Abstract
442 pre-ART, HIV-infected adults were randomized to peer support consisting of structured home visits to promote clinic attendance and preventive care intervention use or standard of care. At baseline, 62% reported previously visiting an HIV clinic, 45% reported taking cotrimoxazole prophylaxis, and 31% were “care-naïve” (no previous clinic visit and not on cotrimoxazole). After one year, intervention participants were more likely to report being in care (92% vs 84%; PRR 1.09, p=0.039), on cotrimoxazole (89% vs 81%; PRR 1.10, p=0.047), and safe water vessel adherence (23% vs 14%; PRR 1.64, p=0.024). The effect was observed only among care-naïve participants (n=139) with 83% intervention vs 53% controls reporting being in HIV care (PRR 1.47, p=0.006), 78% vs 58% on cotrimoxazole (PRR 1.35, p=0.04), and 20% vs 4% safe water vessel adherence (PRR 5.78, p=0.017). Peer support may be an effective intervention to facilitate pre-ART care compliance in this important population.
Keywords: peer support, randomized controlled trial, Uganda, implementation science, linkage
INTRODUCTION
As HIV Counseling and Testing (HCT) increases in low and middle-income countries (LMIC) [1], more people living with HIV (PLHIV) are aware of their serostatus and can be engaged in HIV services [2]. PLHIV who are pre-antiretroviral therapy (ART), either because they are not eligible for treatment, choose to defer ART, or are not receiving appropriate care, can still benefit from being linked and retained for CD4 and clinical monitoring [3]. They also benefit from utilization of preventive care interventions, including cotrimoxazole prophylaxis to prevent bacterial and malarial infections, safe water vessels to prevent diarrheal illnesses, and bednets to prevent malaria [4, 5]. Finally, decreasing risky sexual behaviors may reduce sexually transmitted infections and prevent onward HIV transmission [6].
Unfortunately, there are many challenges to linking and retaining PLHIV in care and limited empiric data on effective intervention strategies to improve engagement in care among pre-ART populations [7, 8]. Peer supporters, PLHIV trained to provide basic counseling, care, and psychosocial support, may be one strategy to address pre-ART needs in an integrated approach that is economical and sustainable. Peers have been used extensively and effectively in many HIV/AIDS programs in LMICs, often as peer educators, but evidence on their impact on pre-ART outcomes is limited [9–11]. Uganda, which in 2013 had an estimated HIV prevalence of 7.4%, 120 000 new HIV infections, 63 000 AIDS-related deaths, and only 40% ART coverage, would likely benefit from additional effective peer-based HIV interventions [12].
We conducted the PeerCARE (Peer Community Assistant in Retention and Engagement) Study, a randomized trial aiming to assess the effectiveness of a peer support intervention on HIV care engagement and preventive care utilization among pre-ART adults in Rakai District, Uganda. Rakai District provides diverse populations in which to explore impact of the peer support intervention, ranging from agrarian communities to high HIV prevalence and incidence fishing communities on Lake Victoria. Here, we report the main quantitative results of the trial.
METHODS
Study design and participants
The study was a two-arm, individually randomized, pragmatically-oriented trial. Participant exclusion criteria were minimal, the intervention was applied flexibly as it would be in normal program implementation, outcomes were patient-oriented, and reporting is per CONSORT guidelines [13–15]. Implementation indicators were also collected [16].
The study setting was the Rakai Health Sciences Program (RHSP), a research institution and PEPFAR implementer in Rakai District, Uganda (population ∼400,000). During the study, RHSP offered HCT services to any “walk-in” patients at RHSP-supervised clinics throughout the District and to participants enrolled in the Rakai Community Cohort Study (RCCS). RCCS is a population-based HIV survey study which includes agrarian and semi-urban communities (referred to as the RCCS general population), as well as fishing communities on Lake Victoria (RCCS fishing community population).
HCT for clinic walk-ins used a point of care rapid HIV test algorithm [17]. RCCS fishing community participants received community-based HCT using the same rapid HIV test algorithm. RCCS general population participants received community-based HCT using a method which maximized specificity [18]; these results were available about two weeks from sample collection and returned through local counselors. All persons found HIV-positive were offered through the HIV clinics a basic care package (BCP) of preventive care interventions (cotrimoxazole prophylaxis, safe water vessels with hypochlorite disinfectant, and insecticide-treated bednets), condoms, health and HIV prevention education, and CD4 monitoring conducted at baseline and every 3 to 6 months thereafter. Persons meeting Ugandan clinical (WHO Stage 4) or CD4 (<350 cells/µl) eligibility criteria were offered ART. All services were free.
The study was conducted from June 2011 to July 2013. Eligibility criteria were HIV-infected adults (≥18 years) who had recently (≤8 weeks) received HCT through RHSP. Potential participants were approached by study staff, typically within 1 week in the clinic or community for walk-ins or within 2–4 weeks for RCCS participants. We included participants who were care-naïve, as well as those who had sought HIV care between the time of HCT and randomization, who may have obtained cotrimoxazole from non-clinic sources (e.g. pharmacies), or who were previously diagnosed with HIV through non-RHSP HCT, excluding only those who had already initiated ART at baseline. After written informed consent, participants were randomized to the peer intervention or standard of care and followed for one year.
