Abstract
Nationally up to 60 % of persons living with HIV are neither taking antiretroviral therapy (ART) nor well engaged in HIV care, mainly racial/ethnic minorities. This study examined a new culturally targeted multi-component intervention to address emotional, attitudinal, and social/structural barriers to ART initiation and HIV care. Participants (N = 95) were African American/Black and Latino adults with CD4<500 cells/mm3 not taking ART, randomized 1:1 to intervention or control arms, the latter receiving treatment as usual. Primary endpoints were adherence, evaluated via ART concentrations in hair samples, and HIV viral load suppression. The intervention was feasible and acceptable. Eight months post-baseline, intervention participants tended to be more likely to evidence “good” (that is, 7 days/week) adherence (60 vs. 26.7 %; p = 0.087; OR = 3.95), and had lower viral load levels than controls (t(22) = 2.29, p = 0.032; OR = 5.20), both large effect sizes. This highly promising intervention merits further study.
Keywords: Antiretroviral therapy, Intervention, HIV/AIDS, Disparities, Motivational interviewing
Introduction
Recent research has produced important advances in the field of HIV/AIDS including treatment as prevention; insights into strategies to seek, test, treat and retain vulnerable populations in care; pre-exposure prophylaxis; new HIV testing algorithms, and improvements in the tolerability and efficacy of antiretroviral therapy (ART) [1–6]. At the same time, however, there is increasing concern about serious gaps in the domestic HIV care continuum [7, 8]. Of the 1.1 million Americans living with HIV, 60 % are not retained in care; 63 % have not been prescribed ART; and only 30 % have undetectable viral loads [9]. Thus overall, approximately 60 % of PLHA are not well engaged in care and not taking ART, mainly African Americans/Blacks and Latinos [9]. In fact, the Centers for Disease Control and Prevention (CDC) has called for improvements in every step along the HIV care continuum, with particular efforts to reduce racial/ethnic disparities [10].
Description of the Multi-Level Factors Driving these Gaps in the HIV Care Continuum
The problems of low uptake of ART and poor engagement in HIV care are related: Those who have declined ART may also avoid HIV care, because they do not wish to discuss or feel pressured to take ART, or because they do not see the need for HIV care if not on ART [11–13], and those not well engaged in HIV care rarely gain access to ART [14]. In fact, the same set of barriers and risk factors foster these two gaps in the HIV care continuum, as described in the next section. The present study conceptualizes barriers to HIV care and ART use through the framework of the Theory of Triadic Influence [15]. This is a multi-level social-cognitive theory focused on three “streams of influence” which act simultaneously to affect health behaviors, namely, individual-, social-, and structural-levels of influence [16, 17]. Moreover, shared cultural and historical experiences among African American/Black and Latino populations, such as past abuses of racial/ethnic minorities by medical research settings, and discrimination and structural racism, may create or foster barriers to health at these levels of influence, such as fear of medications, medical distrust, stigma, and challenges accessing health services, or these types of barriers may resonate more strongly with people of color than Whites as a function of these past and present experiences [18–21]. Thus, identifying culturally relevant barriers to health is critical and creates the foundation for culturally targeted intervention components to reduce health disparities; that is, components focused on group-level characteristics that draw on culturally and socially grounded values, norms, and assets, and which target the most critical barriers to health for these groups [22, 23]. In fact, there is growing awareness targeted intervention components are more effective than more general or nonspecific interventions [24, 25]. In light of the importance of culturally informed approaches to reduce health disparities, the main barriers impeding engagement in HIV primary care and ART uptake for African American/Black and Latino PLHA are described in the following section.
At the individual level of influence, insufficient knowledge, for example about the guidelines regarding frequency of HIV care and the recommended ART initiation thresholds, is a primary barrier to engagement in HIV care and uptake of ART [26–29]. Further, negative health beliefs, including medical distrust, negative expectations about the efficacy of care and/or ART, perceived lack of need for care and/or ART (particularly when one feels healthy), and low levels of perceived “readiness” for ART [28, 30–33] impede care engagement and ART uptake. Negative emotions about care and ART use, primarily fear, for example of being pressured to take ART, side effects, disclosure issues, and the adverse effects of HIV and ART on relationships, are additional serious impediments [34–36]. Moreover, substance use and mental health problems are endemic in this population and interfere with care and ART uptake [37–40]. Finally, substantial proportions of patients lack, or believe they lack, behavioral skills to maintain adherence to ART [41].
Barriers at the social level of influence include prevalent negative social norms regarding health care engagement and taking medications, such as norms that health care systems and medications cannot be trusted, alternative therapies should be tried first, and ART is toxic [5, 11, 12, 42–44]. In addition, high rates of social isolation and low levels of social support impede HIV care and ART use [45, 46], as does HIV stigma or fear of HIV stigma, and challenges managing or avoiding other interdependent co-occurring stigmas related to HIV (e.g., associated with past/ current substance use, sexual minority status, low socioeconomic, racial/ethnic and/or other stigmatized statuses) [47–49].
Structural barriers are aspects of the external environment (economic, social, policy, organizational or other) which limit individuals’ options [50]. At the structural level of influence, barriers to HIV care and ART use encompass challenges negotiating the health care system, including relationships with providers [51], problems with transportation, unstable housing, and poor care access [51]. In particular, challenges accessing services for substance use and mental health concerns impede good HIV health outcomes [12, 13, 28, 52]. Although interventions developed for individual PLHA may not be able to eliminate structural barriers, they can reduce their influence on health outcomes by increasing participants’ options [53].
Barriers at these three levels of influence combine synergistically to reduce PLHA’s motivation, behavioral skills, and access to HIV care and ART. Complicating intervention efforts, barriers at all three levels of influence are commonly rooted in and shaped by poverty [12, 52, 54, 55]. On the other hand, factors facilitating engagement in care and ART initiation operate concurrently with these barriers, and such facilitators can be strengthened by interventions. These include willingness to explore health options despite barriers [12, 56, 57], intrinsic motivation to achieve good health outcomes [58, 59], and social network members who support engagement in HIV care and ART use [60].
Description of the Heart to Heart (HTH) Intervention Evaluated in the Present Study
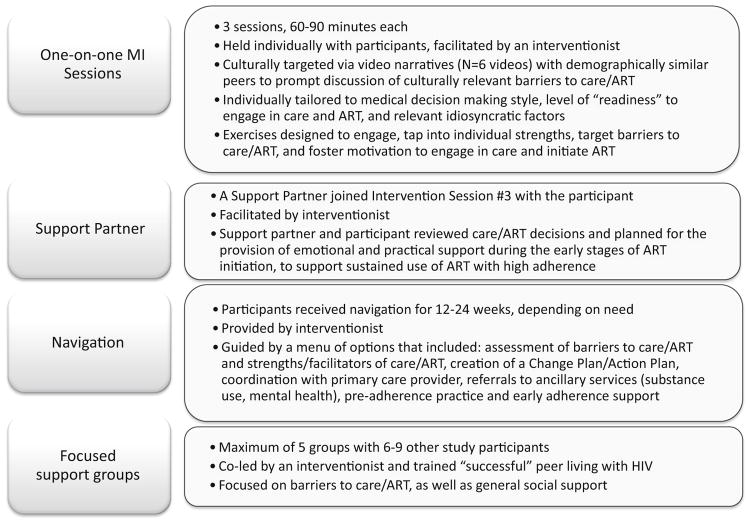
The present study describes a new behavioral intervention designed to address barriers to engagement in HIV care and ART initiation at the three theoretical levels of influence described above, called the “Heart to Heart” (HTH) intervention. Motivational Interviewing (MI) was used as the intervention’s main counseling approach [59]. MI is a flexible, collaborative counseling method that actively engages, focuses, and guides participants in order to elicit and strengthen intrinsic motivation for behavior change [59]. A description of the four main components that made up the HTH intervention—MI sessions, involvement of “support partners,” focused support groups, and patient navigation—is presented in Fig. 1. The HTH intervention’s primary endpoint was ART initiation with high adherence. Further, during the early phases of the study as recruitment procedures were modified, it became apparent participants experienced serious gaps in HIV care engagement, in addition to their not taking ART. In response, poor engagement in HIV care was added as a secondary endpoint and the intervention was modified to also address barriers to HIV care. The cultural targeting of the intervention to African American/Black and Latino PLHA was achieved through brief video narrative segments used during intervention sessions depicting storyline narratives with HIV-infected peers, followed by specific interactive exercises to foster articulation and discussion of culturally grounded barriers to HIV care and ART. These culturally relevant themes were explored further in other intervention components. The HTH intervention was also designed to be flexible and individualized, drawing on individual participants’ strengths as well as highlighting idiosyncratic barriers to HIV care and ART. HTH allowed for individual tailoring of intervention components [61], based on (1) participants’ level of readiness to engage in care and/or initiate ART; (2) medical decision making style [62]; and (3) in response to common but not universal barriers or concerns, such as substance use and mental health problems. With respect to the sequence of activities, MI sessions were conducted before navigation, and support groups were held concurrently with the navigation phase. The intervention was guided by a manual, available from the first author, as is the full protocol.
