Abstract
Background
Work examining the link between lower heart rate variability (HRV) and depression in children and adolescents is lacking, especially in light of the physiological changes that occur during pubertal development.
Method
We investigated the association between spectral measures of resting HRV and depressive symptoms among 127 children and adolescents, ages 10–17. Using spectral analysis, we evaluated (1) the association between relative high frequency (HF) HRV and depressive symptoms; (2) the predictive power of relative HF HRV for depressive symptoms in the context of relative low frequency (LF) and relative very low frequency (VLF) HRV; and (3) the relationship between relative HF, LF, and VLF band activity, age and pubertal maturation.
Results
Consistent with previous work, results revealed that relative HF HRV was negatively associated with self-reported depressive symptoms. As well, relative VLF HRV was positively associated with depressive symptoms. Regression analyses revealed that relative HF HRV and relative VLF HRV significantly predicted self-report depressive symptoms while controlling for age, sex and pubertal maturation, with relative VLF HRV emerging as the strongest indicator of depressive symptoms. Developmental findings also emerged. Age and pubertal maturation were negatively associated with relative HF HRV and positively correlated with relative VLF HRV.
Conclusions
Results provide support for the relationship between HRV and depression and suggest that both HF and VLF HRV are relevant to depression symptom severity. Findings also reinforce the importance of considering pubertal development when investigating HRV-depression associations in children and adolescents.
Limitations
Influences on cardiac control including physical activity levels and exercise patterns could be controlled in future work. Our data speak to a depressive symptom dimension and relative spectral power HRV. Thus, we cannot make strong claims about relative spectral power HRV and clinical depression.
Keywords: Adolescence, depression, heart rate variability, time-frequency analysis
1. Introduction
Depression is a common mental illness, prevalent not only in adults, but also in children and adolescents. According to the National Institute of Mental Health, approximately 11 percent of adolescents have a lifetime prevalence of a depressive disorder, and the risk of developing a depressive disorder increases across adolescence. Interestingly, depressive symptoms in children are strong predictors of later depression in adolescence (Keenan et al., 2009) and also into adulthood (Pine et al., 1999). In addition to its widely studied cognitive symptoms, depression is associated with a number of physical signs and symptoms including pain, fatigue, digestive problems and even changes in the body’s inflammatory response (Bizik et al., 2014; Trivedi, 2004). A growing body of work shows that depression is associated with cardiac function, particularly heart rate variability (HRV) (Carney et al., 2005, 2001), although there is disagreement concerning the cause of HRV abnormalities among those with major depressive disorder (MDD) (Kemp et al., 2011; Licht et al., 2011). Some investigators find that changes in HRV are due to clinical depression itself (Kemp et al., 2010) whereas others report that antidepressant treatment contributes to reductions in HRV (Licht et al., 2010). In their meta-analytic review, Kemp et al. (2010) concluded that among adults without cardiovascular disease, those with MDD had a reduction in HRV (high frequency (HF) spectral measure) and HF HRV was negatively associated with depressive symptom severity. Anti-depressant medications did not affect HRV despite clinical improvement in their depressive symptoms, suggesting that the change in HRV is related to the underlying depression, and not medications (Kemp et al., 2010). Conversely, Licht and colleagues (Licht et al., 2010) report a decrease in HRV among individuals with prolonged use of antidepressants, with partially reversible effects after discontinuing the treatment (see Licht et al. (2015) for similar findings older adults). These findings suggest that medications could cause the decrease in HRV, perhaps more than the depression itself (Licht et al., 2010).
HRV is a non-invasive measure of autonomic nervous system (Evans et al., 2013) function. HRV can reflect the balance between the sympathetic and parasympathetic branches of the ANS. The sympathetic nervous system mediates the body’s fight or flight response, while the parasympathetic nervous system promotes self-regulation and self-soothing (Porges, 2007). Polyvagal theory links autonomic regulation of the heart to the individual’s ability to adapt to challenges in the environment (Porges, 1995b). The ANS, and parasympathetic activity in particular, have been associated with cardiac vagal tone, reflecting input of the vagus nerve to the heart (Porges, 1995c). Vagal control affects the beat-to-beat pattern of the heart, and the amount of variability between heartbeats. HRV is therefore a useful indicator of vagal tone (Friedman, 2007). HRV has been measured by respiratory sinus arrhythmia (RSA), an index of vagal activity that reflects naturally occurring variation in heart rate that occurs during a breathing cycle (Berntson et al., 1997, 1993). Previous work found that low HRV suggests decreased parasympathetic or increased sympathetic activity (Michels et al., 2013; Task Force, 1996). Furthermore, HRV and the related metric, RSA, have been linked to emotion regulation ability across age groups (Berntson et al., 1997, 1993; Porges, 1995a, 1994a, 1996). Consistent with the link between vagal tone and emotion regulation (Berntson et al., 1997, 1993; Porges, 1995a, 1994a, 1996), low vagal tone at baseline is associated with negative emotional traits (Beauchaine, 2001; Thayer and Lane, 2000). Further, a number of studies in adult samples find an association between low HRV and depressive disorders (Carney et al., 2005; Gorman and Sloan, 2000; Licht et al., 2008; Rechlin et al., 1995, 1994; Roose et al., 1989).
