Abstract
This study was undertaken to investigate stem cells in adult mouse ovary, the effect of chemotherapy on them and their potential to differentiate into germ cells. Very small embryonic-like stem cells (VSELs) that were SCA-1+/Lin−/CD45−, positive for nuclear octamer-binding transforming factor 4 (OCT-4), Nanog, and cell surface stage-specific embryonic antigen 1, were identified in adult mouse ovary. Chemotherapy resulted in complete loss of follicular reserve and cytoplasmic OCT-4 positive progenitors (ovarian germ stem cells) but VSELs survived. In ovarian surface epithelial (OSE) cell cultures from chemoablated ovary, proliferating germ cell clusters and mouse vasa homolog/growth differentiation factor 9-positive oocyte-like structure were observed by day 6, probably arising as a result of differentiation of the surviving VSELs. Follicle-stimulating hormone (FSH) exerted a direct stimulatory action on the OSE and induced stem cells proliferation and differentiation into premeiotic germ cell clusters during intact chemoablated ovaries culture. The FSH analog pregnant mare serum gonadotropin treatment to chemoablated mice increased the percentage of surviving VSELs in ovary. The results of this study provide evidence for the presence of potential VSELs in mouse ovaries and show that they survive chemotherapy, are modulated by FSH, and retain the ability to undergo oocyte-specific differentiation. These results show relevance to women who undergo premature ovarian failure because of oncotherapy.
Keywords: ovary, stem cells, VSELs, FSH, OCT-4
Introduction
The last 10 years have been very exciting in the field of female reproductive biology, with many researchers providing compelling evidence for the existence of female germ line stem cells that contribute to postnatal oogenesis. This has challenged the long-held dogma in female biology that mammalian females are born with fixed and nonrenewing pool of germ cells that decrease with age due to ovulation or atresia leading to menopause. Prof Jonathan Tilly’s group1 through studies in adult mice demonstrated that the discordance between changes in the number of nonatretic follicles and rate of atresia did not explain fixed germ cell theory. They showed the presence of mitotically active germ cells and cells expressing meiotic markers in adult mouse ovary. Following this report, several studies have shown the presence of germ line stem cells and potential postnatal oogenesis using various techniques in mice as well as higher animal species including women.2–7 The stem cells have been isolated using different strategies, propagated in vitro for several generations and their ability to differentiate into oocytes in vivo has been demonstrated.4,8,9 Despite these emerging reports from independent laboratories, many are still skeptical about the presence of stem cells in adult mammalian ovary.10–12 Recently, using an endogenous genetic approach, it was shown that mouse postnatal ovaries do not possess mitotically active mouse vasa homolog (MVH; also termed DDX-4) expressing female progenitor cells.13 However, the experimental model used by them did not differentiate small immature oocytes from female progenitors/stem cells, both of which express MVH. Also, the results were reported based on a study of fluorescent MVH expressing cells of 10 to 15 μm diameter, which are larger than the reported size of ovarian germ line stem cells.2,8 It corresponds more to the size of primordial follicle oocytes.14 The study also failed to show the identity of MVH-expressing cells based on other marker expression. More explanations for existing criticisms against the concept of ovarian stem cells and postnatal oogenesis were recently reviewed.15,16
Our group has been working in the area of gonadal stem cells, and we have shown that they include 2 distinct populations, namely, the relatively “quiescent” very small embryonic-like stem cells (VSELs) and the more committed “active” progenitor stem cells, which include ovary germ stem cells (OGSCs) in ovary2,17 and spermatogonial stem cells in testis.18 The concept is very similar to the presence of quiescent and active stem cell population proposed in other organs like gut, skin, and bone marrow.19,20 We have recently reviewed how VSELs and the progenitors are implicated in reproductive biology.21 The 2 populations can be differentiated from each other based on their size, expression of octamer-binding transforming factor 4 (OCT-4) isoforms, and their ability to proliferate2,18 The VSELs express nuclear OCT-4A isoform, which is known to be critically required for maintaining pluripotency in embryonic stem cells and primordial germ cells.22 The progenitors express OCT-4 in cytoplasm (OCT-4B) and subsequently with differentiation, the expression of OCT-4 is lost in the differentiated germ cells. The VSELs were initially described in bone marrow by Prof Ratajczak’s group23 in 2006 and subsequently in various adult mouse organs.24 They express several markers of migratory primordial germ cells and their developmental origin is attributed to epiblast/germ line origin.25 The VSELs are quiescent, and mitogenic growth response signaling has been reported to play a crucial role in its quiescence.26 Their pluripotent status was shown by the presence of open chromatin structure with active transcription of OCT-4 promoter27 and differentiation into 3 germ layers in mice23 as well as in humans.28
In ovary, the 2 populations of stem cells including the VSELs and OGSCs are localized in the surface epithelium (OSE) of mice, rabbits, sheep, monkeys, and women.2,29 Our detailed protocols to isolate both the population of ovarian stem cells from adult mammalian ovaries are published recently.30 Our group has shown that ovarian stem cells (1) spontaneously differentiate into oocyte-like structures in culture;2 (2) exhibit process of cytoplasmic streaming, germ cell nest, and Balbiani body formation in vitro17; (3) express follicle-stimulating hormone receptors (FSHRs) and are modulated by FSH and bFGF.31,32 We have also shown that the alternative FSHR isoform—FSHR3 (a growth factor type 1 receptor)33 is involved in the activation of ovarian stem cells by FSH and results in germ cell differentiation31,34 and pregnant mare serum gonadotropin (PMSG—a FSH analog) treatment augments the process of neo-oogenesis and primordial follicle assembly in mice.35 Other groups have also supported the presence of pluripotent stem cells in ovary and their ability to form oocytes in vitro.6,36–38
Intriguingly, the ovarian stem cells are present in ovaries of perimenopausal women (including a 60-year-old woman) with no naturally present follicles or oocytes2,6,36 and aged mouse.39 This made us consider the presence of stem cells in cases of premature ovarian failure (POF) and early menopause induced by oncotherapy or myeloablative treatments prior to bone marrow transplantation. In the present study, multiple experiments (Figure 1) were undertaken to investigate VSELs and OGSCs in mouse ovary and to understand the effect of chemotherapy (a combination of busulfan and cyclophosphamide) on them. The VSELs were studied by flow cytometry as SCA-1+/Lin−/CD45—as described in the literature.24 The VSELs were observed to survive chemotherapy in mouse ovary and hence further studies were undertaken to investigate the functionality of surviving VSELs and the role of FSH towards the same. Our results provide additional support to the concept of postnatal oogenesis. Also, the data generated show that the surviving VSELs in chemoablated ovary retain their ability to differentiate into oocyte-like structures. Thus, the study may have potential applications in the field of fertility treatment for POF and early menopause induced by oncotherapy, or myeloablative treatments prior to bone marrow transplantation.
Figure 1.
An overview of the study.
Materials and Methods
All experimental protocols used in the present study were approved by Institutional Animal Ethics Committee of NIRRH. Eight-week-old adult female Swiss mice, maintained in the institute experimental animal facility, were used for the study. They were housed in a temperature and humidity controlled room on a 12-hour light/12-hour darkness cycle with free access to food and water.
Details of Various Experiments Undertaken in the Present Study
Various studies undertaken in the present study are depicted in Figure 1.
Effect of Chemotherapy on Mouse Ovarian Follicular Reserve and Stem Cells
Chemotherapy
Mice were initially treated with 4 different regimen of chemotherapy including (1) 25 mg/kg body weight, (2) 15 mg/kg busulfan and 100 mg/kg cyclophosphamide, (3) 12 and 120 mg/kg cyclophosphamide, and (4) 10 mg/kg daily for 4 days and 100 mg/kg cyclophosphamide on first 2 days (n = 3 per group) to select a dose that resulted in complete germ cell depletion. Busulfan (Sigma-Aldrich, Missouri) was dissolved in dimethyl sulfoxide (Sigma-Aldrich), diluted with equal volume of water and injected via intraperitoneal route. Cyclophosphamide (Baxter, India) was dissolved in sterile injection grade water and also injected via intraperitoneal route after 1 to 2 hours of busulfan injection. The animals were sacrificed 1 month after the treatment by cervical dislocation and ovaries collected and used for analysis or further in vitro culture studies. Initial histological examination revealed that the treatment regimen (4) led to complete loss of follicles in various stages of development observed in normal ovaries. Few rare follicles even if present showed signs of atresia and therefore treatment regimen (4) was chosen for further experiments. Chemoablated ovaries were appropriately processed for flow cytometry (to study SCA-1+/Lin−/CD45− VSELs), histology (to study the effect on follicular reserve), immunolocalization studies on sections as well as smears (to study germ cells-specific marker deleted in azoospermia-like (DAZL), and stem cells-specific marker (OCT-4), RNA extraction, and quantitative polymerase chain reaction (Q-PCR; for Dazl and Oct-4). Prior to processing for various studies, the ovaries were cleaned carefully from the adhering extraneous tissue under a stereomicroscope with the help of microscissors. Details of various methods used are described subsequently.
Effect of Chemotherapy on Stem Cells in Isolated OSE
Ovary surface epithelium isolation and detection of stem cells in OSE smears
The OSE was isolated following a method published earlier by Symonds et al40 with slight modifications. The dissected ovaries were rinsed with Dulbecco’s modified Eagle medium (DMEM) high glucose (DMEM-HG; Life Technologies, Grand Island, New York) medium. Ovaries were placed singly in a 1.5-mL tube containing 0.25 mL of DMEM-HG and 100 IU/mL of collagenase type IV (Life Technologies) and incubated at 37°C for 30 minutes. The tubes were vortexed for 2 minutes and DMEM-HG media containing 15% fetal bovine serum (FBS; Life Technologies) were added to stop the reaction. The ovaries were removed and fixed in 4% paraformaldehyde (PFA) to check whether the method results in complete removal of OSE and also to confirm that the underlying cortex remains undisturbed. The supernatant with isolated OSE cells was centrifuged at 1000g for 10 minutes and washed once with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, Missouri). The resultant cell pellet was either suspended in PBS for making smears or in extraction buffer for RNA extraction. The OSE smears were fixed with 4% PFA for immunolocalization studies. As the cells were directly fixed immediately after collagenase digestion, certain degree of morphological changes to cells could not be avoided and this explains altered shape of cells in our results. RNA from the cells was isolated using Arctrus Picopure RNA Isolation Kit (Life Technologies). The stem cells were characterized by studying markers OCT-4 and stage-specific embryonic antigen 1 (SSEA-1) using immunofluorescence and pluripotent transcripts Oct-4A, Oct-4, Sca-1, and Nanog using PCR. Details of various methods used are mentioned subsequently.
