabilize the Af2 surface of the receptor that is known to drive classical agonism, yet perturbs the surface of the ligand binding domain to induce a conformation that is not susceptible to phosphorylation of S273. Importantly, because ML244 binds PPARγ rather than directly inhibiting Cdk5, a kinase that mediates S273 phosphorylation of PPARγ, or any other kinase, the probe does not interfere with phosphorylation of other substrates in the proteome.
In mice fed a high-fat diet, ML244 reduced Cdk5-mediated phosphorylation of S273 on PPARγ, normalized serum glucose levels, and improved insulin sensitivity. In the leptin-deficient ob/ob mouse model of severe obesity, ML244 reduced S273 PPARγ phosphorylation and improved glucose tolerance as effectively as rosiglitazone. However unlike rosiglitazone, ML244 did not cause weight gain or fluid retention in these mice or reduce bone-cell mineralization in culture. Ongoing efforts are now focusing on increasing the drug-like properties of the ML244 scaffold and testing the impact of the resulting compounds following once-a-day oral administration in obese mice.
Allosteric modulators of muscarinic receptors
About 30% of currently marketed drugs modulate GPCRs. The vast majority of GPCR-targeted therapeutics act at an orthosteric site, These direct-acting ligands can have a number of potential drawbacks, including inducing toxicity and receptor desensitization or internalization. In addition, identifying selective orthosteric ligands has often been difficult (Conn et al., 2009a). Muscarinic acetylcholine receptors (mAChRs) have attracted significant interest as therapeutic targets in multiple neurological disorders. In Phase-II and Phase-III clinical trials, pan-orthosteric agonists of the five human mAchRs led to cognitive improvements and had anti-psychotic effects in Alzheimer's disease (AD) and schizophrenia. However, adverse events, which were believed to result from activation of the M2 mAChR and M3 mAChR in the periphery, prevented further clinical development of the compounds. Despite significant effort, selectively targeting the acetylcholine binding site of the mAchRs did not prove possible (Conn et al., 2009b) due to the high evolutionary conservation across subtypes for the endogenous acetylcholine ligand.
In contrast, allosteric sites are less evolutionarily conserved across receptor subtypes and have afforded exciting opportunities to achieve true subtype selectivity, as well as diverse pharmacology by stabilizing unique, activated conformations of the receptors. Moreover, allosteric sites are generally lipophilic pockets, which could targeted by unique small-molecule chemotypes that are structurally distinct from endogenous ligands (Conn et al., 2009b; Wenthur et al., 2014). With this in mind, the MLP undertook screening efforts to identify selective positive allosteric modulators (PAMs) of mAChRs that could be used to dissect the individual contributions of these receptors to the therapeutic efficacy of pan-mAChR agonists. These efforts were aided by employing functional assays that enabled the identification of PAMs and negative allosteric modulators (NAMs) in a single kinetic calcium assay. While the HTS afforded hits, these compounds did not have the physiochemical properties and CNS penetration needed for in vivo target validation experiments. Achieving high-quality small-molecule probes required significant medicinal chemistry optimization of the initial screening hits, leading to the identification of an M1-selective PAM (ML169; Fig. 3) (Bridges et al., 2010; Reid et al., 2011; Tarr et al., 2012), an M4-selective PAM (ML253; Fig. 3) (Le et al., 2013; Niswender et al., 2010) and an M5-selective PAM (ML326; Fig. 3) (Gentry et al., 2010; Gentry et al., 2013). All of these probes had submicromolar EC50s, at least ten-fold selectivity over the other receptor subtypes and, importantly, were brain penetrant.
In a mouse model of AD, the M1-selective probe ML169 potentiated chemically-induced excitation in the striatum, which is believed to mediate anti-psychotic effects, and favored the production of soluble, rather than amyloid, APP (Tarr et al., 2012). In a mouse model of schizophrenia, M4-selective ML253 reversed amphetamine-induced hyperlocomotion, recapitulating the effects of known anti-psychotic agents (Le et al., 2013). Further experiments are now focusing on using these probes to explore the biology and potential therapeutic relevance of these receptors, including investigating the role of M5, about which little is currently known.
Small-molecule activators of a metabolic enzyme
Tumor growth is associated with metabolic adaptations that facilitate cell-autonomous nutrient uptake, an increased rate of aerobic glycolysis, and a shift towards more anabolic metabolism to support cancer cell growth and proliferation. Emerging mechanistic insights into how metabolic pathways are regulated in cancer are pointing towards potential new therapeutic targets; however, which targets represent true cancer dependencies versus nodes where metabolic plasticity will allow cancer cells to adapt and to escape metabolism-targeting therapeutics remains an open question. Small-molecule probes are urgently needed to study how metabolism is altered to support tumor growth and to identify cancer metabolism targets that could be exploited therapeutically.