Randomization and masking
A list of random numbers with block sizes of 2, 4, and 6 was computer-generated. This allocation sequence was detailed on cards in sequentially numbered, opaque, sealed, and stapled envelopes. After completing the baseline survey, the next envelope was opened to obtain arm assignment. Randomization was generally by individual in a 1:1 ratio. However, couples in the same household were randomized together if both consented and had mutually disclosed their HIV serostatus. Participants were necessarily unmasked. Outcome assessment was also unblinded because intervention-specific responses were required in interview questionnaires.
Intervention description
The intervention design and evaluation was based on a situated Information, Motivation, and Behavioral Skills (sIMB) conceptual framework [19]. In this theoretical framework, relevant information, motivation, and behavioral skills factors interact to determine engagement in treatment and prevention services [20]. Our framework contextualizes the sIMB model to our setting through consideration of structural (e.g. access to care) and clinical factors (e.g. patient’s health status) which may have a role in explaining intervention effects [21]. Peers (n=12) were selected from current pre-ART patients; eligibility requirements included good BCP adherence and literacy. Peers underwent a two-day residential training on confidentiality, disclosure, counseling techniques, motivational interviewing principles and skills, engagement in care, the BCP, ART, and condoms [22–24].
Peers were asked to make an initial visit to assigned participants within two weeks of randomization, and then visit each participant monthly. Visits typically occurred in the home, but could be arranged at other locations (e.g. work sites). During visits, peers performed 3 categories of activities: (1) Assessment-Peers assessed participants’ clinical status, clinic attendance, BCP adherence, and sexual behaviors using a structured interview form; (2) Support-Peers provided psychosocial support, encouragement, information on and reminders of clinic appointments and the BCP, and counseling on reducing risky sexual behaviors; (3) Access-Peers provided triage to higher level care if necessary.
Peers were assigned a mean of ∼20 participants (range 4–33) based largely upon proximity, and provided with supplies including a mobile phone. Peers were remunerated (∼10 USD/month) and given a transport stipend (∼1 USD/participant visited) for what was considered part-time work. They were supervised by two part-time coordinators who also regularly conducted field-based skills reinforcement trainings.
Standard of care arm description
The Control Arm consisted of the current standard of pre-ART care provided by RHSP. For RCCS general and fishing community population participants, this meant that upon learning their HIV-positive result, they were given a referral form to visit an RHSP clinic for assessment of ART eligibility and to begin pre-ART care. Walk-ins testing HIV-positive were immediately given the opportunity to engage in pre-ART services the same day, including BCP receipt and CD4 monitoring.
Outcomes
A structured survey questionnaire was administered in person by study staff at baseline and after one year of follow-up. The survey included demographics, sexual behaviors, HIV clinic attendance and HIV preventive care intervention utilization. To cross-verify survey data, RHSP clinical data, including clinic visit dates, ART initiation dates, and CD4 cell counts among those in care, was abstracted from patient charts. CD4 counts assessing ART eligibility were measured only through routine clinical procedures and thus were not available for participants who had not accessed care and CD4 monitoring.
The intervention was intended to affect multiple aspects of HIV care and the primary endpoints were: engagement in care (defined as HIV clinic attendance, either self-reported or based upon clinical records), BCP preventive care use, risky sexual behaviors, and ART initiation. We treated these outcomes as independent hypotheses and did not adjust for multiple comparisons as they potentially had independent mechanisms according to our a priori sIMB conceptual framework. Survey-based, self-reported outcomes (see Table 2 footnotes for details) included currently being in HIV care at an HIV clinic (as perceived by the participant), currently taking ART, current adherence to each of the components of the BCP, and condom use. Ownership of bednets and water vessels, any sexual activity, and number of sexual partners were also assessed. Clinic-based outcomes included first entry into care at an RHSP clinic, CD4 cell counts, and ART initiation. Assessment of appointment adherence and retention using clinic records was not feasible because of secular changes in appointment tracking record-keeping and limited follow-up time. Analyses were by modified intention-to-treat wherein all participants who had data at follow-up were included in analyses. A per protocol analysis of outcomes was also performed, excluding all intervention arm participants who did not receive a home visit and control arm patients who inadvertently received a home visit.
Table II.
Survey-based study outcomes at one year by randomization arm for all participants (n=393).
| Outcome | Intervention n/N (%) |
Control n/N (%) |
Prevalence Risk Ratio (95% CI) |
p value |
|---|---|---|---|---|
| In HIV carea | 178/194 (92%) | 167/199 (84%) | 1.09 (1.00–1.19) | 0.039 |
| On ARTb | 71/194 (37%) | 71/199 (36%) | 1.03 (0.78–1.34) | 0.85 |
| On cotrimoxazolec | 173/194 (89%) | 161/199 (81%) | 1.10 (1.00–1.21) | 0.047 |
| Own bednet | 131/194 (68%) | 135/199 (68%) | 1.00 (0.84–1.18) | 0.96 |
| Bednet adherentd | 91/194 (47%) | 94/199 (47%) | 0.99 (0.79–1.25) | 0.95 |
| Own water vessel | 74/194 (38%) | 49/199 (25%) | 1.55 (1.09–2.20) | 0.014 |
| Water vessel adherente | 45/194 (23%) | 28/199 (14%) | 1.64 (1.07–2.55) | 0.024 |
| Basic care package adherentf | 31/194 (16%) | 18/199 (9%) | 1.77 (1.04–3.00) | 0.035 |
| Any sex past 12 months | 170/194 (88%) | 174/199 (87%) | 1.00 (0.90–1.11) | 0.97 |
| Multiple sexual partnersg | 61/170 (35%) | 51/174 (29%) | 1.22 (0.89–1.68) | 0.21 |
| Any condom useh | 107/170 (63%) | 102/174 (59%) | 1.07 (0.86–1.34) | 0.53 |
Currently? Yes/No;
Currently? Yes/No;
Currently? Yes/No;
Always Use vs Other;
Always Use vs. Other;
On cotrimoxazole and bednet adherent and water vessel adherent vs. Other;
>1 Partner vs. 1 Partner, among sexually active only;
Any condom use past 12 months vs. Other, among sexually active only.