In Vitro Culture of Isolated OSE to Study Differentiation of VSELs
It has been earlier reported that 3 weeks culture of OSE cells scraped from rabbit, sheep, monkey, and human ovaries lead to spontaneous differentiation of oocyte-like structures.2,17,36 Hence, to test the differentiation potential of mouse ovarian stem cell to spontaneously differentiate into oocyte-like structures, isolated OSE cells from control and chemoablated mice were cultured in DMEM-HG media containing 20% FBS and 10 mIU FSH (see subsequently for details) in 4-chambered slides (BD Biosciences, San Jose, California) for 48 hours or longer. Cultures were monitored and images were captured using Eclipse TE 2000-S NIKON inverted microscope. To demonstrate proliferation of cells in vitro, a final concentration of 10 μmol bromodeoxyuridine (BrdU; 550891; BD Biosciences) was added to the culture medium 24 hours prior to collection. The cells were fixed in 4% PFA. At 48-hour time point, dual immunofluorescence was carried out using 2 of the markers OCT-4, MVH, BrdU, and proliferating cell nuclear antigen (PCNA) to demonstrate proliferating germ/stem cells. At 6-day time point, immunocytochemistry was carried out for MVH and growth differentiation factor 9 (GDF-9) to characterize oocyte-like structures. In addition, RNA from day 0 sample was collected to check for absence of any oocytes through presence or absence of diplotene-oocyte marker Msy2. Details of various methods used are described subsequently.
In Vitro Culture of Chemoablated Intact Ovaries and Effect of FSH
Chemoablated ovaries were used for the intact ovary culture. Intact ovary culture retains the 3-dimensional structure of ovary, which is important for differentiation of germ cells.41 Effect of FSH on chemoablated ovaries was studied for 2 reasons. First, FSH is traditionally known to act as survival factor in ovarian cortical cultures,42 although the underlying mechanism is not known. Second, as mentioned in Introduction section, we have reported that FSH modulates ovarian stem cells.31,32,35 Ovaries were isolated from surrounding tissue in an sterile environment and rinsed 2 to 3 times sequentially in sterile Dulbecco PBS, α-minimum essential medium (αMEM; Life Technologies) containing 1× penicillin–streptomycin (Pen-Strep; Life Technologies) and finally in serum-free culture medium αMEM containing 3 mg/mL bovine serum albumin (BSA; Sigma-Aldrich), 5× insulin transferrin and selenium (Sigma-Aldrich), 2 mmol/L sodium pyruvate (Sigma-Aldrich), linoleic acid (Sigma-Aldrich), and 1× Pen-Strep. Ovaries were cultured on 0.4-μm pore size and 12 mm diameter Millicell inserts (EMD Millipore, Billerica, Massachusetts) in culture medium with 10 mIU FSH (FSH-plus group) or without FSH (FSH-minus group). Human urinary FSH (Utrofol, Kuanart Pharmaceuticals, India) was dissolved in PBS containing 0.1% BSA for the study. Ovaries were randomly allotted to each of the 2 groups and studied. Partial media change was given every alternate day. On day 7, ovaries from each group were fixed in 4% PFA for histology and immunolocalization studies (for proliferation marker BrdU, germ cells-specific marker MVH, and premeiotic marker STRA-8). Ovaries (3 ovaries were pooled as single sample) were also frozen in TRIzol for RNA extraction (for studying pluripotent Oct-4A, Sca-1; germ cells Oct-4, Dazl, meiosis Scp3, and Fshr transcripts). The experiment was repeated 4 times and representative data are presented. In additional experiments, 3 ovaries per group were also collected and processed for protein isolation and Western analysis (for OCT-4). To confirm that the cells proliferate in vitro, BrdU at a final concentration of 10 μmol/L was added to culture medium on days 4 and 6 and the ovaries were collected and fixed in PFA on days 5 and 7, respectively. The percentage of BrdU positive cells was calculated from 10 to 12 sections from 3 ovaries for each group and t test was used to calculate significance. Details of various methods used are mentioned subsequently.
Effect of PMSG Treatment to Chemoablated Mice
We have earlier reported that PMSG treatment to normal adult mice increases stem cells activity and augments neo-oogenesis and primordial follicle.35 Similarly, to study the effect of PMSG on VSELs in chemoablated ovary, 5 IU PMSG (National Hormone & Peptide Program, Harbor-UCLA Medical Center, California) was injected subcutaneously 1 month after animals were treated with busulfan and cyclophosphamide. Ovaries were collected after 48 hours of PMSG treatment for analysis by flow cytometry to study effect of PMSG on SCA-1+/Lin−/CD45− VSELs. Details of method used are mentioned subsequently.
Details of Various Methods Used in the Present Study
Histology
Ovaries were collected and fixed in neutral-buffered formalin for histology and immunolocalization studies. Fixed ovarian tissue samples were processed and embedded in paraffin using standard protocols; 5-μm thick sections of the embedded ovaries were prepared. Histoarchitecture of ovarian sections was studied by staining with hematoxylin and eosin (H&E). The representative areas were photographed using Nikon 90i microscope (Nikon) and data were recorded.
Whole ovary smears
These were prepared by fine chopping of the ovary with surgical blade followed by filtration through sieves of 100 and 40 µm sizes and made smears on polylysine-coated glass slides. The smears were fixed with 4% PFA and slides stored at 4°C until use.
Flow cytometry
Whole ovaries from normal, chemoablated, and PMSG-treated mice were used for flow cytometry to enumerate SCA+/Lin−/CD45− cells. A single cell suspension of ovaries was prepared by incubating minced ovaries in DMEM medium containing 750 IU/mL of collagenase type IV (Life Technologies) at 37°C for 20 minutes. After stopping the enzymatic reaction with DMEM medium containing 20% FBS (Life Technologies), cells were filtered through 40 μm cell strainer (BD Biosciences). The cells were blocked with mouse-specific FcR blocking antibody and stained with fluorescein isothiocyanate (FITC) conjugated rat anti-mouse SCA-1 (1 μg/million cells; 553335; BD), PE rat anti-mouse CD45 (2 μg/million cells; 553081; BD), and APC mouse Lineage antibody cocktail (25 μL/mL of cells; 51-9003632; BD). The stained cells were run on FACS Aria, and the results were analyzed using FACS Diva software (BD). Each sample was prepared by pooling both ovaries from a single mouse. t Test was used to calculate significance.
Immunolocalization Studies
Immunohistochemistry
Paraffin sections were used to carry out immunohistochemical studies using methods described earlier.35 Briefly, sections were dehydrated in graded methanol series, endogenous peroxide was blocked in methanol, and antigen retrieval was performed using microwave in citrate buffer with a pH of 6. These steps were followed by blocking, primary antibody incubation, and detection using appropriate Vecta ABC elite detection kits from Vector Laboratories. The primary antibodies used were anti-MVH (1:400; goat polyclonal; AF2030; R&D Biosystems, Minneapolis, Minnesota), anti-STRA-8 (1:400; rabbit polyclonal; ab49602; Abcam, Cambridge, United Kingdom), anticytokeratin-8 (1:10; rabbit polyclonal; ab59400; Abcam), and anti-BrdU antibody (1:500; mouse monoclonal; B8434; Sigma). For BrdU staining, an additional step of incubating sections with 1 N HCl for 10 minutes after antigen retrieval was done and blocking/detection was carried out using M.O.M. detection Kit (BMK-2202; Vector Laboratories, Inc, Burlingame, California; kit for using mouse monoclonal antibody on mouse tissues) according to the manufacturer’s instructions. Mouse testicular sections were used as positive control for detection of STRA-8. The protocol for negative control was exactly identical, except that the addition of primary antibody was omitted and replaced by blocking solution. The representative areas were photographed using Nikon 90i microscope (Nikon, Tokyo, Japan) and data recorded. Prominent dark staining above the background was considered positive.
Immunofluorescence
The smears or cultured cells were first washed thrice with tris-buffered saline (TBS) buffer containing 0.5% bovine serum albumin (Sigma) and 0.5 mmol/L EDTA. Permeabilization of the cells with 0.5% Triton-X in TBS for 7 minutes was carried out for OCT-4 and all dual staining. Only for BrdU staining, culture slides were incubated with 2 N HCL for 30 minutes at room temperature (to denature DNA) followed by 2 washes with TBS prior to permeabilization step. The smears were then blocked for 1 hour at room temperature with 1% BSA and 5% normal goat serum (NGS) for OCT-4 and SCA-1. The blocking for mouse monoclonal antibodies for both single and dual staining was done using M.O.M. detection Kit (mentioned previously) according to the manufacturer’s instructions (steps 7-9 in their protocol). Various primary antibodies used in the study included anti-OCT-4 (rabbit polyclonal; Ab19857 from Abcam or Ab3209 from Millipore), anti-SSEA-1 (mouse monoclonal; MAB4301 from Millipore), anti-SCA-1 (FITC tagged; 553335; BD), anti-Connexin 43 (rabbit polyclonal; Ab11370 from Abcam), E-cadherin (rat monoclonal; ab11512; Abcam), anti-BrdU (mouse monoclonal; B8434; Sigma), anti-PCNA (mouse monoclonal; P8825 from Sigma), and anti-MVH (goat polyclonal; AF2030; R&D Biosystems). After the blocking step, the smears were incubated overnight at 4°C with primary antibody (OCT-4 1:400, SSEA-1 1:100, CNX-43 1:100, MVH 1:400, SCA-1 1:50 dilution, BrdU 1:500, and PCNA 1:2500). For dual immunofluorescence, both primary antibodies were mixed and incubated. Excess primary antibody was removed by 3 washes with TBS and then the cells were incubated with respective fluorescent-tagged secondary antibody (alexaflour-488[green]/568[red]; Life Technologies) for 2 hours at room temperature in dark. The smears were washed thrice with TBS and counterstained with nuclear stains propidium iodide (PI; 0.5 μg/mL) for single staining or 4′,6-diamidino-2-phenylindole (DAPI; 1.47 μmol) for dual staining. All images were captured by laser scanning confocal microscope (Carl Ziess) at magnification of 63×. The conditions were adjusted using respective negative controls (same cells treated identically except the addition of primary antibody).
Immunocytochemistry
GDF-9 and MVH were studied on cultured cells by using a similar protocol as described earlier for immunofluorescence except that the wash buffer used was PBS and additional peroxide block was carried out. The secondary antibody and detection were done using antigoat IgG Vecta ABC Kit (PK 6105; Vector Laboratories) and 3,3′ diaminobenzidine (DAB). The primary antibodies used were anti-GDF-9 (1:10; goat polyclonal; sc12244; Santa Cruz Biotechnology, Dallas, Texas) and anti-MVH (goat polyclonal; AF2030; R&D Biosystems). The representative areas were photographed using Nikon 90i microscope (Nikon) and data recorded.
