Key Points
miR-17-92 is required for T cells to mediate GVHD but not the GVL effect.
Targeting miR-17-92 with antagomirs efficiently alleviates GVHD.
Abstract
MicroRNAs (miRs) play important roles in orchestrating many aspects of the immune response. The miR-17-92 cluster, which encodes 6 miRs including 17, 18a, 19a, 20a, 19b-1, and 92-1, is among the best characterized of these miRs. The miR-17-92 cluster has been shown to regulate a variety of immune responses including infection, tumor, and autoimmunity, but the role of this cluster in T-cell response to alloantigens has not been previously explored. By using major histocompatibility complex (MHC)-matched, -mismatched, and haploidentical murine models of allogeneic bone marrow transplantation (allo-BMT), we demonstrate that the expression of miR-17-92 on donor T cells is essential for the induction of graft-versus-host disease (GVHD), but dispensable for the graft-versus-leukemia (GVL) effect. The miR-17-92 plays a major role in promoting CD4 T-cell activation, proliferation, survival, and Th1 differentiation, while inhibiting Th2 and iTreg differentiation. Alternatively, miR-17-92 may promote migration of CD8 T cells to GVHD target organs, but has minimal impact on CD8 T-cell proliferation, survival, or cytolytic function, which could contribute to the preserved GVL effect mediated by T cells deficient for miR-17-92. Furthermore, we evaluated a translational approach and found that systemic administration of antagomir to block miR-17 or miR-19b in this cluster significantly inhibited alloreactive T-cell expansion and interferon-γ (IFNγ) production, and prolonged the survival in recipients afflicted with GVHD while preserving the GVL effect. Taken together, the current work provides a strong rationale and demonstrates the feasibility to target miR-17-92 for the control of GVHD while preserving GVL activity after allo-BMT.
Introduction
Despite the significant improvements in the field of allogeneic hematopoietic cell transplantation (allo-HCT), graft-versus-host disease (GVHD) remains the major cause of transplant-related morbidity and mortality.1 Multiple cell types, cytokines, chemokines, and signaling pathways involved in the innate and adaptive immune response are implicated in the development of GVHD.2 Further understanding of the molecular mechanisms that regulate the pathophysiology of GVHD is highly desirable.
MicroRNAs (miRs) are endogenous single-stranded and noncoding RNAs of 19 to 22 nucleotides.3,4 The seed sequence in miRs can bind to the partially complementary sequence in their target mRNAs, resulting in degradation of these target mRNAs and translational repression.3,4 The miRs regulate almost every known cellular process and play crucial roles in numerous biological and pathologic responses. Pertaining to miRs’ relation to GVHD, an elegant preclinical study demonstrated that a specific miR-mRNA network regulates allogeneic T-cell responses.5 A recent clinical study showed that miR-423, miR-199a-3p, miR-93, and miR-377 were upregulated in the plasma of patients with acute GVHD, and were then validated as biomarkers to predict GVHD occurrence.6 Other studies have indicated that miR-100,7 miR-34a,8 and miR-1559 play a potentially significant role in GVHD. Specific targeting of miR-155 using locked nucleic acid (LNA)-modified oligonucleotides (also known as antagomirs) effectively mitigates GVHD,9 leading to a current clinical trial to further establish the significance of miR-155 in GVHD (ClinicalTrials.gov identifier #NCT01521039). Evidently, miRs not only regulate the development of GVHD, but may also potentially be used as biomarkers and therapeutic targets.10
The miR-17-92 cluster, one of the best characterized miRs, encodes 6 miRs including miR-17, 18a, 19a, 20a, 19b-1, and 92-1 (supplemental Figure 1). Originally, this miR cluster was found to be involved in tumorigenesis.11 However, recent research has uncovered unexpected roles of its members in a wide variety of settings including normal development, cardiovascular disease, and aging.12,13 Furthermore, the miR-17-92 cluster plays critical roles in early B-cell development; homeostasis; and T-cell differentiation, survival, and function14-16; and its regulatory functions have been explored in anti-infection, antitumor, and autoimmune responses.13-15,17 However, the role this cluster plays in modulating T-cell alloresponses in GVHD and graft-versus-leukemia (GVL) activity has not been elucidated. In the current study, we have investigated how the miR-17-92 cluster regulates T-cell response to alloantigens in murine models of allogeneic bone marrow transplantation (allo-BMT), and revealed that absence or blockade of miR-17-92 significantly reduces GVHD while preserving the GVL effect.
Material and methods
Mice
C57BL/6 (B6; H-2b, CD45.2), B6.Ly5.2 (CD45.1), BALB/c (H-2d), and (B6×DBA2)F1 (BDF1, H-2b/d) were purchased from the National Cancer Institute (Frederick, MD). The founders of C.B10-H2b/LilMcdJ (BALB.B; H-2b) and Thy1.1 B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB.B mice were bred at Medical University of South Carolina (MUSC, Charleston, SC). T-cell conditional miR-17-92 knockout (KO) strain (miR-17-92fl/fl CD4-Cre+) was previously generated and crossed to the B6 background in the laboratory at Duke University,14 and the colony was maintained at MUSC. All animals were housed in the American Association for Laboratory Animal Care–accredited Animal Resource Center at MUSC. Experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of MUSC.
Bone marrow transplant models
T-cell purification from spleen and lymph nodes was done by negative depletion using magnetic beads as previously described.18 Major histocompatibility complex (MHC)-mismatched (B6→BALB/c), MHC-matched but minor histocompatibility antigen (MiHA)-mismatched (B6→BALB.B), and haploidentical (B6→BDF1) bone marrow transplant (BMT) models were used as previously established.19 Recipients were monitored for survival and body weight loss twice per week. The GVL models with A20 B-cell lymphoma or p815 mastocytoma were established as previously described.20 Representative samples of GVHD target organs were excised from recipients 14 days post-BMT and subjected to pathology scoring as previously described.18,20
Treatment with LNA antagomirs
The anti-miR-17 and anti-miR-19 antagomirs are 10-mer seed-targeting LNA oligonucleotides, which were designed as shown in supplemental Figure 1 and synthesized in Exiqon (Woburn, MA). Recipient mice were treated with anti-miR-17 or anti-miR-19, or scrambled at a loading dose of 25 mg/kg at day 0 followed by 5 mg/kg IV twice weekly up to day 21 after BMT.
Flow cytometry and serum cytokine detection
Mononuclear cells were isolated from recipient spleen, liver, gut, and lung as previously described18,20,21 and stained for surface molecules and intracellular cytokines using standard flow cytometry protocols. Stained cells were analyzed using Diva software, LSR II (BD Biosciences, San Jose, CA) and FlowJo (TreeStar, Ashland, OR). Cytokine levels in recipient serum were quantified using a cytometric bead assay according to the manufacturer’s instructions (BD Biosciences, San Jose, CA).22
Statistics
For comparison of recipient survival among groups in GVHD experiments, the log-rank test was used to determine statistical significance. To compare body weight changes, pathology scores, cytokine levels, and receptor levels, a Student t test was performed.