Fig. 1.
Description of the Heart to Heart intervention components
Aims
The study’s primary aim was to examine the acceptability and feasibility of procedures and the intervention components, and explore evidence of intervention efficacy on the following: (1) biological endpoints assessed with biomarkers, namely biological assessment of ART adherence by an objective measure (hair analysis) and HIV-1 viral load responses and (2) HIV care continuum and behavioral endpoints, namely, rates of ART initiation and ART adherence measured by self-report, and engagement in HIV primary care assessed from the medical record. We also explored whether intervention acceptability, feasibility, and evidence of efficacy differed based on participant characteristics such as sex, race/ethnicity, sexual minority status [men who have sex with men (MSM) vs. non-MSM], and substance use.
Methods
Recruitment
Participants (N = 95) were recruited in 2012–2013 using two methods: direct recruitment in hospital-based HIV clinics and a community-based hybrid approach comprised of targeted sampling in community-based organizations and peer-to-peer recruitment (referred to as the “community-recruited sample” below). As we described elsewhere, the study was designed initially to enroll PLHA in hospital-based HIV clinics who were already engaged HIV care and who were medically eligible for ART but who had delayed, declined, or discontinued ART [40]. Yet we identified relatively few patients in the hospital-based HIV clinics who met inclusion criteria (3.1 %), and therefore the community recruitment approach was added. Overall, 63.2 % (60/95) of participants were community-recruited and 36.8 % (35/95) were recruited in the hospital-based HIV clinics [40]. This was a “real world” sample that included PLHA with heterogeneous socio-demographic and background characteristics. Health care and laboratory data were obtained from the medical record, as described below [40]. All procedures were approved by the Institutional Review Board of New York University and the two collaborating hospital sites, and the study was registered with Clinicaltrials.gov (NCT02086630). The study was not designed as a definitive trial of this new intervention. As such, the sample size was not determined by power analysis to detect a particular effect size. Instead, the sample size was the largest that was feasible in the context of a small study of a novel intervention.
Eligibility Criteria
The eligibility criteria were: age 18 years or older; HIV infected for ≥6 months; African-American/Black or Latino/Hispanic race/ethnicity; last CD4 count ≤500 cells/ mm3; had never taken ART or took ART in the past but on fewer than 60 days in the past 6 months and not at all in the past 30 days (to recruit those who stopped ART, but to exclude those taking ART with inconsistent adherence); able to conduct research activities in English; medically eligible for ART for ≥3 months; active patient at the HIV clinic, that is, had seen a provider at the clinic at least once in the past year (clinic-recruited cohort only); not having any condition that in the opinion of the primary care provider would interfere with provision of informed consent or make it unsafe to participate in this study.
Procedures and Study Design
Screening for Eligibility and Baseline Medical Report Form
To foster participant involvement and facilitate accurate self-report of ART status, and to determine preliminary eligibility, the initial screening visit was comprised of a structured assessment and interactive exercises to orient and engage participants into the study. Participants were compensated for the screening visit ($20). Medical (CD4 cell counts, HIV viral load) and past-year health care appointment attendance information were obtained from the medical record using a Medical Report Form (MRF). For the community-recruited participants, MRFs were obtained from the health care provider either directly by the study, with participant signed consent, or obtained from the provider by the participant him/herself, or by staff at the participating HIV clinics for that subgroup. Participants in the community-recruited cohort who obtained MRFs from providers received compensation ($25). MRFs included the signature and license number of the health care provider to confirm veracity of medical information. Study staff used active outreach methods to obtain MRFs in a timely fashion. In keeping with the exploratory nature of the study with this under-studied population, participants who reported not being engaged in HIV health care in the past year and therefore without a baseline (BL) MRF at the time of screening were permitted to remain in the study. These participants were referred to HIV care. In some cases, the BL MRF was obtained several months after the participant was enrolled and in others it was not possible to obtain the MRF. MRF data were entered into an electronic data file and checked for accuracy.
BL Assessment
Participants who met eligibility criteria provided signed informed consent to be enrolled in the study, and participated in a structured BL interview with reliable and valid instruments on HIV knowledge and attitudes, HIV history, mental health, substance use, and health care use patterns, lasting approximately 1 h. The assessment was conducted in computer-assisted personal interviewing (CAPI) and audio, computer-assisted self-interviewing (ACASI) formats, and was focused both on the past 6 months and over the lifespan. Assessments took place at the HIV clinics and a project field site. Participants received $20 for their time and fare for local public transportation.
Randomization, Intervention, and Follow-up
Participants were randomized by the study’s principal statistician at a 1:1 ratio to an intervention or control arm in permuted blocks [63] varying in size from two to six allocations and with stratification by race/ethnicity (African-American vs. Latino) and clinic. Field staff reached the principal statistician by telephone or email when an assignment was needed, and assignments were stored in a password-protected file only available to the principal statistician.
Participants in the control arm received treatment as usual. Treatment as usual in the hospital-based HIV clinics included high quality comprehensive health, mental health, social (e.g. case management, peer support), and ancillary services (e.g., yoga, dental care, acupuncture), including the opportunity to receive intensive support for ART adherence (a program called “Care Coordination”). Almost a third of those in community-recruited cohort were treated in hospital-based HIV clinics [40]. Treatment as usual among the remaining community-recruited participants varied, with some participants having no access to HIV care or any other kind of services (approximately another third of participants), and the remainder receiving social services in community-based organizations, but not regular HIV primary care, or comprehensive HIV care and services in community-based settings. Thus overall, participants had or potentially had access to a high quality of HIV care and supportive services, but typically declined ART-related services, for the reasons described in the Introduction. The HTH intervention was carried out by trained, experienced Master’s level clinicians. Intervention activities were conducted at the hospital sites and field site. Staff and participants were not blind to intervention arm assignment. Follow-up (FU) assessments, comprised of a structured assessment battery using the same measures as the BL assessment, were conducted at Time 2 (T2; 3 months post-intervention, focused mainly on the past 3 months) and Time 3 (T3; 9 months post-intervention, focused mainly on the past 6 months). FU assessments were conducted mainly at the project field site. Participants received $30 for each FU assessment. At the T3 assessment a second MRF was obtained to assess the dates of health care appointments attended, CD4 cell counts, and HIV viral loads for up to 1 year after the baseline interview (an interval that included both the intervention and FU period).
Hair Analysis
Hair concentrations of antiretroviral (ARV) medications serve as an objective biomarker of adherence and exposure. The present study assessed ARV concentrations in hair samples among those who had initiated and were taking ART for at least 6 weeks at the T3 FU visit. Study collaborating partners at the University of California, San Francisco (UCSF) have developed methods to analyze lopinavir (LPV), ritonavir (RTV), atazanavir (ATV), nevirapine (NVP), efavirenz (EFV), darunavir (DRV), raltegravir (RAL), tenofovir (TFV) and emtricitabine (FTC) in small samples of extracted human hair using sensitive methods employing liquid chromatography/tandem mass spectrometry (LC/MS/MS) [64–67]. These hair assays have been validated with good linearity (R2 ≥0.99) and reproducibility [coefficient of variation (CV) <15 %] [66, 67]. Hair collection is noninvasive and does not require specific skills, sterile equipment, or specialized storage conditions, and high rates of acceptability and feasibility of collecting hair samples for hair ART monitoring have been found in rural African settings, and domestically in the Women’s Interagency HIV study [65, 68, 69]. Hair levels of ART have been found to be stronger predictors of treatment outcomes than self-reported adherence [65, 69] or single plasma ART concentrations [69], and there is a strong correlation between administered TFV dose and concentrations of TFV in hair [64]. In the present study, among those reporting taking ART at T3, we first assessed the specific ART regimen from pill bottles or prescriptions. Next, 100 strands of hair were collected, packaged, and shipped to the UCSF HIV Pharmacology laboratory for analyses. In the present study, all participants happened to be taking a TFV-based regimen, so the hair samples were all screened for TFV concentrations (40 % were on a regimen that included Truvada, 14 % were on Atrilpla, 11 % on Complera, 9 % on Stribild, all of which include TFV, and the remainder on another regimen that included TFV).
