Abstract
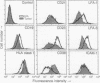
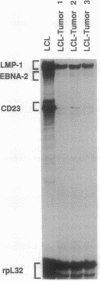
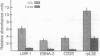
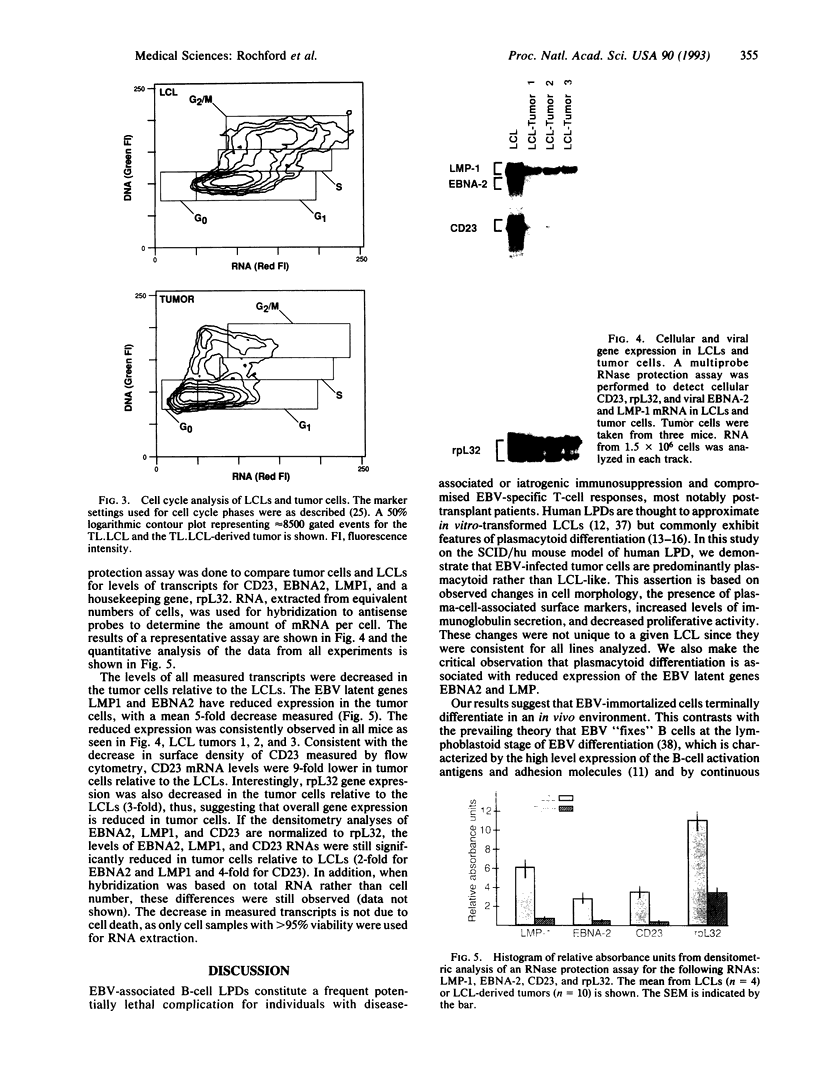
The Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders that arise in immunosuppressed individuals are considered to resemble EBV-transformed in vitro lymphoblastoid cell lines (LCLs) with a mature activated B-cell phenotype. In this study of human lymphoproliferative disorders in the severe combined immunodeficiency mouse model, however, we demonstrate that EBV-infected tumor cells are not LCL-like but are predominantly plasmacytoid and that this phenotype correlates with reduced expression of EBV latent genes. B-cell tumors developed within 3-6 weeks after injection of LCLs into severe combined immunodeficiency mice. The tumors and the injected LCLs were analyzed by flow cytofluorometry for B-cell differentiation and activation markers and by ribonuclease protection assay for cellular and viral gene expression. No differences in the expression of CD19 and CD21 were observed. However, a decrease in CD23, CD11a (lymphocyte function-associated antigen LFA-1), and CD58 (LFA-3) expression and an increase in CD38 (a plasma-cell-associated antigen), CD54 (intracellular adhesion molecule ICAM-1), and HLA class I in the tumor cells relative to the LCLs was observed. Two-color flow cytofluorometric analysis showed that the predominant population (> 80%) in LCLs was CD23hi/CD38lo and that the major population in LCL-derived tumors was CD23lo/CD38hi. Cell cycle analysis showed that, in contrast to actively cycling LCLs, the majority of tumor cells had exited the cell cycle and were restricted to G0/G1 phase. Finally, and most important, a reduction in mRNA for the EBV latent genes EBV nuclear antigen 2 (EBNA2) and latent membrane protein (LMP1) was observed in the tumors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahsmann E. J., Lokhorst H. M., Dekker A. W., Bloem A. C. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma. Blood. 1992 Apr 15;79(8):2068–2075. [PubMed] [Google Scholar]
- Anderson K. C., Park E. K., Bates M. P., Leonard R. C., Hardy R., Schlossman S. F., Nadler L. M. Antigens on human plasma cells identified by monoclonal antibodies. J Immunol. 1983 Mar;130(3):1132–1138. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Dunn S. M., Fecondo J. V., Culvenor J. G., Dührsen U., Burns G. F., Wawryk S. O. Regulation of expression of a human intercellular adhesion molecule (ICAM-1) during lymphohematopoietic differentiation. Blood. 1989 May 15;73(7):1896–1903. [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Pisa P., Fox R. I., Cooper N. R. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest. 1990 Apr;85(4):1333–1337. doi: 10.1172/JCI114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Ando I. EB virus induction is associated with B-cell maturation. Immunology. 1986 Nov;59(3):405–409. [PMC free article] [PubMed] [Google Scholar]
- Crocker J. Proliferation indices in malignant lymphomas. Clin Exp Immunol. 1989 Sep;77(3):299–308. [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Wang F., Hennessy K., Woodland E., Rickinson A., Kieff E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J Virol. 1986 Aug;59(2):453–462. doi: 10.1128/jvi.59.2.453-462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst D. N., McQuitty D. N., Weigle W. O., Hobbs M. V. Expression of membrane activation antigens on murine B lymphocytes stimulated with lipopolysaccharide. Cell Immunol. 1988 Jun;114(1):161–173. doi: 10.1016/0008-8749(88)90263-8. [DOI] [PubMed] [Google Scholar]