Fewer studies have examined the relation between HRV and depression in adolescents and children. Adolescent studies have focused mainly on females with clinical diagnoses. Henje Blom and colleagues (Henje Blom et al., 2010) found that adolescent females diagnosed with anxiety disorders, major depressive disorder (MDD), or both, had lower HRV compared with healthy controls. In the same study, healthy control participants who reported more depressive symptoms on the Beck Depressive Inventory exhibited less HF HRV (Henje Blom et al., 2010). Similarly, Tonhajzerova et al. (2010) found that female adolescents with MDD showed significantly decreased HRV magnitude compared to a control cohort (Tonhajzerova et al., 2010). Lower RSA has also been linked to MDD in adolescent females (Tonhajzerova et al., 2009). In preadolescents, somatic symptoms were negatively related to HRV, while cognitive symptoms were positively related to HRV (Bosch et al., 2009).
Studies in children more often examine the link between broad-band internalizing problems (spanning anxiety and depression) and HRV. In a study of 3–9-year old children, most with a parent history of child onset depression, low resting RSA was related to internalizing problems (Forbes et al., 2006). Similarly, in a sample of 8–12 year old children at risk for depression and conduct problems, low levels of RSA conferred significant risk for depression (Shannon et al., 2007). Dietrich and colleagues (2007) found that low basal RSA predicted internalizing symptoms in children (Dietrich et al., 2007), while Hinnant and El-Sheikh (2009) observed that lower levels of basal RSA predicted both internalizing and externalizing symptoms, although under stress greater internalizing symptoms predicted RSA suppression whereas greater externalizing symptoms predicted RSA augmentation (Hinnant and El-Sheikh, 2009). Taken together, these studies suggest lower basal RSA or RSA “at rest” is associated with greater depressive and internalizing symptomatology in children and adolescents.
Much of the previous work has used RSA as an index of HRV (Berntson et al., 1997, 1993). The overall variability that composes HRV can also be broken down into the frequency components. Frequency domain analysis involves taking heart rate inter beat interval measures and computing a spectral analysis with the Fourier transform, with multi-second frequency bands (high, low, and very low) serving as indices of HRV (Akselrod et al., 1981). Spectral analysis allows for the examination of the level and type of rhythmic activity of underlying physiological systems that support cardiac flexibility. From this perspective, high frequency (HF) HRV has been used as an index of parasympathetic activity. RSA corresponds to the respiratory frequency, 0.15–0.4 Hz, equivalent to the HF band range. Low frequency (LF: 0.04–0.15 Hz) HRV is thought to reflect both sympathetic and parasympathetic effects on HRV (Berntson et al., 1997; Silva et al., 2009). Less is known about the controlling factors of very low frequency (VLF: 0.0033–0.04 Hz) HRV. Some studies suggest a relationship with humoral factors such as thermoregulation and the renin-angiotensin system (Bernardi et al., 1996; Lindqvist et al., 1990; Taylor et al., 1998), while others consider temperature, metabolic and hormonal influences (Friedman, 2007).
Relatively few studies employ spectral analysis to examine the relationship between HRV and depression in adolescents and children. For instance, Tonhajzerova and colleagues (2009) found that female adolescent participants with MDD had significantly less HF HRV compared to healthy matched subjects (Tonhajzerova et al., 2012). At the same time, some recent work reports links between VLF and treatment outcomes in patients with MDD (Jain et al., 2014). Results indicate that lower baseline relative power of VLF predicted improvement in depressive symptoms. Greater relative power of VLF has also been connected with higher depression severity in depressed patients (Davydov et al., 2007). These results suggest VLF may also be a potential correlate of depressive pathophysiology.
Importantly, developmental factors need attention when considering individual differences in HRV among children and adolescents and HRV as a correlate of depressive pathophysiology. The onset of pubertal maturation is a time for changes in stress responsiveness and emotional reactivity that connect to a heightened risk for psychopathology during adolescence (Dahl and Gunnar, 2009; Evans et al., 2013; Stroud et al., 2009; Van den Bos et al., 2014). An association between depression and pubertal status has been documented (Joinson et al., 2012). As well, starting in adolescence, females appear to be more susceptible to depression than males (Costello et al., 2003). This increased risk for females may reflect sex differences in physiological reactivity emerging during adolescence (Ordaz and Luna, 2012). Correspondingly, some work specifically indicates that age and sex might inform the relationship between HRV and depression (Greaves-Lord et al., 2007). More generally, age and puberty have been linked to changes in HRV, such that adolescents exhibited lower HF HRV than pre-adolescents (Tanaka et al., 2000).
The present study examined spectral HRV markers for predicting depressive symptoms among children and adolescents 10–17 years of age. We used 3 commonly used spectral metrics of HRV, the relative power of high frequency (HF: 0.15–0.4 Hz), low frequency (LF: 0.04–0.15 Hz) and very low frequency (VLF: 0.0033–0.04 Hz) bands. The primary aim of this study was to evaluate HF band activity in a typically developing child-adolescent sample, as a correlate of depressive symptoms, after considering age, puberty and sex. We hypothesized that adolescents with greater levels of depressive symptoms would have less relative HF band activity even after controlling for demographic and developmental factors. The second goal of this study was to evaluate the predictive power of relative HF in the context of relative LF and VLF HRV. We hypothesized that reduced relative HF HRV would more strongly predict depressive symptoms than LF or VLF. As our third goal, we examined the associations between HRV (relative HF, LF and VLF), sex, age and puberty.