Study Variables
Assessment Battery
Socio-demographic characteristics were assessed with a structured measure [70]. We assessed HIV history, including year of HIV diagnosis, time between diagnosis and first engagement in health care, and ART use, with a measure from the HIV Cost and Services Utilization Study (HCSUS) [71]. We assessed whether participants had an established HIV primary care provider at baseline, coded as yes/no based on the successful completion of the BL MRF by the provider; some participants reported no engagement in HIV care, could not name a provider, or in some cases the provider identified by the participant was not familiar with the patient or did not complete the form despite staff reminders, often because of lack of familiarity with the patient. Symptoms of depression were assessed with the Center for Epidemiologic Studies—Depression Scale (CES-D8), and those with a score of 7 or higher were coded as having depressive symptoms at a clinically significant level [72]. Alcohol and drug use were assessed with the Risk Factors Questionnaire [73], which assessed the frequency of cigarettes, and eight different substances over the lifetime and past 6 months, as well as injection drug use. Substance use data were coded to indicate whether participants had, over the past 6 months, used drugs, smoked cigarettes daily, used alcohol use four or more times a week, and used drugs daily. We assessed whether they had injected drugs ever and if so, in the past 6 months.
HTH Intervention
Acceptability
Acceptability of the HTH intervention was evaluated with a 7-item scale on participant satisfaction with aspects of the intervention, assessed on a Likert-type scale (Cronbach’s α = 0.74) [74].
Feasibility
Feasibility of study procedures and intervention components were assessed via rates of: participation in intervention activities; hair samples obtained (which were collected if the participant was taking ART in at least the 6 weeks before the T3 assessment); completion of MRFs at BL and T3; and retention to FU assessments. To capture the fact that some participants did not engage in HIV care in the study period, we coded the BL and T3 MRF as “completed or resolved” if the MRF was either returned to the study, or the participant could not identify an HIV care facility or provider, or the participant did provide clinic contact information, but the clinic reported the client was not considered a patient or did not have relevant information. MRFs were incomplete/unresolved if the participant could not be contacted (relevant to T3 only) or the MRF was provided to the clinic but the clinic or provider did not respond with the information.
Main Study Endpoints
Biological Endpoints
ART Adherence by an Objective Measure (Hair Analysis)
Hair samples were used to assess adherence via an objective measure over the past 6 weeks using methods developed by Gandhi and colleagues [65]. Levels of ART in hair reflect uptake from the systemic circulation over weeks to months [75]. The laboratory provided the normalized concentration (by weight) of the core drug tenofovir present in each hair sample. Tenofovir concentrations were provided as quantitative variables (range 0.008–0.053 ng/mg) and then coded based on pre-determined cut-off values as a categorical variable (7 days a week adherence, yes/no) [64]. These cut-off values were extrapolated from a study that examined hair concentrations of tenofovir in HIV-negative individuals dosed with tenofovir at a frequency of 2 days a week, 4 days a week, and then 7 days a week [64]. For example, adherence of 2 days per week is correlated with a tenofovir concentration in hair of 0.008–0.021 ng/mg.
HIV-1 Viral Load Levels
HIV-1 viral load levels were obtained with the MRF at BL and T3. A base ten logarithmic transformation was applied to raw HIV-1 RNA levels prior to summary and analysis. To account for variability on the timing of T3 MRFs, viral load at T3 was operationalized as the most recent value conducted at least 90 days after the BL interview (to allow for initiation of ART for at least 6 weeks). On average, the most recent T3 viral load, which was used in analysis, was 239 days (SD = 64 days) or 7.9 months after the BL interview, or 117 days (approximately 4 months) post-intervention.
HIV Care Continuum and Behavioral Endpoints
ART Initiation
ART initiation (yes/no) was assessed by self-report at T2 and T3, as was ART continuation, that is, whether the participant sustained ART over the FU periods (yes/no).
ART Adherence
ART adherence by self-report focused on the past month at T2 and T3, using the CASE adherence index [76], a simple composite measure of self-reported ART adherence that correlates strongly with 3-day adherence reports and HIV outcomes. The index assesses self-reported frequency of difficulty taking HIV medications on time, average number of days per week at least one dose of HIV medications was missed, and last time missed at least one dose of HIV medications. Items are scored such that higher values indicate better adherence, and the maximum total score is 16. Scores of 11 or higher on this index indicate good adherence (Cronbach’s α = 0.79).
Engagement in HIV Primary Care at T3
There is no gold standard for the assessment of engagement of care [77]. Based on national guidelines, we assessed whether the participant attended at least one HIV primary care visit in the previous 6 months from the T3 MRF [78].
Data Analysis
Fisher’s exact test was used to compare intervention and control conditions on all categorical outcomes. Independent-samples t tests, without assuming equality of variance, were used to compare intervention and control conditions on continuous measures for which no baseline assessment was available, or, in the case of viral loads, when there was substantial missing data on the BL MRF (N = 40 participants were missing viral load results in the year before baseline), because forms could not be completed or results were unavailable. Analysis of covariance was used to compare intervention and control conditions on continuous measures, with baseline included as a covariate. Interval estimates of effect size were calculated to convey effect magnitude and uncertainty. To put all effect sizes in the same metric, interval estimates of standardized mean differences were converted to odds ratios using the formula in Chinn [79]. The R statistical computing environment was used for all analyses [80].
Results
Participants
As presented in Table 1, participants at enrollment were 48.0 years old (SD = 8.88 years), and had lived with HIV for 14.7 years (SD = 8.73 years), on average, and 56.8 % had taken ART previously. Most were African-American/ Black (76.8 %), and 23.2 % were Latino/Hispanic. Almost two thirds (61.1 %) were male, and more than half of males identified as gay or bisexual (56.9 %). All (99 %) were from low socioeconomic status backgrounds (operationalized as being eligible for food stamps and/or government medical benefits such as Medicaid, and/or having been unable to pay for necessities in the past 6 months). About two-thirds rated their health as “good” or better (67.4 %). Participants’ average CD4 count in the year before baseline was 313.21 cells/mm3 (SD = 156.20 cells/mm3) and average log10 viral load in the year before baseline was 3.35 (SD = 1.41). Most (65.3 %) had an established health care provider in the past year and therefore, a BL MRF could be obtained, but a full 34.7 % had not received HIV primary care in the past year or longer. Substance use was widespread (53.7 % had used drugs in the past 6 months), with 22.1 % using daily, and 28.4 % having attended substance use treatment in the past 6 months. Further, depression at a clinically significant level (42.6 %) was common. There were no statistically significant differences at BL between those randomly assigned to intervention and control arms on demographic, background, or health variables.
Table 1.
Socio-demographic and Health Characteristics at Baseline, by Intervention Arm (M [SD] or %)
| Intervention arm (N = 47) | Control arm (N = 48) | Total (N = 95) | t(df) | p | |
|---|---|---|---|---|---|
| Age in years | 47.37 (8.83) | 48.66 (8.97) | 48.02 (8.88) | 0.71 (93) | 0.481 |
| Male sex | 53.19 | 68.75 | 61.05 | – | 0.144 |
| If male, identify as gay or bisexual | 56.0 | 57.6 | 56.9 | – | 1.000 |
| If female, identify as lesbian, gay, or bisexual | 9.1 | 13.3 | 10.81 | – | 1.000 |
| African-American/Black, Not Hispanic | 78.72 | 75.00 | 76.84 | – | 0.809 |
| Latino/Hispanic | 21.28 | 25.00 | 23.16 | – | 0.809 |
| Transgender | 6.38 | 4.17 | 5.26 | – | 0.677 |
| High school graduate or equivalent | 72.34 | 60.42 | 66.32 | – | 0.279 |
| Low socioeconomic status | 97.87 | 100.00 | 98.95 | – | 0.495 |
| Currently employed | 14.89 | 18.75 | 16.84 | – | 0.785 |
| Years since HIV diagnosis | 12.91 (8.78) | 16.35 (8.42) | 14.65 (8.73) | 1.95 (93) | 0.055 |
| Ever taken ART in the past | 48.94 | 64.58 | 56.84 | – | 0.149 |
| Number of times started/stopped ART | 2.65 (2.16) | 6.42 (8.83) | 4.70 (6.88) | 2.02 (26) | 0.054 |
| Health self-rating “good” or better | 65.96 | 68.75 | 67.37 | – | 0.829 |
| ≥6 months between initial HIV diagnosis and first HIV care appointment | 36.17 | 43.75 | 40.00 | – | 0.532 |
| Had an established HIV care provider over the past year (MRF) | 70.21 | 60.42 | 65.26 | – | 0.390 |
| Receives care in hospital-based HIV clinic | 61.7 | 52.08 | 56.84 | – | 0.409 |
| Satisfaction with health care (0–3) | 1.86 (0.74) | 2.01 (0.72) | 1.94 (0.73) | 0.94 (85) | 0.351 |
| Average CD4 in the year before baseline (MRF) | 308.28 (168.62) | 318.61 (143.92) | 313.21 (156.20) | 0.27 (63) | 0.791 |
| Average log10 viral load in the year before baseline (MRF) | 3.26 (1.40) | 3.44 (1.45) | 3.35 (1.41) | 0.47 (52) | 0.642 |
| Health care provider recommended ART (lifetime) | 100.00 | 97.92 | 98.95 | – | 1.000 |
| Number of serious co-morbid health conditions (0–12) | 2.79 (2.28) | 2.27 (1.76) | 2.53 (2.04) | 1.23 (86) | 0.221 |
| Depression screener at a clinically significant level (CES-D8) | 43.48 | 41.67 | 42.55 | – | 1.000 |
| Substance use | |||||
| Smoked cigarettes daily past 6 months | 57.45 | 54.17 | 55.79 | – | 0.837 |
| Any drug use past 6 months | 53.19 | 54.17 | 53.68 | – | 1.000 |
| Daily drug use past 6 months | 19.15 | 25.0 | 22.11 | – | 0.622 |
| Alcohol use 4+ times a week past 6 months | 6.38 | 8.33 | 7.37 | – | 1.000 |
| Substance use treatment past 6 months | 20.00 | 33.33 | 28.42 | – | 0.115 |
| Ever injected drugs (lifetime) | 29.79 | 29.17 | 29.47 | – | 1.000 |
| Injected drugs in the past 6 months | 6.38 | 4.17 | 5.26 | – | 0.677 |
| Enrolled in MMTP past 6 months | 8.57 | 15.00 | 12.63 | – | 0.070 |
None of the differences between intervention and control participants were statistically significant
Acceptability
Intervention acceptability was high: at T2, the assessment proximal to the intervention, participants who attended intervention activities and completed the T2 assessment (N = 41/47; 87 %) found activities to be “very good” or excellent (85.29 %), the information they received was “helpful” or “very helpful” (100 %), their questions were answered “most” or “all of the time” (97.1 %), the project staff treated them as an individual with unique needs and concerns “most” or “all of the time” (100 %), privacy was respected “most” or “all of the time” (100 %), staff were seen to understand the needs of people of the participant’s racial, ethnic or cultural group “most” or “all of the time” (97.1 %), and, if the participant was female, staff were reported to have understood the needs of women most or all of the time (100 %). There were no adverse or unintended effects reported during the intervention or FU assessments.