Statistical analyses
Study power was initially calculated anticipating recruitment of 125 participants per arm with a two-sided α=0.05 to detect a difference between study arms for engagement in care (15% estimated improvement, 80% power), BCP use (20% estimated improvement, 90% power), and condom use (14% estimated improvement, 78% power). After several months of recruitment, due to concerns that secular changes were resulting in healthier patients than anticipated being enrolled who were less likely to reach study outcomes, we increased our recruitment goal to 225 participants per arm.
For dichotomous outcomes, we used Poisson regression with cluster robust standard errors accounting for clustering effects by peer assignment to estimate Prevalence Risk Ratios (PRR) with 95% Confidence Intervals (CIs). At baseline, patients may have already received formal HIV care prior to randomization. As our conceptual framework suggested that the intervention may impact participants differently based upon their prior HIV care experience, we disaggregated the population into “care-naïve” if they both stated that they were not in care and were not taking cotrimoxazole at baseline, or “care-experienced” if they reported being in care and were taking cotrimoxazole. Walk-ins were all classified as care-experienced since they were offered HIV care at time of HCT and prior to randomization. Stratified analyses explored effects by baseline care status, gender, and patient recruitment source. Possible “dose-response” CHW effects were evaluated by including number of visits by CHWs as an independent variable.
Clinic-based outcomes of entry into care at an RHSP clinic and ART initiation were assessed as proportions. Kaplan-Meier survival analyses were conducted using log-rank tests to evaluate differences between study arms, and Cox’s proportional hazards modeling was used to estimate hazard ratios (HR) and 95% CIs of study outcomes. Person-time was accrued from date of randomization to the event of interest (date of entry into care or date of ART initiation) or censoring due to administrative censoring at one year following randomization or loss to follow up. To assess entry into care, participants were classified as “care-experienced” and excluded if there was any clinic record indicating they had visited an RHSP clinic prior to study randomization; all others participants were classified as “care-naïve” and included. We also conducted a subgroup analysis further excluding participants who self-reported being in care or taking cotrimoxazole on the baseline survey to assess intervention effects among participants most likely to have never received formal HIV care. CD4 cell counts were compared with Wilcoxon rank sum test.
Participants randomized as a couple were analyzed as individuals as they constituted a small minority of all participants. A sensitivity analyses was conducted excluding those randomized as a couple.
This trial is registered at ClinicalTrials.gov (NCT01366690) and was approved by the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, and the Johns Hopkins Medicine Institutional Review Board.
RESULTS
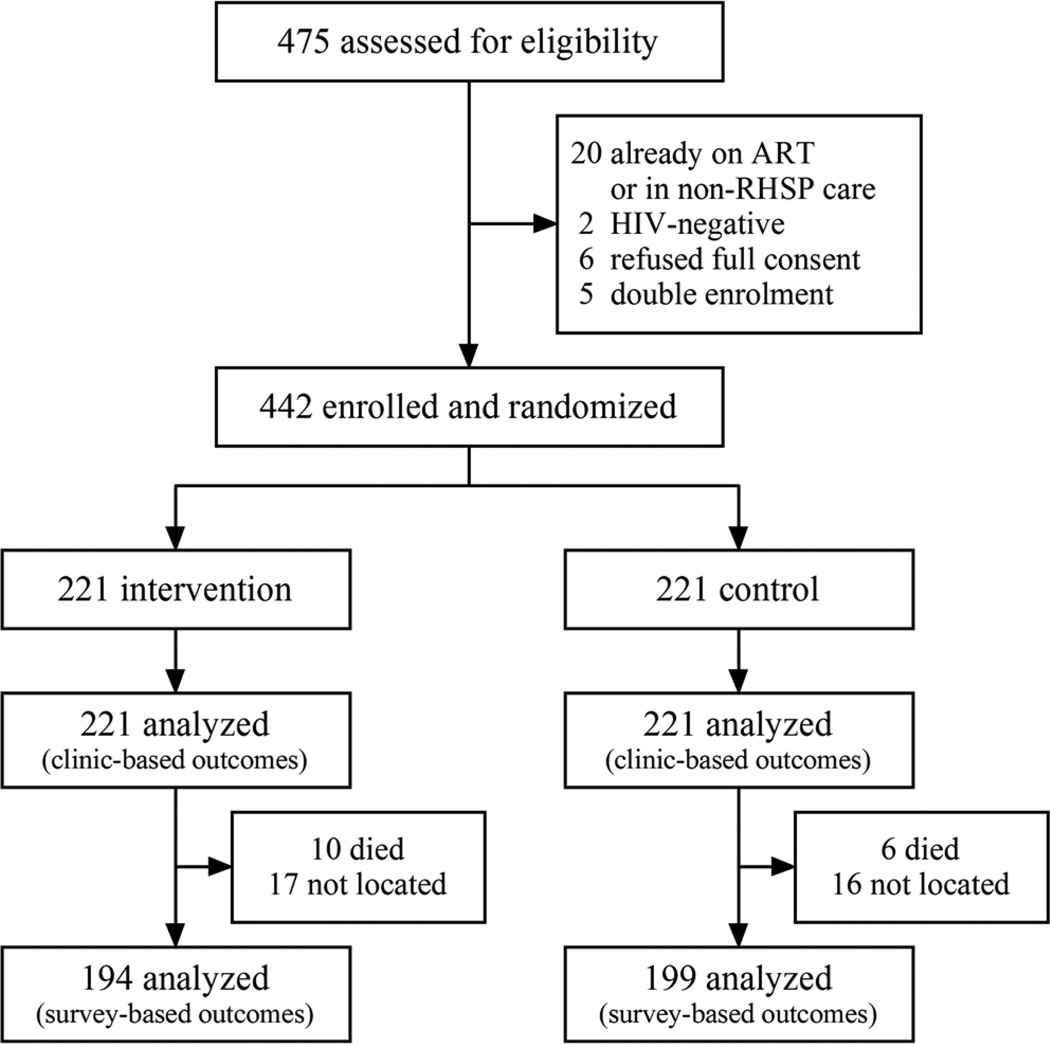
Between June 2011 to July 2013, 442 eligible participants were enrolled and followed (221 intervention and 221 control) (Figure 1). Of 33 out of 475 individuals screened and found ineligible, 20 (4.2%) were found to be on ART and/or receiving care from non-RHSP providers, 5 were doubly enrolled, 2 patients were HIV-negative on confirmatory tests, and 6 declined full consent. 20 participants (4.5%) were randomized together as a couple. Ten intervention participants (4.4%) died compared to 6 (2.7%) control participants (PRR 1.43, 95% CI 0.59–3.43, p=0.43). We were unable to locate and endline survey seventeen (7.7%) intervention participants versus 16 (7.2%) control participants.