Cancer cells in general adopt a less differentiated cell state and, analogous to less differentiated non-cancerous cells (e.g., adult stem cells), tend to rely on aerobic glycolysis. Pyruvate kinase, which catalyzes the final step in glycolysis, is derived from two gene products that each possesses two alternate splice isoforms. Cancer cells often express the M2 isozyme of pyruvate kinase (PKM2), which promotes aerobic glycolysis. In contrast, the M1 isozyme (PKM1) promotes glucose oxidation and is expressed in tissues with high ATP requirements such as muscle, heart and brain. The level of regulation of these isozymes is highly divergent. PKM1 has high constitutive activity, whereas PKM2 can adopt an active or inactive conformation in response to signaling inputs. PKM1 expression increases oxidative glucose metabolism and can suppress both cell proliferation and tumor growth (Christofk et al., 2008; Israelsen et al., 2013; Lunt et al., 2015). However, the extent to which ectopic PKM1 expression recapitulates smallmolecule activation of PKM2 was unknown, leaving uncertain whether small-molecule modulation of PKM2 activity would lead to changes in tumor growth or viability.
More rigorously testing the potential of this therapeutic approach and better understanding the role of pyruvate kinase activity in cancer biology required small-molecule activators of PKM2, yet activating protein function is considered a tall order for small molecules. To address this gap, the MLP conducted a screen to identify molecules that increased PKM2 activity in a biochemical assay. Three different chemotypes were identified and confirmed to be bona fide activators of PKM2. Subsequent optimization of these hits resulted in the identification of ML203 (DASA-58; Fig. 3) and ML265 (TEPP-46; Fig. 3), the first two reported PKM2 activators (Boxer et al., 2010). Further characterization demonstrated that the probes increase activity by stabilizing formation of the PKM2 tetramer, the more active form of the enzyme. This tetramer stabilization is further enhanced in the presence of fructose-1,6-bisphosphate (FBP), an endogenous activator of PKM2. Structural studies revealed that the synthetic small-molecule activators occupy a binding site at a monomer-monomer interface distinct from the allosteric site bound by FBP (Anastasiou et al., 2012). Thus, these MLP probes enhance enzyme activity by stabilizing a protein-protein interaction – a mechanism of action generally viewed as challenging to accomplish (Thiel et al., 2012).
Next, the probes were used to explore the cancer contexts in which activating PKM2 might provide a therapeutic benefit. In cancer cell lines grown in culture, ML203 and ML265 reduced cellular proliferation and had an impact on metabolite levels distinct from the changes observed with ectopic PKM2 expression (Anastasiou et al., 2012). A change in serine metabolism was among the most dramatic changes observed, and PKM2 activation can synergize with serine deprivation to slow the growth of cancer cells (Kung et al., 2012). Pyruvate kinase activation can also impair the growth of xenograft tumors with oral dosing of ML265 causing increased tumor latency and reduced tumor size (Anastasiou et al., 2012). Although these results provide initial evidence that activating PKM2 is tumor suppressive, the effect was relatively modest and the molecules had the highest efficacy in slowing tumor initiation.
A critical next step will be determining if there are established tumor contexts that are particularly dependent on low PK activity or if there are combination treatments that confer particular sensitivity to PKM2 activation, for instance modulators of serine metabolism. Beyond its role in tumors, regulated PK activity is also found in non-cancerous tissues, including the liver and red blood cells. Thus, activation of PKM2 or another PK isoform using small molecules could be beneficial in both cancer and non-cancer disease states. To this end, PK activators are currently being explored as therapeutics for pyruvate kinase deficiency, an inborn error of metabolism that specifically affects the red blood cell isoform of PK (Zanella et al., 2007).
Stabilizing a folded lysosomal enzyme while retaining its activity
Gaucher's disease is a rare, autosomal disorder that results from a deficiency in the lysosomal enzyme glucocerebrosidase (GCase). Currently approved treatments for the disease include enzyme-replacement therapy, which is effective for some patients who have the most common form of the disease; however it is also expensive (Ortolano et al., 2014). When enzyme-replacement therapy is not an option, patients may be treated with a small molecule inhibitor of glucosylceramide synthetase, decreasing production of glucocerebrosides, the substrate of GCase that accumulates in disease. This therapy is associated with a number of side effects including weight loss, tremors and peripheral nerve damage (Larsen et al., 2012). Neither of these treatments is effective against neuropathic forms of the disease (Suzuki, 2013).
Many GCase mutants found in Gaucher’s patients are enzymatically active, but are improperly folded by endoplasmic reticulum (ER) chaperone proteins, causing ER accumulation and degradation before the protein reaches the lysosome (Orvisky et al., 2002). As a result, current drug-discovery efforts are focusing on identifying chemical chaperones that bind mutant GCase to increase the fraction of properly folded enzyme and promote greater trafficking to the lysosome. Chemical chaperones identified to date are transition-state mimetics that stabilize the enzyme, but also inhibit enzymatic activity (Suzuki, 2013). The most advanced chemical GCase chaperone, isofagomine, was recently tested in Phase-II clinical trials. Isofagomine increased GCase levels in the white blood cells of all patients treated, demonstrating its capacity to increase trafficking. However, the treatment only led to a clinical improvement in one of 18 patients who completed the study, probably due to isofagomine’s inhibition of GCase activity (Panicker et al., 2012).
