Abstract
As transcription factors of the lines (LIN)-11/Islet (Isl)-1/mitosis entry checkpoint (MEC)-3 (LIM)-homeobox subfamily, LIM homeobox (Lhx)6 and -8 are remarkably conserved and involved in the morphogenesis of multiple organ systems. Lhx6 and -8 play overlapping and distinctive roles, but in general act as cell fate mediators and in turn are regulated by several transcriptional factors, such as sonic hedgehog, fibroblast growth factors, and wingless-int (Wnt)/β-catenin. In this review, we first summarize Lhx6 and -8 distributions in development and then explore how Lhx6 and -8 act as transcription factors and coregulators of cell lineage specification. Known Lhx6 and -8 functions and targets are outlined in neurogenesis, craniofacial development, and germ cell differentiation. The underlying mechanisms of Lhx6 and -8 in regulating cell fate remain elusive. Whether Lhx6 and -8 affect functions in tissues and organs other than neural, craniofacial, oocytes, and germ cells is largely unexplored. Taken together, Lhx6 and -8 are important regulators of cell lineage specification and may act as one of the pivotal mediators of stem cell fate. Undoubtedly, future investigations of Lhx6 and -8 biology will continue to yield fascinating insights into tissue development and homeostasis, in addition to their putative roles in tissue regeneration and ageing.—Zhou, C., Yang, G., Chen, M., He, L., Xiang, L., Ricupero, C., Mao, J. J., Ling, J. Lhx6 and Lhx8: cell fate regulators and beyond.
Keywords: LIM homeobox genes, neurogenesis, tooth development, palatogenesis, transcriptional regulation
Organogenesis involves complex cellular activities and requires precise coordination of spatial and temporal gene expression patterns. Over the past 30 yr, homeobox genes have been recognized as key regulators of tissue patterning (1). Many homeobox genes are highly conserved from Caenorhabditis elegans to humans and characterized by a specific homeobox DNA sequence that encodes a highly variable protein domain, the homeodomain (HD), which serves as the DNA-binding domain and controls embryogenesis by regulating downstream target genes (2).
Homeotic genes, also known as the homeotic gene complex (HOM-C; Hox, homeotic gene), are typical homeobox gene clusters in genomic loci that form distinct complexes. Other homeobox genes disperse outside the HOM-C/Hox clusters and frequently encode transcription factors with additional domains besides the HD. For example, LIM [named after its founding members lines (LIN)-11, Islet (Isl)-1, and mitosis entry checkpoint (MEC)-3] HD transcription factors consist of the LIM domain in addition to the HD. These LIM HD transcription factors are encoded by genes that contain both LIM and homeobox: the LIM-homeobox (Lhx) genes (3–5). The Lhx gene family has a central role in cell fate specification and subsequent differentiation in invertebrates (6–14) and vertebrates (15–18).
Lhx6 AND -8: AN Lhx SUBFAMILY
HDs and LIM domains are 2 key functional domains of Lhx proteins. Based on similar amino acid HD sequences and overall amino acid homology, individual Lhx proteins are divided into 6 subgroups: Apterous, Isl, LIM homeobox (Lmx), Lim3, Lin-11, and Lhx6 and -8 (3–5). During development, cells express different combinations of Lhx genes and produce an LIM code that defines the fate of cell specification. For example, in Drosophila, all of the intersegmental nerve (ISN)b-specific motoneurons (MNs) express both isl and lim3, whereas MNs that project in ISNd express only isl. ISNd MNs expressing isl may have been redirected to ISNb upon misexpressing lim3 (19). Nevertheless, the LIM code consists of the distinct interactions between Lhx family members or other distinct transcriptional regulators that together play a role in regulating cell fate.
The HDs among Lhx family members share similarities in comparison with other HD-containing proteins, suggesting a common origin of all Lhx proteins and distinctive DNA-binding specificity (3). The LIM domain is a specialized double–zinc-finger motif and mediates the interaction with other proteins, such as other LIM-domain–containing proteins (20–25). The LIM domain and its interaction with other functional regulators are essential for the function of Lhx family members. For example, loss of function of the LIM domains in Xenopus Lim1/Lhx1, by either mutation or deletion, activates transcription factors, suggesting that the LIM domains act as a negative regulator of Lhx proteins (26). Conversely, overexpression of the LIM domain of Islet-3 inhibits the binding of the HD to DNA, leading to ocular and cerebellar defects in zebrafish (27). Accordingly, a model of negative regulation of Lhx6 and -8 by the resident LIM domains has been proposed (28). In the developing CNS, Lhx6 and -8 mRNAs are primarily expressed in the ventral telencephalon, specifically in the medial ganglionic eminence (MGE) of the basal forebrain and in subsets of neurons in the striatum, which is also confirmed in our data (Fig. 1A, B).
Figure 1.
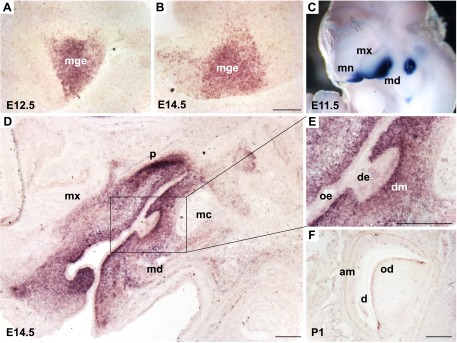
In situ hybridization of Lhx8 expression during mouse development. Lhx8 expression in the central nervous system was restricted to MGE of basal forebrain at E12.5 (A) and E14.5 (B) mouse embryo. C) During tooth morphogenesis, Lhx8 expression was detected primarily in BA1 at E11.5 by wholemount in situ hybridization. D, E) Lhx8 was further detected in neural crest–derived mesenchyme and was especially robust in the dental mesenchyme at E14.5 (cap stage). F) Lhx8 mRNA was restricted postnatally in the odontoblast layer. am, ameloblast; d, dentin; de, dental epithelium; dm, dental mesenchyme; mc, Meckel’s cartilage; md, mandibular process; mn, medial nasal process; mx, maxillary process; od, odontoblast; oe, oral epithelium; p, palate. Scale bars, 200 µm.
Both Lhx6 and -8 proteins contain 2 LIM domains (LIM-1 and -2) followed by an HD (Fig. 2). Lhx6 is 75% homologous with Lhx8 and has the most conserved regions in the HD and LIM domains (95 and 74%, respectively) (28). The Lhx8 gene, previously known as L3 and Lhx7, contains 9 exons and 8 introns, and encodes 367 amino acids (29–35). The first LIM domain is encoded by exons 2 and 3, whereas the second is encoded only by exon 4. The HD is encoded by exons 6, 7, and 8. The Lhx8 gene is located in the distal region of mouse chromosome 3 (32). The homologs in that region map to human chromosome 4, region q25-31m, a region that has been linked to craniofacial clefting (36). However, human LHX8 is located in chromosome 1, region p31.1, suggesting that other important genes involved in craniofacial development reside in chromosome 4, region q25-31m. Mouse Lhx6 is located in chromosome 2B2 and has 13 exons, whereas human LHX6 is located in chromosome 9, region q33.2, and also has 13 exons.
Figure 2.
