Abstract
Purpose
The threat of radiation exposure requires a mechanistic understanding of radiation-induced immune injury and recovery. The study objective was to evaluate responses to ionizing radiation in ovariectomized (surgically post-menopausal) female cynomolgus macaques.
Materials and methods
Animals received a single total-body irradiation (TBI) exposure at doses of 0, 2 or 5 Gy with scheduled necropsies at 5 days, 8 weeks and 24 weeks post-exposure. Blood and lymphoid tissues were evaluated for morphologic, cellular, and molecular responses.
Results
Irradiated animals developed symptoms of acute hematopoietic syndrome, and reductions in thymus weight, thymopoiesis, and bone marrow cellularity. Acute, transient increases in plasma monocyte chemoattractant protein 1 (MCP-1) were observed in 5 Gy animals along with dose-dependent alterations in messenger ribonucleic acid (mRNA) signatures in thymus, spleen, and lymph node. Expression of T cell markers was lower in thymus and spleen, while expression of macrophage marker CD68 (cluster of differentiation 68) was relatively elevated in lymphoid tissues from irradiated animals.
Conclusions
Ovariectomized female macaques exposed to moderate doses of radiation experienced increased morbidity, including acute, dose-dependent alterations in systemic and tissue-specific biomarkers, and increased macrophage/T cell ratios. The effects on mortality exceeded expectations based on previous studies in males, warranting further investigation.
Keywords: Radiation, non-human primate, immunology, molecular profiling, total body irradiation, acute effects, macrophage, cytokines
Introduction
Threat of accidental or malicious exposure of the public or the military to ionizing radiation necessitates development of effective pre- and post-exposure countermeasures. Development of radiation countermeasures also provides potentially valuable strategies for avoidance of normal tissue damage in patients given radiation therapy. A critical component of ionizing radiation damage is loss of protective immune function caused by depletion of nascent and developing immune cells. Immune system compromise in turn can result in significant morbidity and mortality. Detailed examination of the complex processes underlying response to, and recovery from, acute ionizing radiation exposure requires use of small and large animal models.
Rodent models are widely used for assessment of immune function; however, they differ significantly from humans in several characteristics, such as functional markers of natural killer and T cells, proteins involved in antigen processing and presentation and genomic responses to inflammatory diseases (Seok et al. 2013). Old world monkeys such as the cynomolgus macaque have close evolutionary relationships to humans and in some contexts are more appropriate models for evaluating immune system function and host response to infectious diseases, having similar susceptibility to common pathogens such as measles and influenza, and immunosuppressive lentiviruses (Palermo et al. 2013). The utility of the rhesus macaque non-human primate (NHP) model has been well-established to study immune system homeostasis and function, general physiology, and a wide range of pathologic diseases and conditions (Messaoudi et al. 2011) and its utility in radiation countermeasure research is clear (Chen et al. 2010, Robbins et al. 2011, Farese et al. 2012, MacVittie et al. 2012).
The purpose of the present study was to define cellular and molecular changes occurring in critical central and peripheral immune compartments in response to moderate levels of acute total body ionizing radiation exposure in an alternative species of macaque; the cynomolgus macaque (Macaca fascicularis). The cynomolgus macaque is a well-established NHP model of health and disease which provides some advantages over rhesus in terms of availability and size.
Specifically we determined the effects of radiation and recovery time on general health and clinico- pathologic indices; plasma cytokines; and expression of markers of individual cell types in immune compartments (thymus, spleen, and lymph node). Time- and dose-dependent acute systemic responses to ionizing radiation exposure, as well as tissue specific biomarker responses, were observed. The results provide a multi-dimensional profile of responses of female cynomolgus monkeys to graded doses of radiation that will help inform future studies utilizing cynomolgus macaques investigating the effects of radiation exposure.
Methods
Subjects and study design
Fifteen ovariectomized adult female cynomolgus monkeys (Macaca fascicularis) weighing a median of 3.03 kg (2.56–4.59 kg) were obtained from the Institut Pertanian Bogor and had completed prior studies of dietary protein (from soy and milk sources) with varying soy isoflavone content (Wood et al. 2006, Adams et al. 2008) at the Wake Forest School of Medicine Center for Comparative Medicine Research. These well-characterized, ‘late-middle aged’ female monkeys with an estimated median age of 20 years (15–24 years) were screened for exclusion of simian retroviruses and were in excellent health prior to the study. All experimental procedures were in compliance with the Wake Forest University Institutional Animal Care and Use Committee and the Guide for Care and Use of Laboratory Animals. Animals were screened prior to irradiation by clinical examination, complete blood count, and serum chemistry panels. Monkeys were randomized to three groups (0, 2 or 5 Gy), with timed necropsy evaluations planned at 5, 60 and 180 days post-irradiation. Complete animal demographic information can be found in Table I.
Table I.