Chemical chaperones that stabilize the enzyme without inhibiting activity may provide a therapeutic approach with improved efficacy. To identify such compounds, the MLP developed a high throughput dose response assay that used a spleen homogenate solution from a patient with Gaucher’s disease, thus creating an assay using mutant enzyme in a physiological environment. This assay enabled the identification of a pyrazolopyrimidine-based molecule that was optimized to the small-molecule probe ML198 (Fig. 3) (Goldin et al., 2012; Rogers et al., 2010). In three patient-derived dermal fibroblast lines with distinct genotypes, ML198 increased the activity and lysosomal localization of mutant GCase. Moreover, in macrophages from Gaucher's patients, ML198 reduced aberrant lipid accumulation and normalized phagocytic and efferocytosis function. The MLP probe also displayed good pharmacokinetics and brain penetration in mice (Patnaik et al., 2012).
Innovating small-molecule discovery
Human biology is providing new insights into therapeutic targets. For example, human genetics is revealing risk and protective variants of genes across a range of diseases. Biochemical investigations of variant proteins can provide a blueprint or recipe to follow that defines the activity that a drug should confer on the more common version of the protein. However, the activities suggested by these 'experiments of nature’ (Plenge et al., 2013) are challenging and generally unfamiliar to historical drug discovery. For example, rather than pointing to the need for inhibiting a specific protein kinase, they might highlight the need for stabilizing a specific protein such as APP or ApoE to reduce levels of a post-translational modification (Jonsson et al., 2012; Mahley and Huang, 2012). Drugs don’t yet exist that have these mechanisms of action. To realize the promise of precision medicine there is a great need to innovate chemistry, chemical biology and other areas of science and engineering related to therapeutics discovery if we are to uncover the kinds of mechanism of action compounds suggested by human biology.
The MLP has been an early pioneer in efforts to develop small-molecule probes with novel mechanisms of action. One of the lessons learned was the power of close collaborations of academic biologists and clinicians with chemists and chemical biologists for developing effective and informative probes that facilitate studies of emerging biology. Another insight gained was the critical importance of devoting sufficient resources to chemical optimization. Hits straight from screens rarely have the potency and selectivity necessary to be effective tool compounds – and if used with insufficient caution can lead to non-robust conclusions (Begley and Ellis, 2012). Some percentage of hits identified in screens are likely promiscuous compounds unlikely to ever yield tool compounds (Baell and Walters, 2014). Other hits are good starting points, but require a substantial investment in “hit-to-probe” chemistry. When this investment is made, the resulting compounds can provide unique avenues for exploring biology. For instance, the MLP has discovered many small-molecule probes that modulate targets via mechanisms that are difficult or impossible to explore by genetic perturbations, such as altering the rate of protein modifications (noted above), disrupting protein–protein interactions, increasing protein stability and inhibiting protein dimerization (Supplementary Table 1).
The MLP has not been alone in recognizing the critical experimental niche filled by small-molecule probes (Edwards et al., 2015; Edwards et al., 2011; Frye, 2010; Workman and Collins, 2010). Numerous academic and industrial efforts, including public–private partnerships such as the one led by the Structural Genomics Consortium, now focus on identifying and publishing potent and selective small molecules. As a result, the use of small-molecule probes has become a more established part of the biologist’s toolkit.
Once high-quality small-molecule probes are developed, one of the biggest current hurdles to translation is that preclinical models do not accurately predict efficacy and toxicity in humans. Academically initiated human experiments, for example, involving Phase-0 (the FDA exploratory IND path) or Phase-I trials – if performed with safe compounds – could be used to look for target engagement and the modulation of biomarkers likely to be predictive of efficacy. Testing disease hypotheses emerging from human biology with on-mechanism small molecules in humans, although insufficient for drug approval, would almost certainly increase the probability of successful drug development and eventual approval.
In addition to enabling advances in biological understanding, small-molecule probes are more directly impacting therapeutic discovery. For instance, in multiple cases, biotechnology or pharmaceutical companies have licensed the intellectual property around MLP probes (Table 1). This investment speaks to the potential for academic small molecule probe discovery to impact drug discovery efforts. However, there remains an alarming disconnect between the pace at which potential therapeutic targets are uncovered and our ability to develop small molecules with the desired activity.
In contrast to a decade ago, the infrastructure and expertise for small molecule discovery is firmly rooted in academic settings. Innovative small molecule science is now embedded in academia alongside deep and rapidly advancing biomedical knowledge (Reed et al., 2012). Advancing small-molecule discovery in this environment, particularly when methods, data and small-molecule probes are made openly available, may provide an effective bridge towards more rapidly identifying small molecules that modulate the challenging mechanisms illuminated by human biology to accelerate the testing of emerging therapeutic hypotheses.