Feasibility of Procedures and Intervention Components
Feasibility of Recruitment
As we described elsewhere, the hybrid community recruitment approach using targeted sampling and peer-to-peer recruitment was substantially more feasible than recruitment through clinics [40]. This appears to be in part because PLHA with serious barriers to ART do not stay engaged with hospital-based HIV clinics. Instead, they can be found in various community-based and AIDS service locations in community settings, including substance use treatment settings, and are also positioned in larger social networks with other PLHA who can then recruit them for participation in research studies.
Feasibility of MRFs
As presented in Table 2, at BL, MRFs were completed for 65.3 % of study participants. At BL, MRF data were returned on all but one clinic-recruited participant, but obtaining MRF data on the community-recruited cohort was challenging. Regarding the T3 MRF, 87.4 % (83/95) were completed or resolved, and MRF data were received on 68.4 % (65/95) of study participants. Overall, obtaining HIV care and medical information from health care providers or settings using an MRF was feasible, but labor intensive for both the study and providers, and rates of missing data were unacceptably high.
Table 2.
Feasibility of study procedures and intervention components
| % | N | |
|---|---|---|
| BL MRF completed | 65.26 | 62/95 |
| Engaged in ≥1 intervention activity | 93.6 | 44/47 |
| Intervention sessions attended | ||
| Attended ≥1 session | 93.62 | 44/47 |
| No sessions | 6.38 | 3/47 |
| One session | 2.13 | 1/47 |
| Two sessions | 74.47 | 35/47 |
| Three sessions | 17.02 | 8/47 |
| Enrolled a support partner into the intervention | 23.40 | 11/47 |
| Attended ≥1 support group (among those eligible) | 58.97 | 23/39 |
| Engaged in ≥1 navigation activity | 85.1 | 40/47 |
| T2 FU assessment complete | 89.5 | 85/95 |
| T3 FU assessment complete | 80.0 | 76/95 |
| Hair sample collected (if taking ART in 6 wks before T3) | 79.5 | 35/44 |
| T3 MRF completed/resolved | 87.37 | 83/95 |
| T3 MRF completed with data | 68.42 | 65/95 |
Feasibility of Intervention Components
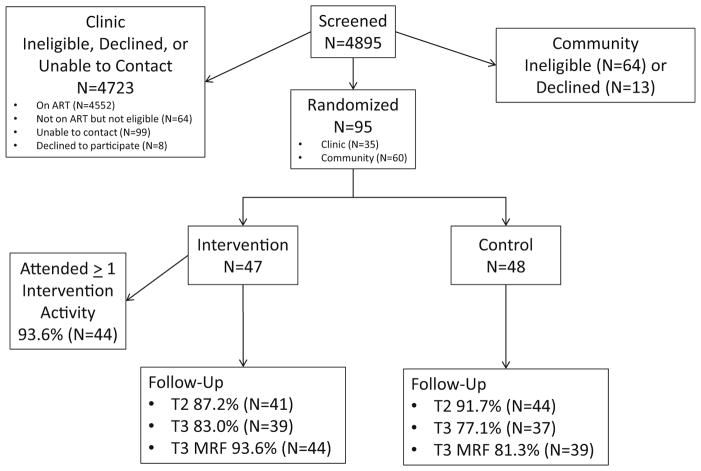
The majority of intervention components were feasible, as shown in Table 2. Almost all participants (93.6 %) engaged in intervention activities, including MI intervention sessions (93.6 %), navigation (85.1 % participated; M contacts 5.53 [SD = 4.04 contacts], min. = 0, max. = 14 contacts), and support groups (59.0 %). Support groups were feasible, but attendance was reduced somewhat because of scheduling issues. The support partner component was not feasible, reducing attendance at MI session 3; pre-adherence issues were instead addressed in navigation. (See CONSORT diagram, Fig. 2, which presents retention by intervention arm. There were no differences in retention between intervention arms.)
Fig. 2.
HTH consort diagram
Feasibility of FU Assessments and Hair Samples
Most participants completed the T2 (89.5 %) and T3 (80.0 %) FU assessments. Most participants who presented for the T3 assessment and were taking ART for at least 6 weeks had sufficient hair for a hair sample: N = 35/44 or 79.5 % of those who were taking ART at T3. All of those with sufficient hair who were asked to provide a sample did so. Thus, retention was high and hair sample collection was feasible and acceptable.
Evidence of Efficacy
Table 3 shows estimates of the impact of the HTH intervention on biological and behavioral endpoints. As noted above, this exploratory study was not powered for definitive null hypothesis significance testing, but as the first test of this new intervention, was designed to explore evidence of efficacy. Findings that nonetheless reached (p < 0.05) or approached (p < 0.10) a statistically significant level are highlighted.
Table 3.