Complete animal demographic information of study to explore responses to ionizing radiation in ovariectomized female cynomolgus macaques.
| ID | Radiation dose (Gy) | Days survived post irradiation | Death comments | Estimated age at death (yrs) | Baseline body weight (kg) | Body weight at death (kg) |
|---|---|---|---|---|---|---|
| A | 0 | – | Scheduled | 20.2 | 2.9 | 3.0 |
| B | 0 | – | Scheduled | 18.9 | 4.0 | 3.5 |
| C | 0 | – | Scheduled | 19.7 | 2.9 | 2.8 |
| D | 2 | 5 | Scheduled | 21.7 | 4.7 | 4.6 |
| E | 2 | 5 | Scheduled | 15.2 | 2.9 | 2.6 |
| F | 2 | 20 | Unexpected | 21.8 | 2.9 | 2.8 |
| G | 2 | 20 | Unexpected | 23.7 | 3.5 | 3.0 |
| H | 2 | 53 | Scheduled | 20.8 | 3.6 | 3.3 |
| I | 2 | 165 | Scheduled | 20.2 | 3.4 | 3 |
| J | 5 | 5 | Scheduled | 22.7 | 2.9 | 2.8 |
| K | 5 | 5 | Scheduled | 18.7 | 3.1 | 3.0 |
| L | 5 | 15 | Unexpected | 20.8 | 3.1 | 3.1 |
| M | 5 | 16 | Unexpected | 19.8 | 3.3 | 3.3 |
| N | 5 | 17 | Unexpected | 20.8 | 3.9 | 3.8 |
| O | 5 | 53 | Scheduled | 14.9 | 4.6 | 4.0 |
| Mean | 0 | 74 | – | 19.6 | 3.3 | 3.1 |
| Mean | 2 | 45 | – | 20.6 | 3.5 | 3.2 |
| Mean | 5 | 19 | – | 19.6 | 3.5 | 3.3 |
Rows (A-O) are individual animal demographics. Mean rows are dose group means. Gy, Gray; Yrs, years; kg, kilograms.
Diet
Following a three-month period of consumption of a laboratory chow diet (Purina LabDiet® Monkey Diet 5037, Richmond, IN, USA), animals were transitioned to a diet designed to mimic a typical Western diet a minimum of two weeks prior to irradiation and maintained on this diet for the duration of the study. This diet was produced in house and contained 34% of calories as fat, 18% of calories as protein, and 48% of calories as carbohydrates, with a moderately atherogenic cholesterol content of 0.20 mg/Cal. Protein was derived solely from animal sources (casein, lactalbumin). Of the fat, fatty acid composition was 40% saturated, 34% monounsaturated and 26% polyunsaturated. Monkeys were supplemented with fresh fruits and vegetables and water ad libitum.
Irradiation
Monkeys were anesthetized with ketamine (10 mg/kg) (Vedco Inc., St Joseph, MO, USA) and irradiated to single doses of 0, 2 or 5 Gy to the mid-plane using two opposed lateral fields of 6 MV X-rays at a dose rate of 60 cGy/min from a Varian 2100C linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA, USA). The radiation source to skin distance was 120 cm.
Clinical monitoring and husbandry
On the day of irradiation, animals were given parenteral prophylactic antibiotic treatment consisting of enrofloxacin (5 mg/kg) (Bayer HealthCare LLC, Shawnee Mission, KS, USA) and ceftazidime (30 mg/kg) (Patterson Veterinary, Devens, MA, USA), continuing until the white blood cell count returned to normal range (6.1–12.5 × 103 cells/ul) or until clinical illness (if present) was resolved. Control animals were maintained on antibiotics for the duration of the study. Clinical assessments were performed daily to monitor for signs of radiation-induced illness including vomiting, diarrhea, weight loss, hemorrhage, and alopecia. Signs of hematologic failure or infection were monitored using the methods of Uckun et al. (1997) as modified from the Children's Cancer Group Clinical Toxicity Criteria. Supportive care was provided when clinically necessary and included, parenteral fluids and supplemental nutrition. Blood transfusions were not given. In the event that animals became moribund, or reached the level of Grade 4 toxicity, as previously used in primate trials at Wake Forest (Hotchkiss et al. 1999, Cohen et al. 2004), they were humanely euthanized by intravenous pentobarbital overdose.
Hematology
Complete blood counts (hematocrit, red blood cells, total and differential leukocyte counts, hemoglobin, platelets) and chemistry panels (serum blood urea nitrogen, creatinine, total protein, albumin, bilirubin, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, potassium, sodium, chloride, total CO2) were collected and analyzed three times weekly for a period of one month following irradiation. Analyses were conducted in-house with a Heska Hematrue analyzer (Heska, Loveland, CO, USA) and by an external commercial veterinary laboratory (Antech Diagnostics, South Haven, MS, USA).
Necropsy/histopathology
All animals were humanely euthanized for collection and evaluation of major organs/tissues, with a particular focus on thymus, spleen, lymph nodes, and bone marrow. Thymus and spleen were collected and portions were snap frozen and stored at − 80°C, minced and incubated in RNAlater® (Qiagen, Austin, TX, USA) until freezing, or fixed with 4% paraformaldehyde followed by paraffin embedding and sectioning for histopathology. Similarly, small pieces of axillary, inguinal and submandibular lymph node tissues were snap frozen for analysis as described below.
Molecular assessment of thymic output
T-cell receptor (TCR) gene rearrangement and thymopoiesis were determined by quantification of signal joint TCR excision circles (sjTREC) (Lynch and Sempowski 2013). Recombinant sjTREC (NHP) plasmid control was generously provided by Dr. Danny Douek (VRC-NIAID).