Figure 1. Trial profile.
Baseline characteristics and process results
There were no significant differences in participant characteristics at baseline (Table 1). At baseline, 62% (n=275) of participants reported already visiting an RHSP clinic, the majority of whom were walk-ins and 45% (n=198) reported currently taking cotrimoxazole prophylaxis. These care-experienced individuals were comparable between study arms. 31% (n=139) had no history of either a RHSP clinic visit or current cotrimoxazole use and were classified as care-naïve at baseline, and were comparable between study arms (Table 1b).
Table I.
Baseline characteristics by study arm (n=442).a
| Characteristic | Subcharacteristic | Intervention (n=221) |
Control (n=221) |
|---|---|---|---|
| Female, n (%) | 133 (60) | 147 (67) | |
| Age in years, median (IQR, interquartile range) |
30·0 (26–37) | 30·0 (27–36) | |
| Marital Status, n (%) | |||
| Married | 120 (54) | 121 (55) | |
| Divorced/Separated | 55 (25) | 63 (29) | |
| Widowed | 13 (6) | 13 (6) | |
| Single | 33 (15) | 24 (11) | |
| Religion, n (%) | |||
| Christian | 200 (91) | 192 (87) | |
| Muslim | 20 (9) | 28 (13) | |
| Other | 1 (<1) | 1 (<1) | |
| Recruitment background, n (%) | |||
| RCCS general population |
82 (37) | 64 (29) | |
| RCCS fishing community population |
70 (32) | 78 (35) | |
| Clinic walk-ins, non-cohort |
69 (31) | 79 (36) | |
| Travel time in minutes to nearest clinic, median (IQR) |
25 (10–40) | 25 (10–50) | |
| Already visited RHSP clinic, n (%) | 132 (60) | 143 (65) | |
| Own bednet, n (%) | 130 (59) | 136 (62) | |
| Always sleep under bednet, n (%) | 74 (34) | 92 (42) | |
| Slept under bednet last night, n (%) | 88 (40) | 111 (50) | |
| Own safe water vessel, n (%) | 14 (6) | 12 (5) | |
| Taking cotrimoxazole, n (%) | 92 (42) | 106 (48) | |
| Any sex past 12 months, n (%) | 200 (91) | 198 (90) | |
| Multiple sexual partners past 12 months, n (%) |
86 (39) | 89 (40) | |
| Any condom use past 12 months, n (%) | 95 (43) | 97 (44) |
| Ia. Baseline characteristics of participants care-naïve at baseline by study arm (n=139).a | |||
|---|---|---|---|
| Characteristic | Subcharacteristic | Intervention (n=78) |
Control (n=61) |
| Female, n (%) | 45 (58) | 41 (67) | |
| Age in years, median (IQR, interquartile range) |
30·0 (25–37) | 30·0 (26–34) | |
| Marital Status, n (%) | |||
| Married | 46 (59) | 36 (59) | |
| Divorced/Separated | 18 (23) | 13 (21) | |
| Widowed | 2 (3) | 1 (2) | |
| Single | 12 (15) | 11 (18) | |
| Religion, n (%) | |||
| Christian | 66 (85) | 52 (85) | |
| Muslim | 12 (15) | 8 (13) | |
| Other | 0 (0) | 1 (2) | |
| Recruitment background, n (%) | |||
| RCCS general population |
47 (60) | 32 (53) | |
| RCCS fishing community population |
31 (40) | 29 (48) | |
| Travel time in minutes to nearest clinic, median (IQR) |
20 (15–30) | 20 (10–30) | |
| Own bednet, n (%) | 46 (59) | 39 (64) | |
| Always sleep under bednet, n (%) | 25 (32) | 27 (44) | |
| Slept under bednet last night, n (%) | 32 (41) | 32 (53) | |
| Own safe water vessel, n (%) | 0 (0) | 0 (0) | |
| Any sex past 12 months, n (%) | 71 (91) | 58 (95) | |
| Multiple sexual partners past 12 months, n (%) |
34 (44) | 31 (51) | |
| Any condom use past 12 months, n (%) | 30 (39) | 30 (49) | |
No statistically significant differences between arms.