Results
miR-17-92 promotes allogeneic T-cell responses in vivo
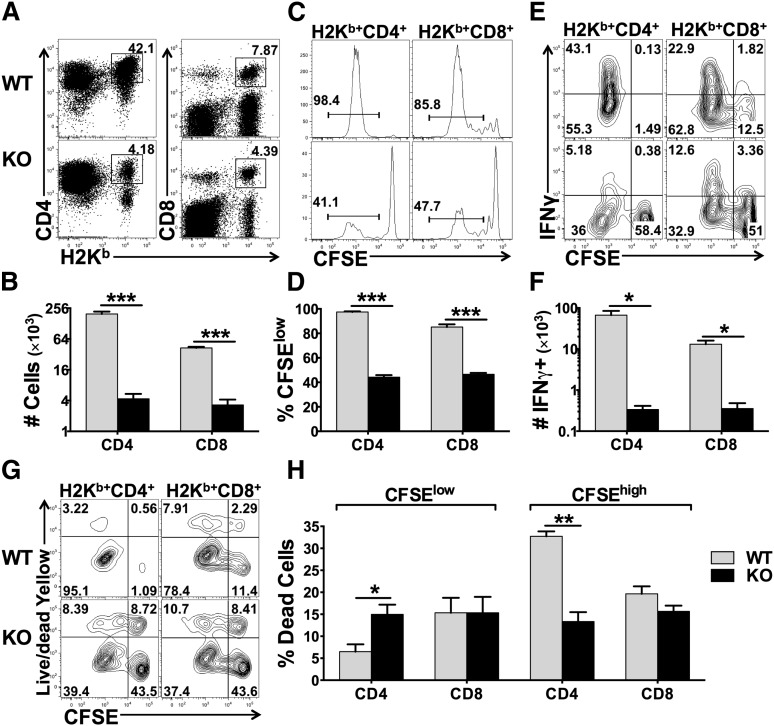
The miR-17-92 cluster promotes T-cell proliferation, enhances Th1 differentiation, protects T cells from activation-induced cell death, and suppresses the generation of induced regulatory T cells (iTregs) under polyclonal stimulation in vitro.14 Therefore, we hypothesized that this miR cluster plays an essential role in T-cell alloresponses. To test this, we used B6 mice with miR-17-92 conditional KO on the T-cell lineage (miR-17-92fl/fl CD4-Cre+). The T-cell subsets including CD4, CD8, Tregs, naïve, and memory T cells were comparable between wild-type (WT) and KO mice (data not shown). We then compared the responses of WT and KO T cells after adoptively transferring them into lethally irradiated allogeneic recipients. We observed that the KO T cells had a substantially reduced ability to proliferate and produce IFNγ compared with WT counterparts, reflected by percentage and number of donor T cells (Figure 1A-B), carboxyfluorescein succinimidyl ester (CFSE) dilution (Figure 1C-D), and percentage and number of IFNγ+ cells in donor T cells (Figure 1E-F). Interestingly, the KO CD4 T cells had an increased rate of cell death among fast-dividing cells (CFSElow) but a decreased rate of cell death among slow-dividing cells (CFSEhigh) compared with their WT counterparts (Figure 1G-H). Decreased rate of cell death in KO CD4 T cells was also observed after being transferred into syngeneic recipients where T cells were undergoing homeostatic proliferation (data not shown). Conversely, miR-17-92 had no effect on cell death of CD8 T cells, regardless of cell division (Figure 1G-H). These results suggest that miR-17-92 enhances T-cell proliferation and activation in response to alloantigens. Furthermore, miR-17-92 differentially regulates CD4 vs CD8 T cells and homeostatic vs antigen-driven responses, which mainly protects antigen-driven CD4 T cells from undergoing cell death.
Figure 1.
Effects of miR-17-92 on T-cell proliferation, activation, and survival. Purified T cells from WT or miR-17-92 KO mice (miR-17-92fl/fl CD4-Cre+) on B6 background were labeled with CFSE and transferred into lethally irradiated BALB/c mice at 2 × 106/mouse. Four days after cell transfer, recipient spleens were collected and subjected to cell counting and fluorescence-activated cell sorting (FACS) staining. (A-B) Percentage (as showed by representative flow figures) and absolute numbers of donor-derived (H2Kb+) CD4 and CD8 T cells on gated live cells; (C-D) representative flow figures of CFSE dilution and percentage of CFSE-diluted cells on gated donor cells; (E-F) percentage and absolute numbers of IFNγ+ cells on gated donor CD4 and CD8 T cells; (G) cell death along with cell division on gated donor T cells; and (H) percentage of dead cells among fast-dividing (CFSElow) or slow-dividing (CFSEhigh) on gated donor T cells. Data were shown of 1 representative mouse (A,C,E,G) or mean ± standard deviation (SD) of 4 mice per group (B,D,F,H). Two replicate experiments were performed with a total of 8 mice. *P < .05, **P < .01, ***P < .001.
miR-17-92 is essential for T cells to induce GVHD
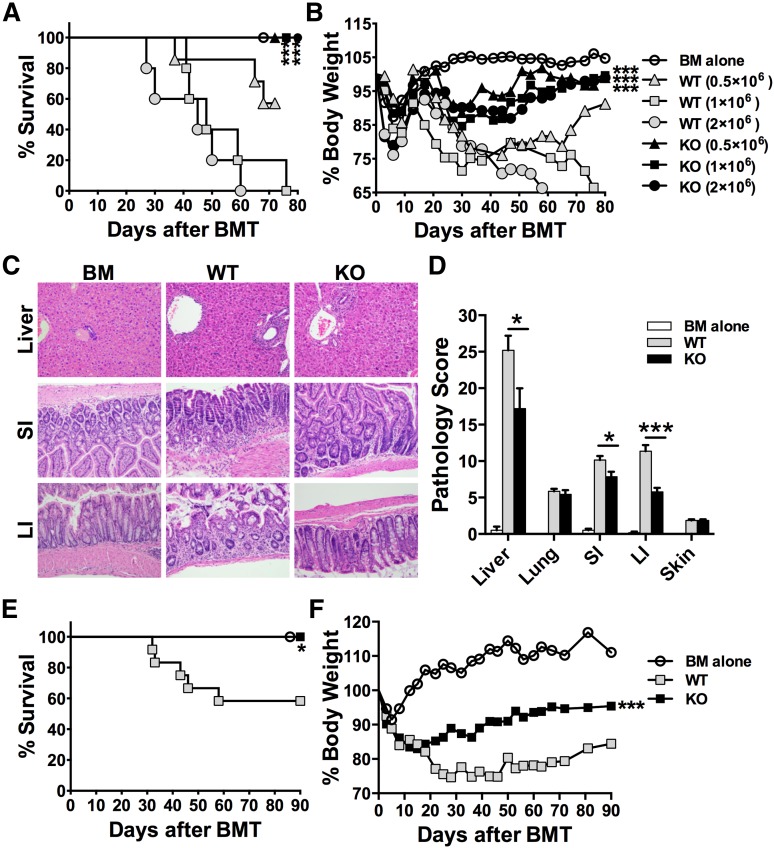
Because miR-17-92 is critical for T-cell proliferation, IFNγ production, and survival in response to alloantigens (Figure 1), we further hypothesized that KO T cells would fail to induce GVHD. Using a MHC-mismatched B6→BALB/c model and titrating the dose of donor T cells, we found that recipients of WT T cells developed severe and lethal GVHD in a dose-dependent manner, whereas recipients of KO T cells all survived long term (Figure 2A, P < .001), with significantly less weight loss (Figure 2B) and milder pathologic injuries in the liver and gut (Figure 2C-D). Thirty days after BMT, recipients of KO T cells had significantly improved donor-derived B-cell reconstitution in the peripheral blood compared with recipients of WT T cells (supplemental Figure 2A). Although the majority of donor-derived T cells originated from mature T cells (H2Kb+Ly5.1–) in the recipients with WT T cells, the majority of donor T cells were BM-derived T cells (H2Kb+Ly5.1+) in the recipients with KO T cells (supplemental Figure 2B). Consistently, 80 days post-BMT, the recipients of KO T cells had significantly more donor BM-derived T and B cells but much fewer transferred T cells in their spleens than those of survived WT T-cell recipients (supplemental Figure 3), indicating that recipients of KO T cells survived long term without severe GVHD.