- Frizzera G., Hanto D. W., Gajl-Peczalska K. J., Rosai J., McKenna R. W., Sibley R. K., Holahan K. P., Lindquist L. L. Polymorphic diffuse B-cell hyperplasias and lymphomas in renal transplant recipients. Cancer Res. 1981 Nov;41(11 Pt 1):4262–4279. [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Simmons R. L. Epstein-Barr virus, immunodeficiency, and B cell lymphoproliferation. Transplantation. 1985 May;39(5):461–472. doi: 10.1097/00007890-198505000-00001. [DOI] [PubMed] [Google Scholar]
- Hatfull G., Bankier A. T., Barrell B. G., Farrell P. J. Sequence analysis of Raji Epstein-Barr virus DNA. Virology. 1988 Jun;164(2):334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- Ho M., Miller G., Atchison R. W., Breinig M. K., Dummer J. S., Andiman W., Starzl T. E., Eastman R., Griffith B. P., Hardesty R. L. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985 Nov;152(5):876–886. doi: 10.1093/infdis/152.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalesnik M. A., Jaffe R., Starzl T. E., Demetris A. J., Porter K., Burnham J. A., Makowka L., Ho M., Locker J. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988 Oct;133(1):173–192. [PMC free article] [PubMed] [Google Scholar]
- Picchio G. R., Kobayashi R., Kirven M., Baird S. M., Kipps T. J., Mosier D. E. Heterogeneity among Epstein-Barr virus-seropositive donors in the generation of immunoblastic B-cell lymphomas in SCID mice receiving human peripheral blood leukocyte grafts. Cancer Res. 1992 May 1;52(9):2468–2477. [PubMed] [Google Scholar]
- Pisa P., Cannon M. J., Pisa E. K., Cooper N. R., Fox R. I. Epstein-Barr virus induced lymphoproliferative tumors in severe combined immunodeficient mice are oligoclonal. Blood. 1992 Jan 1;79(1):173–179. [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Randhawa P. S., Yousem S. A., Paradis I. L., Dauber J. A., Griffith B. P., Locker J. The clinical spectrum, pathology, and clonal analysis of Epstein-Barr virus-associated lymphoproliferative disorders in heart-lung transplant recipients. Am J Clin Pathol. 1989 Aug;92(2):177–185. doi: 10.1093/ajcp/92.2.177. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Rowe M., Hart I. J., Yao Q. Y., Henderson L. E., Rabin H., Epstein M. A. T-cell-mediated regression of "spontaneous" and of Epstein-Barr virus-induced B-cell transformation in vitro: studies with cyclosporin A. Cell Immunol. 1984 Sep;87(2):646–658. doi: 10.1016/0008-8749(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989 Jun;63(6):2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstappen L. W., Johnsen S., Segers-Nolten I. M., Loken M. R. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 1990 Nov 1;76(9):1739–1747. [PubMed] [Google Scholar]
- Thomas J. A., Allday M. J., Crawford D. H. Epstein-Barr virus-associated lymphoproliferative disorders in immunocompromised individuals. Adv Cancer Res. 1991;57:329–380. doi: 10.1016/s0065-230x(08)61003-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Hotchin N. A., Allday M. J., Amlot P., Rose M., Yacoub M., Crawford D. H. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990 May;49(5):944–953. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel-Hansen V., Rosén A., Klein G. EBV-transformed lymphoblastoid cell lines down-regulate EBNA in parallel with secretory differentiation. Int J Cancer. 1987 Mar 15;39(3):404–408. doi: 10.1002/ijc.2910390322. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Young J. A., Trowsdale J. A processed pseudogene in an intron of the HLA-DP beta 1 chain gene is a member of the ribosomal protein L32 gene family. Nucleic Acids Res. 1985 Dec 20;13(24):8883–8891. doi: 10.1093/nar/13.24.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]