Supplementary Material
Acknowledgements
The authors wish to thank Matthew Boxer, Sivaraman Dandapani, Richard Y Ebright, Juan Marugan, Andrew Phillips, Craig Thomas, Matthew Vander Heiden, and Bridget Wagner for thoughtful discussion and critical reading of the manuscript and Katie Ris-Vicari for assistance with figure design. Centers in the MLPCN were funded via the NIH grants U54 MH084681-01 (NIH Chemical Genomics Center), 1 U54 HG005033-01 (Conrad Prebys Center for Chemical Genomics), U54 MH084512-01 (The Scripps Research Institute Molecular Screening Center), 1 U54 HG005032-01 (Broad Institute Probe Development Center), 1 U54 HG005034-01 (Southern Research Specialized Biocontainment Screening Center), U54 MH084690-01 (University of New Mexico Center for Molecular Discovery), U54 MH084691-01 (Johns Hopkins School of Medicine Ion Channel Center), 1 U54 HG005031-01 (University of Kansas Specialized Chemistry Center) and U54 MH084659-01 (The Vanderbilt Specialized Chemistry Center for Accelerated Probe Development).
HR is a scientific founder of and consultant to Receptos, which has licensed RPC-1063. BFC is a scientific founder of and advisor to Abide Therapeutics, a company that is interested in developing serine hydrolase inhibitors as therapeutic agents. LAS and BE are scientific cofounders of Intellicyt, which sells instrumentation for high-throughput flow cytometry. All of the authors from Vanderbilt University receive funding from Janssen, Bristol-Myers Squibb and AstraZeneca for the development of GPCR allosteric modulators for CNS disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adibekian A, Martin BR, Chang JW, Hsu KL, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, Ferguson J, Hodder PS, Rosen H, Cravatt BF. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J Am Chem Soc. 2012;134:10345–10348. doi: 10.1021/ja303400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011a;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. Trial watch: phase III and submission failures, 2007–2010. Nat Rev Drug Discov. 2011b;10:87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010;12:716–721. doi: 10.1111/j.1463-1326.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–1139. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- Bachovchin DA, Brown SJ, Rosen H, Cravatt BF. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat Biotechnol. 2009;27:387–394. doi: 10.1038/nbt.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachovchin DA, Mohr JT, Speers AE, Wang C, Berlin JM, Spicer TP, Fernandez-Vega V, Chase P, Hodder PS, Schurer SC, Nomura DK, Rosen H, Fu GC, Cravatt BF. Academic cross-fertilization by public screening yields a remarkable class of protein phosphatase methylesterase-1 inhibitors. Proc Natl Acad Sci USA. 2011;108:6811–6816. doi: 10.1073/pnas.1015248108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J, Walters MA. Chemistry: Chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- Bern C. Antitrypanosomal therapy for chronic Chagas' disease. N Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- Boxer MB, Jiang JK, Vander Heiden MG, Shen M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park HW, Inglese J, Cantley LC, Auld DS, Thomas CJ. Evaluation of substituted N,N'-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. J Med Chem. 2010;53:1048–1055. doi: 10.1021/jm901577g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges TM, Reid PR, Lewis LM, Dawson ES, Weaver CD, Wood MR, Lindsley CW. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Discovery and development of a second highly selective M1 Positive Allosteric Modulator (PAM) [PubMed] [Google Scholar]
- Carmody LC, Germain AR, Engel JC, Gut J, Kaiser M, Jewett I, LeQuemen S, Marie JC, Dandapani S, Rodriguez A, Perez JR, McKerrow JH, Palmer MAJ, Munoz B, Schrieber SL. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Identification of Diversity-Oriented Synthesis Derived Small Molecule, ML341, with Cidal Activity Against Trypanosoma cruzi. [PubMed] [Google Scholar]
- Chen JK, Lane WS, Schreiber SL. The identification of myriocin-binding proteins. Chem Biol. 1999;6:221–235. doi: 10.1016/S1074-5521(99)80038-6. [DOI] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schurer SC, Vidovic D, Shulman GI, Spiegelman BM, Griffin PR. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Choi SS, Kim ES, Jedrychowski MP, Yang YR, Jang HJ, Suh PG, Banks AS, Gygi SP, Spiegelman BM. Thrap3 docks on phosphoserine 273 of PPARgamma and controls diabetic gene programming. Genes Dev. 2014;28:2361–2369. doi: 10.1101/gad.249367.