Figure 2.
Role of miR-17-92 on donor T cells in the induction of GVHD. BALB/c mice were lethally irradiated and transplanted with 5 × 106/mouse T cell–depleted marrow cells (TCD-BM, Ly5.1+) or plus purified T cells (Ly5.2+) from WT or KO mice on B6 background at the doses indicated. Recipient mice were monitored for survival (A) and body weight change (B) until 80 days after transplant. Five to seven mice were included in each group at each cell dose. Statistical analysis was performed to compare the same dose of WT and KO T cells. In separate experiments, pathology injuries (C) and scores (D) were shown 14 days post-BMT; N = 14 per group. SI, small intestine; LI, large intestine. BALB/b mice were lethally irradiated and transplanted with TCD-BM (Ly5.1+) alone or plus purified T cells at 3 × 106/mouse from WT or KO mice on B6 background. Recipient mice were monitored for survival (E) and body weight change (F) until 90 days after BMT. Data shown were pooled from 2 replicated experiments with 10 to 12 mice per group. *P < .05, **P < .01, ***P < .001.
In clinical hematopoietic cell transplantation, most patients receive grafts from MHC-matched but MiHA-mismatched donors. In an effort to extend our studies to better mimic a clinical scenario, we used the B6→BALB.b (both H2b) model with at least 29 different MiHA loci.23 Although the recipients of WT T cells had modest GVHD with <60% survival and severe weight loss, the recipients of KO T cells had mild GVHD with 100% long-term survival (P < .05) and minimal weight loss (Figure 2E-F). In parallel, the reconstitution of donor BM–derived T and B cells in spleen was significantly better in the recipients of KO T cells compared with those of WT T cells (supplemental Figure 4A-B). In addition, a significantly improved thymus cellularity and function, reflected by the number of total and CD4+CD8+ thymocytes, were found in recipients of KO T cells (supplemental Figure 4A, C). Given that the thymus is a sensitive GVHD target organ, which is important for immune reconstitution, our data indicate that KO T cells did not cause severe damage in recipient thymus or obvious immune deficiency that is typically associated with GVHD. Taken together, these data suggest that miR-17-92 is required for donor T cells to induce GVHD.
miR-17-92 regulates T-cell expansion and migration
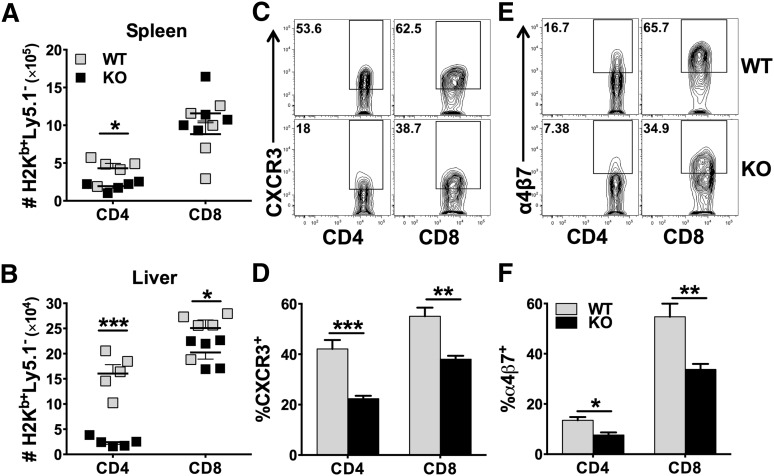
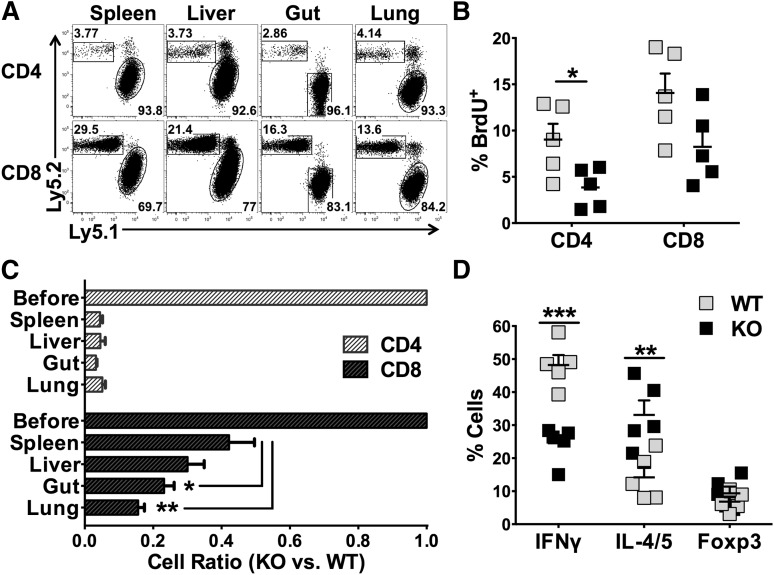
Development of GVHD requires donor T-cell expansion in lymphoid organs and migration into target organs through upregulating chemokine receptors and integrins.24 We examined the presence of donor T cells in recipient spleens (secondary lymphoid organ) and livers (GVHD target organ) 14 days after allo-BMT. We found that KO CD4 T cells were fewer than WT CD4 T cells in the recipient spleen (Figure 3A), and the difference was much more dramatic in the liver (Figure 3B). Although WT and KO CD8 T cells were comparable in the spleen, KO cells were significantly fewer than WT cells in the liver (Figure 3A-B). We observed that both CD4 and CD8 KO T cells had significantly reduced expression of CXCR3 and α4β7 compared with their WT counterparts (Figure 3C-F). In addition, KO T cells expressed significantly lower levels of CXCR3 in the liver (data not shown) and of α4β7 in the gut and mesenteric lymph node (MLN) (supplemental Figure 5A). These data suggest that miR-17-92 is required for CD4, but not CD8, T-cell expansion after allo-BMT. In addition, miR-17-92 may promote T-cell migration to GVHD target organs.