2. Methods
2.1. Participants
Participants in this study were part of a larger study of 160 adolescents. Included in this report were 127 healthy adolescents aged 10–17 years (66 boys, 70 girls) who provided sufficient resting heart rate (HR) data for analysis. The 33 not included reflected 10 who could not be used due to equipment failure and 18 who did not provide data of sufficient quality as detailed below. Additionally, five participants were excluded who, at the time of their physiology visit, were found to have started on a medication that could affect their ECG. These included two participants who were taking Ritalin, and one participant each who were taking Doxofylline, Lamotrigine and Sertraline. Families were recruited via mass mailings to New Haven, CT and surrounding towns within a 20-mile radius of the study research offices. Children were fluent in English and had no evidence of serious mental illness (psychosis, autism, bipolar disorder) assessed via a parental telephone screen. Children were considered ineligible for the study if their caregiver, typically their mother, reported that the child in question was being treated for, or carried a diagnosis of psychosis, autism, or bipolar disorder. Children were intellectually in the normal range based on results from the Vocabulary and Similarities subscales of the Wechsler Abbreviated Scale of Intelligence (verbal IQ mean = 112.15, SD = 12.13, range 82–139) (Wechsler, 1999). The mean age of the children was 13.92 years. (SD = 2.19 years, range = 10.08–17.79 years). The ethnicity of participants were: 72.7% Caucasian, 8.8% Hispanic or Latino, 8.1% African American, 5.9% Asian, 2.2% American Indian or Alaskan, and 2.2% Other. This research was approved by the Yale University School of Medicine Human Investigation Committee and was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2. Procedure
This study was part of a larger study on stress, reward and risk-taking in adolescents (Crowley et al., 2013a, 2013b), which included three visits typically across three weeks. Visit one included self-report assessments completed by youth, behavioral tasks and a brief IQ screen. The psychophysiology session (visit 2) began at 4:05 pm to control for daily hormonal fluctuations. During the visit, participants completed questionnaires and interviews testing cognitive, intellectual and emotional functioning. Adolescents also completed various computer and social tasks while heart rate and neural signals were recorded. Questionnaires were administered and salivary cortisol was recorded at intervals throughout the session. During the initial 7 min of the visit, the period of interest in this report, physiological data was collected as a baseline measure at rest. Informed child and adolescent assent and parental consent were obtained before the start of the study. Participants were compensated $60 as part of a study visit lasting three hours.
2.3. Measures
2.3.1. Adolescent cardiovascular response
Adolescent heart rate was recorded using a 3-lead Coulburn Instruments Holter electrocardiogram (ECG). Three leads were placed on the participant, one on the left arm as a ground, one on the right upper back and one on the left lower back. Prior to this, the area where the electrode was placed was cleaned with an alcohol pad, then Nuprep abrasive, and then alcohol again. ECG Biopac leads were pre-gelled and self-adhesive. ECG data was recorded using WinDaq Pro data acquisition software. The sampling rate was 1000 Hz. Data from the entire visit was processed by Matlab to segment the data into the appropriate task sections. The heart rate data was imported into QRSTool, where peak heartbeats were selected by hand and inter-beat intervals (IBIs) were extracted. Data with movement artifact inhibiting calculation of inter beat intervals was considered to be of insufficient quality. Artifact and ectopic beats were noted and excluded appropriately. Metrics were then derived by CMetX software. To be included, participants had to have at least 6 min of artifact free data. Participants had a mean of 6:57 min of artifact free data (SD = 00:07). Artifact free IBI data was inputted into the Kubios HRV program for spectral analysis. Three spectral frequencies, relative power of very low frequency (VLF: 0.0033–0.04 Hz), low frequency (LF: 0.04–0.15 Hz) and high frequency (HF: 0.15–0.4 Hz) in fast Fourier transform (FFT) were extracted. Relative power was computed as the percentage of total power in each frequency band (Yeragani et al., 1997).
2.3.2. Adolescent emotional response
Adolescents completed the Children’s Depression Inventory (CDI) (Kovacs, 1992). The CDI contains 27 items scored from 0 to 2, with a total score ranging from 0 to 54, and is a commonly used self-report measure for children and adolescents to determine the severity of depressive symptoms. Participants also completed the Early Adolescent Temperament Questionnaire Depressive Mood Scale (EATQ DM) (Capaldi and Rothbart, 1992). The EATQ DM contains 6 items scored from 1 to 5, with a higher score indicating lowered mood, loss of interest and enjoyment in activities and a more unpleasant affect (Capaldi and Rothbart, 1992). The CDI measures clinical depression whereas the EATQ DM is used to assess depressive mood as a normative temperamental characteristic. We combined these measures to assess depressive symptoms from both a normative developmental perspective (EATQ-DM) and a clinical perspective (CDI).
2.3.3. Adolescent pubertal status
Adolescents completed the Pubertal Development Scale (PDS) Self Report, while the primary caregiver completed the PDS Parent Report (Petersen et al., 1988). Both the PDS Self Report and Parent Report contain 5 items scored from 1 to 4. The PDS estimates the pubertal status of the adolescent based on the presence or absence of developmental features such as growth spurt, pubic hair growth and skin changes in both boys and girls, as well as gender specific pubertal changes. Caregiver and self-reports on the PDS were highly correlated (r = 0.853). For the purposes of this study we used the mean of these two measures in regression analyses below.