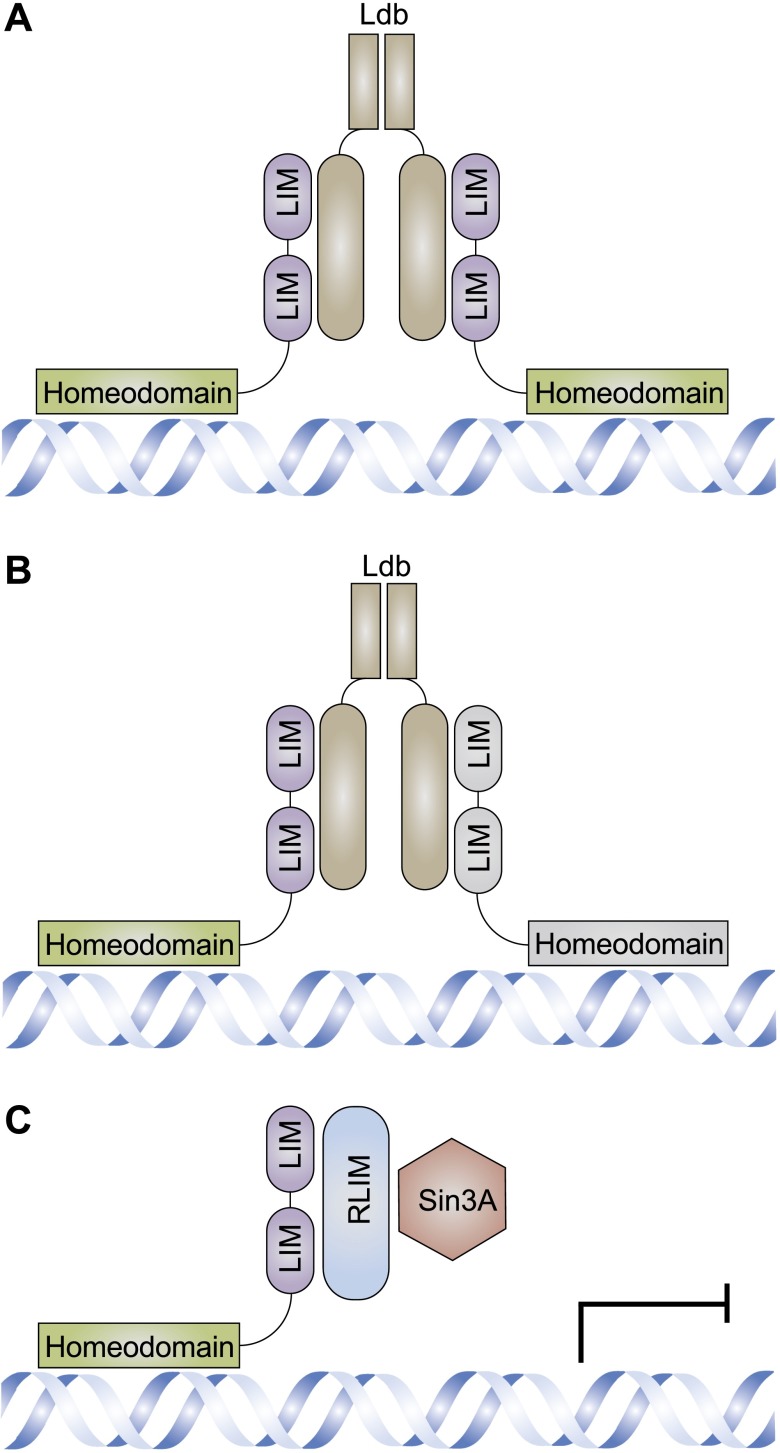
Lhx families function through protein–protein interactions. The N-terminal region of Ldb mediates the dimerization, whereas the C-terminal region mediates the interactions between dimerized Lhx proteins that are the same (A) or different (B) through LIM domains. C) Lhx proteins can also interact with RLIM through the LIM domain, which in turn recruits Sin3A or other epigenetic regulators.
Lhx6 and -8 expression is spatiotemporally regulated in the developing embryo (30). In the nervous system, Lhx6 and -8 are expressed in sensory neurons, MNs, and interneurons. Lhx6 and -8 are also expressed in the ectomesenchyme of the maxillary and mandibular processes, suggesting their putative functions in the patterning of the first branchial arch (BA1) and tooth morphogenesis. In fact, Lhx6 and -8 double-knockout mice have a wide range of craniofacial defects. These include defects in the cranial skeleton, absent molars, duplicate incisors, and cleft palate (37). In addition, Lhx8 is expressed in the forebrain, especially the preoptic ventricular zone, subventricular zone, and mantle. Lhx8-null mice have a visible defect and lose basal forebrain cholinergic neurons (FCNs) (38, 39). Lhx8 is also expressed in oocytes and germ cells within the mouse ovary, and Lhx8-knockout females lose oocytes within 7 postnatal days (40, 41).
Unique combinations of LIM-HD proteins are thought to form the transcriptional LIM code, which consists of the distinct interactions between Lhx family members or other distinct transcriptional regulators. Most of the known Lhx protein interactions are mediated by the LIM domains with cofactors, among which LIM domain binding (Ldb) protein has been intensively studied (Fig. 2A, B). Dimerization of Ldb and its interactions with both Lhx and other regulatory proteins enable Lhx proteins and distinct transcriptional regulators, such as orthodenticle homeobox (Otx) proteins (19) and any given Lhx protein, to form homomeric or heteromeric complexes (42). Besides Ldb, Lhx proteins may also interact with ring finger protein, LIM domain interacting (RLIM), which in turn recruits the sex-lethal interactor (Sin)3A/histone deacetylase corepressor complex, resulting in gene expression repression (43) (Fig. 2C). The LIM code plays an essential role in regulating cell fate. For example, Ldb1 is expressed in natural killer 2 homeobox 1 (Nkx2-1)–positive cells during embryonic development and in mature neurons. Conditional Ldb1 deletion causes a reduction in the number of both GABAergic and cholinergic neurons in the telencephalon, similar to those in Lhx6 or -8 mutant mice (44). For another example, in the Drosophila, all of the ISNb-specific s express both isl and lim3, whereas MNs that project in ISNd express only isl. ISNd MNs expressing isl may have been redirected to ISNb upon misexpressing lim3 (45). Different from the above indirect interactions assisted by cofactors, there are direct interactions between the Pit-Oct-Unc (POU) protein uncoordinated (UNC)-86 and the LIM HD protein MEC-3, which increases DNA binding stability and specificity and specifies neuronal cell fate in the nematode C. elegans (25). As the key elements of the LIM code, Lhx6 and -8 translocate into the nucleus and interact with other transcriptional regulators when activated, regulating specific targets. The homodimerization of Lhx6 and -8 or heterodimerization with other Lhx family members has been intensively studied recently. In addition to the Sin3A/histone deacetylase corepressor complex, SUV39H1 (suppressor of variegation 3-9 homolog 1) has been found by yeast 2-hybrid assay to interact with Lhx8 (46).
Lhx6 AND -8 IN NEUROGENESIS
In the CNS, cholinergic neurons, which use mainly the neurotransmitter acetylcholine to send their messages, are located in diverse regions and have broad functions. In the spinal cord, cholinergic MNs control locomotion, whereas in the forebrain, they are involved in cognitive processes. Defects in the function or survival of cholinergic neurons result in severe diseases, including those associated with impaired motor function and cognitive disorders resulting from the loss of FCNs (47). Lhx family members are pivotal in the regulation of cholinergic neurons and have been intensely studied. For example, in the developing spinal cord, Lhx3, together with Isl1, another Lhx family member, specifies MN fate (48).