Figure 3.
Role of miR-17-92 on donor T-cell migration and expression of chemokine receptor and integrin. BMT was carried out as outlined in Figure 2A, in which 1 × 106/mouse donor T cells were transplanted. Fourteen days post-BMT, recipient spleens and livers were collected and mononuclear cells were isolated and subjected to cell counting and FACS staining. Absolute numbers of donor H2Kb+Ly5.1–CD4+ or CD8+ WT (gray symbols) and KO (black symbols) T cells were shown in spleen (A) or liver (B). % CXCR3+ cells were shown on gated H2Kb+Ly5.1–CD4+ or CD8+ T cells in spleen (C-D). % α4β7+ cells were shown on gated H2Kb+Ly5.1–CD4+ or CD8+ T cells in spleen (E-F). Data shown were pooled from 2 replicated experiments (N = 10/group). *P < .05, **P < .01, ***P < .001.
miR-17-92 controls T-cell differentiation
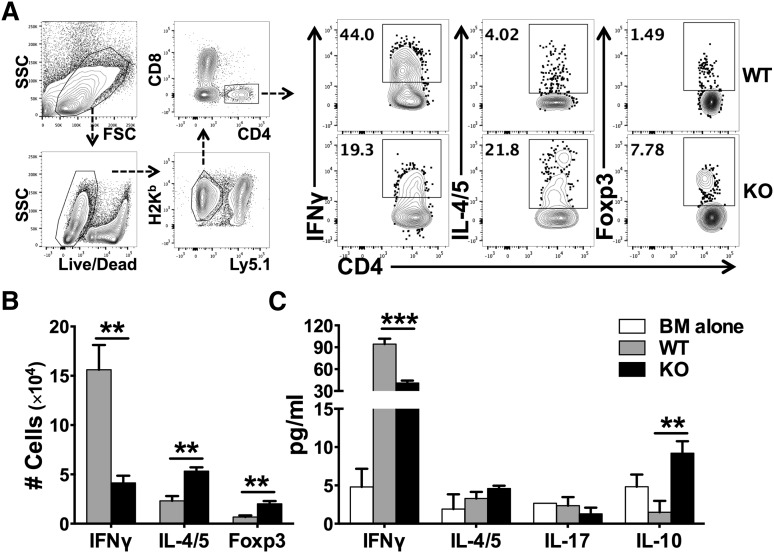
Naïve CD4 T cells can differentiate into various T-cell subsets including Th1, Th2, Th17, and Treg cells. With regard to GVHD, Th1 and Th17 cells are considered to be pathogenic, whereas Tregs are suppressive.25 We therefore evaluated how miR-17-92 affects T-cell differentiation after allo-BMT. We found that WT T cells differentiated predominantly into IFNγ-producing Th1 cells, with quite few IL-4/5–producing Th2 or Foxp3+ iTregs presented in recipient spleens (Figure 4A-B). On the contrary, KO T cells differentiated into significantly fewer Th1 cells and toward Th2 and iTregs cells. The effect of miR-17-92 on T-cell differentiation was also evident in GVHD target organs such as the liver (supplemental Figure 6). In the absence of miR-17-92, KO CD4 T cells also produced significantly lower levels of IFNγ and IL-17 in recipient gut and MLN, which is consistent with the alleviated gut damage in the recipients of KO T cells (supplemental Figure 5B-C). The intracellular cytokine expression was further supported by a similar cytokine production pattern in the serum. The levels of IFNγ were significantly reduced, whereas the levels of IL-10 were significantly increased in recipients transferred with miR-17-92 KO T cells, compared with those with WT T cells (Figure 4C). We reason that decreased Th1, but increased Th2 and iTregs, contributed to the reduced ability of KO T cells to induce GVHD. To test whether iTregs deficient for miR-17-92 are functional, we generated iTregs from WT or KO CD4+CD25– T cells under iTreg-polarizing culture conditions in vitro, and found that the rate of iTreg generation in KO T cells was comparable in transforming growth factor-β (TGFβ) condition, yet was lower in TGFβ plus retinoic acid (RA) condition compared with WT T cells (supplemental Figure 7A). Importantly, the suppressive ability of KO iTregs was similar to that of WT iTregs (supplemental Figure 7B). Thus, we conclude that iTregs generated from KO T cells likely contributed to the reduction of GVHD.
Figure 4.
Effects of miR-17-92 on the differentiation of donor T cells after allo-BMT. BMT was carried out as outlined in Figure 3. Two weeks post-BMT, recipient spleens were collected and subjected to cell counting and FACS staining. (A) The expression of IFNγ (Th1 cytokine), IL-4/5 (Th2 cytokine), and Foxp3 (Treg markers) were shown on gated donor H2Kb+Ly5.1–CD4+ cells as shown in the gating strategy; (B) absolute numbers of IFNγ+, IL-4/5+, or Foxp3+ cells among donor H2Kb+Ly5.1–CD4+ cells were shown. Few cells differentiated into IL-17–producing Th17 cells and there was no difference between WT and KO T cells (data not shown). (C) The levels of cytokines in recipient serum were measured using cytokine beads assay and displayed as mean ± SD. Data shown were pooled from 2 replicated experiments (N = 10/group). **P < .01, ***P < .001.
miR-17-92 regulates T-cell response intrinsically
Our data indicate that deficiency of miR-17-92 in T cells strongly inhibits CD4 T-cell expansion, Th1 differentiation, and T-cell migration to target organs. To test the endogenous defects of these KO T cells, we used a competitive assay to directly compare the responses of WT vs KO T cells in the same recipient after allo-BMT. Although WT and KO T cells were mixed 1:1 before adoptive transfer, there were >90% of WT cells (Ly5.1+) among total donor CD4 T cells in recipient spleen 2 weeks post-BMT, indicating that the WT CD4 T cells ousted their KO counterparts under the competitive environment (Figure 5A). In contrast, the deficiency of miR-17-92 had much less effect on the expansion of CD8 T cells (Figure 5A). Consistently, the percentage of BrdU-labeled cells was significantly reduced in KO CD4 T cells, but comparable in KO CD8 T cells compared with WT counterparts in recipient spleens (Figure 5B). We also calculated the ratio of KO/WT T cells in recipients to evaluate the impact of miR-17-92 on donor T-cell expansion in the spleen and migration into GVHD target organs including the liver, gut, and lung. The ratio of KO/WT CD4 T cells was dramatically reduced in allogenic recipients, but the ratio in each of those target organs was comparable with that in the spleen (Figure 5C), suggesting that miR-17-92 affects CD4 T-cell expansion. On the other hand, the ratio of KO/WT CD8 T cells was significantly reduced in the gut and lung compared with the spleen (Figure 5C). Together with reduced expression of CXCR3 and of α4β7 on the KO T cells (Figure 3 and supplemental Figure 5), these data suggest that miR-17-92 may also promote CD8 T-cell migration. Under the same inflammatory environment, KO CD4 T cells still had significantly reduced IFNγ+ and increased IL-4/5+ cells (Figure 5D). These data indicate that deficiency of miR-17-92 in T cells mainly impairs the expansion of CD4 T cells and modifies their differentiation. Conversely, KO CD8 T cells might impair migration but did not have substantially reduced expansion ability or cytolytic potential (tumor necrosis factor-alpha [TNFα], IFNγ, granzyme B, and CD107; data not shown). These data suggest that miR-17-92 affects T-cell responses intrinsically and regulates CD4 vs CD8 in a different manner.