Evidence of intervention efficacy
| Intervention
|
Control
|
Total
|
t(df) | p | Odds ratio | Odds ratio 95 % CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean or % | SD | Mean or % | SD | Mean or % | SD | |||||
| Biomarker endpoints | ||||||||||
| ART adherence—hair analysis (for those who continued ART at T3; N = 35) | ||||||||||
| Tenofovir (TFV) concentration | 0.05 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 1.35 (33) | 0.188 | 2.30 | 0.67–7.80 |
| “Good” (7 days/weeks) adherence assessed by hair samples | 60.00 | 26.67 | 45.71 | – | 0.087 | 3.59 | 0.80–23.54 | |||
| Conservative analysis of “good” (7 days/weeks) adherence assessed by hair samples (n = 23) | 66.67 | 36.36 | 52.17 | – | 0.220 | 3.30 | 0.48–27.28 | |||
| Most recent log10 HIV viral load at T3 (MRF) | ||||||||||
| All participants (n = 56) | 2.35 | 1.43 | 2.75 | 1.55 | 2.53 | 1.49 | 0.98 (50) | 0.331 | 1.61 | 0.62–4.19 |
| Participants who initiated ART during follow-up (n = 44) | 1.63 | 0.67 | 2.51 | 1.55 | 2.05 | 1.24 | 2.39 (27) | 0.024 | 3.70 | 1.21–11.10 |
| Conservative analysis of participants who initiated ART during follow-up (n = 26) | 1.78 | 0.84 | 2.92 | 1.66 | 2.44 | 1.47 | 2.29 (22) | 0.032 | 5.20 | 1.16–22.55 |
| Most recent HIV viral load was undetectable at T3 (MRF) | ||||||||||
| All participants (n = 56) | 54.84 | 40.00 | 48.21 | – | 0.296 | 1.80 | 0.55–6.08 | |||
| Participants who initiated ART during follow-up (n = 44) | 73.91 | 47.62 | 61.36 | – | 0.121 | 3.03 | 0.75–13.50 | |||
| Conservative analysis of participants who initiated ART during follow-up (n = 26) | 63.64 | 33.33 | 46.15 | – | 0.233 | 3.32 | 0.53–24.41 | |||
| HIV care continuum and behavioral endpoints | ||||||||||
| ART initiation (T2, T3) | ||||||||||
| Provider recommended ART | 88.10 | 88.10 | 88.10 | – | 1.000 | 1.00 | 0.21–4.74 | |||
| Provider wrote prescription | 66.67 | 69.77 | 68.24 | – | 0.818 | 0.87 | 0.31–2.39 | |||
| Initiated ART in study period | 57.45 | 58.33 | 57.89 | – | 1.000 | 0.96 | 0.39–2.36 | |||
| Continued to take ART, for those who initiated | 96.43 | 86.67 | 91.38 | – | 0.354 | 4.06 | 0.37–211.75 | |||
| ART adherence—self-report (for those who continued ART) | N = 27 | N = 26 | N = 53 | |||||||
| Case Adherence Index Score (3–16) | 12.11 | 3.29 | 11.92 | 2.95 | 12.02 | 3.10 | 0.22 (51) | 0.827 | 1.12 | 0.41–3.02 |
| Case Adherence Index Classification “Good Adherence” | 62.96 | 61.54 | 62.26 | – | 1.000 | 1.06 | 0.30–3.72 | |||
| Engagement in HIV primary care past 6 months (T3) | ||||||||||
| Appointment attended in the 6 month period before T3 (MRF) | 59.57 | 47.92 | 53.68 | – | 0.306 | 1.59 | 0.66–3.91 | |||
Biological Endpoints
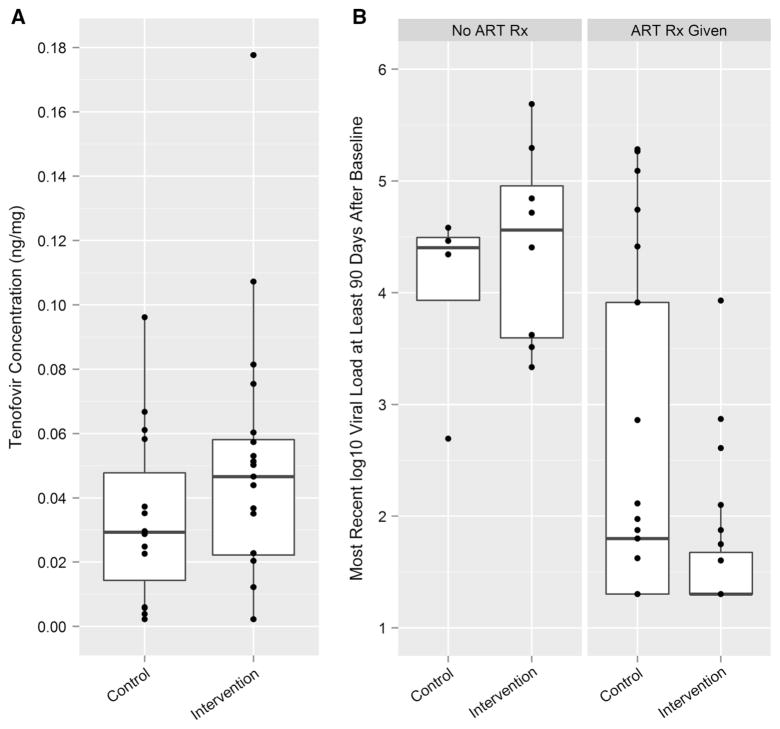
As noted above, we used pre-determined empirically based cut-off values for TFV concentrations to code the continuous, quantitative data as a categorical variable, namely, ART use 7 days a week, on average (yes/no), referred to as “good adherence.” Biomarker-based “good adherence” was more likely among intervention than control participants, a difference approaching statistical significance, with a large effect size (60 vs. 26.7 %; p = 0.087; OR = 3.95). The most recent HIV viral loads assessed at T3 were substantially lower among intervention participants when compared with controls. This was true for all participants who had at least one viral load result more than 90 days after the BL interview (N = 56), and even more so for the subset of participants who initiated ART during the follow-up period (N = 44), with a large effect size [M = 1.63 (SD = 0.67) vs. M = 2.51 (SD = 1.55); t(27) = 2.39; p = 0.024; OR = 3.70]. In Fig. 3, we depict TFV concentration levels in hair in the two arms and HIV viral load levels among those who did and did not get an ART prescription.
Fig. 3.
Intervention impact on tenofovir (TFV) concentration in hair samples and on viral load. a Tenofovir concentration in hair by treatment condition. b Viral load by ART prescription and treatment condition
HIV Care Continuum and Behavioral Endpoints
As shown in Table 3, provider recommendations to start ART and filling of ART prescriptions were both equally likely in the two study arms. Intervention participants were about as likely to receive a prescription from the provider and to initiate ART as controls (57.5 vs. 58.3 %). The timing of ART initiation was similar across arms as well, with median days on ART 139 among intervention participants and 182 among controls (t(51) = 0.63, p = 0.530; not shown on Table 3). Among those who initiated ART, the majority continued to take ART through the FU period (intervention arm 96.4 %, control arm 86.7 %). Among those continuing to take ART at FU interviews, self-reported adherence was similar (62.3 % reporting good adherence) in both arms. About half of participants engaged in at least one HIV care visit (intervention arm 59.6 %, control arm 47.9 %).
We explored whether there were differences in intervention acceptability, feasibility or evidence of efficacy by sex, race/ethnicity, sexual minority status, previous ART experience, recruitment in a clinic (vs. community) and substance use but found no differences.
Sub-analysis Excluding Those with Low HIV Viral Load at BL, that is, the “Conservative Analysis”
As noted above, some MRFs were (unexpectedly) received after a participant had begun intervention activities. In keeping with the exploratory nature of the study, participants were not excluded at that time. In 21 cases (14 intervention arm, 7 control), MRF data indicated HIV viral load levels ≤50 copies/mL within 90 days before or 50 days after the BL interview, indicating these individuals may have entered the study with very low or undetectable viral loads, suggesting the possibility of ART use at the time of enrollment despite self-reports to the contrary. To account for this possibility, we re-conducted the analysis of the viral load endpoint after excluding 18 of these participants (12 intervention, 6 control), leaving a total of N = 26 who initiated ART during the follow-up period. Results from this conservative analysis (shown on Table 3) were consistent with those for the larger group of ART initiators: participants in the intervention arm who had initiated ART evidenced lower log10 HIV viral load levels than controls, with a large effect size [M = 1.78 (SD = 0.84) vs. M = 2.92 (SD = 1.66); t(22) = 2.29; p = 0.032; OR = 5.20]. Because it is possible some of these participants excluded in the conservative analysis were actually not on ART at BL, or stopped and re-initiated ART during the study period, we discuss the main analyses, but not the conservative analyses, below.
Discussion
In this paper we explored a novel behavioral intervention targeted to a population at high risk for poor health outcomes: PLHA from African American/Black and Latino racial/ethnic backgrounds who were not taking ART, nor typically well engaged in HIV care, recruited mainly though peers. We found the multi-component HTH intervention designed to ameliorate barriers to ART initiation and engagement in HIV care was feasible, highly acceptable, and showed evidence of efficacy on biological endpoints, including the critical endpoint of reduction in HIV viral load levels. While past studies have evaluated interventions to improve readiness for adherence [81], ART adherence levels [82–84], and engagement in HIV primary care [85], this is the first behavioral intervention designed for PLHA who have delayed, declined, or discontinued ART, many of whom also experience serious barriers to HIV care [13].
Improvements in ART Adherence and HIV Viral Load Suppression Assessed with Biomarkers
Participants in the intervention arm were more than three times more likely to evidence good adherence (that is, taking ART 7 days a week) than controls (60 vs. 27 %), assessed via ART concentrations in hair samples, at a level approaching statistical significance. Moreover, those in the intervention arm evidenced significantly lower HIV viral load levels than controls at follow up (a difference of 0.88 log10 HIV viral load), at a statistically significant level and a large effect size, based on medical record data. Studies of adherence often rely on self-reported adherence measures to assess intervention efficacy. However, recall bias, forgetfulness, or social desirability bias can limit the utility of such self-reports [86, 87]. The present study found support for the feasibility hair sample collection and analysis to assess levels of ART adherence, and the use of objective biomarkers to evaluate these crucial intervention endpoints were strengths of the study.