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009a;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009b;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandapani S, Germain AR, Jewett I, le Quement S, Marie JC, Muncipinto G, Duvall JR, Carmody LC, Perez JR, Engel JC, Gut J, Kellar D, Siqueira-Neto JL, McKerrow JH, Kaiser M, Rodriguez A, Palmer MA, Foley M, Schreiber SL, Munoz B. Diversity-Oriented Synthesis Yields a New Drug Lead for Treatment of Chagas Disease. ACS Med Chem Lett. 2014;5:149–153. doi: 10.1021/ml400403u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MB, Bachovchin DA, Brown SJ, Finn MG, Rosen H, Cravatt BF, Mowen KA. Novel inhibitors for PRMT1 discovered by high-throughput screening using activity-based fluorescence polarization. ACS Chem Biol. 2012;7:1198–1204. doi: 10.1021/cb300024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AM, Arrowsmith CH, Bountra C, Bunnage ME, Feldmann M, Knight JC, Patel DD, Prinos P, Taylor MD, Sundstrom M, Barker P, Barsyte D, Bengtson MH, Bell C, Bowness P, Boycott KM, Buser-Doepner C, Carpenter CL, Carr AJ, Clark K, Das AM, Dhanak D, Dirks P, Ellis J, Fantin VR, Flores C, Fon EA, Frail DE, Gileadi O, O'Hagan RC, Howe T, Isaac JT, Jabado N, Jakobsson PJ, Klareskog L, Knapp S, Lee WH, Lima-Fernandes E, Lundberg IE, Marshall J, Massirer KB, MacKenzie AE, Maruyama T, Mueller-Fahrnow A, Muthuswamy S, Nanchahal J, O'Brien C, Oppermann U, Ostermann N, Petrecca K, Pollock BG, Poupon V, Prinjha RK, Rosenberg SH, Rouleau G, Skingle M, Slutsky AS, Smith GA, Verhelle D, Widmer H, Young LT. Preclinical target validation using patient-derived cells. Nat Rev Drug Discov. 2015;14:149–150. doi: 10.1038/nrd4565. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- Fidock DA. Microbiology. Eliminating malaria. Science. 2013;340:1531–1533. doi: 10.1126/science.1240539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye SV. The art of the chemical probe. Nat Chem Biol. 2010;6:159–161. doi: 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Bridges TM, Daniels JS, Lamsal A, Vinson PN, Niswender CM, Hodder PS, Rosen H, Conn PJ, Engers J, Lindsley CW, Wood MR. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Development and characterization of a highly selective M5 PAM probe molecule with improved potency. [PubMed] [Google Scholar]
- Gentry PR, Bridges TM, Lamsal A, Vinson PN, Smith E, Chase P, Hodder PS, Engers JL, Niswender CM, Daniels JS, Jeffrey Conn P, Wood MR, Lindsley CW. Discovery of ML326: The first sub-micromolar, selective M5 PAM. Bioorg Med Chem Lett. 2013;23:2996–3000. doi: 10.1016/j.bmcl.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard B, Duvall JR, Lowe JT, Murillo T, Wei J, Akella LB, Marcaurelle LA. Synthesis of a stereochemically diverse library of medium-sized lactams and sultams via S(N)Ar cycloetherification. ACS Comb Sci. 2011;13:365–374. doi: 10.1021/co2000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E, Zheng W, Motabar O, Southall N, Choi JH, Marugan J, Austin CP, Sidransky E. High throughput screening for small molecule therapy for Gaucher disease using patient tissue as the source of mutant glucocerebrosidase. PLoS One. 2012;7:e29861. doi: 10.1371/journal.pone.0029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, Schaeffer MT, Chapman J, Cameron M, Guerrero M, Roberts E, Rosen H. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M, Poddutoori R, Pinacho-Crisostomo F, Schaeffer MT, Brown SJ, Spicer T, Chase P, Ferguson J, Roberts E, Sanna G, Hodder P, Rosen H. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010a. Probe Development Efforts for an Allosteric Agonist of the Sphingosine 1-phosphate Receptor 3 (S1P3) [PubMed] [Google Scholar]
- Guerrero M, Urbano M, Velaparthi S, Schaeffer MT, Brown SJ, Crisp M, Ferguson J, Hodder P, Rosen H, Oldstone M, Roberts E. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010b. Identification of a Novel Agonist of the Sphingosine 1-phosphate Receptor 4 (S1P4) [PubMed] [Google Scholar]
- Guerrero M, Urbano M, Velaparthi S, Zhao J, Schaeffer MT, Brown S, Rosen H, Roberts E. Discovery, design and synthesis of the first reported potent and selective sphingosine-1-phosphate 4 (S1P4) receptor antagonists. Bioorg Med Chem Lett. 2011;21:3632–3636. doi: 10.1016/j.bmcl.2011.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M, Urbano M, Zhao J, Crisp M, Chase P, Hodder P, Schaeffer MT, Brown S, Rosen H, Roberts E. Discovery, design and synthesis of novel potent and selective sphingosine-1-phosphate 4 receptor (S1P(4)-R) agonists. Bioorg Med Chem Lett. 2012;22:537–542. doi: 10.1016/j.bmcl.2011.