Figure 5.
miR-17-92 affects T-cell response intrinsically. Lethally irradiated BALB/c mice were transplanted with 5 × 106/mouse TCD-BM (Thy1.1+) together with 1 × 106 /mouse WT (Ly5.1+ Thy1.2+) and 1 × 106 /mouse miR-17-92 KO T cells (Ly5.2+ Thy1.2+) from B6 mice. Fourteen days after BMT, recipient mice were killed and spleen, liver, lung, and intestine were harvested. Mononuclear cells were isolated from these organs and subjected for flow staining. (A) The percentage of KO (Ly5.2+) and WT (Ly5.1+) CD4 or CD8 T cells on gated on H2Kb+Thy1.2+ donor cells were shown in the representative flow figures. (B) BrdU was injected into recipients (2 mg/mouse) 24 hours before euthanasia of the recipients. Percentage of BrdU+ cells in WT and KO cells T cells were displayed on gated Ly5.1+ or Ly5.2+ cells as shown in (A). (C) Based on the percentage of WT and KO T cells in (A), we calculated the ratio of KO to WT T cells in spleen as well as in GVHD target organs. The first bar indicates 1:1 mixture of WT and KO T cells before BMT. (D) Percentages of IFNγ+, IL-4/5+, and Foxp3+ cells among WT or KO CD4+ T cells are shown. Data shown are from 1 of 2 replicate experiments (N = 10/group). *P < .05, **P < .01, ***P < .001.
miR-17-92 is dispensable to the GVL activity and CD8 T-cell cytolytic function
We next asked the critical question of whether T cells deficient for miR-17-92 maintain their GVL activity. To test this, we infused host-type B-cell lymphoma (A20) together with BM or BM plus T cells into lethally irradiated allogeneic recipients. As expected, all the recipients without T-cell infusion died of lymphoma relapse within 30 days after BMT (supplemental Figure 8A). When receiving 0.5 × 106/mouse T cells, all the recipients of WT T cells died of GVHD (except one who died from tumor relapse) based on their survival (supplemental Figure 8A), body weight loss (supplemental Figure 8B), and tumor signal (supplemental Figure 8C). In contrast, the recipients of KO T cells at the same dose survived without GVHD and were largely free from tumor relapse. Furthermore, when receiving 1.0 × 106/mouse of T cells, all the recipients of WT T cells died of GVHD without tumor relapse, whereas all the recipients of KO T cells had long-term survival without severe GVHD or tumor relapse (supplemental Figure 8D-E). Taken together, these data suggest that the KO T cells had preserved GVL activity in the MHC-mismatched BMT model.
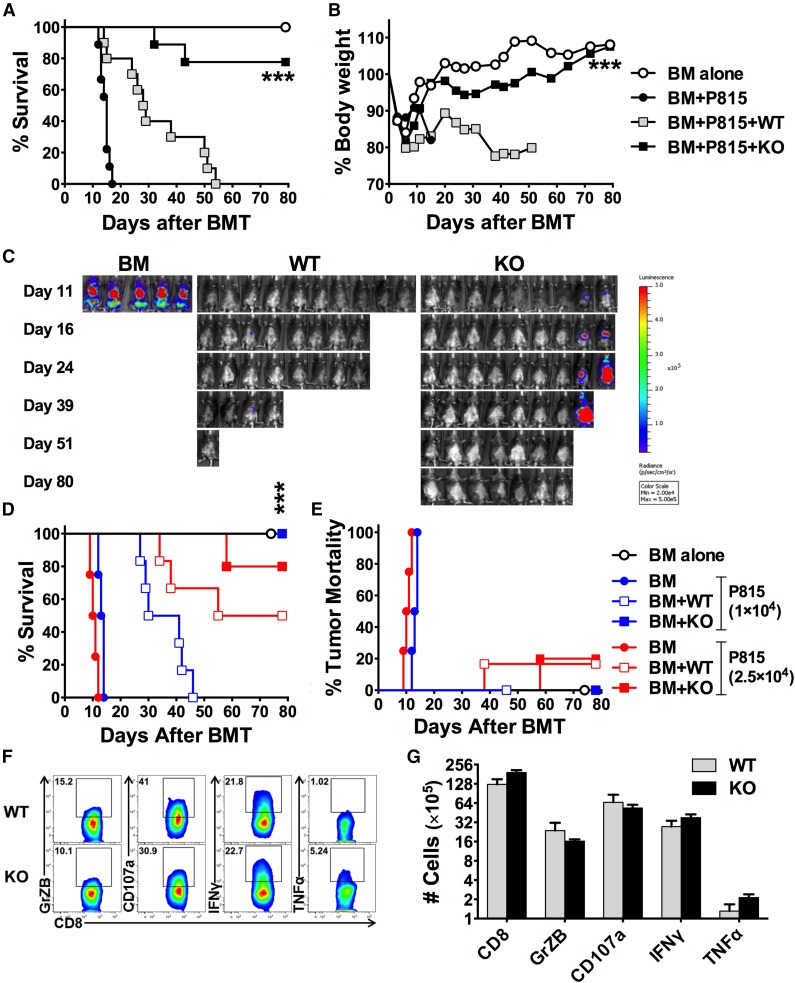
To exclude the possibility that the preserved GVL activity is model-specific, we used a haploidentical B6→BDF1 BMT model with more aggressive p815 mastocytoma. All the recipients without T-cell infusion died of leukemia relapse within 20 days after BMT (Figure 6A), whereas the recipients of WT T cells died of GVHD, reflected by 100% lethality (Figure 6A), severe weight loss (Figure 6B), and no tumor signal (Figure 6C). In contrast, the vast majority of the recipients transplanted with KO T cells survived without GVHD or tumor relapse. To confirm the GVL activity, we tested higher doses of p815 that caused accelerated tumor relapse in the recipients without T-cell infusion (Figure 6D-E). Although the mortality rate caused by tumor relapse was comparable regardless of T-cell type (Figure 6E), the recipients of KO T cells had improved survival (Figure 6D) compared with those of WT T cells. These results indicate that miR-17-92 is essential for donor T cells to induce GVHD but not to mediate the GVL effect. To understand the potential mechanisms by which T cells deficient for miR-17-92 had impaired ability to induce GVHD while preserving GVL activity, we examined the expression of cytolytic molecules on donor CD8 T cells. Compared with WT T cells, the KO CD8 T cells expressed lower levels of granzyme B or CD107a, similar levels of IFNγ, and higher levels of TNFα proportionally (Figure 6F). Furthermore, the absolute numbers of KO CD8 T cells expressing those effector molecules in recipient spleens were comparable with WT cells (Figure 6G). We reason that residual granzyme B, IFNγ, and/or TNFα in KO CD8 T cells are responsible for their preserved GVL activity.