RNA extraction, reverse transcription, and PCR
All procedures including RNA extraction from tissues frozen in TRIzol (Life Technologies) or from the OSE cells using Arctrus Picopure RNA Isolation Kit, DNase treatment using DNase I (Amersham Biosciences, Buckinghamshire, United Kingdom), and reverse transcription using iScript complementary DNA (cDNA) synthesis Kit (Bio-Rad, Hercules, California) were carried out according to manufacturer’s instructions. RNA of 1 μg was used for cDNA synthesis. Both real-time PCR (RT-PCR) and Q-PCR were done using iQSYBR Green SuperMix (Bio-Rad). The primers used and their annealing temperatures are mentioned in Table 1. All the primers had efficiency close to 100% and all primers except Gapdh and Nanog were exon–exon spanning primers. The RT-PCR was carried out in G-STORM thermocycler using standard protocols of 35 PCR cycles. The Q-PCR was carried out in a CFX96 Real-time PCR system (Bio-Rad) using SYBR Green chemistry (Bio-Rad). Various transcripts analyzed included stem cell markers (Oct-4A, Oct-4, Sca-1, and Nanog), proliferating germ cell marker (Dazl), oocyte-specific marker (Msy2), and Fshr. The expression levels of these transcripts were studied in relation to housekeeping transcripts 18s or Gapdh. The amplification conditions included initial denaturation at 94°C for 3 minutes followed by 40 cycles comprising of denaturation at 94°C for 30 seconds, primer annealing for 20 seconds, and extension at 72°C for 30 seconds. The final step included incubation at 94°C for 20 seconds to remove any secondary structures followed by melt curve analysis. The fluorescence emitted at each cycle was collected during the extension step of each cycle. The homogeneity of the PCR amplicons was verified by running the products on 2% agarose gels and also by studying the melt curve. All PCR amplifications were carried out in duplicate. Mean Ct values generated in each experiment using the CFX Manager software (Bio-Rad) were used to calculate the messenger RNA (mRNA) expression levels. Since ΔCt is inversely proportional to relative mRNA expression levels, the levels were calculated manually by the ΔCt method or by standard ΔΔCt method. t Test was used for calculating significance and 3 to 5 samples for each condition or experiment were used.
Table 1.
Primers Used for RT-PCR and Q-PCR.
| Gene | Primer | Annealing Temp, °C |
|---|---|---|
| Fshr | F: TGGAGGCGGCAAACCTCTGAAC R: TCTGGCTTTGGCGAGCAGGTC | 65 |
| Oct-4A | F: CCATGTCCGCCCGCATACGA R: GGGCTTTCATGTCCTGGGACTCCT | 61 |
| Oct-4 | F: CCTGGGCGTTCTCTTTGGAAAGGTG R: GCCTGCACCAGGGTCTCCGA | 61 |
| Nanog4 | F: CAGGAGTTTGAGGGTAGCTC R: CGGTTCATCATGGTACAGTC | 61 |
| Scp3 | F: TGTTGCAGCAGTGGGAACTGGAT R: CCATCTCTTGCTGCTGAGTTTCCA | 68 |
| Sca-1 | F: AGAGGAAGTTTTATCTGTGCAGCCC R: TCCACAATAACTGCTGCCTCCTGA | 66 |
| Dazl4 | F: GTGTGTCGAAGGGCTATGGAT R: ACAGGCAGCTGATATCCAGTG | 61 |
| Msy28 | F: CCTCCCCACTTTCCCATAAT R: AATGGGTGGGGAAGAAAAAC | 55 |
| 18s | F:GGAGAGGGAGCCTGAGAAAC R: CCTCCAATGGATCCTCGTTA | 61 |
| Gapdh4 | F: GTCCCGTAGACAAAATGGTGA R: TGCATTGCTGACAATCTTGAG | 58 |
Abbreviations: Q-PCR, quantitative polymerase chain reaction; RT-PCR, real-time polymerase chain reaction.
Western blotting
Protein extraction was done using ice cold 1× cell lysis buffer containing 50 mmol Tris (SRL, Mumbai, India), 1 mmol EDTA (Fischer Scientific, New York), 150 mmol NaCl (Sigma-Aldrich), 1 mol sodium fluoride (Fischer Scientific, Qualigens, Mumbai), 0.1% sodium dodecyl sulfate (SDS; Fischer Scientific), 1% Triton X-100 (Sigma Aldrich), 2 mmol phenylmethylsulfonyl fluoride (Sigma-Aldrich), and containing 4× protease inhibitor cocktail (Roche Diagnostics, Manheim, Germany). Whole ovaries were minced and homogenized by passing through 20G, 22G, and 26G hypodermic needles. The resultant cell lysate was agitated on ice for 30 minutes followed by centrifugation at 21 000g for 30 minutes to collect the supernatant. Protein concentration was estimated by Folin-Lowry method using spectrophotometer (Beckman Coulter Inc, Indianapolis, Indiana). The extracted protein was incubated in Laemmli buffer for 10 minutes at 95°C. Protein of 50 μg was resolved using 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Amersham Biosciences, Bucks, United Kingdom). The blot was blocked with 5% nonfat dry milk (NFDM) in 1× TBS with Tween 20 overnight at 4°C. Membrane was incubated with anti-OCT-4 (1:500; Ab3209, Millipore) antibody at room temperature for 2 hours followed by incubation with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000, Millipore) for 2 hours at room temperature. The bands on blots were detected using Super Signal West Femto substrate (Thermo Scientific, Waltham, Massachusetts) on photographic films (Eastman Kodak Co, Rochester, New York). Later blot was stripped using stripping buffer (62.5 mmol Tris, 2% SDS, 100 mmol β-mercaptoethanol) for 10 minutes at 60°C to detect housekeeping protein. Actin was used as housekeeping protein and detected using antibody (1:5000, MAB1501; Millipore), and human embryonic stem cell extract was used as positive control. Quantitative analysis of bands was done from 3 blots using Image J software, and t test was used to calculate significance.
Results
Effect of Chemotherapy on Mouse Ovarian Follicular Reserve and Stem Cells
Ovaries of mice treated with a combination of busulfan (10 mg/kg body weight for 4 days) and cyclophosphamide (100 mg/kg body weight for first 2 days) were studied to understand the effect of chemotherapy on follicular reserve and stem cells. Effect on follicular reserve was studied by histology, immunolocalization/Q-PCR for germ cell-specific marker DAZL. Effect on stem cells was studied by immunolocalization/Q-PCR for stem cell marker OCT-4 and flow cytometry analysis for SCA-1+/Lin−/CD45− VSELs in both normal and chemoablated ovaries.
Chemotherapy leads to depletion of mouse ovarian follicular reserve
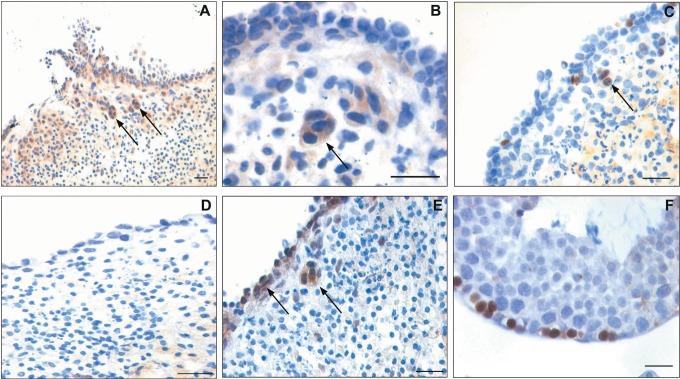
The treatment caused an apparent reduction in size of the ovaries compared to ovaries from untreated control mice (Figure 2A). The ovaries from control animals had normal ovarian histoarchitecture showing follicles in different stages of development and fresh corpus luteum (Figure 2B). The ovaries from treated animals showed depletion of ovarian reserve of follicles including primordial and other developing follicles (Figure 2C). The presence of follicles in the chemoablated ovaries was rare and if present was mostly atretic (data not shown). Interestingly the surface epithelium showed marked change after treatment (Figure 2B inset). It appeared prominent, more striated and multilayered with columnar epithelial cells in contrast to the mostly flat, cuboidal, or squamous epithelial cells that surrounded the ovaries from control animals. Compared to the ovaries from control mice, where DAZL was localized in the cytoplasm of oocytes (Figure 2E), chemotherapy resulted in almost complete loss of DAZL positive oocytes (Figure 2F). Loss of DAZL correlated well with significant reduction (P < .01) of Dazl transcripts in the chemoablated ovaries (Figure 2D).
Figure 2.
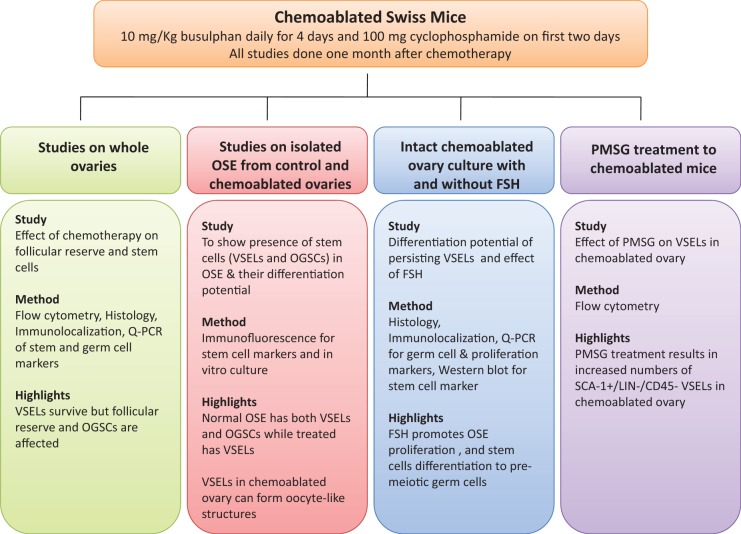
Effect of chemotherapy (busulfan and cyclophosphamide) on mouse ovary and ovarian stem cells. A, Chemoablated ovaries are smaller in size compared to control. B, Hematoxylin and eosin (H&E)-stained control ovarian sections with multiple follicles including (B inset) primordial follicles and single layer of surface epithelial cells (OSE). C, Chemoablated ovary is completely devoid of follicles; however, OSE appeared multilayered (inset). Effect of chemotherapy on oocytes was studied using a germ cell marker DAZL. D, Note significant reduction (P < .01) in Dazl transcripts in chemoablated ovary compared to control with respect to Gapdh by Q-PCR analysis. Immunolocalization using anti-DAZL antibody revealed several oocytes in control ovarian sections (E) compared to no oocytes in chemoablated ovary (F). Effect of chemotherapy on ovarian stem cells was studied using OCT-4 as a marker. Two population of stem cells stained positive for OCT-4 in control ovarian smears (G-I) including small stem cells (VSELs) with nuclear OCT-4 (arrows) and slightly larger cells (OGSCs) with cytoplasmic OCT-4 (asterisks). Occasionally, the OGSCs appeared as clusters (G) and inset represents cluster negative for OCT-4. Negative control for anti-OCT-4 antibody (inset I). In contrast to control, only small nuclear OCT-4 positive VSELs were observed in chemoablated ovarian smears (J-L, arrows). Cytoplasmic OCT-4 positive cells were rarely observed and had relatively low intensity of staining (M, asterisks). The images in (G-I) and (J-M) are merge of green (OCT-4), red (PI), and DIC channels. The Q-PCR results for Oct-4A transcript (representative of nuclear OCT-4 positive VSELs) and total Oct-4 (representative of all Oct-4 isoforms including cytoplasmic OCT-4 positive OGSCs) correlated well with immunolocalization results. Control ovaries revealed lesser expression of Oct-4A compared to total Oct-4 (N), implying the rarity of VSELs compared to OGSCs. In chemoablated ovaries, total Oct-4 transcripts were significantly lower (P < .05; O), however percentage of Oct-4A within the total Oct-4 was significantly higher (P < .05; P) compared to control, and implying predominance of VSELs. Oct-4 and Oct-4A levels represented here were normalized using Gapdh as housekeeping gene. Bar in (B), (C), (E), and (F) equals 20 μm. DAZL indicates deleted in azoospermia like; OCT-4, octamer-binding transforming factor 4; OGSC, ovary germ stem cell; OSE, ovarian surface epithelial; Q-PCR, quantitative polymerase chain reaction; VSELs, very small embryonic-like stem cells. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Very small embryonic-like stem cells persist after chemotherapy
Effect of chemotherapy on the ovarian stem cells was studied by various approaches.