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsdottir SM, Ljosa V, Sokolnicki KL, Anthony Wilson J, Walpita D, Kemp MM, Petri Seiler K, Carrel HA, Golub TR, Schreiber SL, Clemons PA, Carpenter AE, Shamji AF. Multiplex cytological profiling assay to measure diverse cellular States. PLoS One. 2013;8:e80999. doi: 10.1371/journal.pone.0080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- Heidebrecht RW, Jr, Mulrooney C, Austin CP, Barker RH, Jr, Beaudoin JA, Cheng KC, Comer E, Dandapani S, Dick J, Duvall JR, Ekland EH, Fidock DA, Fitzgerald ME, Foley M, Guha R, Hinkson P, Kramer M, Lukens AK, Masi D, Marcaurelle LA, Su XZ, Thomas CJ, Weiwer M, Wiegand RC, Wirth D, Xia M, Yuan J, Zhao J, Palmer M, Munoz B, Schreiber S. Diversity-Oriented Synthesis Yields a Novel Lead for the Treatment of Malaria. ACS Med Chem Lett. 2012;3:112–117. doi: 10.1021/ml200244k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, Kidd MJ, King AM, Meyer MR, Slade D, Lum PY, Stepaniants SB, Shoemaker DD, Gachotte D, Chakraburtty K, Simon J, Bard M, Friend SH. Functional discovery via a compendium of expression profiles. Cell. 2000;102:109–126. doi: 10.1016/s0092-8674(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, DePinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di Vizio D, Mills GB, Cantley LC, Vander Heiden MG. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E, Bhhatarai B, Repetto E, Guerrero M, Riley S, Brown SJ, Kohno Y, Roberts E, Schurer SC, Rosen H. Novel selective allosteric and bitopic ligands for the S1P(3) receptor. ACS Chem Biol. 2012;7:1975–1983. doi: 10.1021/cb300392z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Kamenecka TM, Busby SA, Kumar N, Choi JH, Banks AS, Vidovic D, Cameron MD, Schurer SC, Mercer BA, Hodder P, Spiegelman BM, Griffin PR. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Potent Anti-Diabetic Actions of a Novel Non-Agonist PPARgamma Ligand that Blocks Cdk5-Mediated Phosphorylation. [PubMed] [Google Scholar]
- Knuckley B, Jones JE, Bachovchin DA, Slack J, Causey CP, Brown SJ, Rosen H, Cravatt BF, Thompson PR. A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem Commun (Camb) 2010;46:7175–7177. doi: 10.1039/c0cc02634d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C, Hixon J, Choe S, Marks K, Gross S, Murphy E, DeLaBarre B, Cianchetta G, Sethumadhavan S, Wang X, Yan S, Gao Y, Fang C, Wei W, Jiang F, Wang S, Qian K, Saunders J, Driggers E, Woo HK, Kunii K, Murray S, Yang H, Yen K, Liu W, Cantley LC, Vander Heiden MG, Su SM, Jin S, Salituro FG, Dang L. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19:1187–1198. doi: 10.1016/j.chembiol.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Larsen SD, Wilson MW, Abe A, Shu L, George CH, Kirchhoff P, Showalter HD, Xiang J, Keep RF, Shayman JA. Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J Lipid Res. 2012;53:282–291. doi: 10.1194/jlr.M021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le U, Melancon BJ, Bridges TM, Vinson PN, Utley TJ, Lamsal A, Rodriguez AL, Venable D, Sheffler DJ, Jones CK, Blobaum AL, Wood MR, Daniels JS, Conn PJ, Niswender CM, Lindsley CW, Hopkins CR. Discovery of a selective M(4) positive allosteric modulator based on the 3-amino-thieno[2,3-b]pyridine-2-carboxamide scaffold: development of ML253, a potent and brain penetrant compound that is active in a preclinical model of schizophrenia. Bioorg Med Chem Lett. 2013;23:346–350. doi: 10.1016/j.bmcl.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LH, Wu LF, Altschuler SJ. Image-based multivariate profiling of drug responses from single cells. Nat Methods. 2007;4:445–453. doi: 10.1038/nmeth1032. [DOI] [PubMed] [Google Scholar]
- Lowe JT, Lee MDt, Akella LB, Davoine E, Donckele EJ, Durak L, Duvall JR, Gerard B, Holson EB, Joliton A, Kesavan S, Lemercier BC, Liu H, Marie JC, Mulrooney CA, Muncipinto G, Welzel-O'Shea M, Panko LM, Rowley A, Suh BC, Thomas M, Wagner FF, Wei J, Foley MA, Marcaurelle LA. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J Org Chem. 2012;77:7187–7211. doi: 10.1021/jo300974j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens AK, Heidebrecht RW, Jr, Mulrooney C, Beaudoin JA, Comer E, Duvall JR, Fitzgerald ME, Masi D, Galinsky K, Scherer CA, Palmer M, Munoz B, Foley M, Schreiber SL, Wiegand RC, Wirth DF. Diversity-Oriented Synthesis Probe Targets Plasmodium falciparum Cytochrome b Ubiquinone Reduction Site and Synergizes With Oxidation Site Inhibitors. J Infect Dis. 2014 doi: 10.1093/infdis/jiu565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, Christen S, Elia I, Dinh AT, Stephanopoulos G, Manalis SR, Yaffe MB, Andrechek ER, Fendt SM, Vander Heiden MG. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MDt, Liu H, Lowe JT, Marie JC, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh BC, Wei J, Young DW, Akella LB, Ross NT, Zhang YL, Fass DM, Reis SA, Zhao WN, Haggarty SJ, Palmer M, Foley MA. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: discovery of macrocyclic histone deacetylase inhibitors. J Am Chem Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano DP, Chang MR, Corzo CA, Goswami D, Lam VQ, Pascal BD, Griffin PR. The therapeutic potential of nuclear receptor modulators for treatment of metabolic disorders: PPARgamma, RORs, and Rev-erbs. Cell Metab. 