Figure 6.
Role of miR-17-92 on donor T cell–mediated GVL and cytolytic activities. BDF1 mice were lethally irradiated and transplanted with 5 × 106/mouse TCD-BM alone or plus purified T cells from WT or KO B6 mice at 4 × 106/mouse. Recipient mice were also infused with 5 × 103 luciferase-transduced p815 cells at the day of BMT. Recipients were monitored for survival (A) and body weight change (B) until 80 days after transplant. (C) Tumor growth was monitored using bioluminescence imaging on the dates indicated. Data were pooled from 2 replicate experiments (N = 5-10/group). Using the same BMT model, we increase the dose of p815 cells to 1 or 2.5×104/mouse, respectively. Recipients were monitored for survival (D) and mortality caused by tumor relapse (E) until 80 days after transplant (N = 6/group). In a separate experiment, recipient spleens were collected 14 days post-BMT and stained for the expression of granzyme B (GrZB), CD107a, IFNγ, and TNFα on gated CD8+ donor T cells. Representative flow figures were shown on gated donor CD8 T cells (F), and the bar graph indicates absolute numbers of total CD8 T cells or CD8 T cells that express each of these molecules (G). ***P < .001.
Targeting miR-17 or -19b using specific LNA antagomirs inhibits T-cell alloresponse and alleviates GVHD
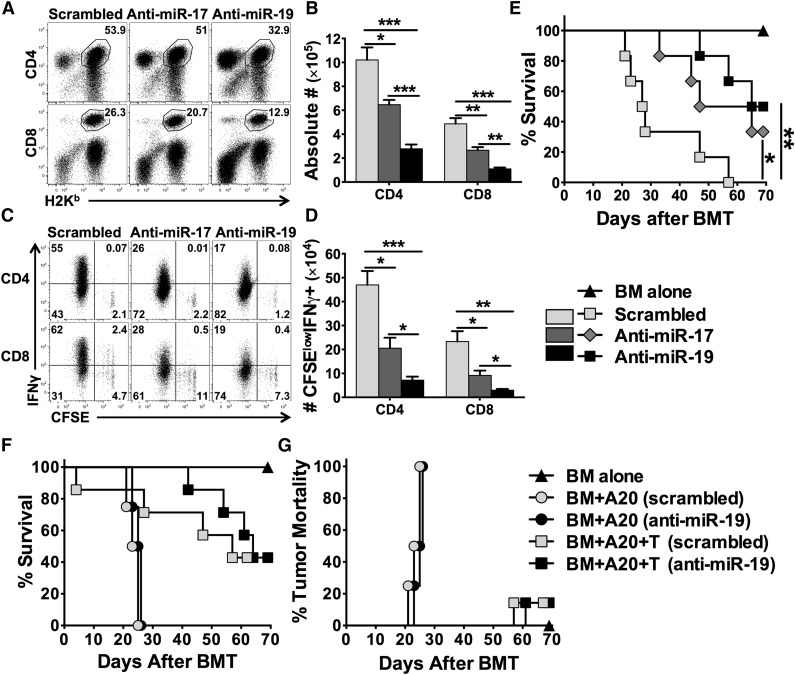
Strong evidence demonstrates that LNA antagomirs can specifically and effectively block target miRs.9,26 Furthermore, the LNA antagomirs specific for the members in the miR-17-92 cluster have been tested and shown to be effective in blocking the function of this cluster and inhibiting tumor progression.27,28 By reconstituting miR-17-92 whole-cluster–deficient T cells with individual miRs, the study by Jiang et al reveals that miR-17 and miR-19b among miR-17-92 cluster are the key players in controlling Th1 responses.14 We thus designed LNA antagomirs that can specifically target miR-17 seed family (miR-17 and miR-20a) or miR-19 seed family (miR-19a and miR-19b-1) (sequences highlighted in supplemental Figure 1). We then tested the effect of these LNA antagomirs on T-cell expansion and activation in allogeneic recipients. Compared with scrambled control, anti-miR-17 significantly reduced donor CD4 and CD8 T-cell expansion (Figure 7A-B) and IFNγ production (Figure 7C-D). The effect of anti-miR-19 was more profound than that of anti-miR-17 (Figure 7A-D). Furthermore, systemic treatment with anti-miR-17 or anti-miR-19 significantly reduced recipient mortality compared with the scrambled control group (Figure 7E; P < .05 and P < .01, respectively). Because blocking miR-19 was more effective to alleviate GVHD, we further evaluated the effect of anti-miR-19 on the GVL activity (Figure 7F-G and supplemental Figure 9). Without T-cell infusion, the recipients had similar tumor relapse and mortality after the treatment with the scrambled and anti-miR-19 (Figure 7F-G and supplemental Figure 9), suggesting that anti-miR-19 did not have a direct effect on tumor growth. With T-cell infusion, the recipients treated with anti-miR-19 showed prolonged survival (Figure 7F) and comparable mortality as a result of tumor relapse as those with scrambled antagomir (Figure 7G and supplemental Figure 9). These data suggest that pharmacologically blocking the key member of the miR-17-92 cluster efficiently constrains T-cell alloresponses and thus attenuates GVHD while preserving GVL activity.
Figure 7.
Effects of anti-miR-17 or anti-miR-19 LNA antagomirs on donor T-cell alloresponse and GVHD. BALB/c mice were lethally irradiated and transplanted with 2 × 106 purified and CFSE-labeled T cells from WT B6 mice. Recipient mice were injected IV with scrambled, anti-miR-17, or anti-miR-19 at 25 mg/kg on day 0 and 5 mg/kg on day 2. Recipients were killed on day 4 after cell transfer, and splenocytes were counted and stained for surface expression of H2Kb (donor marker), CD4, CD8 on gated live cells (A), and intracellular IFNγ along with CFSE profile gated on H2Kb+ CD4+ or CD8+ cells (C). Absolute numbers of CD4+ or CD8+ donor T cells (B), and CFSE-diluted IFN-γ+ cells (D) were shown. Four mice were used in each group. In a separate experiment, BALB/c mice were lethally irradiated and transplanted with 5 × 106 TCD-BM alone or with T cells from B6 mice at 1 × 106/mouse. Recipient mice were treated with individual antagomirs as indicated twice per week from 25 mg/kg on day 0 and then 5 mg/kg until day 21 after BMT. Recipient survival (E) was shown on 6 mice per group. Using the same BMT model, recipient mice were also infused with 5000 luciferase-transduced A20 cells at the day of BMT, and treated with individual antagomirs as indicated. Recipient survival (F) and mortality caused by tumor relapse (G) were shown on 4 to 7 mice per group. *P < .05, **P < .01, ***P < .001.