Control ovary
The smears prepared from whole ovaries of control animals showed 2 types of cells in OCT-4 immunochemical study using polyclonal antibody (Abcam) in agreement with our earlier findings.2 Nuclear OCT-4 positive cells were small (3-6 μm) and rarely present (Figure 2I). The cytoplasmic OCT-4 positive cells were larger in size (7-10 μm) and present more frequently than nuclear OCT-4 positive cells (Figure 2G and H asterisks). The later type occasionally appeared as clusters of cytoplasmic OCT-4 positive cells (Figure 2G) probably suggesting their active state similar to actively dividing germ cell cysts well studied during fetal stage.43 However, most of the other cell clusters representing adhering somatic cells were negative for OCT-4 (Figure 2G inset). These results correlated well with Oct-4 mRNA levels in the whole ovary. Oct-4A, the nuclear isoform-specific Oct-4 transcript, was lower than transcript levels of all isoforms put together (represented as Oct-4; Figure 2N).
Chemoablated ovary
In smears prepared from chemoablated ovaries, the nuclear OCT-4 positive cells were observed (Figure 2J-L). The appearance of cytoplasmic OCT-4 positive cells was rare and if present the intensity of cytoplasmic staining of OCT-4 was fainter than controls (Figure 2M). The results suggest that nuclear OCT-4 positive cells persist whereas the cytoplasmic OCT-4 cells are selectively destroyed by chemotherapy. Gene transcript analysis for Oct-4 in chemoablated ovaries shows a significant reduction (P < .05) in levels compared to control ovaries (Figure 2O). However, the percentage of Oct-4A transcript level within the total Oct-4 remained significantly higher (P < .05) after chemotherapy (Figure 2P), suggesting a selective decrease in cytoplasmic Oct-4 isoform.
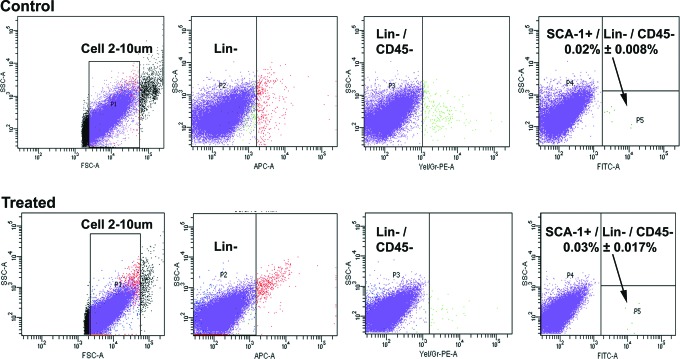
The presence of nuclear OCT-4 positive cells in ovarian smears from both treated and control mice was also confirmed by independent OCT-4A-specific antibody (Supplemental Figure 1). Based on these results and our earlier studies,2,17,18 we propose in mouse ovary that small nuclear OCT-4 positive cells represent VSELs, while the slightly larger cytoplasmic OCT-4 positive cells represent OGSCs. To further confirm that VSELs are present in mouse ovary, flow cytometry experiment based on SCA-1+/Lin−/CD45− expression unique to VSELs24 was carried out. The percentage of SCA-1+/Lin−/CD45− VSELs in whole ovarian population was 0.02% ± 0.01% in control and 0.03% ± 0.017% in chemoablated ovaries (Figure 3). Flow cytometry results provided further evidence that VSELs are present in mouse ovary and they survive chemotherapy.
Figure 3.
Flow cytometry analysis of SCA-1+/Lin−/CD45− VSELs in ovaries from control and chemotreated mice. Cells between 2 and 10 µm were gated using size calibration beads (1) followed by sequential selection for LIN negative population (2), CD45 negative population (3), and then SCA-1 positive population (4) in both control and treated mice. The average percentage of SCA-1+/Lin−/CD45− VSELs with standard deviation from minimum of 4 animals is reported. VSELs indicates very small embryonic-like stem cells.
Effect of Chemotherapy on Stem Cells in Isolated OSE
To confirm that the above-mentioned observations were indeed because of stem cells lodged in ovarian surface epithelium (OSE) and to characterize the stem cells more specifically, additional studies were carried out on isolated OSE preparations. The isolated OSE cells were characterized for their epithelial nature by using epithelial cell-specific markers cytokeratin-8 (CK-8) and E-cadherin (E-Cad). The cells were found positive for both CK-8 (Supplemental Figure 2A) and E-Cad (Supplemental Figure 2B) confirming the epithelial nature. The H&E staining of OSE smears showed the presence of few small spherical cells of 2 sizes with dark nucleus and high nucleocytoplasmic ratio in addition to the epithelial cells (Supplemental Figure 2C and D; arrows and asterisks), which are characteristic of ovarian VSELs and OGSCs reported by us in other mammalian species.18 The removal of OSE was also confirmed using H&E (Supplemental Figure 2E and F) and CK-8 staining (Supplemental Figure 2G and H) of ovaries fixed after removal of the OSE. In general, the number of OSE cells obtained from ovaries of control mice was always higher than treated animals probably because of the differences in size of control and chemoablated ovaries.
Ovarian stem cells are localized to OSE
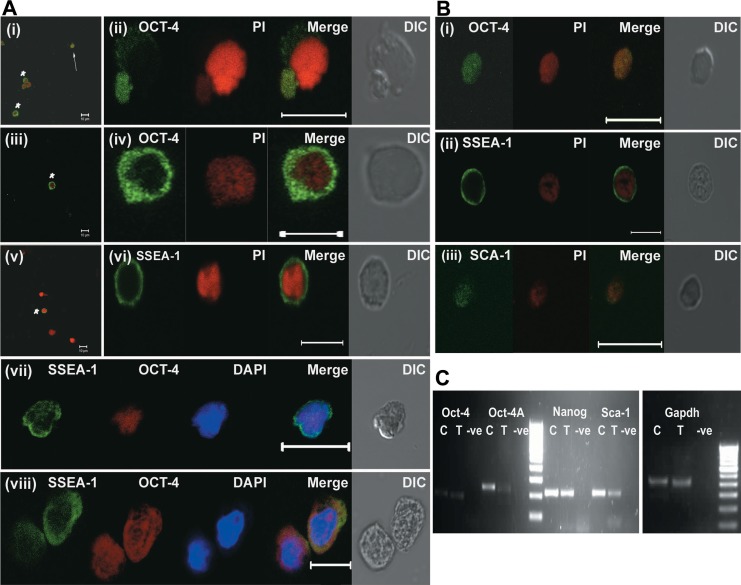
Stem cells in OSE smears were characterized using OCT-4 (Millipore) and SSEA-1 antibodies. Both nuclear OCT-4 (3-6 µm) and cytoplasmic OCT-4 (7-10 µm) were detected by immunofluorescence staining (Figure 4A). SSEA-1 showed positive cell surface localization in cells ranging from 5 to 10 μm size probably constituting both stem cell types (Figure 4A). Dual immunofluorescence of SSEA-1 and OCT-4 confirmed that both nuclear OCT-4 positive and cytoplasmic OCT-4 positive cells were positive for SSEA-1 (Figure 4A). The RT-PCR analysis of control OSE cells showed presence of pluripotent stem cell transcripts Oct-4A, Oct-4, Sca-1, and Nanog (Figure 4C). These results further suggests that the 2 types of cells are VSELs and OGSCs observed in earlier reports and they are localized to OSE similar to other mammals.
Figure 4.
Immunofluorescence characterization of stem cells in OSE smears and their differentiation potential. A, Characterization of stem cells in OSE from control mice. (i-iv) Immunofluorescence using anti-OCT-4 antibody (green) with propidium iodide (red) as counterstain. Note the presence of 2 types of OCT-4 positive cells similar to those observed in whole ovarian smears (Figure 2). The smaller VSELs are positive for nuclear OCT-4 (i-arrows; ii) and slightly larger OGSCs are positive for cytoplasmic OCT-4 (i and iii-asterisks; iv). v-vi, Immunofluorescence using anti-SSEA-1 antibody (green) and PI (red) as counterstain on control OSE smears. The size of SSEA-1 positive cells ranged from 5 to 10 μm probably representing both VSELs and OGSCs. vi, Represents zoomed image of potential VSEL. vii-viii, Dual labeling with anti-OCT-4 (red) and anti-SSEA-1 (green) antibody counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Both nuclear OCT-4 positive VSELs (vii) and cytoplasmic OCT-4 positive OGSCs (viii) were positive for SSEA-1. B, Characterization of stem cells in OSE from treated mice: Immunofluorescence analysis of OSE preparation from chemoablated ovary for OCT-4 (i), SSEA-1 (ii), and SCA-1 (iii) counterstained with propidium iodide (PI; red). Note that OCT-4 shows nuclear localization. C, Real-time polymerase chain reaction (RT-PCR) analysis of RNA isolated from OSE. Image shows presence of Oct-4A, Oct-4, Sca-1, and Nanog in both control and chemoabalated mice. Gapdh was used as housekeeping gene. In the image, C represents control, T represents OSE from chemoablated ovaries, and −ve represents PCR negative control. OCT-4 indicates octamer-binding transforming factor 4; OGSC, ovary germ stem cell; OSE, ovarian surface epithelial; SSEA-1, stage-specific embryonic antigen 1; VSELs, very small embryonic-like stem cells. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
OSE from chemoablated mice contains cells that express VSELs-specific markers
The OSE cell smears isolated from chemoablated mice showed presence of nuclear OCT-4, SSEA-1, and SCA-1 positive cells (Figure 4B), markers expressed by VSELs as reported earlier.24 The RT-PCR analysis showed presence of Oct-4A, Oct-4, Sca-1, and Nanog transcripts in OSE preparations from chemoablated mice similar to control (Figure 4C), confirming the presence of VSELs in OSE after chemotherapy.
Results of this section show that VSELs and OGSCs are present in mouse ovary like other mammals are similarly localized to OSE and chemotherapy affects OGSCs. However, VSELs that are known to be quiescent are spared of the cytotoxic insult. Though the VSELs persist in chemoablated ovary, they were unable to differentiate and form new oocytes. The inability of VSELs to further differentiate may be due to the compromised somatic niche of stem cells as a result of treatment (more details are given in the Discussion section). Thus, following experiments were carried out in vitro to test the functionality of surviving VSELs since inhibitory factors in vivo can be overcome under in vitro conditions.