Molecular characterization of lymphoid tissue cell composition
Quantitative real-time polymerase chain reaction (RTPCR) was used to evaluate the inflammatory cell content of thymus, spleen, and lymph node using previously described methods (Sophonsritsuk et al. 2013; Walker et al. 2008a, 2008b). Levels of mRNA transcripts for a specific marker associated with macrophages cluster of differentiation 68 (CD68), and four markers of T cells T-cell receptor beta, cluster of differentiation 3, 4 and 8 (TCRB, CD3, CD4, CD8) were measured in order to define the cellular composition of the tissue. Total ribosomal nucleic acid (RNA) was purified by homogenization of tissue preserved in RNAlater using TRI Reagent (Molecular Research Center, Inc.; Cincinnati, OH, USA) as described by Chomczynski (1993). RNA content and purity was determined at 260 nm and using A260/A280 ratios obtained on a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA quality was assessed by intensity of 28S and 18S ribosomal RNA bands on an Agilent Microanalyzer. Quantitative Taqman® RT-PCR assays employing cynomolgus-specific primers and Fam-labeled Taqman® MGB probes were used to quantitate mRNA steady-state levels (Sophonsritsuk et al., 2013; Walker et al., 2008a, 2008b). Aliquots of purified total RNA (~ 1–10 ug RNA) were reverse transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) in combination with random hexamers to generate cDNA from which real time RT-PCR quantitative analyses were performed. Aliquots of the cDNA archive were amplified with target-specific primers along with Taqman® probes (for targets described above). Real time quantitative RT-PCR was carried out on an Applied Biosystems ABI PRISM® 7000 Sequence Detection System. PCR was performed in separate wells for each target using a Taqman® Universal Master Mix and associated protocols. For each sample, the threshold cycle (Cts) for the gene of interest and the selected housekeeping gene(s) were determined. The ΔCt [Ct (target gene)-Ct (housekeeping gene(s))] was calculated to provide a measure of relative gene expression as noted above. Target gene expression was normalized to the geometric mean of three control genes [β-actin, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and ribosomal protein, large, P0 (RPLP0)] in each sample.
Acute inflammatory response to radiation
Cytokine analyses were performed on plasma samples collected on days 0, 1, 2, 4 and 6 of the first week, and every other day thereafter for the following 5 weeks, and then at weekly or monthly intervals thereafter. Analyses were carried out using a Luminex bead-based monkey 10-plex assay from Biosource/Life Technologies (Grand Island, NY, USA) for measurement of Interferon-γ, tumor necrosis factor-α, interleukins 2, 4, 8, 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1 alpha and beta, and Regulated on Activation, Normal T Cell Expressed and Secreted (IFNγ, TNFα, IL-2, IL-4, IL-8, IL-10, MCP-1, MIP-1α, MIP-1β, and RANTES). Values at or below the low limit of detection (LLOD) were reported at one-half the values of the LLOD.
Statistics
Statistically, the overall experiment was considered a pilot study with multiple time-points and a small number of subjects in individual cells, thus not lending itself well to traditional statistical approaches. Descriptive statistics were generated for all outcomes across the duration of the study. As noted above, necropsies were conducted at different time-points following irradiation. Some tissue outcomes (e.g., sjTREC analysis, organ weight, cell marker mRNA levels) showed dose-dependent effects which appeared to be only minimally affected by time since irradiation and were pooled across time-points and analyzed by Kruskal-Wallis non-parametric analysis of variance (ANOVA). Spear-man rho and Kendall's tau correlations were determined to estimate trends and dose response relationships with some variables. A p value of < 0.05 was considered significant. As a descriptive analysis, no adjustments for multiplicity were made. Analyses were conducted using JMP Statistical Discovery Software (Version 3.2.2, SAS Institute, Inc., Cary, NC, USA) or Statistica (Statsoft Inc, Tulsa, OK, USA).
Results
Clinical observations
Nausea or vomiting immediately after irradiation was seen in 0/3 controls, 1/6 animals given the 2 Gy dose, and 2/6 animals given the 5 Gy dose. Weight loss (5–15% of pre- irradiation body weight) was seen in 0/3 controls, 2/6 animals given 2 Gy, and 6/6 animals given 5 Gy. Early humane euthanasia was elected in 2/6 animals in the 2 Gy group (at 20 days post-irradiation) and 3/6 animals in the 5 Gy dose group (at 15, 16 and 17 days post-irradiation). due to severe clinical illness. Hemorrhage was seen in most irradiated animals (skin/subcutis, gingiva, lung, epicardium, skeletal muscle); this was attributed to thrombocytopenia.
Clinical pathology findings
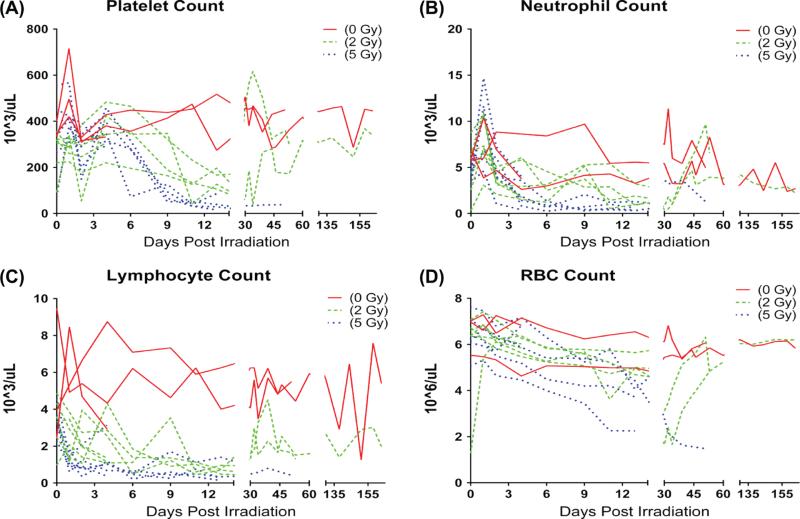
Dose-dependent decreases were seen in platelet counts, white blood cell counts, and red blood cell (RBC) counts. Platelets declined on day 2, reaching a nadir on average of 22,250 cells/ul approximately 14–23 days after irradiation with recovery to an average of > 300,000 cells/ul approximately 30–45 days after irradiation in survivors (Figure 1A). Neutro-phil counts (Figure 1B) increased acutely at day 2, and then declined to an average nadir of ~ 990 cells/ul approximately 6–14 days after irradiation. Lymphocyte counts declined to ~ 1600 cells/ul by day 2, reaching a nadir 6–10 days post irradiation which persisted for at least 6 months (Figure 1C). RBC counts in both irradiated groups declined (Figure 1D). RBC levels for the 5 Gy dose group failed to recover, while the monkeys receiving the 2 Gy dose exhibited a gradual increase in RBC count approximately 30 days after radiation, which eventually returned to non-irradiated control levels (0 Gy). Mortality increased with radiation dose and overall survival time post irradiation decreased with dose (Table I).