Discussion
Primarily using a genetic approach, our data demonstrate that the miR-17-92 cluster intrinsically controls multiple aspects of the T-cell response to alloantigens and regulates CD4 and CD8 T cells in a different manner. The miR-17-92 strongly enhances activation, survival, and proliferation of CD4 T cells. In addition, this cluster is critical for Th1 differentiation, but is suppressive for Th2 and iTreg differentiation. In contrast to CD4 T cells, miR-17-92 has no impact on cell survival and a decreased effect on proliferation and cytolytic function, but may promote migration of CD8 T cells. As a result, the expression of miR-17-92 on donor T cells is essential for the induction of GVHD but not for the mediation of the GVL effect after allo-BMT. Furthermore, pharmacologic blockade of miR-17 or miR-19b is effective in controlling T-cell alloresponses and attenuating GVHD severity.
In the current study, we have provided compelling evidence that miR-17-92 is required for T cells to induce GVHD. T cells deficient for miR-17-92 were essentially unable to mediate GVHD in all 3 allogeneic BMT models tested: MHC-mismatched (Figure 2A-B), MHC-matched but MiHA-mismatched (Figure 2E-F), and haploidentical (Figure 6) models. Mechanistically, we first observed that CD4 T cells deficient for miR-17-92 dramatically decreased expansion both on day 4 (Figure 1A-B) and day 14 (Figure 3A) after allo-BMT. Decreased expansion likely resulted from reduced proliferation (Figure 1C-D) and increased activation-induced cell death (Figure 1G-H) in alloantigen-driven response in vivo. Unexpectedly, the cell death rate was significantly decreased on KO CD4 T cells that were undergoing slow or no cell division (CFSEhigh) (Figure 1G-H). Consistently, the KO CD4 T cells had lower rates of cell death and expressed higher levels of Bcl-2 compared with WT T cells once being transferred into irradiated syngeneic recipients (data not shown). Thus, miR-17-92 protects CD4 T cells from extrinsic apoptosis under alloantigen-driven proliferation, whereas it promotes intrinsic apoptosis under homeostatic proliferation, which is likely because miR-17-92 negatively regulates PTEN during T-cell activation with antigens and may also negatively regulate Bcl-2 during T-cell maintenance.12 As a consequence, it is plausible that inhibition of miR-17-92 would specifically or preferentially suppress an alloantigen-driven response while preserving T-cell homeostasis, which would benefit patients with GVHD without causing broad immune suppression.
In accordance with the published report that miR-17-92 promotes IFNγ production in vitro and Th1 differentiation in delayed-type hypersensitivity in vivo,14 we found that miR-17-92 was essential for optimal differentiation to Th1 cells after allo-BMT (Figure 4). Given that GVHD is primarily driven by Th1 cells, we surmised that compromised Th1 differentiation largely accounts for the impaired ability of miR-17-92 KO T cells to induce GVHD. In parallel to compromised Th1 differentiation, T cells deficient for miR-17-92 substantially enhanced differentiation into less pathogenic Th2 cells and suppressive iTregs (Figure 4). A recent report from Bluestone’s group showed that natural Tregs deficient for miR-17-92 do not retain suppressive function because of a loss of IL-10 production.29 However, we found that iTregs generated from KO and WT CD4 T cells in vitro had comparable suppressive function (supplemental Figure 7). Thus, we reason that iTregs generated from KO T cells likely contributed to the alleviated GVHD.
Donor T-cell infiltration into target organs is a prerequisite for GVHD development; chemokine receptors and integrins play important roles in T-cell migration to GVHD target organs.30 We found that there were significantly fewer KO T cells in recipient livers than their WT counterparts (Figure 3). Both the expansion and migration ability of T cells contribute to the number of donor T cells present in a target organ. In a competitive environment, the deficiency of this cluster dramatically reduced the expansion on CD4 but less on CD8 T cells, and it may further reduce migration of CD8 T cells (Figure 5A-C). Furthermore, miR-17-92–deficient T cells expressed significantly lower levels of CXCR3 and α4β7, the chemokine receptor and integrin that direct T-cell migration to the target organs, such as the liver and gut.31,32 It is not clear whether CXCR3 and α4β7 are directly regulated by miR-17-92, or secondary to T-cell activation and Th1 polarization. However, decreased T-cell migration to the target organs likely contributes to the reduced pathogenicity of miR-17-92–deficient T cells in the induction of GVHD.
Of clinical importance, we demonstrated that miR-17-92 was dispensable for T cell–mediated GVL effect in 2 preclinical BMT models (supplemental Figure 8 and Figure 6). We attribute the preserved GVL effect to largely intact cytotoxic T lymphocyte activation and function in the absence of miR-17-92. There are several lines of evidence to support this:
1. miR-17-92 had no effect on the survival of CD8 T cells after BMT (Figure 1G-H).
2. Although CD8 T cells deficient for miR-17-92 showed decreased proliferation in the early phase of BMT (Figure 1C-D), their expansion was not significantly compromised in the later phase of BMT (Figure 3A). Our observation is consistent with published findings that overexpression of miR-17-92 in lymphocytes induces a major expansion in the CD4 but to a lesser extent in the CD8 T cells.11
3. miR-17-92 deficiency may impair CD8 T-cell migration rather than substantially reduce proliferation (Figures 3 and 5).
4. miR-17-92 had little or no effect on cytolytic activity, reflected by levels of granzyme B and CD107a, and cytokine production, reflected by intracellular expression of IFNγ (Figure 6F-G).
It is worthy to point out that both CD4 (data not shown) and CD8 T cells had increased TNFα production in the absence of miR-17-92 (Figure 6F), which is consistent with a previous report that TNFα is a target of miR-19a.33 Given TNFα is a GVL mediator,34,35 elevated TNFα production by donor T cells may also contribute to the preserved GVL effect.