2014a;19:193–208. doi: 10.1016/j.cmet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Marciano DP, Dharmarajan V, Griffin PR. HDX-MS guided drug discovery: small molecules and biopharmaceuticals. Curr Opin Struct Biol. 2014b;28:105–111. doi: 10.1016/j.sbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R, American Heart A, American Diabetes A. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Rodriguez AL, Sheffler DJ, Utley TJ, Vinson PN, Dawson ES, Jones CK, Wood MR, Daniels JS, Conn PJ, Engers JL, Le UM, Melancon BJ, Hopkins CR, Lindsley CW. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Extended Probe Characterization: Development of an M4 PAM with Improved Activity and Brain Exposure, while Avoiding Species Bias. [PubMed] [Google Scholar]
- Nomura DK, Dix MM, Cravatt BF. Activity-based protein profiling for biochemical pathway discovery in cancer. Nat Rev Cancer. 2010;10:630–638. doi: 10.1038/nrc2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M, Hodder P, Crisp M, Roberts E, Guerrero M, Urbano M, Velaparthi S, Zhao J, Rosen H, Schaeffer MT, Brown S, Ferguson J. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Probe Development Efforts to Identify Novel Antagonists of the Sphingosine 1-phosphate Receptor 4 (S1P4) [PubMed] [Google Scholar]
- Oprea TI, Bologa CG, Boyer S, Curpan RF, Glen RC, Hopkins AL, Lipinski CA, Marshall GR, Martin YC, Ostopovici-Halip L, Rishton G, Ursu O, Vaz RJ, Waller C, Waldmann H, Sklar LA. A crowdsourcing evaluation of the NIH chemical probes. Nat Chem Biol. 2009;5:441–447. doi: 10.1038/nchembio0709-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano S, Vieitez I, Navarro C, Spuch C. Treatment of Lysosomal Storage Diseases: Recent Patents and Future Strategies. Recent Pat Endocr Metab Immune Drug Discov. 2014 doi: 10.2174/1872214808666140115111350. [DOI] [PubMed] [Google Scholar]
- Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol Genet Metab. 2002;76:262–270. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Panicker LM, Miller D, Park TS, Patel B, Azevedo JL, Awad O, Masood MA, Veenstra TD, Goldin E, Stubblefield BK, Tayebi N, Polumuri SK, Vogel SN, Sidransky E, Zambidis ET, Feldman RA. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc Natl Acad Sci U S A. 2012;109:18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik S, Zheng W, Choi JH, Motabar O, Southall N, Westbroek W, Lea WA, Velayati A, Goldin E, Sidransky E, Leister W, Marugan JJ. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J Med Chem. 2012;55:5734–5748. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone PM, Simms B, Nigsch F, Lounkine E, Kutchukian P, Cornett A, Deng Z, Davies JW, Jenkins JL, Glick M. Rethinking molecular similarity: comparing compounds on the basis of biological activity. ACS Chem Biol. 2012;7:1399–1409. doi: 10.1021/cb3001028. [DOI] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- Reed JC, White EL, Aube J, Lindsley C, Li M, Sklar L, Schreiber S. The NIH's role in accelerating translational sciences. Nat Biotechnol. 2012;30:16–19. doi: 10.1038/nbt.2087. [DOI] [PubMed] [Google Scholar]
- Reid PR, Bridges TM, Sheffler DJ, Cho HP, Lewis LM, Days E, Daniels JS, Jones CK, Niswender CM, Weaver CD, Conn PJ, Lindsley CW, Wood MR. Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): the development of ML169, an MLPCN probe. Bioorg Med Chem Lett. 2011;21:2697–2701. doi: 10.1016/j.bmcl.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Patnaik S, Schoenen F, Zheng W, Choi J, Motabar O, Southall N, Westbroek W, Goldin E, Sidransky E, Leister W, Marugan JJ, Aube J. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Discovery, SAR, and Biological Evaluation of Non-inhibitory Chaperones of Glucocerebrosidase. [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev. 2008;223:221–235. doi: 10.1111/j.1600-065X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–662. doi: 10.1146/annurev-biochem-062411-130916. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Satsu H, Schaeffer MT, Guerrero M, Saldana A, Eberhart C, Hodder P, Cayanan C, Schurer S, Bhhatarai B, Roberts E, Rosen H, Brown SJ. A sphingosine 1-phosphate receptor 2 selective allosteric agonist. Bioorg Med Chem. 2013;21:5373–5382. doi: 10.1016/j.bmc.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Niphakis MJ, Cravatt BF. Determining target engagement in living systems. Nat Chem Biol. 2013;9:200–205. doi: 10.1038/nchembio.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B. Fluid retention mediated by renal PPARgamma. Cell Metab. 