In the developing forebrain, FCNs are derived from the MGE in the ventral telencephalon. In the developing CNS, Lhx6 and -8 mRNAs are primarily in the ventral telencephalon, specifically in the MGE of the basal forebrain and in subsets of neurons in the striatum (28, 49, 50). Lhx6 and -8 expression patterns in the developing CNS indicate their involvement in a basal telencephalon territory associated with formation of the MGE (28). Lhx8 may further control the development of distinct neuron subtypes. For example, Lhx8-null mutant mice are deficient in the development of FCNs (51) and lack the nucleus basalis, a major source of cholinergic input to the cerebral cortex. In addition, the number of FCNs is reduced in several other areas of the subcortical forebrain in Lhx8-null mutants, including the caudate putamen, medial septal nucleus, nucleus of the diagonal band, and magnocellular preoptic nucleus (51). Lhx8 expression is necessary for the specification or differentiation of FCNs for spatial learning and memory, as seen by the impairment in spatial learning and memory in Lhx8-deficient mice (52). In Lhx8-deficient mice, FCN progenitors survive but fail to generate cholinergic interneurons in the striatum and cholinergic projection neurons in the basal forebrain (52). In the developing forebrain, the Isl1-Lhx8 hexamer is recruited to the cholinergic gene battery and promotes cholinergic gene expression. Furthermore, the expression of the Isl1-Lhx8 complex enables the acquisition of cholinergic fate for embryonic stem cell-derived neurons (47). Thus, Lhx8, together with Isl1, plays an important role in cholinergic neuron specification in the developing forebrain.
During early development, GABAergic interneuron progenitors arise from the ventral telencephalic area, such as the MGE and caudal ganglionic eminence (CGE), and migrate to target sites where they form synaptic connections. Lhx8 is involved in GABAergic fate determination, likely with Lhx6 (39, 53–55). Notably, tangentially migrating and mature interneurons maintain Lhx6, but not Lhx8, expression. In Lhx6 mutants, tangential migration is slowed along with abnormal neocortical laminar position of interneurons. In addition, somatostatin (SST)+ and parvalbumin (PV)+ cortical interneurons are drastically reduced (56). Blockage of Lhx6 has little effect on stable GABA production or enzyme expression in dissociated MGE neurons in culture, as opposed to Lhx8 (57). Some Lhx6−/− MGE cells acquire a CGE-like fate caused by aberrant expression of aristaless related (Arx) and chemokine (C-X-C motif) receptor (CXCR)-7, which can be rescued by restoration of Arx and CXCR7 (59).
In vitro studies indicate that Lhx8 knockdown in the murine neuroblastoma cell line Neuro2a (N2a), severely reduces cholinergic differentiation and increases GABAergic marker expression, suggesting Lhx8 involvement in the determination of neurotransmitter phenotypes during basal forebrain development (39). Lhx8 suppression leads to a dramatic decrease in the number of cells positive for the cholinergic marker choline O-acetyltransferase (ChAT), and overexpression of Lhx8 rescues this effect. No significant changes have been observed in the number of neuronal class III β-tubulin–positive (Tuj1+) neurons and GABA+ cells (59, 60). In addition, Lhx8 has been found to promote the differentiation of hippocampal neural stem/progenitor cells into cholinergic neurons and Lhx8 overexpression to increase the proportion of ChAT-positive cells in vitro (61).
As an essential regulator for neurogenesis, Lhx family members mediate developmental cues and cell-type–specific gene expression profiles. First, Lhx6 and -8 are simultaneously coexpressed with several signaling molecules and transcription factors. For example, the preoptic ventricular zone expresses sonic hedgehog (Shh) and transcription factors including Nkx2-1, developing brain homeobox (Dbx1), and Lhx8, whereas the subventricular zone and mantle express Shh, Lhx8, and Isl1 (62–65). Nkx2-1 appears to specify the development of the basal telencephalon by positively regulating the expression of Lhx6 and -8 and by repressing other transcription factors (52). At birth, Nkx2-1–deficient mutants lack all cholinergic neurons in the subpallium, including striatal interneurons and basal forebrain projection neurons, and complete loss of Lhx6 and -8 expression in the developing telencephalon (49, 51). On the other hand, Lhx6 and -8 coexpression in early MGE neurons is necessary for the induction of Shh expression (55). Lhx6 and -8 regulate MGE development through autonomous and nonautonomous mechanisms, the latter by promoting Shh expression in MGE neurons, which in turn induces the development of the rostrodorsal MGE (55). All of these transcription factors work together in a network and govern the identity of neurons. Besides its integrative role for Nkx2-1 and Shh in cell lineage specification, Lhx8 is associated with transcriptional regulation of choline acetyltransferase or low-affinity NGF receptor (LNGFR) genes (29).
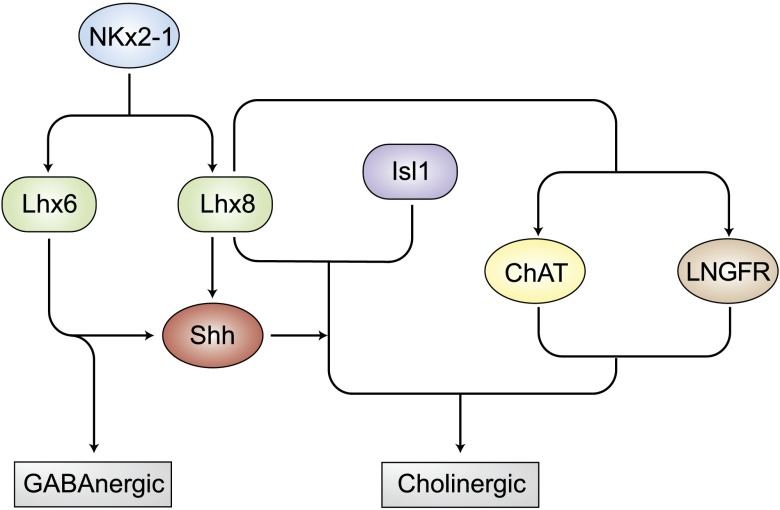
In summary, Lhx6 and -8 are pivotal regulators of cell fate in cholinergic and GABAergic neurons. Lhx8 and Isl1 together specify cholinergic fate (39, 51, 59, 64, 66), whereas Lhx8 and Lhx6 specify GABAergic fate (39, 53, 54). Lhx6 and -8, together with other specific cofactors, regulate downstream cell-specific gene expression patterns responding to upstream developmental cues and form the cell-type–specific LIM code (Fig. 3).
Figure 3.
Lhx6 and -8 in neurogenesis. Neurogenesis-related transcription factor NKx2-1 upregulates Lhx6 and -8. Lhx8 together with Lhx6 promotes GABAergic neuron differentiation, whereas Lhx8 together with Isl1 favors cholinergic differentiation mediated by Shh, ChAT, and LNGFR.
Lhx6 AND -8 IN TOOTH DEVELOPMENT
Lhx6 and -8 are key transcription factors in tooth development. Teeth begin to develop by sequential and reciprocal inductions between the epithelium and mesenchyme tooth germ (67). Crosstalk between the ectoderm-derived epithelium and cranial neural crest–derived mesenchyme regulates the shape, position, and size of the tooth (67).
Tooth development in mice is initiated at embryonic day (E)11 as localized thickening of the epithelium that marks the positions of the teeth. Before the initiation of tooth formation, expression of both Lhx6 and -8 is detected in the presumptive oral and odontogenic mesenchyme of the maxillary and mandibular processes of BA1, symbolizing the establishment of the oral–aboral axis (28). Unlike other regulatory genes, Lhx6 and -8 expression is unique in its restricted expression in the mesenchyme during tooth development (28). Lhx8 transcripts are first detected in the neural crest–derived ectomesenchyme of BA1 in E9.5 mice and are expressed in the dental mesenchyme from the bud (E12.5) to early bell (E16.5) stages of the molar tooth germ (28, 29, 35). A representative expression profile of Lhx8 during tooth development is shown in Fig. 1C–F. Lhx6 shows similar expression patterns during early tooth development (37). Lhx6 expression in dental papilla at the late bell stage (28) coincides with diminishing Lhx8 expression (35). As opposed to their robust expression in molar mesenchyme, Lhx6 and -8 are not or weakly expressed in the incisors (37). Together, Lhx6 and -8 appear to have partially redundant, but sometimes distinctive, roles in tooth morphogenesis (28, 68).