Using a translational approach, we demonstrate that blocking miR-17 or miR-19b by using specific LNA antagomirs significantly reduced T-cell proliferation and activation under the stimulation of alloantigens and further attenuated the severity of GVHD while preserving GVL activity (Figure 7 and supplemental Figure 9). Although not to the extent of miR-17-92 deficiency, the effect of antagomir treatment was quite remarkable, given only a single member of this cluster was inhibited. Our study provides the rationale to establish the significance of miR-17-92 in GVHD and to target this cluster for GVHD prophylaxis in the clinic. As a novel therapeutic strategy, several miR modulators have entered into clinical trials.36 Moreover, antagomirs can be relatively easily delivered into primary lymphocytes ex vivo before BMT,37,38 which may lead to a higher efficiency and specificity in knocking-down miRs in T and B cells before transfer and thus result in effective prevention of GVHD with low toxicity. miR-17-92, also known as polycistronic oncomir-1, is overexpressed in many types of malignancies,13 and a strategy to silence this cluster to suppress solid tumor growth has been established in preclinical models.27,28 Therefore, we anticipate that within the foreseeable future, the translational feasibility of silencing miR-17-92 will be achieved, which will significantly benefit cancer patients, especially those with hematologic malignancies after allo-HCT.
Acknowledgments
The authors appreciate the technical support provided by the Department of Laboratory Animal Research, Flow Cytometry Core and Imaging Core at MUSC.
This work was supported in part by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant NIH R01 AI080285, and National Cancer Institute grants CA118116, CA143812, and CA169116 (X.-Z.Y.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.W. participated in experimental design; performed research; collected, analyzed, and interpreted data; performed statistical analysis; and drafted and revised the manuscript; J.H., D.B., J.F., H.N., S.S., and Y.L. performed research, collected and analyzed data, and edited the manuscript; C.L. performed pathologic analysis; J.J., Q.-J.L., and C.X. participated in experiment design, interpreted data, and edited manuscript; and X.-Z.Y. designed research; collected, analyzed, and interpreted data; and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xue-Zhong Yu, Department of Microbiology & Immunology, Medical University of South Carolina, HCC350, MSC 955, 86 Jonathan Lucas St, Charleston, SC 29425; e-mail: yux@musc.edu; and Changqing Xia, 45 Changchun St, Xicheng District, Beijing 100053, China; e-mail: cqx65@yahoo.com.
References
- 1.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Tawara I, Zhao M, et al. Allogeneic T cell responses are regulated by a specific miRNA-mRNA network. J Clin Invest. 2013;123(11):4739–4754. doi: 10.1172/JCI70013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao B, Wang Y, Li W, et al. Plasma microRNA signature as a noninvasive biomarker for acute graft-versus-host disease. Blood. 2013;122(19):3365–3375. doi: 10.1182/blood-2013-06-510586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonhardt F, Grundmann S, Behe M, et al. Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100. Blood. 2013;121(17):3307–3318. doi: 10.1182/blood-2012-07-442665. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Romero M, Ratajczak P, et al. Increased apoptosis is linked to severe acute GVHD in patients with Fanconi anemia. Bone Marrow Transplant. 2013;48(6):849–853. doi: 10.1038/bmt.2012.237. [DOI] [PubMed] [Google Scholar]
- 9.Ranganathan P, Heaphy CE, Costinean S, et al. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119(20):4786–4797. doi: 10.1182/blood-2011-10-387522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atarod S, Dickinson AM. MicroRNAs: The Missing Link in the Biology of Graft-Versus-Host Disease? Front Immunol. 2013;4:420. doi: 10.3389/fimmu.2013.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olive V, Li Q, He L. mir-17-92: a polycistronic oncomir with pleiotropic functions. Immunol Rev. 2013;253(1):158–166. doi: 10.1111/imr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang S, Li C, Olive V, et al. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118(20):5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AA, Penny LA, Yuzefpolskiy Y, Sarkar S, Kalia V. MicroRNA-17∼92 regulates effector and memory CD8 T-cell fates by modulating proliferation in response to infections. Blood. 2013;121(22):4473–4483. doi: 10.1182/blood-2012-06-435412. [DOI] [PubMed] [Google Scholar]
- 16.Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu SQ, Jiang S, Li C, Zhang B, Li QJ. miR-17-92 cluster targets phosphatase and tensin homology and Ikaros Family Zinc Finger 4 to promote TH17-mediated inflammation. J Biol Chem. 2014;289(18):12446–12456. doi: 10.1074/jbc.M114.550723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Wang D, Liu C, et al. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118(18):5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haarberg KM, Li J, Heinrichs J, et al. Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice. Blood. 2013;122(14):2500–2511. doi: 10.1182/blood-2012-12-471938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y, Liu C, Djeu JY, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111(2):954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela JO, Iclozan C, Hossain MS, et al. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119(12):3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XZ, Albert MH, Anasetti C. Alloantigen affinity and CD4 help determine severity of graft-versus-host disease mediated by CD8 donor T cells. J Immunol. 2006;176(6):3383–3390. doi: 10.4049/jimmunol.176.6.3383. [DOI] [PubMed] [Google Scholar]
- 23.Bailey DW, Mobraaten LE. Estimates of the number of loci contributing to the histoincompatibility between C57BL-6 and BALB-c strains of mice. Transplantation. 1969;7(5):394–400. doi: 10.1097/00007890-196905000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J, Heinrichs J, Yu XZ. Helper T-cell differentiation in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Arch Immunol Ther Exp (Warsz) 2014;62(4):277–301. doi: 10.1007/s00005-014-0284-z. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Varambally S, Maher CA, et al. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117(23):6172–6183. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obad S, dos Santos CO, Petri A, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43(4):371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy BL, Obad S, Bihannic L, et al. Silencing of the miR-17∼92 cluster family inhibits medulloblastoma progression. Cancer Res. 2013;73(23):7068–7078. doi: 10.1158/0008-5472.CAN-13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Kouchkovsky D, Esensten JH, Rosenthal WL, Morar MM, Bluestone JA, Jeker LT. microRNA-17-92 regulates IL-10 production by regulatory T cells and control of experimental autoimmune encephalomyelitis. J Immunol. 2013;191(4):1594–1605. doi: 10.4049/jimmunol.1203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He S, Cao Q, Qiu Y, et al. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J Immunol. 2008;181(11):7581–7592. doi: 10.4049/jimmunol.181.11.7581. [DOI] [PubMed] [Google Scholar]
- 32.Waldman E, Lu SX, Hubbard VM, et al. Absence of the beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107(4):1703–1711. doi: 10.1182/blood-2005-08-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Wang Z, Yang S, et al. TNF-α is a novel target of miR-19a. Int J Oncol. 2011;38(4):1013–1022. doi: 10.3892/ijo.2011.924. [DOI] [PubMed] [Google Scholar]
- 34.Schmaltz C, Alpdogan O, Muriglan SJ, et al. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101(6):2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 35.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soifer HS, Koch T, Lai J, et al. Silencing of gene expression by gymnotic delivery of antisense oligonucleotides. Methods Mol Biol. 2012;815:333–346. doi: 10.1007/978-1-61779-424-7_25. [DOI] [PubMed] [Google Scholar]
- 38.Stein CA, Hansen JB, Lai J, et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38(1):e3. doi: 10.1093/nar/gkp841. [DOI] [PMC free article] [PubMed] [Google Scholar]