The median time from receipt of HIV results to randomization for all participants was 11 days (IQR 6–40 days). After a median follow-up of 363 days in each arm (IQR of 351–378 days), 87% of intervention and 90% of control participants completed the end of study survey (p=0.45). 90% of intervention arm participants received at least one peer visit and the median number of visits received was 7 (IQR 2–13). The median time from randomization to first visit was 19 days (IQR 10–51). Due to administrative error, ten control arm participants received a single peer visit and three control arm patients received multiple visits (11, 13, and 17 visits).
Self-reported outcomes, all participants
At the one year follow-up, a greater proportion of intervention than control arm participants self-reported being currently in HIV care, on cotrimoxazole prophylaxis, and adherent to safe water vessel use, and overall BCP adherent (Poisson regression results shown in Table 2). No intervention effects were observed on ART initiation, bednet use, or sexual behaviors. No effect modification was found by gender or recruitment source (results not shown.) A per protocol analysis of all key outcomes showed similar results (see supplementary Table 4), and results were not affected by excluding individuals randomized as couples. No dose-response effect was seen regarding number of CHW visits received.
Self-reported outcomes, care-naïve and care-experienced subgroups
Table 3 shows subgroup analyses for participants care-naïve at baseline. At one year of follow-up, intervention arm participants were more likely to report being in care at an HIV clinic, on cotrimoxazole, adherent to safe water vessel use, and overall BCP adherent. Subgroup analyses of care-experienced participants found no significant intervention effects (see supplementary Table 5).
Table III.
Survey-based study outcomes at one year by randomization arm for participants care-naïve at baseline (n=126).
| Outcome | Intervention n/N (%) |
Control n/N (%) |
Prevalence Risk Ratio (95% CI) |
p value |
|---|---|---|---|---|
| In HIV carea | 57/69 (83%) | 32/57 (56%) | 1.47 (1.12–1.94) | 0.006 |
| On ARTb | 19/69 (28%) | 12/57 (21%) | 1.31 (0.67–2.56) | 0.43 |
| On cotrimoxazolec | 54/69 (78%) | 33/57 (58%) | 1.35 (1.01–1.81) | 0.04 |
| Own bednet | 51/69 (74%) | 34/57 (60%) | 1.24 (0.94–1.63) | 0.13 |
| Bednet adherentd | 37/69 (54%) | 24/57 (42%) | 1.27 (0.87–1.86) | 0.21 |
| Own water vessel | 24/69 (35%) | 3/57 (5%) | 6.61 (2.06–21.2) | 0.002 |
| Water vessel adherente | 14/69 (20%) | 2/57 (4%) | 5.78 (1.37–24.3) | 0.017 |
| Basic care package adherentf | 13/69 (19%) | 1/57 (2%) | 10.7 (1.46–78.8) | 0.020 |
| Any sex past 12 months | 63/69 (91%) | 53/57 (93%) | 0.98 (0.88–1.10) | 0.75 |
| Multiple sexual partnersg | 21/63 (33%) | 18/53 (34%) | 0.98 (0.61–1.60) | 0.94 |
| Any condom useg | 38/63 (60%) | 29/53 (55%) | 1.10 (0.80–1.53) | 0.56 |
Currently? Yes/No;
Currently? Yes/No;
Currently? Yes/No;
Always Use vs Other;
Always Use vs. Other;
On cotrimoxazole and bednet adherent and water vessel adherent vs. Other;
>1 Partner vs. 1 Partner, among sexually active only;
Any condom use past 12 months vs. Other, among sexually active only.
Clinic-based outcomes
RHSP clinic-based data was available for 94% (n=415) of participants. 60% (n=265) were care-naïve at baseline according to clinic records (kappa=0.43 for agreement with survey-based care-naïve classification). Of these participants, the proportion enrolling into RHSP care was higher in the intervention (75/133=56.4%) compared to the control arm (59/132=44.4%), but this difference was not statistically significant (PRR 1.28, 95% CI 0.95–1.71, p=0.07). CD4 cell counts assessing ART eligibility were available for 44% (n=98) of intervention participants (median 408 cells/µl, IQR 258–717 cells/µl) and 43% (n=96) of control participants (median 332 cells/µl, IQR 206–561 cells/µl), but this difference was not statistically significant (p=0.07). Based on clinic records, there was no difference in the proportion initiating ART in the intervention (69/216=31.9%) compared to the control arm (64/215=29.8%) (PRR 1.09, 95% CI 0.81–1.45, p=0.59). Median CD4 cell counts among the 50 intervention arm participants who initiated ART and had a CD4 cell count available was 300 cells/µl (IQR 222–475 cells/µl) compared to 248 cells/µl (IQR 169–332 cells/µl) among 47 control arm participants (p=0.008).