Lhx6 and -8 are further involved in the crosstalk between the epithelium and mesenchyme in tooth morphogenesis by specifying the fate of ectomesenchymal cells of BA1 (28). In contrast to a lack of craniofacial defects in Lhx6 null mutants, mice homozygous for the Lhx8 mutation develop cleft palate. Lhx6 and -8 double-homozygous knockout mice have multiple craniofacial defects, including cleft palate and missing molars. The absence of molars in Lhx6 and -8 double mutants is caused by failure of specification of molar mesenchyme, rather than by patterning defects in BA1 (37). A single functional copy of either Lhx6 or -8 appears sufficient for molar development. Some of the roles played by Lhx6 and -8 may be interchangeable, although Lhx6 only partially compensates for Lhx8 in tooth development. Thus, Lhx6 and -8 are crucial for craniofacial development and patterning of mammalian dentition.
Oral epithelium initiates tooth development and instructs the underlying mesenchyme to begin odontogenesis by E10.5–11.5 (34, 69). Dental mesenchyme, however, gains tooth induction ability by ∼E12.5 (34, 69). One of the tooth initiation signals in dental epithelium is likely fibroblast growth factor (FGF)-8 (Fig. 4). Previous studies indicate that FGF signaling is necessary for the development of both molars and incisors (33) and controls Lhx6 and -8 expression in a time-dependent manner. Blocking FGF signals by Su5402, an inhibitor of FGF receptor signaling, leads to Lhx8 repression up to E11.0 and Lhx6 repression up to E10.75 (33). In the Fgf8;Nes-cre mutants, which lack FGF8 expression in the ectoderm, Lhx8 expression is attenuated in the BA1, but Lhx6 expression is abolished (70). Specifically, Lhx8 expression responds in a graded fashion to different FGF8 concentrations (34). Spatially, FGF8 from the rostral (oral) epithelium determines the rostral–caudal polarity in BA1 by inducing Lhx8 expression in neural crest–derived mesenchyme (34), whereas in the caudal region, FGF8 concentration is too low to induce Lhx8 expression. Thus, the rostral–caudal polarity is likely determined by interactions between dental epithelium and mesenchyme, rather than by signals inherent in the neural crest (34), leading to tooth morphogenesis (28).
Figure 4.
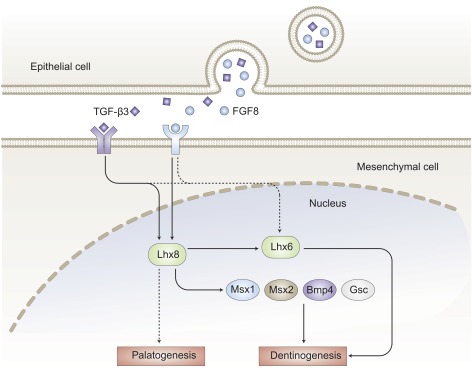
Critical roles of Lhx6 and -8 in epithelium–mesenchyme crosstalk. Epithelium-derived FGF8 and TGF-β3 bind to their receptors in the underlying mesenchyme, resulting in the upregulation of Lhx6 and -8. Lhx8, together with Lhx6, is involved in odontogenesis through regulating Msx1, Msx2, Bmp4, Gsc, and others.
An explant assay is widely used for the study of the mechanisms of tooth development. In one study, mesenchyme cells from E12.5 tooth germ explants are treated with antisense oligodeoxynucleotides (AS-ODNs) against Lhx8 (35). The explants treated with AS-ODNs fail to form tooth germs and produce a large number of undifferentiated epithelial cells and a small number of mesenchymal cells. In addition, the expression of Lhx6, Msh homeobox (Msx)-1, Msx2, bone morphogenic protein (Bmp)4, and goosecoid homeobox (Gsc) is suppressed after the application of AS-ODNs (35). Lhx8 is expressed before Gsc and represses Gsc (34). Paired-like homeodomain (Pitx)-2 and Lhx6 are coexpressed in the oral and dental epithelium and epithelial cell lines (71). Pitx2 activates endogenous Lhx6 expression and the Lhx6 promoter. In turn, Lhx6 directly interacts with Pitx2 to inhibit its transcriptional activities through a dominant repressive effect on the Pitx2 synergistic activation with lymphoid enhancer-binding factor (Lef)-1 and β-catenin cofactors, resulting in aberrant cell migration and differentiation (71).
Lhx8 and -8 are specific to odontogenic mesenchymal cells in the developing tooth germ (72) and may represent hallmarks of odontoblast precursors (73), serving as a reporter for dentinogenesis. Lhx8 and other early odontogenic marker genes are expressed in dentin sialophosphoprotein (DSPP)-overexpressing adipose stromal cells (74). Accordingly, Lhx8 mRNA and Lhx8 proteins are robustly expressed in human odontoma–derived mesenchymal cells compared to those in adult dental mesenchyme stem cells. Thus, Lhx8 may play an important role in odontoma formation, although mechanisms of Lhx8 in odontoma morphogenesis remain unknown (75).
In summary, dental mesenchyme begins to express Lhx6 and -8 and their downstream genes to initiate dentinogenesis in response to FGF8 and possibly other signals from dental epithelium (Fig. 4). Lhx6 and -8 expression changes dynamically during tooth development, suggesting that many of their downstream target genes are mediated and affect tooth development in ways that remain to be unraveled.
Lhx6 AND -8 IN PALATOGENESIS
Cleft palate and cleft lip affects ∼1 in 700 human births (76). Lhx6 and -8 play pivotal roles in palatogenesis. At E13.5, Lhx6 and -8 expression overlaps in the palatal shelves, the developing tongue, and vibrissae follicles (37). Lhx8 expression is persistent in palatal mesenchyme up to the fusion of palatal shelves, whereas Lhx6 is only transiently expressed in E14.5 palatal epithelium (68). Lhx8 expression is also detected in the medial nasal process (37). In Lhx8 homozygous mutant embryos, the palatal shelves are underdeveloped because of attenuated cell proliferation and often fail to fuse, leading to cleft palate. Despite cleft palate in Lhx8 homozygous mutant embryos, other oral structures including the teeth, mandible, tongue, and Meckel’s cartilage, are apparently unaffected (77). Thus, the function of Lhx8 is especially pivotal in palatogenesis, and an aberration in Lhx8 may be involved in those syndromic and nonsyndromic forms of cleft palate in humans (77). To curtail Lhx6 compensation, Lhx8-deficient mice are generated with the LIM domains of Lhx8 expressed from the recombinant allele and are called δHD/δHD mice (38), distinctive from Lhx8 −/− mice (37). In δHD/δHD mice, Lhx8 is deficient due to an absent HD, whereas the remaining LIM domains dominantly block Lhx6 function. As expected, δHD/δHD mice have a greater incidence of cleft palate (∼95%) than do Lhx8−/− mice (∼60%), likely because of the absence of Lhx6 compensation for Lhx8 function (38). Thus, results indicate that both Lhx6 and -8 are involved in palatogenesis. Medical sequencing analyses of candidate genes for nonsyndromic cleft lip and palate suggest that point mutations in Lhx8 are among the rare causes of isolated cleft lip, with or without cleft palate (78). In maxillary prominence, Lhx8 is found to be a downstream target for epithelium-derived FGF8b or TGFβ3 (79) (Fig. 4). To this end, FGF8b or TGFβ3 signaling pathways and other Lhx8-related pathways may by functionally important in palatogenesis. For example, a gene deficiency in a set such as T-box genes and wingless-int (Wnt) (81–84) also results in cleft palate. One of the enhancers (Lhx8_enh1) located upstream of Lhx8 appears to be a direct target of the Wnt/β-catenin pathway. This pathway appears to be essential for Lhx8 expression in cells isolated from the maxillary arch (84).