In Vitro Culture of Isolated OSE Cells to Study Differentiation Potential of VSELs
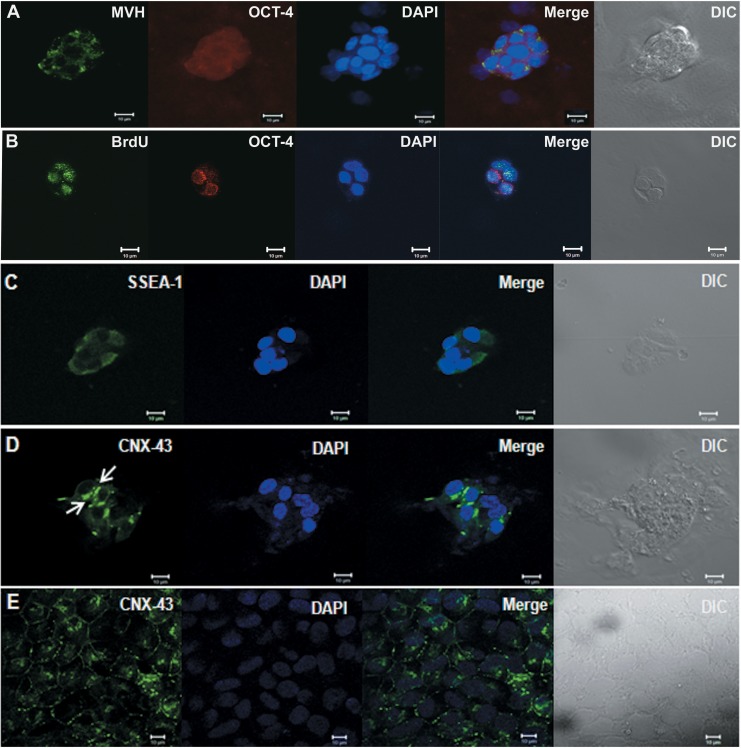
Control mouse OSE cell culture yields germ cell clusters
Clusters of cells with typical ovoid cells were observed after 2 days of control OSE cell culture. Immunofluorescence analysis showed that the clusters were positive for both MVH and cytoplasmic OCT-4 (Figure 5A) suggesting they are germ cell clusters. To confirm that the clusters were formed as a result of rapid cell division as observed in typical germ cell proliferation during fetal ovarian development, incorporation of BrdU was studied. Dual immunolocalization for BrdU and OCT-4 shows that the cytoplasmic OCT-4 positive clusters were positive for BrdU also (Figure 5B). These clusters also expressed SSEA-1 (Figure 5C) and showed characteristic connexin-43 (CNX-43) staining (Figure 5D and Supplemental Figure 3), which are characteristic of germ cell cysts. Results show the differentiation potential of ovarian stem cells into germ cells and formation of proliferating germ cell cyst structures.
Figure 5.
In vitro culture of OSE from control mouse ovaries. When OSEs from control mouse were cultured for 48 hours in the presence of follicle-stimulating hormone (FSH), clusters of cells that were positive for both MVH (green) and cytoplasmic OCT-4 (red; A) were observed. When cultured with BrdU, the OCT-4 positive clusters showed incorporation of BrdU as seen by colocalization of cytoplasmic OCT-4 (red) and BrdU (green; B) suggesting that the ovarian stem cells show the differentiation potential into proliferating germ cells cyst-like structures. Further characterization of the clusters showed expression of germ cell cyst markers stage-specific embryonic antigen 1 (SSEA-1; green; C) and Connexin-43 (CNX-43, green; D). Expression of CNX-43 (green) on human embryonic stem cell line KIND1 (E) was taken as positive control. 4′,6-Diamidino-2-phenylindole (DAPI; blue) was used as nuclear stain. All bars equals 10 μm. BrdU indicates bromodeoxyuridine; MVH, mouse vasa homolog; OCT-4, octamer-binding transforming factor 4; OSE, ovarian surface epithelial. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Chemoablated mouse OSE cell culture ovaries leads to oocyte-like structures
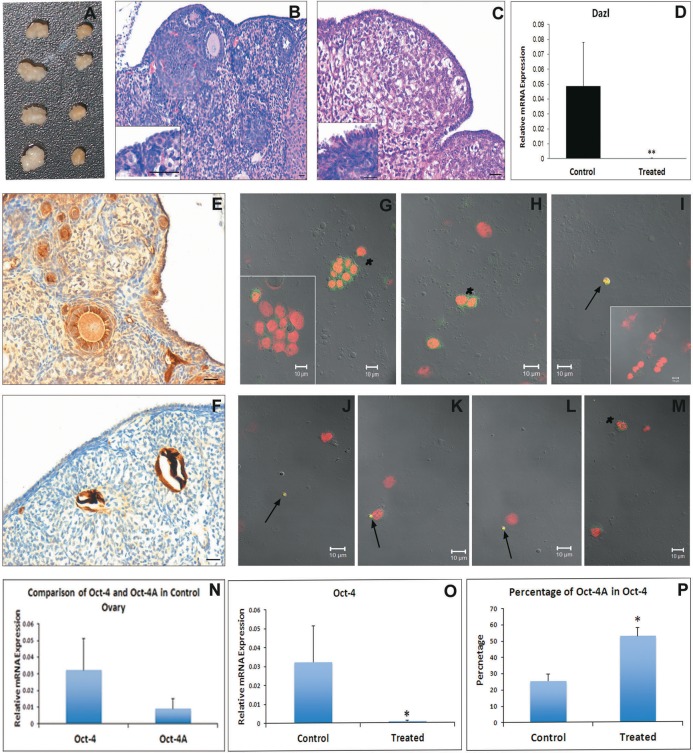
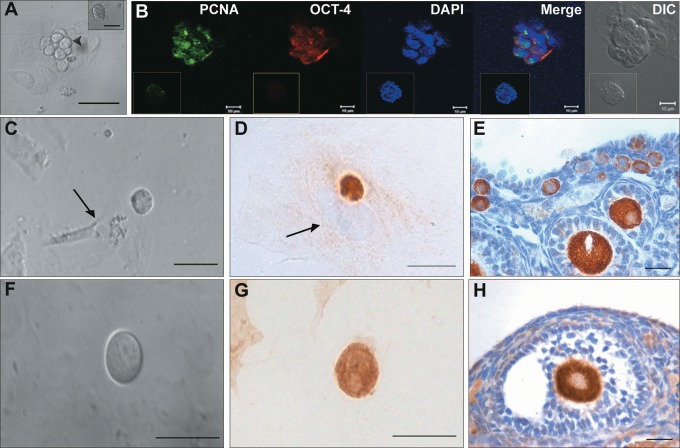
To study differentiation potential of VSELs into oocyte-like cells, cultures of OSE from chemoablated ovaries were studied. Culture of OSE from chemoablated ovary helped to avoid any possible contamination from primordial follicular oocytes, which could potentially contaminate initial control OSE preparations. This was experimentally confirmed by checking for oocyte-specific marker Msy2 gene expression (a marker expressed from primordial follicular diplotene-oocytes to mature oocytes44 in OSE from chemoablated mouse ovaries by RT-PCR on day 0. The day 0 sample was absent for Msy2 expression (Supplemental Figure 4A) indicating that the starting cells for culture was completely devoid of any oocytes. Upon culture, clusters of ovoid cells usually overlying epithelial/fibroblasts cells were observed similar to those observed in control OSE culture on day 2 (Figure 6A). These clusters were positive for cytoplasmic OCT-4 and proliferating cell marker PCNA suggesting they are proliferating stem cell clusters (Figure 6B). They were different from other clusters (formed by cellular aggregation), which usually consisted of a large number of small cells (Figure 6A inset) and were negative for OCT-4 and PCNA (Figure 6B inset). The clusters also incorporated BrdU confirming their proliferating status (Supplemental Figure 4B). On day 6, the clusters were not observed, but single large round oocyte-like cells were observed (Figure 6C and F). Most of the oocyte-like cells observed were found in close association with fibroblasts (Figure 6C arrow). Immunolocalization studies showed the oocyte-like structures were positive for MVH and oocyte-specific marker (from primary follicular stage) GDF-9 (Figure 6D and G, respectively). This experiment shows that persisting VSELs in chemoablated OSE cell culture could differentiate to proliferating progenitors (OCT-4 and PCNA positive germ cell clusters) and to MVH and GDF-9 positive oocyte-like structures in vitro.
Figure 6.
In vitro culture of ovarian surface epithelial (OSE) isolated from chemoablated mouse ovaries. A, Direct microscopic observation of culture on day 2. Clusters of cells (arrowhead) containing fewer cells per cluster with ovoid shape and large nucleus overlying epithelial/fibroblastic cells were observed. Inset represents a different cell cluster with small cells. B, Immunofluorescence analysis shows the cell clusters with ovoid cells were positive for PCNA (green) in nucleus and OCT-4 (red) in cytoplasm suggesting they are proliferating stem cell clusters. Blue represents the nuclear counterstain 4′,6-diamidino-2-phenylindole (DAPI). Insets in respective channels represent nongerm cell cluster showing no staining for OCT-4 and PCNA. C and F, Oocyte-like cells observed on day 6 of culture. They were found in close association with underlying fibroblast cell (arrow). D and G, Immunocytochemical staining of cultured cells harvested on day 6 shows that the oocyte-like cells were positive for germ cell marker MVH (D) and oocyte specific marker GDF-9 (G). In D, arrow represents the nucleus of underlying fibroblast cell. E and H, represents the positive control (mouse ovarian sections) for MVH (E) and GDF-9 (H) that was used to check specificity of the antibodies. Bar equals 20 μm (A and C-H) and 10 μm (B). GDF-9 indicates growth differentiation factor 9; MVH, mouse vasa homolog; OCT-4, octamer-binding transforming factor 4; PCNA, proliferating cell nuclear antigen. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
In Vitro Culture of Chemoablated Intact Ovaries and Effect of FSH
Follicle-stimulating hormone induces proliferation of OSE
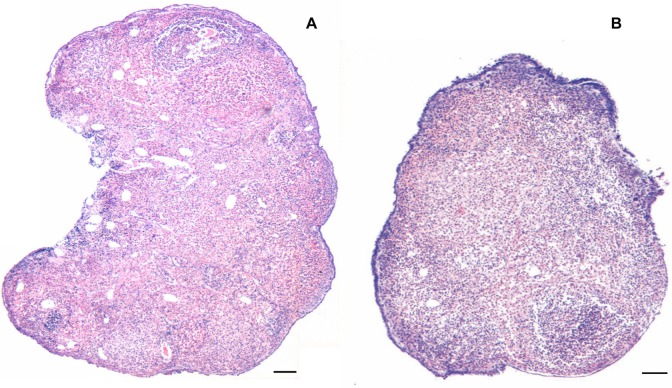
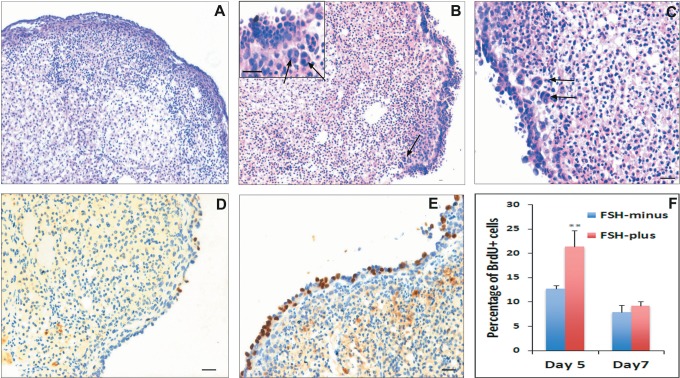
The H&E-stained sections of chemoablated ovaries, studied after 7 days in culture, clearly showed increased thickness of OSE along the periphery in the presence of FSH (FSH-plus group) compared to when FSH was absent (FSH-minus group) in the medium (Figure 7). Higher magnification revealed the presence of multilayered and highly proliferative OSE in FSH-plus group (Figure 8B and C). Frequently, clusters of cells close to OSE were observed only in the FSH-plus group (Figure 8B and C; arrows). To confirm that the OSE proliferated in response to FSH and not because of better survival of cells in the presence of FSH, BrdU incorporation studies were carried out. A higher number of cells in the FSH-plus group incorporated BrdU, indicating that the proliferation of OSE indeed increased in response to FSH (Figure 8D and E). To quantify the number of BrdU positive (BrdU+) cells and total number of OSE cells across the whole section was counted and percentage calculated. There was a significant increase (P < .01) in percentage of BrdU positive cells on day 5 in the FSH-plus group confirming increase in proliferation of OSE (Figure 8F). However, on day 7, there was no difference between the 2 groups and the levels in the FSH-plus group were comparable to that in the FSH-minus group on day 5 suggesting reduced proliferation with time during culture.