Figure 1.
Effects of 2 and 5 Gy TBI on circulating blood components: (A) Platelets, (B) Neutrophils, (C) Lymphocytes, and (D) Erythrocytes. Single data points from individuals plotted across time. This Figure is reproduced in color in the online version of International Journal of Radiation Biology
Impact of ionizing radiation on lymphoid and hematopoietic tissues
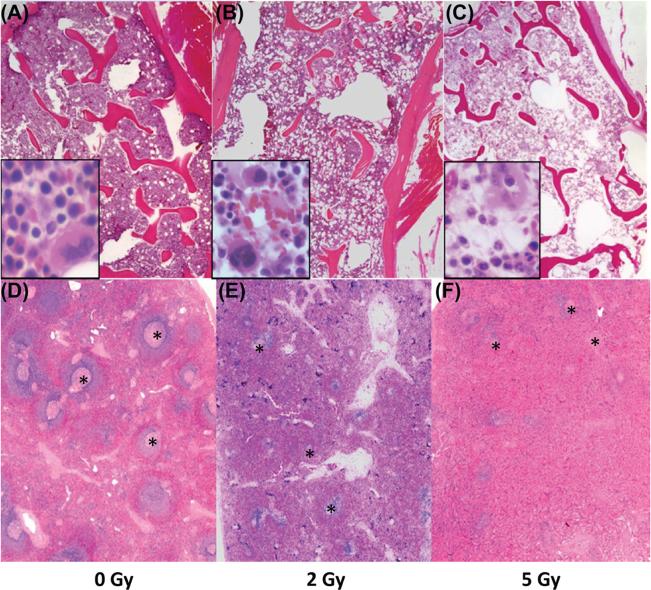
Severe hematopoietic cell depletion was observed across all lineages in the bone marrow of animals that received 2 or 5 Gy radiation (Figure 2A–C). Lymphocyte depletion in the spleen (and lymph node, data not shown) was noted at necropsy in all irradiated animals (Figure 2D–F). One animal in the 5 Gy group developed cellulitis and had bacterial colonies in the lungs, consistent with sepsis.
Figure 2.
Histologic sections of bone marrow (A–C) and spleen (D–F) from a control animal (0 Gy, A and D) and irradiated animals (2 Gy, B and E; and 5 Gy, C and F) five days post radiation exposure. The bone marrow from the irradiated animals was severely hypocellular with necrosis and loss of cells across all hematopoietic lineages. Note the dose-dependent marked necrosis and depletion of lymphoid follicles in the spleens of irradiated animals. Hematoxylin and eosin stain. Magnification 2×; inset magnification; 40×. Areas of splenic white pulp are marked with an (*). This Figure is reproduced in color in the online version of International Journal of Radiation Biology
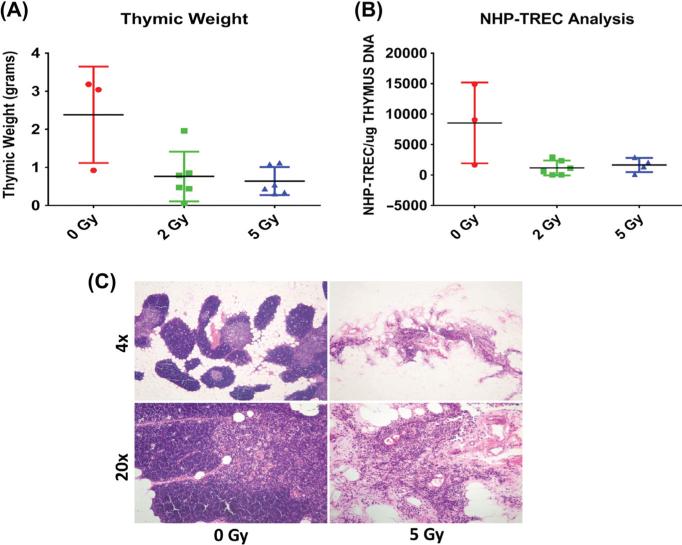
Significantly lower thymic weight was observed in animals receiving radiation compared to controls (both p < 0.04, control vs. 2 Gy p = 0.04, control vs. 5 Gy p = 0.02, Figure 3A), and there was an inverse relationship between thymic weight and radiation dose (Spearman rho = − 0.546, p = 0.05). Thirteen thymic samples yielded good quality genomic DNA capable of generating a thymic sjTREC result; due to the small group size, data from specific doses were grouped independent of time to show the overall impact of irradiation on thymopoiesis. The data show sjTREC, or TCR gene rearrangement, tended be lower with 2 and 5 Gy irradiation (p = 0.053) (Figure 3B), suggesting diminished T cell development, a finding consistent with the histologic demonstration of marked cortical and medullary atrophy in thymic tissues from these animals (Figure 3C).