Clinic-based outcomes, time to care
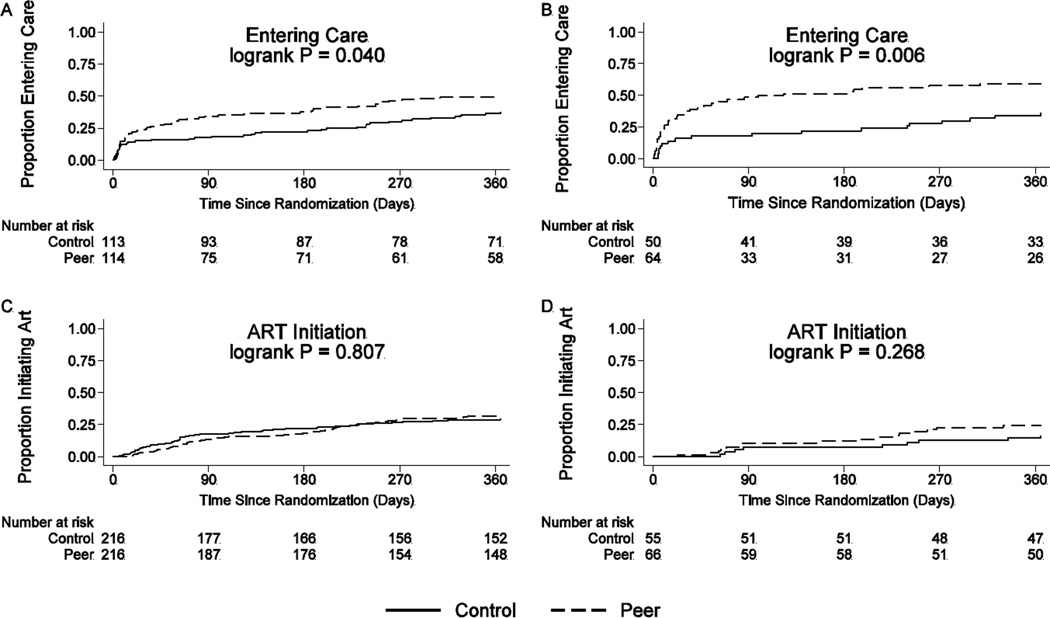
Kaplan-Meier curves for time to entry into care are shown in Figure 2. Among baseline participants care-naïve according to clinic records (n=227), the cumulative probability of entry into RHSP care at one year was 49.1% in the intervention and 37.3% in the control arm (p=0.041); the Cox proportional hazard ratio for entry into care was 1.51 (95% CI 1.01–2.25, p=0.04). In the subgroup of participants care-naïve according to clinic record and baseline survey self-report (n=114), the cumulative probability of entry into RHSP care at one year was 59.4% in the intervention compared to 36.0% in the control arm (p=0.006), and the Cox proportional hazard ratio for entry into care was 2.17 (95% CI 1.23–3.80, p=0.007).
Figure 2. Kaplan-Meier estimates for time to care and ART initiation.
(A) Cumulative proportions entering care (first clinic visit) who were care-naïve at baseline according to clinic data only; (B) Cumulative proportions entering care who were care-naïve at baseline according to clinic and survey data; (C) Cumulative proportions initiating ART who were not on ART at baseline according to clinic data only; (D) Cumulative proportions initiating ART who were not on ART and care-naïve at baseline according to clinic and survey data.
Clinic-based outcomes, time to ART initiation
Kaplan-Meier curves for time to ART initiation are also shown in Figure 2. The cumulative probability of ART initiation at an RHSP clinic was 31.9% in the intervention arm and 29.8% in the control arm for all eligible participants (p=0.81) with a hazard ratio for ART initiation of 1.04 (95% CI 0.74–1.47, p=0.80). Among participants ART and care-naïve according to clinical records and the baseline survey, the cumulative probability of ART initiation at an RHSP clinic was 24.2% in the intervention arm and 16.3% in the control (p=0.27) with a hazard ratio for ART initiation of 1.58 (95% CI 0.70–3.58, p=0.27).
DISCUSSION
In this randomized trial, participants in the peer support intervention arm were more likely to report being in care, on cotrimoxazole prophylaxis, and adhering to safe water vessel use at one year of follow-up. These intervention effects were seen only among participants who were classified as care-naïve at baseline. Clinic data supported survey results, finding that intervention arm participants who were care-naïve at baseline were more likely to enroll into care during follow-up. This study is one of the first randomized evaluations to demonstrate effectiveness of an intervention to promote pre-ART engagement in care [9, 25–35].
Improving engagement in care is important as attrition of patients from HCT to care engagement is common and associated with adverse clinical outcomes [7, 36]. Cotrimoxazole use decreases morbidity and mortality [37], and clean water vessel ownership and use reduces diarrhea morbidity [38]. Thus, the peer intervention which improved these outcomes among care-naïve participants can be expected to have beneficial effects on patient-oriented, clinical outcomes. Additionally, higher CD4 counts at time of ART initiation may suggest that intervention arm participants were obtaining CD4 screening earlier in their HIV disease course or had slower disease progression than control participants.
The intervention did not affect ART use, possibly because of short follow-up with many participants having CD4 cell counts above ART eligibility criteria. Intervention arm participants may have also had improved health and slower CD4 cell count declines as a results of better adherence to preventive care interventions, resulting in decreased likelihood of ART eligibility. However, a lack of CD4 cell count results on all participants limits definitive conclusions. There was a lack of an intervention effect on bednet use which may be due to the non-HIV specific nature of bednets which are commonly used by all adults irrespective of HIV status in this setting. In contrast, water vessels with disinfectant are rarely used by HIV-negative persons and may thus be stigmatizing [39]. Finally, no differential between arms was observed for sexual behavior outcomes. These results will all be further explored in a complementary qualitative evaluation [40].