Lhx6 AND -8 IN OTHER SYSTEMS
In addition to neural and craniofacial systems, Lhx8 is expressed in oocytes and germ cells. Lhx8-knockout ovaries are grossly similar to wild-type ovaries at birth, but begin to lose oocytes within 7 d. Lhx8-knockout ovaries fail to maintain primordial follicles and show a lack of transition from primordial to growing follicles. Lhx8-null ovaries misexpress oocyte-specific genes, such as growth differentiation factor-9 (Gdf9), POU class 5 homeobox (Pou5f1), and newborn ovary homeobox (Nobox) (40). The roles of Lhx8 in oocyte maintenance and differentiation during early oogenesis are fulfilled partially by downregulating the Nobox pathway (40). Lhx8 also appears to be essential in mammalian folliculogenesis, as Lhx8 deficiency disrupts early folliculogenesis (41). Thus, Lhx8 may be a key fertility mediator in humans and a candidate gene for premature ovarian failure (85). However, mutations in Lhx8 exons are uncommon in Caucasians and Koreans with premature ovarian failure (86, 87).
Although an Lhx8 mutation is rare in premature ovarian failure, epigenetic aberration may be a cause. Bisulfite sequencing revealed the methylation dynamics of Lhx8-3′, and yet there is little alteration in Lhx8-5′. H3 acetylation of Lhx8 also increases during primordial follicle assembly and activation. Thus, Lhx8 expression seems to be related to the activation of primordial follicles and correlates with the demethylation of the Lhx8 3′ UTR and the high acetylation of H3 (88), suggesting an epigenetic layer of Lhx8 gene expression regulation. Consistently, Lhx8 expression is undetectable in women with premature ovarian insufficiency (89). Epigenetic aberrations have been found in the Lhx6 promoter region in lung cancer (90) and cervical cancer (91, 92), suggesting a putative role of Lhx6 as a tumor-suppressor gene. Furthermore, Lhx8 is highly expressed in brown and bright preadipocytes, as compared with epididymal fat–derived preadipocytes (93–98), although the precise roles of Lhx8 in adipose tissue are largely unknown. Lhx6 was expressed in lung and acted as a potential tumor-suppressor gene in lung cancer, with epigenetic silencing (99). The epigenetic regulation of Lhx6, as a tumor-suppressor gene and methylation marker, is also associated with head and neck carcinoma (100, 101).
SUMMARY AND FUTURE DIRECTIONS
Lhx6 and -8 are highly conserved transcription factors of the LIM-homeobox subfamily, and like other homeobox genes, play important roles in pattern formation. Lhx6 and -8 are expressed in neural and craniofacial tissues during development, in addition to oocytes and germ cells. Lhx6 and -8 act as robust regulators of cell fate in neurogenesis, tooth morphogenesis, and oogenesis. However, the underlying mechanisms of Lhx6 and -8 in regulating cell fate during development remain largely elusive. Lhx6 and -8 appear to have overlapping, yet distinctive, roles in development where they have both common and unique targets in different organ systems. Lhx family members are transcriptionally activated and interact with additional transcriptional factors in response to FGF8 and other signals during tooth morphogenesis. Identification of the upstream regulators, interacting proteins, and downstream targets of Lhx6 and -8 will undoubtedly improve our understanding of development. Thus far, Lhx6 and -8 have been studied primarily in neural and craniofacial systems. How Lhx6 and -8 regulate development in other organs with abundant Lhx6 and -8 expression warrants new investigations. In short, there is still much to learn about Lhx6 and -8 in during development. In addition, very little is known regarding their putative roles in tissue regeneration and wound healing. Given their pivotal roles in development, one could speculate that Lhx6 and -8 also have important functions in these additional areas that warrant new and exciting investigations.
Acknowledgments
The authors thank R. Birdie, Q. Guo, and J. Melendez (Center for Craniofacial Regeneration, Columbia University Medical Center, New York, NY, USA) for administrative and technical assistance. This article was supported by U.S. National Institutes of Health (NIH) Grant R01AR065023 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, U.S. NIH Grant R01DE023112 from the National Institute of Dental Research (NIDR), and Guangdong Pioneer Grant (52000-3210002) (to J.J.M.); NIDR Grant NIH/K12DE023583 (to M.C.); and National Natural Science Foundation of China Grant 81170932 (to J.L.).
Glossary
- Arx
aristaless related homeobox
- AS-ODN
antisense oligodeoxynucleotide
- BA1
first branchial arch
- Bmp
bone morphogenetic protein
- CGE
caudal ganglionic eminence
- ChAT
choline O-acetyltransferase
- CXCR
chemokine (C-X-C motif) receptor
- E
embryonic day
- FGF
fibroblast growth factor
- Gsc
goosecoid homeobox
- H3
histone 3
- HD
homeodomain
- HOM-C
homeotic gene complex
- Hox
homeotic gene
- Isl
Islet
- ISN
intersegmental nerve
- Ldb
LIM domain binding
- Lef
lymphoid enhancer-binding factor
- Lhx
LIM homeobox
- LIM
lines (LIN)-11/Isl-1/MEC-3
- LNGFR
low-affinity nerve growth factor (NGF) receptor
- MEC
mitosis entry checkpoint
- MGE
medial ganglionic eminence
- MN
motor neuron
- Msx
Msh homeobox
- Nkx2-1
natural killer 2 homeobox 1
- Nobox
newborn ovary homeobox
- Otx
orthodenticle homeobox
- Pitx
paired-like homeodomain
- POU
Pit-Oct-Unc
- RLIM
ring finger LIM protein
- Shh
sonic hedgehog
- Sin3A
sex-lethal interactor (SIN)-3 transcription regulator family member A
- Wnt
wingless-int
REFERENCES
- 1.McGinnis W., Krumlauf R. (1992) Homeobox genes and axial patterning. Cell 68, 283–302 [DOI] [PubMed] [Google Scholar]
- 2.Boncinelli E. (1997) Homeobox genes and disease. Curr. Opin. Genet. Dev. 7, 331–337 [DOI] [PubMed] [Google Scholar]
- 3.Hobert O., Westphal H. (2000) Functions of LIM-homeobox genes. Trends Genet. 16, 75–83 [DOI] [PubMed] [Google Scholar]
- 4.Taira M., Evrard J. L., Steinmetz A., Dawid I. B. (1995) Classification of LIM proteins. Trends Genet. 11, 431–432 [DOI] [PubMed] [Google Scholar]
- 5.Dawid I. B., Toyama R., Taira M. (1995) LIM domain proteins. C. R. Acad. Sci. III 318, 295–306 [PubMed] [Google Scholar]
- 6.Bourgouin C., Lundgren S. E., Thomas J. B. (1992) Apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron 9, 549–561 [DOI] [PubMed] [Google Scholar]
- 7.Ericson J., Thor S., Edlund T., Jessell T. M., Yamada T. (1992) Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 256, 1555–1560 [DOI] [PubMed] [Google Scholar]
- 8.Freyd G., Kim S. K., Horvitz H. R. (1990) Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature 344, 876–879 [DOI] [PubMed] [Google Scholar]
- 9.Thor S., Ericson J., Brännström T., Edlund T. (1991) The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7, 881–889 [DOI] [PubMed] [Google Scholar]