Figure 3.
Effects of 2 and 5 Gy TBI on thymus weight (A), thymic sjTREC levels, (B) and thymic histology (C). Data are mean ± SEM of n = 3–6 per group. Data from samples from each dose at different post-irradiation time-points were pooled for analysis due to the low number of observations and the lack of evidence for a time dependent trend. In the 0 and 5 Gy histologic sections note the marked loss of architecture (almost complete loss of cortical lymphocytes), with relative sparing of medullary epithelial elements, in the thymus from the animal treated with 5 Gy (right panels), compared to an un-irradiated control (left panels). Hematoxylin and eosin stain. Upper panels 4×, lower panels 20×. This Figure is reproduced in color in the online version of International Journal of Radiation Biology
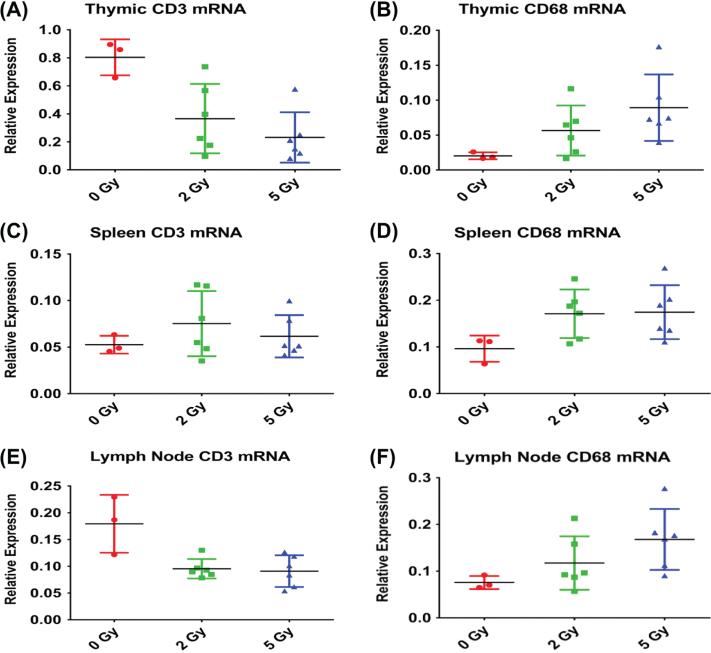
Tissue expression levels of key mRNA transcripts evaluated by qRT-PCR indicated radiation-induced shifts in the relative cell populations present in lymphoid tissues. Expression of T cell markers was reduced with radiation exposure; CD3 mRNA was significantly reduced in thymus (p = 0.006) and lymph node (p < 0.004) but not spleen (p > 0.1) (Figure 4), while CD4 mRNA was significantly reduced in thymus (p = 0.001, data not shown). CD68 expression tended to be elevated in the irradiated thymus (p = 0.07) lymph node (p = 0.09), and spleen (p = 0.11). Radiation exposure was inversely correlated with expression of CD3 in lymph node (tau = − 0.41, p = 0.054) and thymus (tau = − 0.53, p = 0.014), with CD4 in thymus (tau = − 0.598, p = 0.005), and positively correlated with CD68 expression in lymph node (tau = 0.506, p = 0.018) and thymus (tau = 0.55, p = 0.01). Dose-dependent increases in expression ratios of macrophage/T cell markers (CD68/CD3) was observed in Spleen and Lymph Node. An increase in expression of macrophage/T cell marker ratios was also observed in the thymus of animals exposed to 5 Gy (results not shown).
Figure 4.
Effects of 2 and 5 Gy TBI on levels of mRNA for T cell (CD3) and macrophage (CD68) markers as indices of tissue cell populations in lymph node, spleen, and thymus. Data from samples from each dose at different post-irradiation time-points were pooled for analysis due to the low number of observations and the lack of evidence for a time-dependent trend. Error bars indicate the standard error of the mean (SEM) for n = 3–6 per group. This Figure is reproduced in color in the online version of International Journal of Radiation Biology
Acute cytokine response to radiation
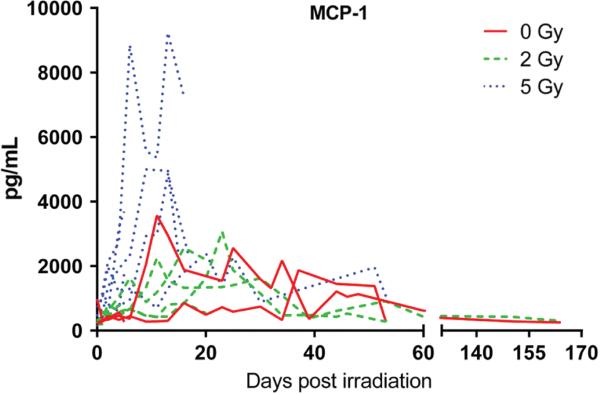
The effects of total body irradiation on systemic levels of plasma cytokines IFNγ, TNFα, IL-2, IL-4, IL-10, MCP-1, MIP-1α, MIP-1β, RANTES and IL-8 were evaluated over time post irradiation. The most consistent radiation-related finding was an increase in plasma MCP-1 levels in the group receiving the 5 Gy dose (Figure 5). Changes over time in other cytokines were variable, and sometimes exhibited changes in controls which could be responses to frequent blood draws or other factors (see Supplementary Figure 1, available online at http://informahealthcare.com/abs/doi/10.3109/09553002.2015.1028597).