2.4. Data analysis
Analysis was accomplished using SPSS statistical software. The main study variables were a depression composite, referred to as our “D-composite” (CDI, EATQ-DM; standardized values, averaged) and spectral power in VLF, LF and HF bands. The CDI and EATQ-DM have been considered together in a composite in previous research on depressed mood in adolescence (Moore et al., 2013). Analysis included linear regression models using the depression composite as the criterion variable and the spectral relative power measures as the predictor variables.1 The association between age, puberty and HRV measures were first analyzed with correlation analyses and further examined within the context of linear regression. Probability level of 0.05 for significance and two-tailed tests were used to evaluate a priori tests.
3. Results
The means and standard deviations for all major study variables are presented in Table 1. Our analytic approach consisted of correlation analyses and hierarchical multiple regression analyses. A priori, we were interested in the association between depressive symptoms and HRV. The strong association between self-reported EATQ depressive mood and CDI total (r = 0.623) justified our creation of an average composite of these two measures. Correlation analyses (Table 2) revealed that HF HRV was negatively associated with the depressive symptom composite. VLF HRV was more strongly positively related to the depressive symptom composite than was HF HRV. LF HRV was unrelated to depressive symptoms (see Fig. 1 for scatter plots of the depressive symptom composite and HF, LF and VLF HRV). Supporting our decision to consider age and puberty in our models, these measures were associated with HF HRV (Table 2). Supporting our decision to consider sex in the model, both the EATQ DM measure and the D-composite were associated with sex (Table 2). Females reported significantly more depressive symptoms than males on our D-composite (t(125) = −2.059, p = 0.042), with females showing a positive mean z-score and males showing a negative mean z-score (Table 1).
Table 1.
Means and standard deviations for major study variables.
| Sex | Age | PDS-self | PDS-parent | Mean-PDS | CDI Total | EATQ DM | D-Composite | VLF | LF | HF |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 14.06 (2.20) | 2.71 (0.38) | 2.60 (0.45) | 2.65 (0.38) | 7.79 (6.13) | 16.10 (4.10) | 0.19 (0.93) | 25.19 (11.04) | 29.13 (10.84) | 40.05 (19.13) |
| Male | 13.79 (2.20) | 2.44 (0.79) | 2.27 (0.85) | 2.35 (0.80) | 6.48 (5.98) | 14.42 (3.83) | −0.14 (0.88) | 27.62 (13.55) | 31.80 (13.61) | 35.14 (18.61) |
| Total | 13.92 (2.19) | 2.57 (0.64) | 2.43 (0.70) | 2.50 (0.64) | 7.13 (6.06) | 15.26 (4.04) | 0.03 (0.91) | 26.42 (12.38) | 30.47 (12.34) | 37.57 (18.95) |
PDS – Pubertal Development Scale, CDI – Child Depression Inventory, EATQ DM – Early Adolescent Temperament Questionnaire Depressive Mood Scale, D-Composite –depression composite (CDI plus EATQ DM mean z-score), VLF – very low frequency relative spectral power HRV; LF – low frequency relative spectral power HRV; HF – high frequency relative spectral power HRV
Table 2.
Correlations among Major Study Variables.2
| Sex | Age | PDS-self | PDS-parent | Mean -PDS | CDI Total | EATQ DM | D-Composite | VLF | LF | HF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 1 | ||||||||||
| Age | 0.062 | 1 | |||||||||
| PDS-self | 0.212* | 0.683** | 1 | ||||||||
| PDS -parent | 0.231* | 0.701** | 0.853** | 1 | |||||||
| Mean-PDS | 0.237** | 0.722** | 0.959** | 0.967** | 1 | ||||||
| CDI Total | 0.108 | 0.349** | 0.202* | 0.139 | 0.181* | 1 | |||||
| EATQ DM | 0.208* | 0.115 | 0.013 | −0.061 | −0.024 | 0.652* | 1 | ||||
| D-Composite | 0.181* | 0.261* | 0.123 | 0.055 | 0.1 | 0.905** | 0.914** | 1 | |||
| VLF | −0.098 | 0.15 | 0.17 | 0.13 | 0.141 | 0.219* | 0.272** | 0.265** | 1 | ||
| LF | −0.109 | 0.113 | 0.112 | 0.092 | 0.108 | 0.054 | 0.033 | 0.048 | 0.049 | 1 | |
| HF | 0.13 | −0.247** | −0.248** | −0.198* | −0.222** | −0.221* | −0.203* | −0.232** | −0.768** | −0.640** | 1 |
PDS – Pubertal Development Scale, CDI – Child Depression Inventory, EATQ DM – Early Adolescent Temperament Questionnaire Depressive Mood Scale, D-Composite –depression composite (CDI plus EATQ DM mean z-score), VLF – very low frequency relative spectral power HRV; LF – low frequency relative spectral power HRV; HF – high frequency relative spectral power HRV
p ≤ 0.01
p ≤ 0.05, uncorrected
Fig. 1.