- 10.Way J. C., Chalfie M. (1988) mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell 54, 5–16 [DOI] [PubMed] [Google Scholar]
- 11.Thor S., Thomas J. B. (1997) The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron 18, 397–409 [DOI] [PubMed] [Google Scholar]
- 12.Blair S. S., Brower D. L., Thomas J. B., Zavortink M. (1994) The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development 120, 1805–1815 [DOI] [PubMed] [Google Scholar]
- 13.Cohen B., McGuffin M. E., Pfeifle C., Segal D., Cohen S. M. (1992) apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 6, 715–729 [DOI] [PubMed] [Google Scholar]
- 14.Lundgren S. E., Callahan C. A., Thor S., Thomas J. B. (1995) Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development 121, 1769–1773 [DOI] [PubMed] [Google Scholar]
- 15.Sheng H. Z., Moriyama K., Yamashita T., Li H., Potter S. S., Mahon K. A., Westphal H. (1997) Multistep control of pituitary organogenesis. Science 278, 1809–1812 [DOI] [PubMed] [Google Scholar]
- 16.Pfaff S. L., Mendelsohn M., Stewart C. L., Edlund T., Jessell T. M. (1996) Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84, 309–320 [DOI] [PubMed] [Google Scholar]
- 17.Riddle R. D., Ensini M., Nelson C., Tsuchida T., Jessell T. M., Tabin C. (1995) Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell 83, 631–640 [DOI] [PubMed] [Google Scholar]
- 18.Vogel A., Rodriguez C., Warnken W., Izpisúa Belmonte J. C. (1995) Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature 378, 716–720 [DOI] [PubMed] [Google Scholar]
- 19.Bach I., Carrière C., Ostendorff H. P., Andersen B., Rosenfeld M. G. (1997) A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 11, 1370–1380 [DOI] [PubMed] [Google Scholar]
- 20.Dawid I. B., Breen J. J., Toyama R. (1998) LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 14, 156–162 [DOI] [PubMed] [Google Scholar]
- 21.Feuerstein R., Wang X., Song D., Cooke N. E., Liebhaber S. A. (1994) The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc. Natl. Acad. Sci. USA 91, 10655–10659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German M. S., Wang J., Chadwick R. B., Rutter W. J. (1992) Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 6, 2165–2176 [DOI] [PubMed] [Google Scholar]
- 23.Leonard J., Serup P., Gonzalez G., Edlund T., Montminy M. (1992) The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc. Natl. Acad. Sci. USA 89, 6247–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeichel K. L., Beckerle M. C. (1994) The LIM domain is a modular protein-binding interface. Cell 79, 211–219 [DOI] [PubMed] [Google Scholar]
- 25.Xue D., Tu Y., Chalfie M. (1993) Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science 261, 1324–1328 [DOI] [PubMed] [Google Scholar]
- 26.Taira M., Otani H., Saint-Jeannet J.-P., Dawid I. B. (1994) Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature 372, 677–679 [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi Y., Segawa H., Tokumoto M., Tsubokawa T., Hotta Y., Uyemura K., Okamoto H. (1997) Ocular and cerebellar defects in zebrafish induced by overexpression of the LIM domains of the islet-3 LIM/homeodomain protein. Neuron 18, 369–382 [DOI] [PubMed] [Google Scholar]
- 28.Grigoriou M., Tucker A. S., Sharpe P. T., Pachnis V. (1998) Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development 125, 2063–2074 [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto K., Tanaka T., Furuyama T., Kashihara Y., Mori T., Ishii N., Kitanaka J., Takemura M., Tohyama M., Wanaka A. (1996) L3, a novel murine LIM-homeodomain transcription factor expressed in the ventral telencephalon and the mesenchyme surrounding the oral cavity. Neurosci. Lett. 204, 113–116 [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K., Tanaka T., Furuyama T., Kashihara Y., Ishii N., Tohyama M., Kitanaka J., Takemura M., Mori T., Wanaka A. (1996) Differential expression of LIM-homeodomain genes in the embryonic murine brain. Neurosci. Lett. 211, 147–150 [DOI] [PubMed] [Google Scholar]
- 31.Wanaka A., Matsumoto K., Kashihara Y., Furuyama T., Tanaka T., Mori T., Tanno Y., Yokoya S., Kitanaka J., Takemura M., Tohyama M. (1997) LIM-homeodomain gene family in neural development. Dev. Neurosci. 19, 97–100 [DOI] [PubMed] [Google Scholar]
- 32.Kitanaka J., Takemura M., Matsumoto K., Mori T., Wanaka A. (1998) Structure and chromosomal localization of a murine LIM/homeobox gene, Lhx8. Genomics 49, 307–309 [DOI] [PubMed] [Google Scholar]
- 33.Mandler M., Neubüser A. (2001) FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev. Biol. 240, 548–559 [DOI] [PubMed] [Google Scholar]
- 34.Tucker A. S., Yamada G., Grigoriou M., Pachnis V., Sharpe P. T. (1999) Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development 126, 51–61 [DOI] [PubMed] [Google Scholar]
- 35.Shibaguchi T., Kato J., Abe M., Tamamura Y., Tabata M. J., Liu J. G., Iwamoto M., Wakisaka S., Wanaka A., Kurisu K. (2003) Expression and role of Lhx8 in murine tooth development. Arch. Histol. Cytol. 66, 95–108 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell L. E., Healey S. C., Chenevix-Trench G. (1995) Evidence for an association between nonsyndromic cleft lip with or without cleft palate and a gene located on the long arm of chromosome 4. Am. J. Hum. Genet. 57, 1130–1136 [PMC free article] [PubMed] [Google Scholar]
- 37.Denaxa M., Sharpe P. T., Pachnis V. (2009) The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev. Biol. 333, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori T., Yuxing Z., Takaki H., Takeuchi M., Iseki K., Hagino S., Kitanaka J., Takemura M., Misawa H., Ikawa M., Okabe M., Wanaka A. (2004) The LIM homeobox gene, L3/Lhx8, is necessary for proper development of basal forebrain cholinergic neurons. Eur. J. Neurosci. 19, 3129–3141 [DOI] [PubMed] [Google Scholar]
- 39.Manabe T., Tatsumi K., Inoue M., Matsuyoshi H., Makinodan M., Yokoyama S., Wanaka A. (2005) L3/Lhx8 is involved in the determination of cholinergic or GABAergic cell fate. J. Neurochem. 94, 723–730 [DOI] [PubMed] [Google Scholar]
- 40.Choi Y., Ballow D. J., Xin Y., Rajkovic A. (2008) Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol. Reprod. 79, 442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y. S., Ballow D., Zhao Y. G., Westphal H., Rajkovic A. (2007) Lhx8 deficiency disrupts early folliculogenesis and oocyte-specific gene expression in the mouse ovary. Biol. Reprod. 77, 91–91 [Google Scholar]
- 42.Jurata L. W., Pfaff S. L., Gill G. N. (1998) The nuclear LIM domain interactor NLI mediates homo- and heterodimerization of LIM domain transcription factors. J. Biol. Chem. 273, 3152–3157 [DOI] [PubMed] [Google Scholar]