Figure 7.
Low magnification comparison of hematoxylin and eosin (H&E)-stained ovarian sections from chemoablated ovaries cultured with and without FSH. The chemoablated ovaries are completely devoid of follicles (similar to that shown in Figure 2C). FSH treatment in vitro for 7 days results in extensive proliferation of ovarian surface epithelial (OSE), which appears thick and multilayered (B) compared to FSH-minus group (A). Bar equals 100 μm. FSH indicates follicle-stimulating hormone.
Figure 8.
Effect of FSH on in vitro cultured chemoablated ovaries: higher magnification of hematoxylin and eosin (H&E)-stained ovarian sections showed presence of multilayered and highly proliferative OSE in FSH-plus group (B and C) compared to FSH-minus group (A). Frequently, clusters of cells (presumably differentiating germ cell clusters, refer Figure 9) close to OSE were observed in FSH-plus group (B and C; arrows). BrdU incorporation studies to investigate the proliferation status of cells showed a higher number of cells incorporate BrdU in the FSH-plus group on day 5 (E) compared to FSH-minus group (D) indicating proliferation of OSE in response to FSH. The percentage of BrdU+ cells was significantly (P < .01) higher on day 5 in FSH-plus group confirming increase in proliferation of OSE (F). However, on day 7, there was no difference between the 2 groups and the levels were comparable to that of percentage in FSH-minus group on day 5 suggesting reduced proliferation with time during culture. Bar equals 20 μm. BrdU indicates bromodeoxyuridine; FSH, follicle-stimulating hormone; OSE, ovarian surface epithelial. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Premeiotic germ cell clusters observed in response to FSH
To investigate if the clusters of cells observed in H&E-stained sections (Figure 8B and C) were indeed germ cell clusters, immunolocalization studies were carried out. These clusters were positive for BrdU indicating that they are proliferating cell clusters and also were found positive for both MVH and premeiotic marker STRA-8 (Figure 9; arrows). In addition, occasionally few STRA-8 positive clusters were observed on the OSE (Figure 9E). The results suggest that the clusters probably represent germ cell cysts similar to that observed in fetal ovarian development43 and similar to the clusters observed in OSE cultures as described previously (Figures 5 and 6B). These clusters were not surrounded by any pregranulosa-like cells as observed in ovigerous cords that consist of germ cell cysts and somatic pregranulosa cells during embryonic ovarian development.43 This suggests that the clusters probably represents premeiotic germ cell cysts (or nests) before ovigerous cord formation.
Figure 9.
Immunolocalization of MVH, STRA-8, and BrdU on in vitro cultured chemoablated ovaries. A and B, Immunolocalization of MVH on ovaries from the FSH-plus group. Note the cluster of cells (arrows) close to OSE is positive for MVH. C, Immunolocalization of BrdU on ovaries from the FSH-plus group. The clusters of cells are also positive for BrdU (arrows) indicating their proliferative state. Immunolocalization of STRA-8 on ovaries from (D) the FSH-minus group and (E) the FSH-plus group. Cell clusters positive (arrows) for Stra8 are observed close to OSE and also on OSE. Mouse testicular section was used as positive control for STRA-8 in which spermatogonia along the basement membrane express STRA-8 (F). Bar equals 20 μm. BrdU indicates bromodeoxyuridine; FSH, follicle-stimulating hormone; MVH, mouse vasa homolog. OSE, ovarian surface epithelial. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Very small embryonic-like stem cells respond to FSH
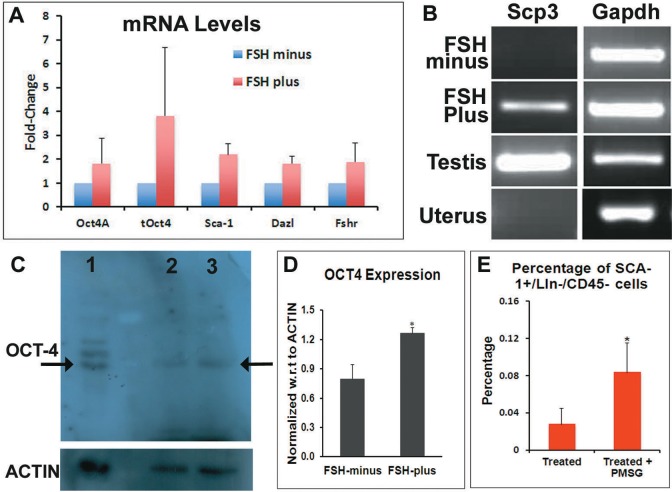
The Q-PCR analysis of pluripotent VSEL markers (Oct-4A, Sca-1), progenitors, and differentiating germ cell markers (Oct-4 and Dazl) showed increase in the FSH-plus group compared to the FSH-minus group (Figure 10A). Fshr (mediator of FSH function) mRNA levels also increased in the FSH-plus group as expected. Through RT-PCR, positive band for Scp3 (meiotic marker) was detected in the FSH-plus group in one of the 4 independent experiments performed (Figure 10B), although SCP3 was not detectable at protein level (data not shown). Western blot analysis using anti-OCT-4 antibody (Millipore) on total cell protein extracts showed a detectable band of OCT-4 in both FSH-plus and minus groups (Figure 10C); although the intensity of the band when normalized with respect to the housekeeping protein ACTIN was significantly more (P < .05) in the FSH-plus group than in the FSH-minus group (Figure 10D). The Q-PCR and Western blot analysis show an increase in stem cell markers suggesting direct stimulatory effect of FSH on stem cells localized in the OSE.
Figure 10.
Analysis of stem/germ cell markers by Q-PCR/Western blot and flowcytometry analysis of SCA-1+/Lin−/CD45− VSELs in ovaries of PMSG-treated chemoablated mice. A, Q-PCR analysis for stem (Oct-4A, Sca-1), germ cell (Oct-4, Dazl) markers, and FSH receptors showed increase in FSH-plus group compared to FSH-minus group. 18s was used for normalization. B, Real-time polymerase chain reaction (RT-PCR) analysis for meiotic marker Scp3 showed the presence of Scp3 mRNA in FSH-plus group and positive control mouse testicular sample, while Scp3 was absent in FSH-minus group and negative control uterine sample. Gapdh was used as housekeeping gene. C, Western blot analysis using anti-OCT-4 antibody on total cell protein extracts from whole ovary showed a band of OCT-4 both in FSH-minus (lane 2) and FSH-plus group (lane 3). Human embryonic stem cells extract was used as positive control (lane 1). Actin was used as housekeeping protein (C lower panel). D, Densitometry analysis of the OCT-4 band with respect to Actin showed the amount of OCT-4 in FSH-plus group is significantly higher (P < .05) compared to FSH-minus group. E, Comparison of SCA-1+/Lin−/CD45− VSELs in ovaries of chemoablated mice (treated) and chemoablated mice treated with PMSG (treated + PMSG) by flowcytometry. The percentage of SCA-1+/Lin−/CD45− was significantly more (P < .1) in treated + PMSG than just treated mice. BrdU indicates bromodeoxyuridine; FSH, follicle-stimulating hormone; PMSG, pregnant mare serum gonadotropin; OCT-4, octamer-binding transforming factor 4; Q-PCR, quantitative polymerase chain reaction; VSELs, very small embryonic-like stem cells. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Effect of PMSG Treatment to Chemoablated Mice
To test the effect of FSH on VSELs in vivo, flow cytometric analysis of SCA-1+/Lin−/CD45− VSELs in chemoablated ovaries exposed to PMSG (an FSH analog) was carried out. The percentage of VSELs increased significantly to 0.08% ± 0.03% (P < .1) in comparison to 0.03% ± 0.017% in chemoablated mice (Figure 10E). The results provide further support to the results obtained on intact ovary culture (with and without FSH, as discussed earlier) that FSH treatment leads to increase in mouse ovarian VSELs activity and our earlier published results.32,35
To summarize, all the above-mentioned results (both OSE and whole ovary culture) suggest that the chemoablated mouse ovaries, which lack oocytes, can be reinstated to have oocyte-specific differentiation from surviving stem cells. As only VSELs were observed to persist in chemoablated ovary, the differentiation to germ cells observed can be presumed to have initiated from surviving VSELs. The results show FSH has an important role in differentiation. It induces VSEL numbers in chemoablated ovaries and initiates their differentiation into germ cells and oocyte-like structures. However, the meiotic block is not overcome by FSH alone and suggests the need for additional factors as discussed in the following section.