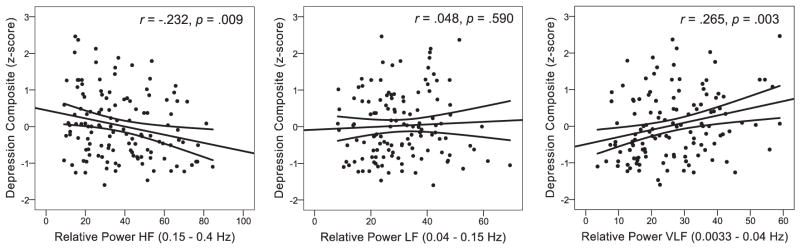
Scatterplots of a self-reported depression composite and relative spectral power heart-rate variability for High Frequency (HF), Low Frequency (LF) and Very Low Frequency (VLF). Fit lines are displayed with 95% confidence intervals around the mean.
To evaluate the predictive power of HF HRV in explaining individual variability in depressive symptoms (D-composite), we used hierarchical linear regression, first entering age, sex and pubertal development (see Table 3). We began a priori with HF, because among spectral measures, HF has the most support in the literature as a correlate of depressive symptomatology. In a first block, age, puberty and sex yielded an overall significant model F (3, 123) = 6.046, p < 0.001, R2 = 0.129, with sex, age and pubertal status all contributing significantly to the model (all ps < 0.05, see Table 3). HF was then added in a second block in a stepwise fashion, again yielding an overall significant model, F(4, 122) = 6.582, p < 0.001, R2 = 0.177, ΔR2 = 0.049. Sex, age and pubertal status all continued to significantly contribute to the model, as did HF (all ps < 0.05, see Table 3). Across our correlation and regression analyses, these data indicate that reduced HF is associated with greater levels of depressive symptoms, even after accounting for other demographic and developmental factors.
Table 3.
Hierarchical regression analysis predicting a depressive symptom composite from heart-rate variability relative spectral power, co-varying for sex, age and pubertal status.
| B | Std. error | β | t | Sig. | R | ΔR | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| (Constant) | −2.179 | 0.551 | −3.958 | <0.001*** | 0.358 | 0.129 | |
| Age | 0.185 | 0.051 | 0.445 | 3.608 | <0.001*** | ||
| Sex | 0.398 | 0.160 | 0.218 | 2.486 | 0.014* | ||
| Puberty | −0.388 | 0.180 | −0.273 | −2.152 | 0.033* | ||
| Model 2 | |||||||
| Step 1 | |||||||
| (Constant) | −1.546 | 0.586 | −2.637 | 0.009** | 0.421 | 0.049 | |
| Age | 0.172 | 0.050 | 0.412 | 3.409 | <0.001*** | ||
| Sex | 0.473 | 0.158 | 0.260 | 2.983 | 0.003** | ||
| Puberty | −0.442 | 0.177 | −0.310 | −2.495 | 0.014* | ||
| Step 2 | |||||||
| HF | −0.011 | 0.004 | −0.233 | −2.695 | 0.008** | ||
| Model 3 | |||||||
| Step 1 | |||||||
| (Constant) | −2.574 | 0.544 | −4.731 | <0.001*** | 0.445 | 0.070 | |
| Age | 0.177 | 0.050 | 0.426 | 3.581 | <0.001*** | ||
| Sex | 0.462 | 0.155 | 0.254 | 2.975 | 0.004** | ||
| Puberty | −0.435 | 0.174 | −0.305 | −2.493 | 0.014* | ||
| Step 2 | |||||||
| VLF | 0.020 | 0.006 | 0.269 | 3.252 | 0.001** | ||
| Excluded | |||||||
| LF | 0.049 | 0.596 | 0.552 | ||||
| HF | −0.053 | −0.405 | 0.686 | ||||
p ≤ 0.001
p ≤ 0.01
p ≤ 0.05 (n = 127)
Next, building on the finding of a moderate VLF depressive symptom association, we evaluated the relative predictive power of our three spectral HRV measures (HF, LF, VLF) for explaining variability in depressive symptoms, after controlling for age, puberty and sex. In this analysis we used hierarchical linear regression, entering age, sex and pubertal development in an initial block and then adding HF, LF and VLF with a forward selection procedure in a second block. In the second block, only VLF remained a significant predictor of the depressive symptom composite, F(4, 122) = 7.532, p < 0.0001, R2 = 0.198, ΔR2 = 0.070. These data suggest that VLF HRV may be a viable risk correlate of depressive pathophysiology in children and adolescents, possibly more predictive than HF or LF HRV. It is worth noting that the simple correlation between VLF and HF was r = −0.768, which is not surprising given that they are relative spectral measures.
It is also worth noting that both age and pubertal status were related to HF and VLF, but not LF HRV. In general, increased age and pubertal maturation were associated with decreases in HF and increases in VLF HRV. However, the association between the depressive symptom composite and HF or VLF remained after considering age and pubertal maturation across 10–17 years of age. Notably, although sex was weakly but significantly related to depressive symptoms, sex was not significantly related to the HRV measures we examined here across the correlation and regression analyses we employed.
4. Discussion
A primary goal of this study was to examine the relationship between depressive symptoms and heart rate variability among typically developing children and adolescents. We relied on a composite measure of depressive symptoms from the self-reported EATQ Depressive Mood scale and the Child Depression Inventory, thus capturing both normative depressive mood characteristics (EATQ DM) and clinical symptoms of depression (CDI) among children and adolescents. Similar composite measures have been applied in other studies of youth depressive symptomatology and allow for inclusion of both the more severe end of the depression dimension with the CDI, as well as the less severe end of the depression continuum with the EATQ DM (Moore et al., 2013). We employed relative power measures of HF, LF and VLF spectral power as used in previous adult studies (Jain et al., 2014).