2005;2:77–78. doi: 10.1016/j.cmet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Chaperone therapy update: Fabry disease, GM1-gangliosidosis and Gaucher disease. Brain Dev. 2013;35:515–523. doi: 10.1016/j.braindev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Tarr JC, Turlington ML, Reid PR, Utley TJ, Sheffler DJ, Cho HP, Klar R, Pancani T, Klein MT, Bridges TM, Morrison RD, Blobaum AL, Xiang Z, Daniels JS, Niswender CM, Conn PJ, Wood MR, Lindsley CW. Targeting selective activation of M(1) for the treatment of Alzheimer's disease: further chemical optimization and pharmacological characterization of the M(1) positive allosteric modulator ML169. ACS Chem Neurosci. 2012;3:884–895. doi: 10.1021/cn300068s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel P, Kaiser M, Ottmann C. Small-molecule stabilization of protein-protein interactions: an underestimated concept in drug discovery? Angew Chem Int Ed Engl. 2012;51:2012–2018. doi: 10.1002/anie.201107616. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Bachovchin DA, Speers AE, Spicer TP, Fernandez-Vega V, Hodder P, Rosen H, Cravatt BF. Potent and selective inhibitors of glutathione S-transferase omega 1 that impair cancer drug resistance. J Am Chem Soc. 2011;133:16605–16616. doi: 10.1021/ja2066972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano M, Guerrero M, Rosen H, Roberts E. Modulators of the Sphingosine 1-phosphate receptor 1. Bioorg Med Chem Lett. 2013;23:6377–6389. doi: 10.1016/j.bmcl.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano M, Guerrero M, Velaparthi S, Crisp M, Chase P, Hodder P, Schaeffer MT, Brown S, Rosen H, Roberts E. Discovery, synthesis and SAR analysis of novel selective small molecule S1P4-R agonists based on a (2Z,5Z)-5-((pyrrol-3-yl)methylene)-3-alkyl-2-(alkylimino)thiazolidin-4-one chemotype. Bioorg Med Chem Lett. 2011a;21:6739–6745. doi: 10.1016/j.bmcl.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano M, Guerrero M, Zhao J, Velaparthi S, Schaeffer MT, Brown S, Rosen H, Roberts E. SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P(4)) receptor. Bioorg Med Chem Lett. 2011b;21:5470–5474. doi: 10.1016/j.bmcl.2011.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Rosen H, Oldstone MB. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol Res. 2011;51:15–25. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Jaramillo DE, Dancik V, Fass DM, Haggarty SJ, Shamji AF, Wagner BK, Schreiber SL, Clemons PA. Automated structure-activity relationship mining: connecting chemical structure to biological profiles. J Biomol Screen. 2014a;19:738–748. doi: 10.1177/1087057114530783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawer MJ, Li K, Gustafsdottir SM, Ljosa V, Bodycombe NE, Parkin M, Sokolnicki KL, Bray MA, Kemp MM, Winchester E, Taylor B, Grant GB, Hon CS-Y, Duvall JR, Wilson AJ, Bittker JA, Dancik V, Narayan R, Subramanian A, Winckler W, Golub TR, Carpenter AE, Shamji AF, Schreiber SL, Clemons PA. Towards performance-diverse small-molecule libraries for cell-based phenotypic screening using multiplexed high-dimensional profiling. Proc Natl Acad Sci U S A. 2014b;111:10911–10916. doi: 10.1073/pnas.1410933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- Weiwer M, Mulrooney C, Massi D, Heidebrecht R, Wiegand R, Lukens AK, Dick J, Wirth D, Ekland E, Cheng CC, Zhao J, Yuan J, Su XZ, Johnson RL, Guha R, Dandapani S, Munoz B, Palmer M, Thomas C, Austin CP, Fidock D, Schreiber SL. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. ML238: An Antimalarial Small Molecule of a Unique Structural Class. [PubMed] [Google Scholar]
- Wenthur CJ, Gentry PR, Mathews TP, Lindsley CW. Drugs for allosteric sites on receptors. Annu Rev Pharmacol Toxicol. 2014;54:165–184. doi: 10.1146/annurev-pharmtox-010611-134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P, Collins I. Probing the probes: fitness factors for small molecule tools. Chem Biol. 2010;17:561–577. doi: 10.1016/j.chembiol.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DW, Bender A, Hoyt J, McWhinnie E, Chirn GW, Tao CY, Tallarico JA, Labow M, Jenkins JL, Mitchison TJ, Feng Y. Integrating high-content screening and ligand-target prediction to identify mechanism of action. Nat Chem Biol. 2008;4:59–68. doi: 10.1038/nchembio.2007.53. [DOI] [PubMed] [Google Scholar]
- Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev. 2007;21:217–231. doi: 10.1016/j.blre.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Zuhl AM, Mohr JT, Bachovchin DA, Niessen S, Hsu KL, Berlin JM, Dochnahl M, Lopez-Alberca MP, Fu GC, Cravatt BF. Competitive activity-based protein profiling identifies aza-beta-lactams as a versatile chemotype for serine hydrolase inhibition. J Am Chem Soc. 2012;134:5068–5071. doi: 10.1021/ja300799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.