Figure 5.
Effects of 2, and 5 Gy TBI on plasma MCP-1. Single data points from individuals plotted across time. This Figure is reproduced in color in the online version of International Journal of Radiation Biology
Discussion
Previous studies of the effects of ionizing radiation in the non-human primate have been primarily done in male animals, with most of the recent work performed in adolescent or young adult male rhesus monkeys (Farese et al. 2001, 2003, 2012, MacVittie et al. 2012, Dörr et al. 2014). The purpose of the present study was to evaluate responses to graded doses of total body radiation in the ovariectomized female cynomolgus monkey, a well-established model of post-menopausal female health. Our major long-term goal is to develop medical countermeasures that minimize the adverse health consequences of unintentional (accidental or terrorism-related) exposures to radiation in men and women across their lifespan. The present study lays some of the groundwork necessary for the development of an alternative and relatively more available animal model for evaluation of radiation mitigation strategies in post-menopausal women.
Cynomolgus macaques are an excellent model for the study of inflammation and cardiovascular disease (Register 2009), cancer risk (Cline and Wood 2009), and other conditions, and may provide a useful addition to our non-human primate animal model portfolio for discovery and development of radiation countermeasures. The effects of radiation exposure on stem cells (Adler and Erbelding 1988), brain electrical activity (Legeza and Turlakov 1991), liver function (Yannam et al. 2014), clinical, cardiac, cortisol stress response (Darenskaia et al. 1992, 2001), and mitigating effects of gonadotropin hormone releasing hormone (GnRH) antagonism on the testes (Shetty et al. 2013) have been studied in this species. Additional studies focusing on immune tissues/organs in this species should facilitate identification of pathways to immune dysfunction and hypothesis generation leading to novel pharmacologic and/or nutritional countermeasures to prevent or treat adverse effects of radiation on at risk organ systems.
In this study ovariectomized female cynomolgus monkeys exposed to a single 2–5 Gy dose of total body ionizing radiation experienced reductions in thymus weight and thymopoiesis, loss of cortical thymocytes, depletion of bone marrow, and peripheral hemorrhage which was attributed to thrombocytopenia. Radiation dose-dependent decreases in platelet, white blood cell (WBC), and red blood cell (RBC) counts were also observed. Similar patterns of neutropenia, thrombocytopenia, along with transient increases in systemic inflammatory cytokines (e.g., IFNγ, MCP-1, and MIP-1), and subsequent recovery have been observed in male rhesus macaques exposed to total body irradiation doses in the range of 7–8.9 Gy, which is consistent with 30–90% lethality at 60 days (Farese et al. 2012).
Altered mRNA signatures in thymus, spleen, and lymph node were observed in our cohort of irradiated cynomolgus female animals. CD3 (a marker of T cells) demonstrated tissue specific responses; exhibiting decreased expression with increasing radiation dose in thymus and lymph node, but not spleen; while expression of the macrophage marker CD68 increased in a dose-dependent manner relative to T cell-specific marker expression. These results suggest exposure to ionizing radiation may induce acute, dose-dependent alterations in systemic and tissue-specific biomarker responses with increases in macrophage/T cell ratios in lymphoid tissues. Histologic evaluations of these tissues suggested an overall reduction in the number of lymphocytes present, so the observed increase in macrophage/T cell marker ratios in these lymphoid tissues may reflect persistence of resident macrophages.
This study was designed to provide pilot data regarding the effects of sub-lethal and theoretically low to moderate single doses of total body irradiation at 2 and 5 Gy in ovariectomized adult female cynomolgus monkeys. Radiation dose levels of 2 and 5 Gy were selected largely based upon known responses of pubertal and adult male rhesus monkeys to total body irradiation (Farese et al. 2001, 2003, 2012, Stickney et al. 2006, Hérodin et al. 2007, Burdelya et al. 2008). Ovariectomized female cynomolgus monkeys were more radiosensitive than anticipated, as 2/6 animals receiving the 2 Gy dose and 3/6 of the animals receiving the 5 Gy dose experienced significant health problems and were euthanized ahead of schedule on the basis of our predefined health criteria, indicating that future non-lethal studies utilizing this model of radiation exposure should use total body doses lower than 5 Gy. Estimates of exposures from Nagasaki data suggest that the lethal dose in humans at 60 days for 50% of those exposed (LD50) is approximately 4.1 Gy (Anno et al. 2003).