We observed a significant negative association between depressive symptoms and HF HRV, indicating that adolescents with greater depressive symptoms showed less relative power HF band activity. This is consistent with previous research in children, finding that lower HF power, and therefore lower parasympathetic activity, was associated with sadness, anxiety, peer problems and anger (Michels et al., 2013). Our findings suggest that children and adolescents with greater depressive tendencies may have less relative parasympathetic influence on their HRV.
Interestingly, VLF HRV showed a strong positive association with depressive symptoms, stronger than HF HRV. To date, a limited number of studies examine VLF HRV and depression—with even fewer examining VLF HRV in a child and adolescent sample. One previous study investigated the relationship between relative power VLF HRV at baseline with depressive symptoms, finding that lower baseline relative power VLF predicted improvement in depressive symptoms after treatment (Jain et al., 2014). Another study investigating the effect of depression in an adult population found that the log of VLF was lower among depressed patients compared to non-depressed patients (Carney et al., 2005). The difference in these results compared to our own could reflect a developmental effect of depression on the VLF component of HRV —although differences in this adult sample could also reflect their history of myocardial infarction, clinically depressed status, the index examined, or that Carney et al. (2005) measured VLF levels over a 24-h period which is a considerably longer data length.
What is compelling about our data is that, even after controlling for sex, age and puberty, decreased HF and increased VLF continued to predict greater levels of depressive symptoms in our sample. While we examined HF and VLF as relative measures that have ~50% of their variance in common, VLF accounted for a greater proportion of the variance in depressive symptoms (6.9%) than did HF (3.8%). Other recent work highlights the emerging importance of specifically considering greater VLF and depression risk. In a sample of middle-aged male twins, Vaccarino and colleagues (2008) documented a common genetic factor linking depression with decreased HRV and therefore autonomic dysfunction. Their study suggests that genetic background explains most of the variance in VLF power, while environmental factors mostly explain the variance in HF power HRV. Furthermore, Vaccarino and colleagues found that VLF HRV was among the HRV measures most highly correlated with depression (Vaccarino et al., 2008). Understanding that there is an underlying genetic component linking HRV to depression, and that VLF band provides a strong association for this link, suggests that adolescents with greater relative VLF HRV are at increased risk for depression due in part to heritable biological processes reflected in this aspect of autonomic function.
While emerging work suggests that VLF HRV is relevant in depression pathophysiology, the physiological mechanisms and generation of VLF HRV have not been as clearly defined as for HF and LF HRV components. Single neuron recording work in dogs by Amour and colleagues (Armor, 2003) indicates that VLF HRV is part of an intrinsic cardiac system, produced by the heart itself (Shaffer et al., 2014), and modulated by efferent sympathetic activity (Kember et al., 2001). Fundamental to health and wellbeing, VLF HRF is more strongly associated with all cause mortality than are LF HRV or HF VLF HRV itself (Shaffer et al., 2014). VLF HRV is associated with thermoregulation, the renin-angiotensin system and metabolic processes (Bernardi et al., 1996; Friedman, 2007; Lindqvist et al., 1990; Taylor et al., 1998). Moreover decreased VLF HRV is also associated with chronic inflammation, as seen by increased IL-6 and CRP levels (Carney et al., 2007; Janszky et al., 2004; Lampert et al., 2008). Carney et al. (2007) interestingly found this connection to be present particularly in those with depression. Recently Jain et al. (2014) speculated that dysregulation of biological rhythms in the VLF frequency range, such as energy metabolism, might also affect fatigue as seen in depression.
5. Limitations and future directions
Our findings should be considered in light of study limitations. We did not study clinical levels of depressive symptoms. Thus we cannot make strong claims about relative spectral power HRV and clinical depression. In the current study, adolescents were not excluded for hypertension or other somatic disorders that could have potentially influenced HRV, although no subjects were taking anti-hypertensive medications at the time of participation. Some relevant influences on cardiac control including physical activity levels and exercise patterns could also be controlled in future work. As well, although we obtained at least one cycle of data encompassing the lowest frequency range for VLF (0.0033 Hz) by requiring at least 6 min of ECG data, a longer data collection period, perhaps in the 12-min range, would likely yield a more stable estimate of VLF HRV.
While existent data already indicates that HF HRV is negatively associated with depression and anxiety (Pittig et al., 2013), including an adult sample would be useful to determine whether or not patterns of VLF HRV and depressive symptoms continue into adulthood. Future work will need to examine spectral HRV measures prospectively vis-à-vis depression risk. HRV is viewed as a correlate of emotion regulation ability (Berntson et al., 1997, 1993; Porges, 1995a, 1994a, 1996) and some clinical treatments for depression such as mindfulness (Teasdale et al., 1995) and yoga (Shapiro et al., 2007) target aspects of emotion regulation particularly relevant to heart-rate variability. Future work could examine whether VLF HRV and HF HRV are measures impacted by these types of treatments and whether such changes track symptom improvement.
Supplementary Material
Acknowledgments
Funding
This research was supported by NARSAD Young Investigator Award (MJC), Yale Interdisciplinary Research Consortium on Stress, Self-Control and Addiction Pilot project funding (MJC) through 1UL1RR024925-01 (R. Sinha); NIDA grants K01 DA034125 (MJC), RO1-DA-06025 (LCM), DA-017863 (LCM) and KO5 (LCM), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
We are grateful to for the help of Christopher Bailey, Max Greger-Moser, Amanda Ng, and Jessica Walthall for their help with data collection.