Discussion
In the present study, we have identified 2 populations of potential stem cells in mouse ovaries—VSELs and ovarian germ stem cells (OGSCs), which are localized in the ovary surface epithelium (OSE). Culture of enzymatically isolated OSE shows formation of proliferating germ cell cysts similar to fetal ovary.43 Chemotherapy destroyed the ovarian follicular reserve as expected and also the potential OGSCs. However, VSELs survived possibly because they are quiescent. In OSE cultures from chemoablated ovaries, proliferating germ cell clusters and MVH and GDF-9 positive oocyte-like structures were observed, which probably formed from differentiation of the surviving VSELs. Further, studies on intact chemoablated ovary culture and in vivo PMSG treatment showed that FSH stimulates surviving VSELs, as evident by formation of proliferating germ cell clusters and increase in their numbers. To the best of our knowledge, this is the first report showing survival of stem cells (VSELs) in mouse ovary which otherwise undergoes premature failure due to chemotherapy. Similar POF is frequently observed in women cancer survivors since recent advances in treatment options have considerably improved survival rates in patients with cancer.45 The present study provides hope that these surviving VSELs could help restore normal ovarian function or be manipulated to yield autologous oocytes for use during assisted reproduction in future for cancer survivors. This may preclude the need to bank oocytes, embryos, or ovarian cortical tissue prior to oncotherapy—since stem cells survive oncotherapy and may be manipulated when required to restore ovarian function. It is interesting to mention here our earlier report that human and marmoset ovarian cortical tissue besides being a source of primordial follicles is also an excellent source of ovarian stem cells.32
Stem Cells in Adult Mouse Ovary
In agreement with earlier reports from our group, we report the presence of 2 populations of potential stem cells in adult mouse ovary. They include VSELs demonstrated by expression of nuclear OCT-4, cell surface SSEA-1, SCA-1+/Lin−/CD45− and Nanog (Figures 2 –4), and slightly larger progenitors termed OGSCs characterized by cytoplasmic OCT-4 and SSEA-1. Results of the present study differ slightly from the views recently proposed on ovarian stem cells by Woods and Tilly.46 The OSCs described by them are possibly equivalent to the OGSCs described in the present study. The present study shows that adult mouse ovaries also possess more primitive and potentially pluripotent VSELs in addition to the OSCs.
The present study also shows differentiation of ovarian stem cells and formation of proliferating germ cell clusters/cysts both in isolated OSE and whole ovary cultures. This was demonstrated by expression of MVH and OCT-4, SSEA-1, CNX-43, STRA-8, and incorporation of BrdU. Proliferating germ cell cysts area well-documented phenomenon during fetal oogenesis43 and demonstration of such cysts in adult mammalian ovary has been considered as evidence for postnatal oogenesis. This study shows that postnatal stem cells indeed can form nests as are seen during fetal oogenesis prior to follicle formation in controlled conditions and contradicts studies that deny presence of germ cell cysts in adult mammalian ovary.10,13 We have demonstrated similar phenomenon in sheep ovaries17 and have also published explanations for lack of germ cell cysts observed by others recently.16
Effect of Chemotherapy on Mouse Ovarian Follicular Reserve and Stem Cells
The present study demonstrates that VSELs persist in chemoablated mouse ovaries, while the potential progenitors (OGSCs), follicular cells, and differentiated oocytes are severely affected, resulting in depletion of ovarian reserve. This was demonstrated by histology, gene expression, flow cytometry, and immunofluorescence analysis for pluripotent stem cell markers in both whole ovary and OSE preparations post chemoablation. Flow cytometry results showed that the percentage of VSELs in chemoablated ovaries (0.03% ± 0.017%) was comparable to control (0.02% ± 0.01%). The results are similar to our observations in mouse testis47 and in agreement with earlier report48 that showed murine bone marrow VSELs are resistant to total body irradiation while the hematopoietic stem cells (HSCs) are affected. The surviving VSELs proliferate as shown through BrdU uptake but fail to differentiate into HSCs.48 The inability of VSELs to further differentiate may be explained based on compromised somatic niche of stem cells as a result of treatment.
The integrity of the stem cell niche is critical for the balance of self-renewal and differentiation of any stem cell in an organism.49 Park et al50 have reported that the somatic environment of ovary was severely affected after combined busulfan and cyclophosphamide treatment in mice. They showed that the compromised environment could not support oocyte/follicle growth when primordial follicles obtained from immature mouse were transplanted 1 week after treatment. Age-induced reduction in fertility and menopause has also been explained based on compromised niche. Many studies have shown that ovarian stem cells are present in aged mouse and in perimenopausal women.2,6,32,36,39,51 Niikura et al39 demonstrated that the stem cells from aged ovary retain differentiation potential and form oocytes when exposed to young ovarian environment. Other studies have independently reported that the stem cells from perimenopausal women retain potential to differentiate into oocyte-like structures and parthenotes in in vitro conditions in which the inhibitory factors that exist in vivo are overcome.2,36 All these studies have led to conclusions that failure of oocyte replenishment and new follicle formation in the aged ovary is probably due to impairment of the somatic microenvironment rather than depletion/aging of the stem cells.29,52–54 In addition, recovery of atrophied ovarian function and spontaneous pregnancies have been reported after heterotopic transplantation of cryopreserved ovarian cortical tissue or after allogeneic bone marrow.55–58 In these cases, it has been suggested that the heterotopic ovarian transplant or the bone marrow transplantation may have improved the ovarian niche (microenvironment) through endocrine/paracrine factors resulting in the recovery of ovarian function from persisting stem cells.29,59,60 Similar to ovary, reports in male mice have shown that spermatogonial stem cell transplantation in irradiated or chemodepleted testes could only support colonization and not differentiation.61 This has led to a similar conclusion in testis that the compromised somatic niche does not support stem cell differentiation.61,62 We were able to successfully restore endogenous spermatogenesis by providing healthy niche through transplantation of sertoli or mesenchymal cells into chemoablated mouse testis.47 Similar studies should be undertaken in ovaries of higher animal models to restore somatic niche not only in chemoablated ovaries but also in aged ovaries with an aim to restore neo-oogenesis and primordial follicle assembly. This could possibly be achieved by transplanting mesenchymal cells (which are source of growth factors and cytokines) in the ovaries and careful follow-up to study functional restoration.
Differentiation Potential of VSELs That Survive Chemotherapy
In this study, we have shown that under in vitro conditions, chemoablated ovarian cells show formation of germ cell nests which coexpress PCNA and OCT-4, incorporate BrdU, and differentiate to form GDF-9 positive oocytes during OSE cells cultured in vitro (Figure 6). As the results of the present study show that the only stem cell type that survives chemotherapy is VSELs, it can be presumed that the proliferating germ cells and oocytes could have formed from differentiation of surviving VSELs. This ability of VSELs to initiate oocyte-specific differentiation appears to be modulated by FSH. The differentiation observed during OSE culture was in the presence of FSH. The role of FSH was delineated during intact chemoablated ovary culture. Treatment of FSH induced proliferation of OSE (Figures 7 and 8), increased mRNA transcripts of Oct-4A and Sca-1 (Figure 10A), and also OCT-4 (Figure 10C). The PMSG treatment to chemoablated mice resulted in higher percentage of VSELs compared to untreated mice (0.08% ± 0.03% vs 0.03% ± 0.017%). Increase in proliferation of OSE in response to FSH or FSH analogs have been shown earlier both in vivo and in vitro.31,35,63–66 Patel et al31 reported that the sheep ovarian stem cells express FSH receptors and undergo proliferation and clonal expansion in response to FSH via a novel FSHR transcript R3. The potential role of FSH-FSH3 in stem cells with respect to ovarian biology was recently reviewed.34 Similarly Hillard et al63 recently reported that FSH increases mouse OSE proliferation through activation of oncogenic pathways in surface epithelial cells. It is likely that in women with POF and high levels of FSH, stem cells may undergo proliferation and initial differentiation. However, haploid oocyte formation and primordial follicle assembly may require additional factors, which are not available in the compromised somatic niche (due to chemotherapy).
Although oocyte-like structures were observed during in vitro OSE culture, oocyte formation was not observed during intact chemoablated ovary culture. During intact ovary culture, FSH treatment induced the formation of germ cell clusters that incorporate BrdU and are positive for MVH and STRA-8. It also led to an increase in mRNA levels of Dazl, a critical protein involved in early germ cell differentiation,67 suggesting increase in differentiation of stem cells to germ cells. MVH is a germ cell-specific marker68 whose efficient translation depends on interaction with DAZL,69 and STRA-8 is required for meiosis initiation and is expressed by ovarian germ cells just before meiosis initiation.70 Expression of both MVH and STRA-8 and incorporation of BrdU in cell clusters suggest that FSH treatment induced stem cell proliferation and formation of clusters and further differentiation into germ cells. However, the germ cells did not go through meiosis, as meiotic marker SCP3 was not detected at protein level in any of the experiments. Detection of Scp3 mRNA (Figure 10B) in one of the experiments suggests that the new germ cells formed have the potential to undergo meiosis. In addition, there were no obvious ovigerous cord-like structures (ie, germ cells surrounded by granulosa-like somatic cells) observed whose formation normally coincides with meiotic initiation during mouse ovarian development at E13.5.71 Even though there is a possibility of stem cells being genetically altered as a result of chemotherapy, lack of meiosis leading to full differentiation of germ cells to oocytes and new follicle assembly of chemoablated mouse ovaries (may also explain the POF phenotype in women despite high serum FSH levels) observed in this study could be better explained based on (1) requirement of additional factors besides FSH for oogenesis/follicular formation and (2) inability to recruit pregranulosa cells for primordial follicle assembly. We have earlier proposed that neo-oogenesis occurs from the stem cells and surrounding epithelial cells undergo epithelial–mesenchymal transition to result in primordial follicle assembly.2,29 Both these processes appear to be affected by chemotherapy and as mentioned earlier, further research is required in higher animal species to develop strategies to restore these processes in chemoablated ovary.
Clinical evidence exists wherein transplanting frozen ovarian cortical tissue pieces in a heterotopic site restored functionality of the atretic ovary resulting in spontaneous pregnancies and live births.55 The transplanted cortical tissue possibly provides necessary factors to restore the functionality of the surviving stem cells in the atretic ovary as has been speculated earlier.59,60 Thus, it is possible that improving the local niche of the chemoablated ovary may restore its function. As mentioned earlier, we have attempted this by transplanting Sertoli cells/mesenchymal cells into chemoablated testis, which resulted in restoration of testicular function.47 Similar attempts on our part to transplant mesenchymal cells in chemoablated mouse ovary failed for 2 reasons: (1) mouse ovary is small in size and further reduces in size due to chemotherapy and (2) the dose of busulfan and cyclophosphamide used in the present study to ensure complete depletion of follicles does not support long-term survival of the mice for follow-up studies. Few other groups have transplanted mesenchymal cells in chemoablated ovary and reported restored ovarian function.72–76 All these groups have transplanted cells immediately after chemotherapy and have not waited for chemotherapy to completely destroy follicular reserve. Hence the effect appears more protective than restoration of ovarian function.
Future Prospects
Further studies are required in higher animal species to develop novel approaches to restore ovarian function from the surviving VSELs in chemoablated ovary. It is imperative to mention here that future research should focus on developing both in vivo and in vitro strategies to obtain functional oocytes from the persisting VSELs. Such strategies may also benefit existing cancer survivors, including women with POF who were deprived of fertility preservation options prior to oncotherapy and aged women with compromised ovarian function who wish to have a family.
Making gametes for infertile couples by differentiating embryonic and induced pluripotent stem cells remain a distant dream. The major bottle-neck being faced at present is differentiating them into primordial germ cells, although some success has been achieved recently.77 Pluripotent VSELs with a distinct epigenetic profile may provide a better option to obtain gametes, because they are indeed the primordial germ cells that survive in adult ovaries and testes.78
Supplementary Material
Acknowledgments
We thank Department of Science and Technology, Government of India for providing KS with Woman Scientist fellowship under Scheme-A, SR/WOSA/LS-222/2010(G). Thanks to Harshada and Pranesh in SCB Department for their help. We thank Shobha and Reshma for confocal microscopy studies and Dr Srabani Mukherjee, Gayatri, Sushma, and Yuvika for their help in carrying out flow cytometry studies.