The radiosensitivity differences observed here could be due to species, sex, and/or menopausal status. Estrogen has been shown to modulate immune responses is some systems through estrogen receptor interactions with the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (McKay and Cidlowski 1999, Kalaitzidis and Gilmore 2005, De Bosscher et al. 2006), and the lack of endogenous estrogen in these ovariectomized animals may have influenced responses to radiation. Influences of sex or endogenous sex hormones on the responses to radiation have not been well-studied, especially in non-human primate models, although there is evidence in the literature of an effect of sex. In rats exposed to total body radiation, males appeared to exhibit increased survival over females in late stages after exposure (Peters et al. 1981). Markedly dissimilar responses to acute and chronic radiation exposure in the transcriptional profiles of skeletal muscle of male and female mice have also been reported (Kovalchuk et al. 2004). In rhesus monkeys, there appeared to be no difference between males and females in susceptibility to radiation-induced cataract formation (Sonneveld et al. 1979). Women are also at greater risk for developing pneumonitis following radiation to the lung (Baker et al. 2013). Female sex has also recently been shown to be a strong predictor of acute (odds ratio [OR]: 1.72) and late (OR: 3.96) radio toxicities (Meyer et al. 2012). Furthermore, a recent study showed that women had two-fold greater risk for the development of cancer following low-dose ionizing radiation for cardiac imaging than men (Lawler et al. 2013). Women also tend to be at greater risk for future cancer development following total body irradiation. According to the 14th report on mortality in the Life Span Study cohort of atomic bomb survivors, females had excess relative risks (ERR) twice as high as males for total deaths and all solid cancer deaths (Ozasa et al. 2012).
These findings suggest that more investigation is needed to derive definitive conclusions as to the effects of male and female sex, as well as reproductive stage (premenopausal versus post-menopausal), on molecular and cellular effects of total body irradiation (TBI). Future studies addressing these questions, as well as aim how the immune system reconstitutes/rebalances after radiation exposure are warranted.
Supplementary Material
Acknowledgements
This study was supported by the Radiation Countermeasure Center for Research Excellence (NIH U19 AI067798). We thank Jean Gardin, Lisa O'Donnell and J.D. Bottoms (Wake Forest School of Medicine), and Jeff Hale (Duke University, retired) for their technical assistance.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary material available online
Supplementary Figure 1.
References
- Adams MR, Anthony MS, Chen H, Clarkson TB. Replacement of dietary soy protein isolate with concentrates of soy 7S or 11S globulin has minimal or no effects on plasma lipoprotein profiles and biomarkers of coronary risk in monkeys. Atherosclerosis. 2008;196:76–80. doi: 10.1016/j.atherosclerosis.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler ID, Erbelding C. Radiation-induced translocations in spermatogonial stem cells of Macaca fascicularis and Macaca mulatta. Mutat Res. 1988;198:337–342. doi: 10.1016/0027-5107(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–575. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, Dilling TJ. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85:190–195. doi: 10.1016/j.ijrobp.2012.03.041. [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Deoliveira D, Spasojevic I, Sempowski GD, Jiang C, Owzar K, Wang X, Gesty-Palmer D, Cline JM, Bourland JD, Dugan G, Meadows SK, Daher P, Muramoto G, Chute JP, Chao NJ. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PloS One. 2010;5:e11056. doi: 10.1371/journal.pone.0011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- Cline JM, Wood CE. Estrogen/isoflavone interactions in cynomolgus macaques (Macaca fascicularis). Am J Primatol. 2009;71:722–731. doi: 10.1002/ajp.20680. [DOI] [PubMed] [Google Scholar]
- Cohen KA, Liu TF, Cline JM, Wagner JD, Hall PD, Frankel AE. Toxicology and pharmacokinetics of DT388IL3, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human interleukin 3 (IL3), in cynomolgus monkeys. Leuk. Lymphoma. 2004;45:1647–1656. doi: 10.1080/10428190410001663572. [DOI] [PubMed] [Google Scholar]
- Darenskaia NG, Korotkevich AO, Maliutina TS, Nasonova TA. [A possibility to predict the severity of individual damage following the exposure to supralethal radiation dosage. Prediction by early radiation response]. Radiatsionnaia Biol Radioecol Ross Akad Nauk. 2001;41:165–170. [PubMed] [Google Scholar]
- Darenskaia NG, Kuznetsova SS, Korotkevich AO, Nasonova TA. [Comparative radiation sensitivity of monkeys, Macaca rhesus and Macaca fascicularis]. Radiobiologiia. 1992;32:370–376. [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- Dörr H, Lamkowski A, Graessle DH, Bennett A, Shapiro A, Farese AM, Garofalo M, MacVittie TJ, Meineke V. Linking the human response to unplanned radiation and treatment to the non-human primate response to controlled radiation and treatment. Health Phys. 2014;106:129–134. doi: 10.1097/HP.0b013e3182a12de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Casey DB, Smith WG, Vigneulle RM, McKearn JP, MacVittie TJ. Leridistim, a chimeric dual G-CSF and IL-3 receptor agonist, enhances multilineage hematopoietic recovery in a non-human primate model of radiation-induced myelosuppression: Effect of schedule, dose, and route of administration. Stem Cells. 2001;19:522–533. doi: 10.1634/stemcells.19-6-522. [DOI] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, 3rd, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–382. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, MacVittie TJ, Roskos L, Stead RB. Hematopoietic recovery following autologous bone marrow transplantation in a nonhuman primate: Effect of variation in treatment schedule with PEG-rHuMGDF. Stem Cells Dayt Ohio. 2003;21:79–89. doi: 10.1634/stemcells.21-1-79. [DOI] [PubMed] [Google Scholar]