HTH May Signal New Types of Approaches to the Problem of Adherence
Adherence has been called the “Achilles’ heel” of HIV treatment [7]. Past successful adherence interventions have focused on those ready to initiate or those who have already initiated ART, and used cognitive-behavioral, strengths based, skill building, and/or motivational enhancement approaches to help patients overcome barriers to adherence, boost adherence rates, sustain adherence, and improve clinical outcomes [81–83, 88–90]. While many of these programs have been efficacious or effective, they have typically resulted in small to moderate effects which diminished upon the intervention’s cessation [91]. The HTH intervention was a culturally targeted pre-adherence program to address emotional, attitudinal, and social barriers to the initiation of ART and HIV care engagement, and to reduce structural barriers to these health outcomes, targeting the barriers most relevant to African American/ Black and Latino PLHA. Thus HTH was designed to intervene prior to patients’ decisions about whether to initiate ART, and to successfully engage those with past negative experiences with ART, with fears and mistrust of ART, and individuals who were fairly certain they would never initiate ART. Indeed, fear appears to play a primary role in the avoidance of HIV care and ART use [11, 12, 36], particularly among vulnerable and marginalized populations [92]. Fear, a basic human emotion [93], is triggered by the perception of threat and activates the desire for avoidance of the hazard [94, 95]. Thus fear, as well as anxiety, a related emotion, may prompt PLHA to avoid care, ART use, and also to decline behavioral interventions to improve HIV care continuum outcomes [96]. This relationship between fear of ART, specifically fear of toxic side effects, stigma, and disclosure of HIV status, and avoidance of HIV care, has been found in a recent review of international studies [97], and was also highlighted in a recent article by Mayer [5]. In fact, Mayer noted healthcare providers generally underestimate the impact of emotional, rather than circumstantial, barriers that prevent people from seeking testing, care, and treatment for HIV infection. The present study highlights the potential utility of addressing the emotional underpinnings of ART initiation.
The Intervention’s Possible Mechanisms of Action
Participants in the intervention arm evidenced superior HIV viral load outcomes even a number of months after the intervention concluded. These sustained improvements were interpreted mainly in light of the goals of the MI counseling approach, and in the context of the short-lived effects of most adherence interventions noted above. As described in the Introduction, MI is a method for cultivating durable intrinsic motivation for behavior change. The accepted theoretical underpinning of MI is Self Determination Theory, which describes the optimal conditions for fostering the most volitional and high quality forms of motivation among clients or research participants, namely, by providing experiences of autonomy, competence, and relatedness [17, 98, 99]. The HTH intervention approach was consistent with these principles of Self Determination Theory, and we speculate that building high quality intrinsic motivation for behavior change may have been one major driver of intervention efficacy. On the other hand, it is possible enhanced intrinsic motivation was not the main mechanism of action. For example, intervention components may have influenced a primarily cognitive decision-making pathway. Alternately, the amelioration of competing priorities (substance use and mental health problems) and/or reduction of structural barriers may have been pivotal, although these reductions did not reach statistical significance in the present study. As we discuss below, modifications to the HTH intervention are needed for the next phase of this research program, and future studies with a longer follow up period and a study of intervention mediators may shed light on whether the HTH approach has enduring effects, and on its specific mechanisms of action.
Understanding High Rates of ART Initiation
The substantial rates of ART initiation in both treatment arms may highlight the response of HIV care settings to the latest national HIV treatment guidelines. Since 2012, the Department of Health and Human Services (DHHS) has recommended all PLHA initiate ART regardless of CD4 counts, although the strength of the recommendation is strongest for those with CD4 counts less than 500 cells/ mm3 [100]. This recommendation has been gaining traction in health departments and clinical settings [100, 101] since then. The high ART initiation rates may also highlight the success of HIV clinics to build readiness for ART, even with their most vulnerable patients. On the other hand, we found PLHA with serious barriers to ART tend not to engage with hospital-based HIV clinics, underscoring the need for outreach and other creative approaches [40].
Evidence of Efficacy on HIV Care Attendance and Lessons Learned
At the time they entered the study, only 65 % of participants, mainly those recruited from clinics, evidenced engagement in HIV primary care in the prior year, a rate substantially lower than recommended by the DHHS guidelines [100], but consistent with studies of the HIV continuum of care [8, 14]. The HTH intervention did not produce significant improvements in HIV care engagement, and only half (53.7 %) had attended an appointment in the previous 6 months at the follow up. Because rates of engagement in care over the previous 6 months were roughly comparable to rates of ART initiation, it is possible participants who did not wish to initiate ART were not motivated to attend, or actively avoided, HIV care in that period. These findings also suggest PLHA may initiate ART with high adherence despite less than optimal engagement in care, although this is not ideal for patients, given the many benefits to HIV primary care independent of and in conjunction with ART [37].
Implications for Intervention Components to Improve Retention in Care
Recent reviews of interventions to improve engagement in HIV care have noted the importance of strategies that address individual-, interpersonal-, environmental-, and structural-level barriers [85, 102–104]. As we have noted, the HTH intervention also took such a multi-level approach. The HTH intervention model conceptualized engagement in HIV primary care and ART initiation as separate but closely related health care decisions, and emphasized the importance of HIV care, even for those not ready to initiate ART. On the other hand, the intervention addressed these two decision pathways within the same intervention components. We interpret these findings in light of research on medical decision making which suggests decisions to initiate treatment are fundamentally different from those on whether to obtain preventive care [105–108]. Thus HTH intervention components to improve regular engagement in HIV care can be enhanced by disentangling HIV care decisions from ART decisions, building on this literature on medical decision making, and increasing the duration and intensity of the focus on HIV care, drawing on past efficacious approaches [79].
Acceptability, Feasibility, and Evidence of Efficacy with Diverse Subgroups of PLHA
This was a diverse, “real world” sample of PLHA. The intervention was acceptable, feasible, and showed evidence of efficacy for a number of different socio-demographic subgroups, including men and women, African Americans/ Black and Latinos, substance users, and sexual minorities (that is, those who identified as gay or bisexual), providing support for the overall approach to both culturally target and individually tailor intervention components. These promising findings for diverse subgroups are critical given the population of PLHA’s substantial heterogeneity. In particular, African Americans/Blacks experience the most severe burden of HIV, followed by Latinos; gay, bisexual, and other MSM, particularly those from African American/ Black racial backgrounds, have some of the highest HIV incidence rates, but heterosexual women and men make up a substantial minority of those at risk for HIV infection; and substance use problems among PLHA are endemic, chronic, and recurring [109]. However, more exploration of effect moderators is warranted given the pilot study’s small sample size.
Study Limitations
Because this is a largely exploratory study with a modest sample size, estimates of efficacy are imprecise and some differences between study conditions are not statistically significant. However, interval estimates of effect sizes indicate the HTH intervention overall was promising, particularly in its impact on the important outcomes of biological measures of ART adherence and HIV viral suppression. Data on engagement in HIV care lacked detail, and the study may have under-estimated engagement in HIV care by focusing on HIV primary care, when participants may have received HIV care from other types of providers and/or in emergency departments [110, 111]. Indeed, Mugavero and colleagues have noted the “fractured” nature of the health care delivery system, and called for integrated systems that provide feedback about patients system-wide as they move back and forth along the HIV care continuum [51].
Generalizability
Because the sample of African American/Black and Latino PLHA in the present study was diverse with respect to background and socio-demographic characteristics, we speculate findings will generalize to similar populations in urban areas in the US. However, the low proportion of clinic-recruited PLHA suggests those enrolled may not be representative of the larger population of PLHA retained in HIV clinics but not taking ART. Also, because structural barriers to engagement in HIV care and ART vary somewhat across areas of the US, our community-recruited participants may not be representative of PLHA in other locations across the US.
Implications
There is an urgent need for interventions to improve outcomes along the HIV continuum of care for the nation’s most vulnerable PLHA. The present study sheds light on strategies to seek out and engage these populations, who are largely hidden and wary of health care settings. Further it identifies a number of promising intervention components to improve HIV viral suppression, which merit further study. Future studies of ART initiation and HIV care engagement can incorporate objective biomarker measures of efficacy, including hair samples.
Acknowledgments
We wish to thank the men and women who participated in the study, the Peter Krueger Center for Immunological Disorders at Mount Sinai Beth Israel, Spencer Cox Center for Health at Mount Sinai St. Luke’s-Roosevelt Hospital Center, and Gay Men’s Health Crisis for making this study possible. Special thanks to Rob Shiau at the Peter Krueger Center and Zach Hennessey, MA at Spencer Cox for facilitating study implementation. This work would not have been possible without Lisa Sanfilippo, RN; Andrea Wagner, RN; Christopher Hilliard, MPH; Amy Braksmajer, Ph.D.; and Victoria Sharp, MD. The study was supported by the National Institutes of Mental Health (R34MH093352) and the Center for Drug Use and HIV Research (CDUHR; P30 DA011041). Dr. Monica Gandhi was supported by NIAID/NIH RO1AI098472 and we thank Drs. Yong Huang and Howard Horng, directors of the hair analysis laboratory at UCSF. We particularly wish to acknowledge our Program Officer at the National Institute of Mental Health (NIMH), Michael Stirratt, Ph.D., Program Chief at the NIMH Division of AIDS Research for scientific guidance throughout the study.
Contributor Information
Marya Gwadz, Email: mg2890@nyu.edu.