- 43.Bach I., Rodriguez-Esteban C., Carrière C., Bhushan A., Krones A., Rose D. W., Glass C. K., Andersen B., Izpisúa Belmonte J. C., Rosenfeld M. G. (1999) RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nat. Genet. 22, 394–399 [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y., Flandin P., Vogt D., Blood A., Hermesz E., Westphal H., Rubenstein J. L. (2014) Ldb1 is essential for development of Nkx2.1 lineage derived GABAergic and cholinergic neurons in the telencephalon. Dev. Biol. 385, 94–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thor S., Andersson S. G. E., Tomlinson A., Thomas J. B. (1999) A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature 397, 76–80 [DOI] [PubMed] [Google Scholar]
- 46.Weimann M., Grossmann A., Woodsmith J., Özkan Z., Birth P., Meierhofer D., Benlasfer N., Valovka T., Timmermann B., Wanker E. E., Sauer S., Stelzl U. (2013) A Y2H-seq approach defines the human protein methyltransferase interactome. Nat. Methods 10, 339–342 [DOI] [PubMed] [Google Scholar]
- 47.Cho H. H., Cargnin F., Kim Y., Lee B., Kwon R. J., Nam H., Shen R., Barnes A. P., Lee J. W., Lee S., Lee S. K. (2014) Isl1 directly controls a cholinergic neuronal identity in the developing forebrain and spinal cord by forming cell type-specific complexes. PLoS Genet. 10, e1004280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thaler J. P., Lee S. K., Jurata L. W., Gill G. N., Pfaff S. L. (2002) LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell 110, 237–249 [DOI] [PubMed] [Google Scholar]
- 49.Marin O., Anderson S. A., Rubenstein J. L. (2000) Origin and molecular specification of striatal interneurons. J. Neurosci. 20, 6063–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asbreuk C. H., van Schaick H. S., Cox J. J., Kromkamp M., Smidt M. P., Burbach J. P. (2002) The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience 109, 287–298 [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y., Marín O., Hermesz E., Powell A., Flames N., Palkovits M., Rubenstein J. L., Westphal H. (2003) The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc. Natl. Acad. Sci. USA 100, 9005–9010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fragkouli A., Hearn C., Errington M., Cooke S., Grigoriou M., Bliss T., Stylianopoulou F., Pachnis V. (2005) Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur. J. Neurosci. 21, 2923–2938 [DOI] [PubMed] [Google Scholar]
- 53.Bachy I., Rétaux S. (2006) GABAergic specification in the basal forebrain is controlled by the LIM-hd factor Lhx7. Dev. Biol. 291, 218–226 [DOI] [PubMed] [Google Scholar]
- 54.Fogarty M., Grist M., Gelman D., Marín O., Pachnis V., Kessaris N. (2007) Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flandin P., Zhao Y., Vogt D., Jeong J., Long J., Potter G., Westphal H., Rubenstein J. L. (2011) Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neves G., Shah M. M., Liodis P., Achimastou A., Denaxa M., Roalfe G., Sesay A., Walker M. C., Pachnis V. (2013) The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex. Cereb. Cortex 23, 1811–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alifragis P., Liapi A., Parnavelas J. G. (2004) Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J. Neurosci. 24, 5643–5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogt D., Hunt R. F., Mandal S., Sandberg M., Silberberg S. N., Nagasawa T., Yang Z., Baraban S. C., Rubenstein J. L. (2014) Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron 82, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manabe T., Tatsumi K., Inoue M., Makinodan M., Yamauchi T., Makinodan E., Yokoyama S., Sakumura R., Wanaka A. (2007) L3/Lhx8 is a pivotal factor for cholinergic differentiation of murine embryonic stem cells. Cell Death Differ. 14, 1080–1085 [DOI] [PubMed] [Google Scholar]
- 60.Manabe T., Tatsumi K., Inoue M., Matsuyoshi H., Makinodan M., Yamauchi T., Makinodan E., Yokoyama S., Sakumura R., Okuda H., Wanaka A. (2008) Knockdown of the L3/Lhx8 gene suppresses cholinergic differentiation of murine embryonic stem cell-derived spheres. Int. J. Dev. Neurosci. 26, 249–252 [DOI] [PubMed] [Google Scholar]
- 61.Shi J., Li H., Jin G., Zhu P., Tian M., Qin J., Tan X., Zhao S., Wang F., Hua Y., Xiao Y. (2012) Lhx8 promote differentiation of hippocampal neural stem/progenitor cells into cholinergic neurons in vitro. In Vitro Cell. Dev. Biol. Anim. 48, 603–609 [DOI] [PubMed] [Google Scholar]
- 62.Flames N., Pla R., Gelman D. M., Rubenstein J. L., Puelles L., Marín O. (2007) Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García-López M., Abellán A., Legaz I., Rubenstein J. L., Puelles L., Medina L. (2008) Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J. Comp. Neurol. 506, 46–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elshatory Y., Gan L. (2008) The LIM-homeobox gene Islet-1 is required for the development of restricted forebrain cholinergic neurons. J. Neurosci. 28, 3291–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abellán A., Medina L. (2009) Subdivisions and derivatives of the chicken subpallium based on expression of LIM and other regulatory genes and markers of neuron subpopulations during development. J. Comp. Neurol. 515, 465–501 [DOI] [PubMed] [Google Scholar]
- 66.Elshatory Y., Everhart D., Deng M., Xie X., Barlow R. B., Gan L. (2007) Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 27, 12707–12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao J. J., Prockop D. J. (2012) Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell 11, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Mori T., Takaki H., Takeuch M., Iseki K., Hagino S., Murakawa M., Yokoya S., Wanaka A. (2002) Comparison of the expression patterns of two LIM-homeodomain genes, Lhx6 and L3/Lhx8, in the developing palate. Orthod. Craniofac. Res. 5, 65–70 [DOI] [PubMed] [Google Scholar]
- 69.Lumsden A. G. (1988) Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103(Suppl), 155–169 [DOI] [PubMed] [Google Scholar]
- 70.Trumpp A., Depew M. J., Rubenstein J. L., Bishop J. M., Martin G. R. (1999) Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 13, 3136–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z., Gutierrez D., Li X., Bidlack F., Cao H., Wang J., Andrade K., Margolis H. C., Amendt B. A. (2013) The LIM homeodomain transcription factor LHX6: a transcriptional repressor that interacts with pituitary homeobox 2 (PITX2) to regulate odontogenesis. J. Biol. Chem. 288, 2485–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.F. Zhu, R.R. Nie, L. Wu, L. Liu, W. Tang, and W.D. Tian (2008) [Spontaneous odontogenic differentiation and critical gene expression of mouse dental papilla mesenchymal cell in vitro]. In Chinese. Sichuan Da Xue Xue Bao Yi Xue Ban 39, 286-289, 297. [PubMed]
- 73.Priam F., Ronco V., Locker M., Bourd K., Bonnefoix M., Duchêne T., Bitard J., Wurtz T., Kellermann O., Goldberg M., Poliard A. (2005) New cellular models for tracking the odontoblast phenotype. Arch. Oral Biol. 50, 271–277 [DOI] [PubMed] [Google Scholar]