- Hérodin F, Roy L, Grenier N, Delaunay C, Baugé S, Vaurijoux A, Grégoire E, Martin C, Alonso A, Mayol J-F, Drouet M. Antiapoptotic cytokines in combination with pegfilgrastim soon after irradiation mitigates myelosuppression in nonhuman primates exposed to high irradiation dose. Exp Hematol. 2007;35:1172–1181. doi: 10.1016/j.exphem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Hotchkiss C, Hall PD, Cline JM, Willingham MC, Kreitman RJ, Gardin J, Latimer A, Ramage J, Feely T, DeLatte S, Tagge EP, Frankel AE. Toxicology and pharmacokinetics of DTGM, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human granulocyte-macrophage colony-stimulating factor, in cynomolgus monkeys. Toxicol Appl Pharmacol. 1999;158:152–160. doi: 10.1006/taap.1999.8691. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Ponton A, Filkowski J, Kovalchuk I. Dissimilar genome response to acute and chronic low-dose radiation in male and female mice. Mutat Res. 2004;550:59–72. doi: 10.1016/j.mrfmmm.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Lawler PR, Afilalo J, Eisenberg MJ, Pilote L. Comparison of cancer risk associated with low-dose ionizing radiation from cardiac imaging and therapeutic procedures after acute myocardial infarction in women versus men. Am J Cardiol. 2013;112:1545–1550. doi: 10.1016/j.amjcard.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Legeza VI, Turlakov IS. [The effect of a high-intensity radiation exposure on the brain function of monkeys. The postradiation changes in brain bioelectrical activity]. Radiobiologiia. 1991;31:97–106. [PubMed] [Google Scholar]
- Lynch HE, Sempowski GD. Molecular measurement of T cell receptor excision circles. Methods Mol. Biol. Clifton NJ. 2013;979:147–159. doi: 10.1007/978-1-62703-290-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, Booth C, McFarland E, Jackson W., 3rd The acute gastrointestinal subsyndrome of the acute radiation syndrome: A rhesus macaque model. Health Phys. 2012;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Estep R, Robinson B, Wong SW. Nonhuman primate models of human immunology. Antioxid Redox Signal. 2011;14:261–273. doi: 10.1089/ars.2010.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Fortin A, Wang CS, Liu G, Bairati I. Predictors of severe acute and late toxicities in patients with localized head-and-neck cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:1454–1462. doi: 10.1016/j.ijrobp.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–243. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- Palermo RE, Tisoncik-Go J, Korth MJ, Katze MG. Old world monkeys and new age science: the evolution of nonhuman primate systems virology. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 2013;54:166–180. doi: 10.1093/ilar/ilt039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Sailer U, Peters K. [Variability of the dose effect in the radio-biologic experimentation on animals. Third communication: Influence of breed, sex, age as well as radiation dose on body weight and survival time in days after whole-body irradiation of Wistar and Siv-50 rats (author's transl)]. Strahlentherapie. 1981;157:124–133. [PubMed] [Google Scholar]
- Register TC. Primate models in women's health: Inflammation and atherogenesis in female cynomolgus macaques (Macaca fascicularis). Am J Primatol. 2009;71:766–775. doi: 10.1002/ajp.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175:519–525. doi: 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty G, Uthamanthil RK, Zhou W, Shao SH, Weng CC, Tailor RC, Hermann BP, Orwig KE, Meistrich ML. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology. 2013;1:886–898. doi: 10.1111/j.2047-2927.2013.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld P, Peperkamp E, van Bekkum DW. Incidence of cataracts in rhesus monkeys treated with whole-body irradiation. Radiology. 1979;133:227–229. doi: 10.1148/133.1.227. [DOI] [PubMed] [Google Scholar]
- Sophonsritsuk A, Appt SE, Clarkson TB, Shively CA, Espeland MA, Register TC. Differential effects of estradiol on carotid artery inflammation when administered early versus late after surgical menopause. Menopause. 2013;20:540–547. doi: 10.1097/GME.0b013e31827461e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickney DR, Dowding C, Garsd A, Ahlem C, Whitnall M, McKeon M, Reading C, Frincke J. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 2006;6:1706–1713. doi: 10.1016/j.intimp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Yanishevski Y, Tumer N, Waurzyniak B, Messinger Y, Chelstrom LM, Lisowski EA, Ek O, Zeren T, Wendorf H, Langlie MC, Irvin JD, Myers DE, Fuller GB, Evans W, Gunther R. Pharmacokinetic features, immunogenicity, and toxicity of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin in cynomolgus monkeys. Clin Cancer Res. 1997;3:325–337. [PubMed] [Google Scholar]
- Walker SA, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008a;196:106–113. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SE, Register TC, Appt SE, Adams MR, Clarkson TB, Chen H, Isom S, Franke AA, Kaplan JR. Plasma lipiddependent and independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for post-menopausal atherosclerosis burden. Menopause. 2008b;15:950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Clarkson TB, Appt SE, Franke AA, Boue SM, Burow ME, McCoy T, Cline JM. Effects of soybean glyceollins and estradiol in postmenopausal female monkeys. Nutr Cancer. 2006;56:74–81. doi: 10.1207/s15327914nc5601_10. [DOI] [PubMed] [Google Scholar]
- Yannam GR, Han B, Setoyama K, Yamamoto T, Ito R, Brooks JM, Guzman-Lepe J, Galambos C, Fong JV, Deutsch M, Quader MA, Yamanouchi K, Kabarriti R, Mehta K, Soto-Gutierrez A, Roy-Chowdhury J, Locker J, Abe M, Enke CA, Baranowska-Kortylewicz J, Solberg TD, Guha C, Fox IJ. A nonhuman primate model of human radiation-induced venocclusive liver disease and hepatocyte injury. Int J Radiat Oncol Biol Phys. 2014;88:404–411. doi: 10.1016/j.ijrobp.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.