Charles M. Cleland, Email: cmc13@nyu.edu.
Elizabeth Applegate, Email: eaa6@nyu.edu.
Mindy Belkin, Email: mjf223@nyu.edu.
Monica Gandhi, Email: monica.gandhi@ucsf.edu.
Nadim Salomon, Email: nsalomon@chpnet.org.
Angela Banfield, Email: adb12@nyu.edu.
Noelle Leonard, Email: nrl4@nyu.edu.
Marion Riedel, Email: mr108@columbia.edu.
Hannah Wolfe, Email: hannahlahozwolfe@gmail.com.
Isaiah Pickens, Email: isaiah.pickens@gmail.com.
Kelly Bolger, Email: bolgerkelly9@gmail.com.
DeShannon Bowens, Email: dkbow@yahoo.com.
David Perlman, Email: perlman@chpnet.org.
Donna Mildvan, Email: dmildvan@chpnet.org.
References
- 1.Granich R, Williams B, Montaner J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Curr Opin HIV AIDS. 2013;8(1):41–9. doi: 10.1097/COH.0b013e32835b80dd. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Liu AY. CROI 2014: new tools to track the epidemic and prevent HIV infections. Top Antivir Med. 2014;22(2):579–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Doshi RK, Malebranche D, Bowleg L, Sangaramoorthy T. Health care and HIV testing experiences among Black men in the South: implications for “Seek, Test, Treat, and Retain” HIV prevention strategies. AIDS Patient Care STDS. 2013;27(2):123–33. doi: 10.1089/apc.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer KH. Introduction: linkage, engagement, and retention in HIV care: essential for optimal individual- and community-level outcomes in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52(Suppl 2):S205–7. doi: 10.1093/cid/ciq043. [DOI] [PubMed] [Google Scholar]
- 6.Montague BT, Vuylsteke B, Buve A. Sustainability of programs to reach high risk and marginalized populations living with HIV in resource limited settings: implications for HIV treatment and prevention. BMC Public Health. 2011;11:701. doi: 10.1186/1471-2458-11-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachega JB, Uthman OA, Del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;59(Suppl 1):S21–7. doi: 10.1093/cid/ciu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: hIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention. [Accessed 1 Dec 2014];HIV in the United States: the stages of care. 2014 Nov; Available at: http://www.cdc.gov/nchhstp/newsroom/docs/HIV-Stages-of-Care-Factsheet-508.pdf.
- 11.Beer L, Fagan JL, Garland P, et al. Medication-related barriers to entering HIV care. AIDS Patient Care STDS. 2012;26(4):214–21. doi: 10.1089/apc.2011.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias CR, Cunningham W, Cabral HD, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21(6):426–34. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 13.Kalichman SC, Graham J, Luke W, Austin J. Perceptions of health care among persons living with HIV/AIDS who are not receiving antiretroviral medications. AIDS Patient Care STDS. 2002;16(5):233–40. doi: 10.1089/10872910252972285. [DOI] [PubMed] [Google Scholar]
- 14.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–44. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 15.Flay BR, Snyder F, Petraitis J. The theory of triadic influence. In: DiClimente RJ, Kegler MC, Crosby RA, editors. Emerging theories in health promotion practice and research. New York: Jossey-Bass; 2009. pp. 451–510. [Google Scholar]
- 16.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227–68. [Google Scholar]
- 17.Vansteenkiste M, Sheldon KM. There’s nothing more practical than a good theory: integrating motivational interviewing and self-determination theory. Br J Clin Psychol. 2006;45(Pt 1):63–82. doi: 10.1348/014466505X34192. [DOI] [PubMed] [Google Scholar]
- 18.van Ryn M. Research on the provider contribution to race/ethnicity disparities in medical care. Med Care. 2002;40(1 Suppl):I140–51. doi: 10.1097/00005650-200201001-00015. [DOI] [PubMed] [Google Scholar]
- 19.Whetten K, Leserman J, Whetten R, et al. Exploring lack of trust in care providers and the government as a barrier to health service use. Am J Public Health. 2006;96(4):716–21. doi: 10.2105/AJPH.2005.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogart LM, Thorburn S. Are HIV/AIDS conspiracy beliefs a barrier to HIV prevention among African Americans? J Acquir Immune Defic Syndr. 2005;38(2):213–8. doi: 10.1097/00126334-200502010-00014. [DOI] [PubMed] [Google Scholar]
- 21.Shavers VL, Shavers BS. Racism and health inequity among Americans. J Natl Med Assoc. 2006;98(3):386–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Kreuter MW, Skinner CS, Steger-May K, et al. Responses to behaviorally vs culturally tailored cancer communication among African American women. Am J Health Behav. 2004;28(3):195–207. doi: 10.5993/ajhb.28.3.1. [DOI] [PubMed] [Google Scholar]
- 23.Schneider TR, Salovey P, Apanovitch AM, et al. The effects of message framing and ethnic targeting on mammography use among low-income women. Health Psychol. 2001;20(4):256–66. doi: 10.1037//0278-6133.20.4.256. [DOI] [PubMed] [Google Scholar]
- 24.Barrera M, Jr, Castro FG, Strycker LA, Toobert DJ. Cultural adaptations of behavioral health interventions: a progress report. J Consult Clin Psychol. 2013;81(2):196–205. doi: 10.1037/a0027085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreuter MW, Lukwago SN, Bucholtz DC, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 2003;30(2):133–46. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 26.Fagan JL, Beer L, Garland P, et al. The influence of perceptions of HIV infection, care, and identity on care entry. AIDS Care. 2012;24(6):737–43. doi: 10.1080/09540121.2011.630360. [DOI] [PubMed] [Google Scholar]
- 27.Jenness SM, Myers JE, Neaigus A, Lulek J, Navejas M, Raj-Singh S. Delayed entry into HIV medical care after HIV diagnosis: risk factors and research methods. AIDS Care. 2012;24(10):1240–8. doi: 10.1080/09540121.2012.656569. [DOI] [PubMed] [Google Scholar]
- 28.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS. 2013;27(14):2271–9. doi: 10.1097/QAD.0b013e328362fdde. [DOI] [PubMed] [Google Scholar]
- 29.Kalichman SC, Catz S, Ramachandran B. Barriers to HIV/AIDS treatment and treatment adherence among African-American adults with disadvantaged education. J Natl Med Assoc. 1999;91(8):439–46. [PMC free article] [PubMed] [Google Scholar]
- 30.Tugenberg T, Ware NC, Wyatt MA. Paradoxical effects of clinician emphasis on adherence to combination antiretroviral therapy for HIV/AIDS. AIDS Patient Care STDS. 2006;20(4):269–74. doi: 10.1089/apc.2006.20.269. [DOI] [PubMed] [Google Scholar]
- 31.Bogart LM, Wagner G, Galvan FH, Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Syndr. 2010;53(5):648–55. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandelowski M, Voils CI, Chang Y, Lee EJ. A systematic review comparing antiretroviral adherence descriptive and intervention studies conducted in the USA. AIDS Care. 2009;21(8):953–66. doi: 10.1080/09540120802626212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordqvist O, Sodergard B, Tully MP, Sonnerborg A, Lindblad AK. Assessing and achieving readiness to initiate HIV medication. Patient Educ Couns. 2006;62(1):21–30. doi: 10.1016/j.pec.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Alfonso V, Bermbach N, Geller J, Montaner JS. Individual variability in barriers affecting people’s decision to take HAART: a qualitative study identifying barriers to being on HAART. AIDS Patient Care STDS. 2006;20(12):848–57. doi: 10.1089/apc.2006.20.848. [DOI] [PubMed] [Google Scholar]
- 35.Gold RS, Hinchy J, Batrouney CG. The reasoning behind decisions not to take up antiretroviral therapy in Australians infected with HIV. Int J STD AIDS. 2000;11(6):361–70. doi: 10.1258/0956462001916065. [DOI] [PubMed] [Google Scholar]
- 36.Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45(3):334–41. doi: 10.1097/QAI.0b013e31806910e3. [DOI] [PubMed] [Google Scholar]
- 37.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 38.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45(1):127–30. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 39.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008;22(3):233–43. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- 40.Gwadz M, Applegate E, Cleland C, et al. HIV-Infected individuals who delay, decline, or discontinue antiretroviral therapy: comparing clinic- and peer-recruited cohorts. Front Public Health. 2014;2:81. doi: 10.3389/fpubh.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 42.Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Longitudinal relationships between antiretroviral treatment adherence and discrimination due to HIV-serostatus, race, and sexual orientation among African-American men with HIV. Ann Behav Med. 2010;40(2):184–90. doi: 10.1007/s12160-010-9200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollini RA, Blanco E, Crump C, Zuniga ML. A community-based study of barriers to HIV care initiation. AIDS Patient Care STDS. 2011;25(10):601–9. doi: 10.1089/apc.2010.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen-Smith A, McCarty F, Hankerson-Dyson D, Diclemente R. Prevalence and predictors of complementary and alternative medicine use in African-Americans with acquired immune deficiency syndrome. Focus Altern Complement Ther. 2012;17(1):33–42. doi: 10.1111/j.2042-7166.2011.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catz SL, McClure JB, Jones GN, Brantley PJ. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11(3):361–73. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- 46.Waldrop-Valverde D, Guo Y, Ownby RL, Rodriguez A, Jones DL. Risk and protective factors for retention in HIV care. AIDS Behav. 2014;18(8):1483–91. doi: 10.1007/s10461-013-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol. 2013;68(4):225–36. doi: 10.1037/a0032705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao KC, Enriquez M, Gantt TC, et al. Nonengagement in HIV care: a descriptive and qualitative study in hospitalized patients and community-based analysis. J Int Assoc Provid AIDS Care. 2013;12(3):178–84. doi: 10.1177/2325957412472058. [DOI] [PubMed] [Google Scholar]