Authors’ Note: NIRRH Accession number for this article RA/36/10-2013.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Department of Science and Technology, Government of India under Woman Scientist Scheme-A [SR/WOSA/LS-222/2010(G)] and NIRRH core support provided by Indian Council of Medical Research, Government of India, New Delhi
Supplemental Material: The online data supplements are available at http://rs.sagepub.com/supplemental.
References
- 1. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428 (6979):145–150. [DOI] [PubMed] [Google Scholar]
- 2. Parte S, Bhartiya D, Telang J, et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011;20 (8):1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 2010;79 (3):159–170. [DOI] [PubMed] [Google Scholar]
- 4. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11 (5):631–636. [DOI] [PubMed] [Google Scholar]
- 5. Zhang D, Fouad H, Zoma WD, Salama SA, Wentz MJ, Al-Hendy A. Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci. 2008;15 (2):139–146. [DOI] [PubMed] [Google Scholar]
- 6. Virant-Klun I, Zech N, Rozman P, et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76 (8):843–856. [DOI] [PubMed] [Google Scholar]
- 7. Bukovsky A, Caudle MR, Svetlikova M, Upadhyaya NB. Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod Biol Endocrinol. 2004;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18 (3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Y, Bai Y, Chu Z, et al. GSK3 inhibitor-BIO regulates proliferation of female germline stem cells from the postnatal mouse ovary. Cell Prolif. 2012;45 (4):287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci. 2013;110 (21):8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oatley J, Hunt PA. Of mice and (wo) men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod. 2012;86 (6):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Notarianni E. Reinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserve. J Ovarian Res. 2011;4 (1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109 (31):12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J Exp Clin Assist Reprod. 2006;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woods DC, White YA, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci. 2013;20 (1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhartiya D, Sriraman K, Parte S, Patel H. Ovarian stem cells: absence of evidence is not evidence of absence. J Ovarian Res. 2013;6 (1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parte S, Bhartiya D, Patel H, et al. Dynamics associated with spontaneous differentiation of ovarian stem cells in vitro . J Ovarian Res. 2014;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhartiya D, Kasiviswanathan S, Unni SK, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem. 2010;58 (12):1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327 (5965):542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Rosa L, De Luca M. Cell biology: dormant and restless skin stem cells. Nature. 2012;489 (7415):215–217. [DOI] [PubMed] [Google Scholar]
- 21. Bhartiya D, Unni S, Parte S, Anand S. Very small embryonic-like stem cells: Implications in reproductive biology. Biomed Res Int. 2013;2013:682326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281 (44):33554–33565. [DOI] [PubMed] [Google Scholar]
- 23. Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4 (+) SSEA-1(+) Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20 (5):857–869. [DOI] [PubMed] [Google Scholar]
- 24. Zuba-Surma EK, Kucia M, Wu W, et al. Very small embryonic-like stem cells are present in adult murine organs: Image stream-based morphological analysis and distribution studies. Cytometry A. 2008;73A(12):1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin DM, Liu R, Klich I, et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24 (8):1450–1461. [DOI] [PubMed] [Google Scholar]
- 26. Mierzejewska K, Heo J, Kang JW, et al. Genome-wide analysis of murine bone marrow-derived very small embryonic-like stem cells reveals that mitogenic growth factor signaling pathways play a crucial role in the quiescence and ageing of these cells. Int J Mol Med. 2013;32 (2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin DM, Zuba-Surma EK, Wu W, et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4 (+) very small embryonic-like stem cells. Leukemia. 2009;23 (11):2042–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Havens AM, Sun H, Shiozawa Y, et al. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo . Stem Cells Dev. 2014;23 (7):689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhartiya D, Sriraman K, Parte S. Stem cell interaction with somatic niche may hold the key to fertility restoration in cancer patients. Obstet Gynecol Int. 2012;2012:921082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parte S, Patel H, Sriraman K, Bhartiya D. Isolation and characterization of stem cells in adult mammalian ovary. Methods Mol Biol. 2015;1235:203–229. [DOI] [PubMed] [Google Scholar]
- 31. Patel H, Bhartiya D, Parte S, Gunjal P, Yedurkar S, Bhatt M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. J Ovarian Res. 2013;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Parte S, Bhartiya D, Manjramkar DD, Chauhan A, Joshi A. Stimulation of ovarian stem cells by follicle stimulating hormone and basic fibroblast growth factor during cortical tissue culture. J Ovarian Res. 2013;6 (1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Babu PS, Krishnamurthy H, Chedrese PJ, Sairam MR. Activation of extracellular-regulated kinase pathways in ovarian granulosa cells by the novel growth factor type 1 follicle-stimulating hormone receptor. Role in hormone signaling and cell proliferation. J Biol Chem. 2000;275 (36):27615–27626. [DOI] [PubMed] [Google Scholar]
- 34. Bhartiya D, Singh J. FSH-FSHR3-stem cells in ovary surface epithelium: basis for adult ovarian biology, failure, aging and cancer. Reproduction. 2015;149 (1):R35–R48. [DOI] [PubMed] [Google Scholar]
- 35. Bhartiya D, Sriraman K, Gunjal P, Modak H. Gonadotropintreatment augments postnatal oogenesis and primordialfollicleassembly in adult mouseovaries? J Ovarian Res 2012;5 (1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Virant-Klun I, Rozman P, Cvjeticanin B, et al. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009;18 (1):137–149. [DOI] [PubMed] [Google Scholar]
- 37. Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol. 2005;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong SP, Lee ST, Lee EJ, et al. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil Steril. 2010;93 (8):2594–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging. 2009;1 (12):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Symonds DA, Tomic D, Miller KP, Flaws JA. Methoxychlor induces proliferation of the mouse ovarian surface epithelium. Toxicol Sci. 2005;83 (2):355–362. [DOI] [PubMed] [Google Scholar]
- 41. Lei L, Zhang H, Jin S, et al. Stage-specific germ–somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208 (3):640–647. [DOI] [PubMed] [Google Scholar]
- 42. Wright CS, Hovatta O, Margara R, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14 (6):1555–1562. [DOI] [PubMed] [Google Scholar]
- 43. Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143 (2):139–149. [DOI] [PubMed] [Google Scholar]
- 44. Gu W, Tekur S, Reinbold R, et al. Mammalian male and female germ cells express a germ cell specific Y-box Protein, MSY2. Biol Reprod. 1998;59 (5):1266–1274. [DOI] [PubMed] [Google Scholar]
- 45. Stovall DW, McGee EA. How chemotherapy harms ovarian function: and how to assess your patients’ risk and reproductive status. SRM. 2010;8(3):21–28. [Google Scholar]
- 46. Woods DC, Tilly JL. An evolutionary perspective on adult female germline stem cell function from flies to humans. Semin Reprod Med. 2013;31 (1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anand S, Bhartiya D, Sriraman K, Patel H, Manjramkar DD. Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J Stem Cell Res Ther. 2014;4(7):216. [Google Scholar]
- 48. Ratajczak J, Wysoczynski M, Zuba-Surma E, et al. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011;39 (2):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132 (4):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park MR, Choi YJ, Kwon DN, et al. Intraovarian transplantation of primordial follicles fails to rescue chemotherapy injured ovaries. Sci Rep. 2013;3:1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Virant-Klun I, Skutella T. Stem cells in aged mammalian ovaries. Aging. 2010;2 (1):3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bukovsky A. Ovarian stem cell niche and follicular renewal in mammals. Anat Rec. 2011;294 (8):1284–1306. [DOI] [PubMed] [Google Scholar]
- 53. Massasa E, Costa XS, Taylor HS. Failure of the stem cell niche rather than loss of oocyte stem cells in the aging ovary. Aging. 2010;2 (1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15 (7):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oktay K, Türkçüoğlu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril. 2011;95 (2):804. e7–e10. [DOI] [PubMed] [Google Scholar]
- 56. Demeestere I, Simon P, Buxant F, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21 (8):2010–2014. [DOI] [PubMed] [Google Scholar]
- 57. Salooja N1, Szydlo RM, Socie G, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358 (9278):271–276. [DOI] [PubMed] [Google Scholar]
- 58. Schimmer AD, Quatermain M, Imrie K, et al. Ovarian function after autologous bone marrow transplantation. J Clin Oncol. 1998;16 (7):2359–2363. [DOI] [PubMed] [Google Scholar]
- 59. Oktay K, Goswami S, Darzynkiewicz Z. Manipulating ovarian aging: a new frontier in fertility preservation. Aging. 2011;3 (1):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21 (6):1345–1348. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Z, Shao S, Meistrich ML. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211 (1):149–158. [DOI] [PubMed] [Google Scholar]
- 62. Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92 (2):577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hilliard TS, Modi DA, Burdette JE. Gonadotropins activate oncogenic pathways to enhance proliferation in normal mouse ovarian surface epithelium. Int J Mol Sci. 2013;14 (3):4762–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burdette JE, Kurley SJ, Kilen SM, Mayo KE, Woodruff TK. Gonadotropin-induced superovulation drives ovarian surface epithelia proliferation in CD1 mice. Endocrinology. 2006;147 (5):2338–2345. [DOI] [PubMed] [Google Scholar]
- 65. Stewart SL, Querec TD, Gruver BN, O’Hare B, Babb JS, Patriotis C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J Cell Physiol. 2004;198 (1):119–124. [DOI] [PubMed] [Google Scholar]
- 66. Davies BR, Finnigan DS, Smith SK, Ponder BA. Administration of gonadotropins stimulates proliferation of normal mouse ovarian surface epithelium. Gynecol Endocrinol. 1999;13 (2):75–81. [DOI] [PubMed] [Google Scholar]
- 67. Kerr CL, Cheng L. The dazzle in germ cell differentiation. J Mol Cell Biol. 2010;2 (1):26–29. [DOI] [PubMed] [Google Scholar]
- 68. Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93 (1-2):139–149. [DOI] [PubMed] [Google Scholar]
- 69. Reynolds N, Collier B, Maratou K, et al. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet. 2005;14 (24):3899–3909. [DOI] [PubMed] [Google Scholar]
- 70. Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2005;103 (8):2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nicholas CR, Haston KM, Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev Biol. 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10 (4):353–363. [DOI] [PubMed] [Google Scholar]
- 73. Liu T, Huang Y, Guo L, Cheng W, Zou G. CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int J Med Sci. 2012;9 (7):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takehara Y, Yabuuchi A, Ezoe K, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93 (2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sun M, Wang S, Li Y, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4 (4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang S, Yu L, Sun M, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Irie N, Weinberger L, Tang WWC, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160 (1-2):253–268. doi:10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bhartiya D, Hinduja I, Patel H, Bhilawadikar R. Making gametes from pluripotent stem cells—a promising role for very small embryonic-like stem cells. Reprod Biol Endocrinol. 2014;12:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.