- 74.Wu L., Zhu F., Wu Y., Lin Y., Nie X., Jing W., Qiao J., Liu L., Tang W., Zheng X., Tian W. (2008) Dentin sialophosphoprotein-promoted mineralization and expression of odontogenic genes in adipose-derived stromal cells. Cells Tissues Organs 187, 103–112 [DOI] [PubMed] [Google Scholar]
- 75.Kim J. Y., Jeon S. H., Park J. Y., Suh J. D., Choung P. H. (2011) Comparative study of LHX8 expression between odontoma and dental tissue-derived stem cells. J. Oral Pathol. Med. 40, 250–256 [DOI] [PubMed] [Google Scholar]
- 76.Dixon M. J., Marazita M. L., Beaty T. H., Murray J. C. (2011) Cleft lip and palate: understanding genetic and environmental influences. Nat. Rev. Genet. 12, 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y., Guo Y. J., Tomac A. C., Taylor N. R., Grinberg A., Lee E. J., Huang S., Westphal H. (1999) Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proc. Natl. Acad. Sci. USA 96, 15002–15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vieira A. R., Avila J. R., Daack-Hirsch S., Dragan E., Félix T. M., Rahimov F., Harrington J., Schultz R. R., Watanabe Y., Johnson M., Fang J., O’Brien S. E., Orioli I. M., Castilla E. E., Fitzpatrick D. R., Jiang R., Marazita M. L., Murray J. C. (2005) Medical sequencing of candidate genes for nonsyndromic cleft lip and palate. PLoS Genet. 1, e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inoue M., Kawakami M., Tatsumi K., Manabe T., Makinodan M., Matsuyoshi H., Kirita T., Wanaka A. (2006) Expression and regulation of the LIM homeodomain gene L3/Lhx8 suggests a role in upper lip development of the chick embryo. Anat. Embryol. (Berl.) 211, 247–253 [DOI] [PubMed] [Google Scholar]
- 80.Matsumura K., Taketomi T., Yoshizaki K., Arai S., Sanui T., Yoshiga D., Yoshimura A., Nakamura S. (2011) Sprouty2 controls proliferation of palate mesenchymal cells via fibroblast growth factor signaling. Biochem. Biophys. Res. Commun. 404, 1076–1082 [DOI] [PubMed] [Google Scholar]
- 81.Meng L., Bian Z., Torensma R., Von den Hoff J. W. (2009) Biological mechanisms in palatogenesis and cleft palate. J. Dent. Res. 88, 22–33 [DOI] [PubMed] [Google Scholar]
- 82.Zirzow S., Lüdtke T. H., Brons J. F., Petry M., Christoffels V. M., Kispert A. (2009) Expression and requirement of T-box transcription factors Tbx2 and Tbx3 during secondary palate development in the mouse. Dev. Biol. 336, 145–155 [DOI] [PubMed] [Google Scholar]
- 83.Xiang L., Chen M., He L., Cai B., Du Y., Zhang X., Zhou C., Wang C., Mao J. J., Ling J. (2014) Wnt5a regulates dental follicle stem/progenitor cells of the periodontium. Stem Cell Res. Ther. 5, 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landin Malt A., Cesario J. M., Tang Z., Brown S., Jeong J. (2014) Identification of a face enhancer reveals direct regulation of LIM homeobox 8 (Lhx8) by wingless-int (WNT)/β-catenin signaling. J. Biol. Chem. 289, 30289–30301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzumori N., Pangas S. A., Rajkovic A. (2007) Candidate genes for premature ovarian failure. Curr. Med. Chem. 14, 353–357 [DOI] [PubMed] [Google Scholar]
- 86.Qin Y., Zhao H., Kovanci E., Simpson J. L., Chen Z. J., Rajkovic A. (2008) Analysis of LHX8 mutation in premature ovarian failure. Fertil. Steril. 89, 1012–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeon S., Won H. J., Kim Y. S., Lyu S. W., Seok H. H., Kim N. K., Lee W. S., Shim S. H., Yoon T. K., Choi Y. (2010) Novel single-nucleotide polymorphisms of LHX8 gene in Korean women with premature ovarian insufficiency. Genes Genomics 32, 397–400 [Google Scholar]
- 88.Zhang L. J., Pan B., Chen B., Zhang X. F., Liang G. J., Feng Y. N., Wang L. Q., Ma J. M., Li L., Shen W. (2012) Expression and epigenetic dynamics of transcription regulator Lhx8 during mouse oogenesis. Gene 506, 1–9 [DOI] [PubMed] [Google Scholar]
- 89.Jagarlamudi K., Rajkovic A. (2012) Oogenesis: transcriptional regulators and mouse models. Mol. Cell. Endocrinol. 356, 31–39 [DOI] [PubMed] [Google Scholar]
- 90.Liu W. B., Jiang X., Han F., Li Y. H., Chen H. Q., Liu Y., Cao J., Liu J. Y. (2013) LHX6 acts as a novel potential tumour suppressor with epigenetic inactivation in lung cancer. Cell Death Dis. 4, e882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung S., Jeong D., Kim J., Yi L., Koo K., Lee J., Kim C. J., Kim C. H., An S., Yang Y., Lim J. S., Kim K. I., Lee M. S. (2011) Epigenetic regulation of the potential tumor suppressor gene, hLHX6.1, in human cervical cancer. Int. J. Oncol. 38, 859–869 [DOI] [PubMed] [Google Scholar]
- 92.Jung S., Jeong D., Kim J., Yi L., Koo K., Lee J., Lee S. D., Park J. W., Chang B., Kim C. H., Kim C. J., Lee M. S. (2010) The role of hLHX6-HMR as a methylation biomarker for early diagnosis of cervical cancer. Oncol. Rep. 23, 1675–1682 [DOI] [PubMed] [Google Scholar]
- 93.Carey A. L., Vorlander C., Reddy-Luthmoodoo M., Natoli A. K., Formosa M. F., Bertovic D. A., Anderson M. J., Duffy S. J., Kingwell B. A. (2014) Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS ONE 9, e91997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jespersen N. Z., Larsen T. J., Peijs L., Daugaard S., Homøe P., Loft A., de Jong J., Mathur N., Cannon B., Nedergaard J., Pedersen B. K., Møller K., Scheele C. (2013) A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 [DOI] [PubMed] [Google Scholar]
- 95.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2010) Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scheele C., Larsen T. J., Nielsen S. (2014) Novel nuances of human brown fat. Adipocyte 3, 54–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Waldén T. B., Petrovic N., Nedergaard J. (2010) PPARalpha does not suppress muscle-associated gene expression in brown adipocytes but does influence expression of factors that fingerprint the brown adipocyte. Biochem. Biophys. Res. Commun. 397, 146–151 [DOI] [PubMed] [Google Scholar]
- 98.Macotela Y., Emanuelli B., Mori M. A., Gesta S., Schulz T. J., Tseng Y.-H., Kahn C. R. (2012) Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61, 1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu W. B., Jiang X., Han F., Li Y.-H., Chen H.-Q., Liu Y., Cao J., Liu J.-Y. (2016) LHX6 acts as a novel potential tumor suppressor with epigenetic inactivation in lung cancer. Cell Death Dis. 4, e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung S., Jeong D., Kim J., Yi L., Koo K., Lee J., Kim C.-J., Kim C.-H., An S., Yang Y., Lim J.-S., Kim K. I., Lee M.-S. (2011) Epigenetic regulation of the potential tumor suppressor gene, hLHX6.1, in human cervical cancer. Int. J. Oncol. 38, 859–869 [DOI] [PubMed] [Google Scholar]
- 101.Estécio M. R. H., Youssef E. M., Rahal P., Fukuyama E. E., Góis-Filho J. F., Maniglia J. V., Goloni-Bertollo E. M., Issa J. P. J., Tajara E. H. (2006) LHX6 is a sensitive methylation marker in head and neck carcinomas. Oncogene 25, 5018– 5026 [DOI] [PubMed] [Google Scholar]