- 49.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–8. doi: 10.1007/s11606-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS. 2000;14(Suppl 1):S3–10. doi: 10.1097/00002030-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 51.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52(Suppl 2):S238–46. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aidala AA, Lee G, Abramson DM, Messeri P, Siegler A. Housing need, housing assistance, and connection to HIV medical care. AIDS Behav. 2007;11(6 Suppl):101–15. doi: 10.1007/s10461-007-9276-x. [DOI] [PubMed] [Google Scholar]
- 53.Des Jarlais DC. Structural interventions to reduce HIV transmission among injecting drug users. AIDS. 2000;14(Suppl 1):S41–6. doi: 10.1097/00002030-200006001-00006. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham WE, Andersen RM, Katz MH, et al. The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270–81. doi: 10.1097/00005650-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Riley ED, Gandhi M, Hare C, Cohen J, Hwang S. Poverty, unstable housing, and HIV infection among women living in the United States. Curr HIV/AIDS Rep. 2007;4(4):181–6. doi: 10.1007/s11904-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 56.Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14(3):309–18. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 57.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 58.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 59.Miller WR, Rollnick S. Motivational interviewing: helping people change. New York: Guilford Press; 2012. [Google Scholar]
- 60.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–18. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 61.Resnicow K, McMaster F. Motivational Interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act. 2012;9:19. doi: 10.1186/1479-5868-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi: 10.1016/j.pec.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 63.Piantadosi S. Clinical trials: a methodological perspective. New York: Wiley; 2005. [Google Scholar]
- 64.Liu AY, Yang QY, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) Plos One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23(4):471–8. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(21):3401–9. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y, Yang Q, Yoon K, et al. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(6):1923–33. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66(3):311–5. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–75. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Institute on Drug Abuse. [Accessed 3 Feb 2015];Seek, test, treat and retain for vulnerable populations: data harmonization measure (demographics measure) Available at: http://www.drugabuse.gov/sites/default/files/sttrfiles/DemographicsV.pdf.
- 71.Shapiro M, Morton S, McCaffrey D, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281(24):2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 72.Huba GJ, Melchior LA. Module 26B:CES-D8 Form. Culver City: The Measurement Group; 1995. Staff of the Measurement Group, HRSA/ HAB’s SPNS Cooperative Agreement Steering Committee. Available at: www.TheMeasurementGroup.com. [Google Scholar]
- 73.Des Jarlais DC, Friedman SR, Novick DM, et al. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261(7):1008–12. doi: 10.1001/jama.261.7.1008. [DOI] [PubMed] [Google Scholar]
- 74.Huba GJ, Melchior LA Staff of the Measurement Group, HRSA/HAB’s SPNS Cooperative Agreement Steering Committee. Module 11: Client Satisfaction Survey. Culver City: The Measurement Group; 1997. Available at: www.TheMeasurementGroup.com. [Google Scholar]
- 75.Beumer JH, Bosman IJ, Maes R. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55(6):353–7. [PubMed] [Google Scholar]
- 76.Mannheimer SB, Mukherjee R, Hirschhorn LR, et al. The CASE adherence index: a novel method for measuring adherence to antiretroviral therapy. AIDS Care. 2006;18(7):853–61. doi: 10.1080/09540120500465160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–71. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 78.Department of Health and Human Services. [Accessed 21 Sept 2014];Clinical Care Guidelines. Available at: http://aidsinfo.nih.gov/guidelines.
- 79.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 80.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 81.Wagner GJ, Lovely P, Schneider S. Pilot controlled trial of the adherence readiness program: an intervention to assess and sustain HIV antiretroviral adherence readiness. AIDS Behav. 2013;17(9):3059–65. doi: 10.1007/s10461-013-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health. 2011;101(3):531–8. doi: 10.2105/AJPH.2010.197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10(6):515–21. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–52. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- 88.Johnson BT, Michie S, Snyder LB. Effects of behavioral intervention content on HIV prevention outcomes: a meta-review of meta-analyses. J Acquir Immune Defic Syndr. 2014;66(Suppl 3):S259–70. doi: 10.1097/QAI.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27(2):159–69. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 90.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–96. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kemppainen JK, Holzemer WL, Nokes K, et al. Self-care management of anxiety and fear in HIV disease. J Assoc Nurses AIDS Care. 2003;14(2):21–9. doi: 10.1177/1055329002250958. [DOI] [PubMed] [Google Scholar]
- 93.Ortony A, Turner TJ. What’s basic about basic emotions. Psychol Rev. 1990;97(3):315–31. doi: 10.1037/0033-295x.97.3.315. [DOI] [PubMed] [Google Scholar]
- 94.Öhman A. Fear and anxiety: evolutionary, cognitive, and clinical perspectives. In: Haviland-Jones MLJM, editor. Handbook of emotions. New York: The Guilford Press; 2000. pp. 573–93. [Google Scholar]
- 95.Gray JA. The neuropsychology of anxiety. Oxford: Oxford University Press; 1982. [Google Scholar]
- 96.Turner MM. Using emotional appeals in health messages. In: Cho H, editor. Health communication message design. Thousand Oaks: Sage Publications, Inc; 2012. pp. 59–73. [Google Scholar]
- 97.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 98.Deci EL, Ryan RM. Self-determination theory: a macrotheory of human motivation, development, and health. Can Psychol. 2008;49(3):182. [Google Scholar]
- 99.Vansteenkiste M, Williams GC, Resnicow K. Toward systematic integration between self-determination theory and motivational interviewing as examples of top-down and bottom-up intervention development: autonomy or volition as a fundamental theoretical principle. Int J Behav Nutr Phys Act. 2012;9:23. doi: 10.1186/1479-5868-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Department of Health and Human Services. [Accessed 25 Sept 2014];Guidelines for the use of antiretroviral agents in HIV-infected adolescents and adults. 2014 May; Available at: http://aidsinfo.nih.gov/contentfiles/adultandadolescentgl.pdf.
- 101.New York City Department of Health and Mental Hygiene. [Accessed 1 Oct 2014];Antiretroviral Therapy. Available at: http://www.nyc.gov/html/doh/html/living/nyc-hivart.shtml.
- 102.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 103.Liau A, Crepaz N, Lyles CM, et al. Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: a qualitative systematic review, 1996–2011. AIDS Behav. 2013;17(6):1941–62. doi: 10.1007/s10461-013-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JJ, Maulsby C, Kinsky S, et al. The development and implementation of the national evaluation strategy of access to care, a multi-site linkage to care initiative in the United States. AIDS Educ Prev. 2014;26(5):429–44. doi: 10.1521/aeap.2014.26.5.429. [DOI] [PubMed] [Google Scholar]
- 105.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Mak. 1996;16(1):58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 106.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 107.Wills CE, Holmes-Rovner M. Integrating decision making and mental health interventions research: research directions. Clin Psychol. 2006;13(1):9–25. doi: 10.1111/j.1468-2850.2006.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shechter SM, Bailey MD, Schaefer AJ, Roberts MS. The optimal time to initiate HIV therapy under ordered health states. Oper Res. 2008;56(1):20–33. [Google Scholar]
- 109.Centers for Disease Control and Prevention. [Accessed 10 Oct 2014];HIV in the United States: At A Glance. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html.
- 110.Mohareb AM, Rothman RE, Hsieh YH. Emergency department (ED) utilization by HIV-infected ED patients in the United States in 2009 and 2010: a national estimation. HIV Med. 2013;14(10):605–13. doi: 10.1111/hiv.12052. [DOI] [PubMed] [Google Scholar]
- 111.Parashar S, Chan K, Milan D, et al. The impact of unstable housing on emergency department use in a cohort of HIV-positive people in a Canadian setting. AIDS Care. 2014;26(1):53–64. doi: 10.1080/09540121.2013.793281. [DOI] [PMC free article] [PubMed] [Google Scholar]