The main intervention effects were among care-naïve participants. This suggests that peer support may be most effective if targeted at PLHIV who are not yet engaged in care. We previously found that peer support was beneficial for PLHIV who had been on ART for several years, possibly by mitigating treatment fatigue [41]. Longitudinal peer interventions which support PLHIV from HIV-diagnosis to entry in care, ART initiation, and retention versus more targeted approaches could be evaluated in future studies.
This trial had a number of limitations. Some study outcomes relied on self-reported data which are vulnerable to social desirability and recall biases [42]. The non-significant results for self-reported outcomes such as bednet use and sexual behaviors suggest such bias was modest. Baseline participant in care status showed only moderate agreement between clinic records and survey responses, suggesting limitations to self-report and a complex relationship between patients and health system engagement. Clinic records were collected for non-research purposes and may have been incomplete or contained errors. Nevertheless, the general concordance of clinic-based and survey-based outcomes is reassuring and highlights the benefits of data triangulation when evaluating complex interventions.
Due to administrative error, a small number of control patients were visited by peers, and contamination may have occurred during interactions between intervention and control participants. These effects would bias results toward the null, and may have limited power to detect marginally significant differences. However, a per protocol analysis showed similar results, suggesting such bias was minimal.
We were also unable to assess long-term retention due to the one year follow-up period and could not collect clinical data from non-RHSP clinics which may have led to an underestimation of care outcomes. The study setting included populations in which RHSP research and service activities have previously been conducted which may affect generalizability. However, demographic and behavioral characteristics of the RHSP study population are similar to those reported for southwestern Uganda in Demographic and Health Surveys [43], suggesting that the population is not atypical of rural southwestern Uganda.
This study was focused on effectiveness and implementation science research rather than efficacy per se [44]. For example, the actual processes and mechanisms of the peer support intervention were not strictly regulated and instead allowed to adapt to “real-world” conditions. Qualitative, conceptual model, and cost evaluations are being conducted and will add further insights.
This intervention consisted of PLHIV trained as peer supporters. The involvement of PLHIV in their own care has been a longstanding policy of the World Health Organization (WHO), UNAIDS [45], and PEPFAR [46]. PLHIV are a sustainable resource drawn from the local community which can mitigate the shortage of trained health workers. Whether defined as “expert patients”[47], peer health workers [41], or peer educators [11], PLHIV provide a potentially effective, local cadre. Further efforts to include PLHIV in HIV care programs should be encouraged, along with implementation science to evaluate their impact.
In summary, our findings support the effectiveness of peer support to improve engagement in HIV care and preventive care intervention utilization in pre-ART, HIV-infected adults not initially engaged in care. Future evaluations of the economics, sustainability, and scalability of this potentially high impact intervention to other settings will be informative.
Supplementary Material
Acknowledgements
The authors thank Caitlin Kennedy (Johns Hopkins University, USA) for editorial contributions and Megan Hosein (Johns Hopkins University, USA) and Julia Peterson (USA) for data collection contributions. This study was supported by The National Institute of Mental Health, National Institutes of Health (K23MH086338), the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institute of Health, and the Johns Hopkins University Center for AIDS Research (P30AI094189).
References
- 1.Mullinax M, Higgins J, Wagman J, Nakyanjo N, Kigozi G, Serwadda D, et al. Community understandings of and responses to gender equality and empowerment in Rakai, Uganda. Global public health. 2013;8(4):465–478. doi: 10.1080/17441692.2013.768686. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Annals of Internal Medicine. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton EF, Saag MS, Mugavero M. Engagement in Human Immunodeficiency Virus Care: Linkage, Retention, and Antiretroviral Therapy Adherence. Infectious disease clinics of North America. 2014;28(3):355–369. doi: 10.1016/j.idc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Mermin J, Bunnell R, Lule J, Opio A, Gibbons A, Dybul M, et al. Developing an evidence-based, preventive care package for persons with HIV in Africa. Trop Med Int Health. 2005;10(10):961–970. doi: 10.1111/j.1365-3156.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- 5.Peterson K, van Griensven J, Huis in ‘t Veld D, Colebunders R. Interventions to reduce mortality in sub-Saharan Africa among HIV-infected adults not yet on antiretroviral therapy. Expert Review of Anti-infective Therapy. 2012;10(1):43–50. doi: 10.1586/eri.11.151. [DOI] [PubMed] [Google Scholar]
- 6.King R, Katuntu D, Lifshay J, Packel L, Batamwita R, Nakayiwa S, et al. Processes and outcomes of HIV serostatus disclosure to sexual partners among people living with HIV in Uganda. AIDS and behavior. 2008;12(2):232–243. doi: 10.1007/s10461-007-9307-7. [DOI] [PubMed] [Google Scholar]
- 7.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17(12):1509–1520. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. The Lancet Infectious diseases. 2013;13(1):65–76. doi: 10.1016/S1473-3099(12)70273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Scaling up HIV/AIDS care: service delivery and human resource perspectives. 2004 [Google Scholar]
- 11.Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS education and prevention : official publication of the International Society for AIDS Education. 2009;21(3):181–206. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The GAP Report. Geneva: UNAIDS; 2014. [Google Scholar]