Abbreviations
- HRV
Heart Rate Variability
- HF
High Frequency
- LF
Low Frequency
- VLF
Very Low Frequency
- ANS
Autonomic Nervous System
- RSA
Respiratory Sinus Arrhythmia
- MDD
Major Depressive Disorder
- HR
Heart Rate
- ECG
Electrocardiogram
- IBI
Inter-beat Interval
- FFT
Fast Fourier Transform
- CDI
Children’s Depression Inventory
- EATQ DM
Early Adolescent Temperament Questionnaire Depressive Mood Scale
- PDS
Pubertal Developmental Scale
Appendix A. Supplementary Information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jad.2015.06.057.
Footnotes
Log of the relative frequency bands resulted in near identical correlations.
Contributors
MJC, LCM, TMC and REM designed the study. JDM, MJC and LV wrote the first draft of the study. JDM, JW and LV analyzed the heart-rate data. MJC, TMC, JDM, HJR revised the manuscript.
Conflicts of interest
None.
References
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Armour JA. Neurocardiology: Anatomical and Functional Principles. Institute of HeartMath; Boulder Creek: 2003. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Valle F, Coco M, Calciati A, Sleight P. Physical activity influences heart rate variability and very-low-frequency components in Holter electrocardiograms. Cardiovasc Res. 1996;32:234–237. doi: 10.1016/0008-6363(96)00081-8. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bizik G, Bob P, Raboch J, Pavlat J, Uhrova J, Benakova H, Zima T. Dissociative symptoms reflect levels of tumor necrosis factor alpha in patients with unipolar depression. Neuropsychiatr Dis Treat. 2014;10:675–679. doi: 10.2147/NDT.S50197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Dietrich A, Ormel J, Verhulst FC, Oldehinkel AJ. Preadolescents’ somatic and cognitive-affective depressive symptoms are differentially related to cardiac autonomic function and cortisol: the TRAILS study. Psychosomat Med. 2009;71:944–950. doi: 10.1097/PSY.0b013e3181bc756b. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK. Development and validation of an early adolescent temperament measure. J Early Adolesc. 1992;12:153–173. [Google Scholar]
- Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Int Med. 2005;165:1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Miller GE, Steinmeyer B, Rich MW, Duntley SP. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomat Res. 2007;62:463–467. doi: 10.1016/j.jpsychores.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, van Noordt SJ, Wu J, Hommer RE, South M, Fearon RM, Mayes LC. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain and Cognition. 2013a doi: 10.1016/j.bandc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Hommer RE, South M, Molfese PJ, Fearon RM, Mayes LC. A developmental study of the feedback-related negativity from 10–17 years: age and sex effects for reward versus non-reward. Dev Neuropsychol. 2013b;38:595–612. doi: 10.1080/87565641.2012.694512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Davydov DM, Shapiro D, Cook IA, Goldstein I. Baroreflex mechanisms in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:164–177. doi: 10.1016/j.pnpbp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FE, Greaves-Lord K, van Roon AM, Ormel J, Neeleman J, Rosmalen JG. Externalizing and internalizing problems in relation to autonomic function: a population-based study in preadolescents. J Am Acad Child Adolesc Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Tulen JH, Franken IH, Huizink AC. Determinants of physiological and perceived physiological stress reactivity in children and adolescents. PLoS One. 2013;8:e61724. doi: 10.1371/journal.pone.0061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children’s affect regulation during a disappointment: psychophysiological responses and relation to parent history of depression. Biol Psychol. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Sondeijker FE, Dietrich A, Oldehinkel AJ, Rosmalen JG, Ormel J, Verhulst FC. Testing the tripartite model in young adolescents: is hyperarousal specific for anxiety and not depression? J Affect Disord. 2007;102:55–63. doi: 10.1016/j.jad.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99:604–611. doi: 10.1111/j.1651-2227.2009.01657.x. Oslo, Norway: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: the role of individual differences in patterns of RSA responding. J Abnorm Child Psychol. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Jain FA, Cook IA, Leuchter AF, Hunter AM, Davydov DM, Ottaviani C, Tartter M, Crump C, Shapiro D. Heart rate variability and treatment outcome in major depression: a pilot study. Int J Psychophysiol: Off J Int Organ Psychophysiol. 2014 doi: 10.1016/j.ijpsycho.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Janszky I, Ericson M, Lekander M, Blom M, Buhlin K, Georgiades A, Ahnve S. Inflammatory markers and heart rate variability in women with coronary heart disease. J Intern Med. 2004;256:421–428. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- Joinson C, Heron J, Araya R, Paus T, Croudace T, Rubin C, Marcus M, Lewis G. Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychol Med. 2012;42:2579–2589. doi: 10.1017/S003329171200061X. [DOI] [PubMed] [Google Scholar]
- Keenan K, Feng X, Hipwell A, Klostermann S. Depression begets depression: comparing the predictive utility of depression and anxiety symptoms to later depression. J Child Psychol Psychiatry. 2009;50:1167–1175. doi: 10.1111/j.1469-7610.2009.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kember GC, Fenton GA, Armour JA, Kalyaniwalla N. Competition model for aperiodic stochastic resonance in a Fitzhugh-Nagumo model of cardiac sensory neurons. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;63:041911. doi: 10.1103/PhysRevE.63.041911. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Malhi GS. Effects of serotonin reuptake inhibitors on heart rate variability: methodological issues, medical comorbidity, and clinical relevance. Biol Psychiatry. 2011;69:e25–e26. doi: 10.1016/j.biopsych.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory (CDI) Multi-health Systems, Inc; 1992. [Google Scholar]
- Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:e751–e757. 759. doi: 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry. 2010;68:861–868. doi: 10.1016/j.biopsych.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Licht CM, Naarding P, Penninx BW, van der Mast RC, de Geus EJ, Comijs H. The association between depressive disorder and cardiac autonomic control in adults 60 years and older. Psychosomat Med. 2015;77:279–291. doi: 10.1097/PSY.0000000000000165. [DOI] [PubMed] [Google Scholar]
- Licht CMM, Penninx BWJH, de Geus EJC. Reply to: effects of serotonin reuptake inhibitors on heart rate variability: methodological issues. Med Co-morbidity Clin Relevance Biol Psychiatry. 2011;69:e27–e28. doi: 10.1016/j.biopsych.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Jalonen J, Parviainen P, Antila K, Laitinen LA. Effect of posture on spontaneous and thermally stimulated cardiovascular oscillations. Cardiovasc Res. 1990;24:373–380. doi: 10.1093/cvr/24.5.373. [DOI] [PubMed] [Google Scholar]
- Michels N, Sioen I, Clays E, De Buyzere M, Ahrens W, Huybrechts I, Vanaelst B, De Henauw S. Children’s heart rate variability as stress indicator: association with reported stress and cortisol. Biol Psychol. 2013;94:433–440. doi: 10.1016/j.biopsycho.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Moore MN, Salk RH, Van Hulle CA, Abramson LY, Hyde JS, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on rumination, distraction, and depressed mood in adolescence. Clin Psychol Sci. 2013;1:316–322. doi: 10.1177/2167702612472884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37:1135–1157. doi: 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: reliability and validity of a self-report measure. J Youth Adolesc. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Pittig A, Arch JJ, Lam CW, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive-compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol. 2013;87:19–27. doi: 10.1016/j.ijpsycho.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995a;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology Vol. 1995b;32 (32):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory Psychophysiology. 1995c;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monogr Soc Res Child Dev. 1994;59:167–186. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Kaschka WP. Is diurnal variation of mood associated with parasympathetic activity? J Affect Disord. 1995;34:249–255. doi: 10.1016/0165-0327(95)00011-b. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? J Affect Disord. 1994;32:271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Roose SP, Glassman AH, Dalack GW. Depression, heart disease, and tricyclic antidepressants. J Clin Psychiatry. 1989;50 (Suppl):S12–S16. Discussion 17. [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Dev Psychopathol. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D, Cook IA, Davydov DM, Ottaviani C, Leuchter AF, Abrams M. Yoga as a complementary treatment of depression: effects of traits and moods on treatment outcome. Evid-Based Complement Alternat Med: eCAM. 2007;4:493–502. doi: 10.1093/ecam/nel114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GJ, Ushizima MR, Lessa PS, Cardoso L, Drager LF, Atala MM, Consolim-Colombo FM, Lopes HF, Cestari IA, Krieger JE, Krieger EM. Critical analysis of autoregressive and fast Fourier transform markers of cardiovascular variability in rats and humans. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica … [et al] 2009;42:386–396. doi: 10.1590/s0100-879x2009000400012. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Borres M, Thulesius O, Tamai H, Ericson MO, Lindblad LE. Blood pressure and cardiovascular autonomic function in healthy children and adolescents. J Pediatrics. 2000;137:63–67. doi: 10.1067/mpd.2000.108098. [DOI] [PubMed] [Google Scholar]
- Task Force, o.t.E.S.o.C.a.t.N.A.S.o.P.a.E. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JM. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther. 1995;33:25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Ondrejka I, Chladekova L, Farsky I, Visnovcova Z, Calkovska A, Jurko A, Javorka M. Heart rate time irreversibility is impaired in adolescent major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:212–217. doi: 10.1016/j.pnpbp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Ondrejka I, Javorka K, Turianikova Z, Farsky I, Javorka M. Cardiac autonomic regulation is impaired in girls with major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:613–618. doi: 10.1016/j.pnpbp.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Ondrejka I, Javorka M, Adamik P, Turianikova Z, Kerna V, Javorka K, Calkovska A. Respiratory sinus arrhythmia is reduced in adolescent major depressive disorder. Eur J Med Res. 2009;14 (Suppl 4):280–283. doi: 10.1186/2047-783X-14-S4-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH. The link between depression and physical symptoms. Prim Care Companion J Clin Psychiatry. 2004;6:12–16. [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70:628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos E, de Rooij M, Miers AC, Bokhorst CL, Westenberg PM. Adolescents’ increasing stress response to social evaluation: pubertal effects on cortisol and alpha-amylase during public speaking. Child Dev. 2014;85:220–236. doi: 10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; 1999. [Google Scholar]
- Yeragani VK, Sobolewski E, Kay J, Jampala VC, Igel G. Effect of age on long-term heart rate variability. Cardiovasc Res. 1997;35:35–42. doi: 10.1016/s0008-6363(97)00107-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