- 13.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. AHRQ Technical Reviews. Rockville (MD): 2006. Criteria for Distinguishing Effectiveness From Efficacy Trials in Systematic Reviews. [PubMed] [Google Scholar]
- 14.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. Bmj. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Journal of clinical epidemiology. 2009;62(5):464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care. 2012;50(3):217. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galiwango RM, Musoke R, Lubyayi L, Ssekubugu R, Kalibbala S, Ssekweyama V, et al. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. Journal of virological methods. 2013;192(1–2):25–27. doi: 10.1016/j.jviromet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray RH, Makumbi F, Serwadda D, Lutalo T, Nalugoda F, Opendi P, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. Bmj. 2007;335(7612):188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivet Amico K. A situated-Information Motivation Behavioral Skills Model of Care Initiation and Maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. Journal of health psychology. 2011;16(7):1071–1081. doi: 10.1177/1359105311398727. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Current HIV/AIDS reports. 2008;5(4):193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 21.Ware NC, Wyatt MA, Bangsberg DR. Examining theoretic models of adherence for validity in resource-limited settings. A heuristic approach. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S18–S22. doi: 10.1097/01.qai.0000248343.13062.4b. [DOI] [PubMed] [Google Scholar]
- 22.Naar-King S, Parsons JT, Johnson AM. Motivational interviewing targeting risk reduction for people with HIV: a systematic review. Curr HIV/AIDS Rep. 2012;9(4):335–343. doi: 10.1007/s11904-012-0132-x. [DOI] [PubMed] [Google Scholar]
- 23.Miller WR, Rollnick S. Motivational Interviewing. New York: Guilford; 1991. [Google Scholar]
- 24.Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care. 2012;24(5):583–592. doi: 10.1080/09540121.2011.630354. [DOI] [PubMed] [Google Scholar]
- 25.Govindasamy D, Meghij J, Kebede Negussi E, Clare Baggaley R, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings--a systematic review. Journal of the International AIDS Society. 2014;17(1):19032. doi: 10.7448/IAS.17.1.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–e59. doi: 10.1097/QAI.0b013e3182303921. [DOI] [PubMed] [Google Scholar]
- 27.MacPherson P, Lalloo DG, Webb EL, Maheswaran H, Choko AT, Makombe SD, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312(4):372–379. doi: 10.1001/jama.2014.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380(9845):889–898. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhamadi L, Tumwesigye NM, Kadobera D, Marrone G, Wabwire-Mangen F, Pariyo G, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in Eastern Uganda. Trials. 2011;12:184. doi: 10.1186/1745-6215-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 31.Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. Aids. 2011;25(13):1657–1661. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topp SM, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, Reid SE. Integrating HIV treatment with primary care outpatient services: opportunities and challenges from a scaled-up model in Zambia. Health policy and planning. 2012 doi: 10.1093/heapol/czs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermans SM, Castelnuovo B, Katabira C, Mbidde P, Lange JM, Hoepelman AI, et al. Integration of HIV and TB services results in improved TB treatment outcomes and earlier prioritized ART initiation in a large urban HIV clinic in Uganda. J Acquir Immune Defic Syndr. 2012;60(2):e29–e35. doi: 10.1097/QAI.0b013e318251aeb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong'ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012 doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 35.Burtle D, Welfare W, Elden S, Mamvura C, Vandelanotte J, Petherick E, et al. Introduction and evaluation of a ‘pre-ART care’ service in Swaziland: an operational research study. BMJ Open. 2012;2(2) doi: 10.1136/bmjopen-2011-000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson K, van Griensven J, Huis in ‘t Veld D, Colebunders R. Interventions to reduce mortality in sub-Saharan Africa among HIV-infected adults not yet on antiretroviral therapy. Expert review of anti-infective therapy. 2012;10(1):43–50. doi: 10.1586/eri.11.151. [DOI] [PubMed] [Google Scholar]
- 37.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. The Lancet. 364(9443):1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 38.Lule JR, Mermin J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of home-based water chlorination and safe storage on diarrhea among persons with human immunodeficiency virus in Uganda. The American journal of tropical medicine and hygiene. 2005;73(5):926–933. [PubMed] [Google Scholar]
- 39.Nakigozi G, Atuyambe L, Kamya M, Makumbi FE, Chang LW, Nakyanjo N, et al. A qualitative study of barriers to enrollment into free HIV care: perspectives of never-in-care HIV-positive patients and providers in Rakai, Uganda. BioMed research international. 2013;2013:470245. doi: 10.1155/2013/470245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy CE, Medley AM, Sweat MD, O'Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2010;88(8):615–623. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PloS one. 2010;5(6):e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rea LM, Parker RA. Designing and Conducting Survey Research: A Comprehensive Guide. San Francisco: Jossey-Bass; 2005. [Google Scholar]
- 43.Uganda Bureau of Statistics (UBOS) and ICF International Inc. Uganda Demographic and Health Survey 2011. Kampala, Uganda: UBOS and Calverton, Maryland: ICF International Inc; 2012. [Google Scholar]
- 44.Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC medical research methodology. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joint United Nations Programme on HIV/AIDS (UNAIDS) The greater involvement of people living with HIV (GIPA): policy brief. 2007 Contract No.: July 15, 2008. [Google Scholar]
- 46.Kerry J. Involving Civil Society as Part of Country-Level Planning for HIV/AIDS Programming. 2013 Jun 13; [Google Scholar]
- 47.Kielmann K, Cataldo F. Tracking the rise of the “expert patient” in evolving paradigms of HIV care. AIDS Care. 2010;22(sup1):21–28. doi: 10.1080/09540121003721000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.